The feasibility and toxicity of allogeneic stem cell transplantation after nonmyeloablative conditioning including thiotepa, fludarabine, and cyclophosphamide have been investigated in 6 patients with breast cancer and 7 patients with renal cell cancer. The program included the use of escalating doses of donor lymphocyte infusions (DLI) and/or interferon alpha (IFNα) for patients showing no tumor response and no graft-versus-host disease (GVHD). Patients were at high risk of transplant-related mortality (TRM) because of age, advanced stage, and previous treatments. We observed a partial remission in 4 renal cancer and in 2 breast cancer patients (one at the molecular level in the bone marrow), occurring after cyclosporine withdrawal or after DLI and/or IFNα. All the responses were accompanied by the occurrence of acute GVHD. We conclude that reduced-intensity allogeneic stem cell transplantation is a feasible procedure in renal and breast cancer, and that the exploitation of graft-versus-tumor effect after DLI is a promising finding.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) with myeloablative conditioning has been used in few patients with metastatic solid tumors: a graft-versus-tumor (GVT) effect has been postulated for breast cancer (BC)1,2and ovarian cancer.3 Very recently, a nonmyeloablative regimen was employed by Childs et al4 in malignant melanoma and renal cell cancer (RCC). The rationale was based on the concept that HSCT can be considered a form of adoptive immunotherapy in chemoresistant tumors. The results in RCC were encouraging, showing a disease regression in 53% of the patients.4 It is noteworthy that the response was associated with graft-versus-host disease (GVHD) occurrence and full donor T-cell engraftment. It has been shown that nonmyeloablative regimens have a lower treatment-related mortality (TRM),5 and thus can be used also in old and heavily pretreated patients. Donor lymphocyte infusions (DLI) are a well-estabilished treatment for patients with hematologic malignancies relapsing after allogeneic transplantation,6 7 but their potential role in programs of allogeneic transplantation for advanced solid tumors is currently unknown.
We have recently shown that a reduced-intensity conditioning with thiotepa, fludarabine, and cyclophosphamide can provide a successful engraftment with a rather low TRM.8 Here, we report the preliminary results of a pilot study exploring the role of nonmyeloablative conditioning followed by allogeneic HSCT in metastatic BC and RCC. To further enhance the GVT effect, we planned escalating doses of DLI for patients not responding, without any sign of acute GVHD.
Study design
There were 6 patients with advanced BC and 7 patients with RCC, ages 18 to 60 years, with an HLA-identical sibling donor, enrolled in the study from December 1998 to May 2001 (Table1). Patients had metastatic tumors refractory to standard treatment, evaluable disease, and a life expectancy of more than 6 months. Patients with BC had received a median number of 3 previous chemotherapy lines, including autologous transplantation, and 3 patients had bone marrow involvement at trephine biopsy. All RCC patients had undergone nephrectomy and had previously been treated with cytokine-based therapy.
Patient characteristics and outcome of treatment
| Patient no. . | Age, y/ sex . | Diagnosis/ stage/histo . | Site(s) of disease . | Status before Tx . | PD after Tx (day) . | Days to DLI . | DLI dose (CD3+ cells/kg) . | IFNα . | aGVHD grade . | cGVHD . | Response . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40/F | BC/IIB | lung, pleura, skin | PD | + 232 | + 258 | 0.9 × 107/kg | – | I (+ 14) | – | – | 1003+ SD |
| 2 | 52/F | BC/IIA | liver, lung, pleura, bone, BM | PD | + 70 | + 148 | 1 × 107/kg | – | – | – | – | 464 death, PD |
| 3 | 46/F | BC/IIIB | nodes, skin | PD | + 60 | + 111 | 2 × 107/kg | – | II (+ 122) | extensive | PR (+ 225) | 490 death, PR GVHD |
| 4 | 40/F | BC/IIB | bone, BM | SD | + 120 | + 132 | 1 × 107/kg | – | – | – | – | 437+ PD |
| + 171 | 5 × 107/kg | |||||||||||
| + 205 | 1 × 108/kg | |||||||||||
| 5 | 36/F | BC/IIA | lung, bone, nodes | SD | + 41 | – | – | – | – | – | – | 109 death, PD |
| 6 | 44/F | BC/IIA | bone, BM, pleura | PD | + 69 | + 131 | 2 × 107/kg | – | II (+ 206) | extensive | PR (+ 227) | 417+ PR |
| + 164 | 1 × 108/kg | yes | ||||||||||
| 7 | 60/F | RCC/III clear cell | lung, CNS | PD | + 70 | + 367 | 1.2 × 107/kg | – | II (+ 102 and + 559) | extensive | PR (+ 117) | 820 death, PD |
| + 425 | 5 × 107/kg | – | ||||||||||
| + 474 | 1 × 108/kg | yes | ||||||||||
| 8 | 54/M | RCC/III clear cell | lung | PD | + 61 | – | – | – | II (+ 81) | limited | PR (+ 116) | 498+ PD |
| 9 | 44/M | RCC/IV clear cell | lung | PD | – | – | – | – | III (+ 89) | extensive | PR (+ 104) | 263 death, PR aspergillus |
| 10 | 48/F | RCC/IV clear cell | lung, CNS | PD | – | – | – | – | II (+ 12) | – | SD | 235+ SD |
| 11 | 18/M | RCC/III papillary | lung, bone, nodes, CNS | PD | + 30 | + 130 | 7 × 107/kg | – | – | – | – | 140 death, PD |
| 12 | 59/M | RCC/II clear cell | lung | SD | – | – | – | – | II (+ 130) | limited | PR (+ 189) | 208+ PR |
| 13 | 47/M | RCC/IV clear cell | lung | SD | – | – | – | – | II (+ 107) | extensive | SD | 194+ SD |
| Patient no. . | Age, y/ sex . | Diagnosis/ stage/histo . | Site(s) of disease . | Status before Tx . | PD after Tx (day) . | Days to DLI . | DLI dose (CD3+ cells/kg) . | IFNα . | aGVHD grade . | cGVHD . | Response . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40/F | BC/IIB | lung, pleura, skin | PD | + 232 | + 258 | 0.9 × 107/kg | – | I (+ 14) | – | – | 1003+ SD |
| 2 | 52/F | BC/IIA | liver, lung, pleura, bone, BM | PD | + 70 | + 148 | 1 × 107/kg | – | – | – | – | 464 death, PD |
| 3 | 46/F | BC/IIIB | nodes, skin | PD | + 60 | + 111 | 2 × 107/kg | – | II (+ 122) | extensive | PR (+ 225) | 490 death, PR GVHD |
| 4 | 40/F | BC/IIB | bone, BM | SD | + 120 | + 132 | 1 × 107/kg | – | – | – | – | 437+ PD |
| + 171 | 5 × 107/kg | |||||||||||
| + 205 | 1 × 108/kg | |||||||||||
| 5 | 36/F | BC/IIA | lung, bone, nodes | SD | + 41 | – | – | – | – | – | – | 109 death, PD |
| 6 | 44/F | BC/IIA | bone, BM, pleura | PD | + 69 | + 131 | 2 × 107/kg | – | II (+ 206) | extensive | PR (+ 227) | 417+ PR |
| + 164 | 1 × 108/kg | yes | ||||||||||
| 7 | 60/F | RCC/III clear cell | lung, CNS | PD | + 70 | + 367 | 1.2 × 107/kg | – | II (+ 102 and + 559) | extensive | PR (+ 117) | 820 death, PD |
| + 425 | 5 × 107/kg | – | ||||||||||
| + 474 | 1 × 108/kg | yes | ||||||||||
| 8 | 54/M | RCC/III clear cell | lung | PD | + 61 | – | – | – | II (+ 81) | limited | PR (+ 116) | 498+ PD |
| 9 | 44/M | RCC/IV clear cell | lung | PD | – | – | – | – | III (+ 89) | extensive | PR (+ 104) | 263 death, PR aspergillus |
| 10 | 48/F | RCC/IV clear cell | lung, CNS | PD | – | – | – | – | II (+ 12) | – | SD | 235+ SD |
| 11 | 18/M | RCC/III papillary | lung, bone, nodes, CNS | PD | + 30 | + 130 | 7 × 107/kg | – | – | – | – | 140 death, PD |
| 12 | 59/M | RCC/II clear cell | lung | SD | – | – | – | – | II (+ 130) | limited | PR (+ 189) | 208+ PR |
| 13 | 47/M | RCC/IV clear cell | lung | SD | – | – | – | – | II (+ 107) | extensive | SD | 194+ SD |
Tx indicates transplantation; histo, histology; PD, progressive disease; SD, stable disease; BM, bone marrow; PR, partial remission; DLI, donor lymphocyte infusion; IFNα, interferon alpha; aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; BC, breast cancer; RCC, renal cell cancer; CNS, central nervous system.
The preparative regimen consisted of 10 mg/kg thiotepa on day −6 (5 mg/kg was used for RCC); 30 mg/kg cyclophosphamide followed by 30 mg/msq fludarabine on days −4 and −3. Patients received lenograstim-mobilized peripheral blood stem cells from their sibling donor on day 0 (median dose, 5.33 × 106CD34+ cells/kg of recipient's body weight; range, 1.89-7.22).
Cyclosporine A (CSA; target blood levels, 150 ng/mL-300 ng/mL) and short-course methotrexate (MTX; 10 mg/msq day 1; 8 mg/msq days 3 and 6) were used for acute GVHD prophylaxis. CSA was administered at full dose through day +70 and, if GVHD was absent, the dose was tapered by 20% every week. Donor-recipient chimerism analysis was carried out on bone marrow mononuclear cells and on CD3+ and CD13/CD33+ cell subsets obtained by cell sorting from peripheral blood mononuclear cells using polymerase chain reaction (PCR) of informative minisatellite regions.9 In patients with mixed donor/recipient T-lymphocyte chimerism or progressive disease on day +60, CSA was tapered off over a 2-week period. Patients who had stable or progressive disease after the withdrawal of CSA and had no evidence of GVHD were eligible to receive up to 3 infusions of DLI, given monthly in escalating doses (1-2 × 107, 5 × 107, and 1 × 108 CD3+ cells/kg recipient's weight). Patients who had no response to DLI and no GVHD were eligible to receive low-dose subcutaneous interferon alpha (IFNα; 3 × 106 IU × 3 per week).
All patients were evaluated by bone marrow biopsy, total-body computed tomographic (CT) scan, and other examinations depending on particular sites of disease, before transplantation and monthly thereafter. Reverse transcriptase (RT)–PCR analysis of maspin and mammaglobin expression was carried out on bone marrow cells, as originally described.10 11
Results and discussion
All patients had a sustained myeloid and platelet engraftment (neutrophils ≥ 500/mcL median day 12, range 10-14; platelets ≥ 20 000/mcL median day 12, range 8-16 days). On day ±60, bone marrow chimerism was more than or equal to 80% donor in 12 of 13 patients; peripheral blood (PB) myeloid engraftment was more than or equal to 80% in 10 of 11 patients, and PB lymphoid chimerism was more than or equal to 80% in 9 of 11 evaluable patients.
No early TRM was observed during the first 100 days; one patient died of late TRM for lung and brain aspergillus infection. Acute GVHD grades II-III developed in 8 patients (3 after DLI) a median of 95 days after transplantation (range, 12-208 days). Of these 8 patients, 7 progressed to chronic GVHD: 2 limited and 5 extensive forms. The median follow-up was 417 days (range, 194-1003 days) with an overall response rate of 46% (6 partial remission according to RECIST criteria).12 No complete responses were observed; 4 patients responded following CSA withdrawal. DLIs were given to 7 patients progressing after allografting, and 3 achieved a partial remission. All responses occurred after the development of acute GVHD, and with full donor T-cell chimerism.
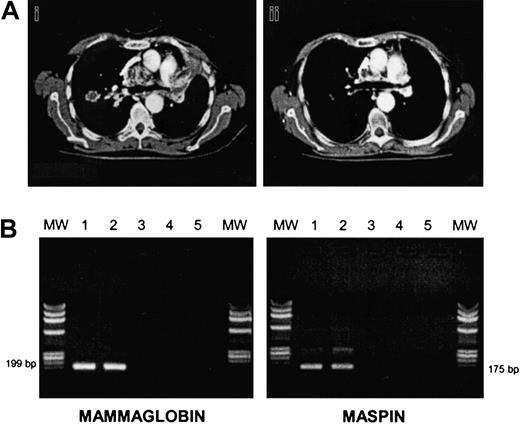
There were 3 of 7 RCC patients who progressed soon after the transplantation (median time, 61 days). There were 4 patients who responded after CSA withdrawal; patient no. 7 relapsed but eventually responded again to DLIs plus IFNα (Figure1A). The other 2 RCC patients had stable disease, and patient no. 11 (papillary RCC) died of progressive disease 10 days after the first DLI. In all BC patients, disease progressed at a median of 69 days after the transplantation (range, 41-232 days). In order to induce a GVT effect, all patients (except patient no. 5 who died of progressive disease at day +109) received escalating doses of DLIs, at a median of 51 days after disease progression (range, 12-78). We observed 2 partial responses (patients no. 3 and no. 6): one after a single-dose DLI, the other after 2 infusions followed by IFNα. Patient no. 3 had an extensive skin and lymph node involvement before allografting that partially regressed along with a grade II acute GVHD developing after DLI. Patient no. 6 was unique since she had bone marrow involved by disease before and after allografting, and bone lytic metastases. BC cells were still present in the marrow before DLI, as assessed by morphology, cytokeratine immunohistochemistry, and by RT-PCR for maspin and mammaglobin genes. After DLI plus IFNα she developed a grade II acute GVHD, and then marrow samples became PCR-negative for maspin and mammaglobin gene expression (Figure 1B). We and others have previously shown that RT-PCR for maspin and mammaglobin is a sensitive and specific assay for detecting occult BC cells.13 This finding suggests a GVT effect at the marrow level.
Responses to allografting in renal cell cancer and breast cancer.
(A) CT thorax scan of patient no. 7 before (i) and after (ii) DLI plus IFNα. (B) RT-PCR analysis of mammaglobin and maspin expression in bone marrow cells of patient no. 6 before and after DLI. Bone marrow mononuclear cells were analyzed for mammaglobin and maspin expression preallograft (lane 1), before DLI (lane 2), and after DLI (lane 3), respectively. Lane 4: negative control; lane 5: no DNA. MW indicates molecular weight marker.
Responses to allografting in renal cell cancer and breast cancer.
(A) CT thorax scan of patient no. 7 before (i) and after (ii) DLI plus IFNα. (B) RT-PCR analysis of mammaglobin and maspin expression in bone marrow cells of patient no. 6 before and after DLI. Bone marrow mononuclear cells were analyzed for mammaglobin and maspin expression preallograft (lane 1), before DLI (lane 2), and after DLI (lane 3), respectively. Lane 4: negative control; lane 5: no DNA. MW indicates molecular weight marker.
Our data confirm the existence of a graft-versus-RCC effect demonstrated by Childs et al.4 There were 4 of 6 patients with renal clear cell carcinoma and a sufficient follow-up who achieved a PR after withdrawal of CSA or after DLI plus IFNα. Clinical evidence of graft-versus-BC effect has been reported in a limited number of patients (2/10) by Ueno et al,2 and in one anecdotal case by Eibl et al.1 However, the study by Ueno et al was different from ours in that it included patients without progressive disease, adopted a myeloablative conditioning regimen with demonstrated antitumor activity, and performed DLI in only one case without response. The dose of T cells to induce a clinical response may vary in different malignancies: Lokhorst et al14 have shown that effective DLI doses for relapsed multiple myeloma are higher than those used for chronic myeloid leukemia. Whether this principle applies to solid tumors, and in particular to BC, is still uncertain. Further, the utilization of specific T-cell subsets, the optimal time interval from allograft to DLI, and the schedule of IFNα administration to enhance a GVT effect remain to be determined.
Supported in part by CNR grant no. 00.00026.ST97 to M.B.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marco Bregni, Bone Marrow Transplant Unit and Gene Therapy Program, Istituto H San Raffaele, Via Olgettina 60, 20132 Milano, Italy; e-mail: marco.bregni@hsr.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal