Follicular lymphoma (FL) grades 1 and 2 are regarded as a distinct disease entity, whereas data suggest that FL grade 3 might be an inhomogeneous tumor category. To define the biologic spectrum of FL, 89 follicular lymphomas were studied for their cytologic composition, antigen expression, mitotic and proliferation indices, cytogenetics, and clinical data. In contrast to the homogeneous appearance of FL grades 1 and 2 (29 and 33 cases, respectively), 2 types of FL grade 3 were recognized. Eleven cases of FL 3a displayed structural features similar to those of FL 1 and 2 and were composed of centroblasts and centrocytes, whereas 16 cases of FL 3b, with (n = 4) or without (n = 12) a diffuse large B-cell lymphoma component (DLBL) (FL 3b ± DLBL), consisted exclusively of blasts. In contrast to FL 3a, FL 3b ± DLBL were CD10+ in only 50% of cases and displayed plasmacytoid differentiation in 44% of cases. Although FL3a was t(14;18)+ in 8 of 11 (73%) cases, only 2 of 16 (13%) FL3b ± DLBLs harbored this translocation. In contrast, chromosomal breaks at 3q27 were encountered in 7 of 16 (44%) FL 3b ± DLBL in contrast to only 2 of 11 (18%) FL 3a, and the spectrum of secondary aberrations in FL 3b ± DLBL was similar to that of diffuse large B-cell lymphoma. We conclude, therefore, that FL grade 3 is a heterogeneous disease group and that the distinction proposed in the new World Health Organization classification between FL 3a (with centrocytes) and FL3b (without centrocytes) is of biologic, and possibly clinical, importance.
Introduction
Follicular lymphoma (FL) and diffuse large B-cell lymphoma are the most frequent types of malignant non-Hodgkin lymphoma (NHL) in the Western world. FL comprises lymphoid neoplasias with a predominantly follicular growth pattern in most patients. It is composed of neoplastic B cells, reactive follicle center-specific T cells, and highly specialized antigen-presenting follicular dendritic cells, and it is thought to arise, in 70% to 90% of patients, on the background of the t(14;18)(q32;q21) chromosome translocation imparting the rearrangement and overexpression of the BCL2gene.1 The World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues1 recommends their subclassification/grading into 3 categories according to the number of centroblasts. In the Kiel classification, however, lymphoid tumors with a follicular growth pattern or a follicular component are not regarded as one entity. FL grades 1 and 2 are, in most of the cases, equivalent to the low-grade centroblastic–centrocytic lymphoma, and WHO grade 3 largely corresponds to follicular (high-grade) centroblastic lymphoma.2,3 Although FLs are commonly regarded as indolent lymphomas, the biologic background of grade 3 tumors is poorly understood. Their clinical and prognostic significance and their proper treatment are still a matter of debate.4-6
In the current work, we sought to characterize the (cyto)morphologic spectrum and antigen expression profile of follicular lymphomas and to correlate these features to their mitotic and proliferative indices and to their cytogenetic aberrations. The relative value of these individual items for the definition of biologic entities and their clinical importance were defined. We were able to conclusively demonstrate that follicular lymphomas grade 3 are a morphologically and biologically inhomogeneous disease group and that their separation into FL 3a (with preserved maturation to centrocytes) and FL 3b (exclusively composed of centroblasts) is of biologic, and possibly clinical, significance.
Materials and methods
Specimen selection
Eighty-nine nodal malignant non-Hodgkin lymphomas (NHLs), diagnosed between 1991 and 1998, with exclusively or partially follicular growth patterns were included in the study. Clinical data were available from referring clinicians of 86 patients, 62 of whom were investigated at the time of primary diagnosis. Twenty-four patients had already been treated at the time bioptic material for the study was received (relapses). In all cases, cytogenetic studies yielded an aberrant karyotype. The cases were reviewed on slides stained for hematoxylin and eosin, Giemsa, periodic acid-Schiff, and Gomori silver impregnation. Follicular structures were present in at least 25% of the infiltrated areas (follicular or follicular and diffuse growth pattern, according to the WHO classification1). Immunophenotyping was performed for diagnostic purposes on paraffin sections using B- (CD20, CD23) and T-cell markers (CD3, CD5) or on frozen sections with anti-CD3, CD5, CD22, CD23, and immunoglobulin light chain antibodies.
Grading
Tumors were graded according to the criteria proposed in the new WHO classification of hematopoietic neoplasms. FLs were diagnosed as grade 1 when the number of centroblasts in 10 randomly selected high-power fields (HPFs, ×40) was 50 or less, as grade 2 when it was between 51 and 150, and as grade 3 when it exceeded 150.1Diffuse large B-cell lymphoma component (DLBL) was diagnosed if there were diffuse sheets of blasts in routine stainings without a clear-cut follicular growth pattern, irrespective of the size of the area.
Mitotic index
Mitotic index was assessed in all cases by counting mitoses in 10 randomly selected HPFs (×40) in Giemsa-stained slides.
Proliferation index and immunohistochemistry
Proliferation indices were estimated by 2 observers in areas with the highest reactivity for the MIB1 antibody (DAKO, Glostrup, Denmark), detecting the Ki67 antigen, on paraffin slides in steps of 10%. Expression of p53 was investigated on paraffin sections using the DO-7 antibody (DAKO). To score a case as positive, strong nuclear expression in at least 20% of cells was required. To assess plasmacytoid differentiation (CIg+), intracytoplasmic immunoglobulin was detected with antibodies to κ and λ light chains (DAKO). Unequivocal monotypic cytoplasmic reactivity for one of the markers was required to score a case as CIg+. CD10 and Bcl-2 expressions were evaluated on paraffin and frozen sections using antibodies to Bcl-2 (DAKO) and CD10 (VILA1; Immunotech, Marseille, France) (NCL-CD10 270; Novocastra, Newcastle, England). If reactions on paraffin sections were negative for either antibody, staining was repeated on frozen sections. All immunohistochemical reactions on paraffin were performed using the peroxidase–anti-peroxidase method after antigen retrieval.7
Cytogenetic studies
Classic cytogenetic studies were carried out according to established protocols. Briefly, unstimulated 10-mL cell cultures were set up in RPMI 1640 medium containing 1 to 2 × 106 cells per milliliter culture medium and were allowed to grow overnight. Metaphase preparation involved a 30-minute exposure to colchicine, hypotonic shock in 0.075 M KCl, and fixation in methanol–acetic glacial acid 3:1. Metaphases were stained using a trypsin–Giemsa standard technique, and results were evaluated according to the International System of Cytogenetic Nomenclature.8
The number of structural aberrations in a given case was counted as the sum of structural aberrations in the karyotype, implying that translocations between 2 chromosomes were counted as one aberration, as were deletions or additions occurring in one chromosome. Numerical aberrations were counted as one aberration, and supernumerary chromosomes with structural aberrations were counted as 2 aberrations.
Statistical analyses
Frequencies in different parameters were compared and analyzed using χ2 analysis, t test for independent variables, or other appropriate tests, respectively, depending on the nature of the data. Overall survival (OS) was measured from the date of diagnosis to the time of death from any cause or to the last follow-up. Failure-free survival (FFS) was defined as the time from diagnosis to the first occurrence of progression under therapy, relapse after complete or partial remission, death from any cause, or last follow-up. OS and FFS distributions were calculated according to the methods described by Kaplan and Meier.9Time-to-event distributions were compared using the log-rank test. Multivariate analysis to determine independent predictors of survival was carried out using the Cox proportional hazards model for survival analysis.10
Results
Histology
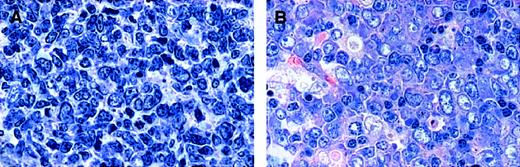
On the basis of the number of blasts in 10 unselected HPFs and in keeping with the recommendations of the new WHO classification,1 29 lymphomas were classified as grade 1 (FL 1), 33 as grade 2 (FL 2), 15 as grade 3 (FL 3), and 12 as grade 3 with DLBL (FL 3 + DLBL). In all cases a follicular growth pattern was observed in at least 25% of the area infiltrated. The cytologic composition of FL 3, however, was heterogeneous. Among FL grade 3, 11 tumors consisting of centroblasts and centrocytes were designated as FL 3a. In these cases, centrocytes were often large, and the distinction between large centrocytes and small centroblasts was not always clear-cut (Figure 1A). Four FL grade 3 tumors were exclusively composed of large and small blasts, without residual centrocytes. These were classified as FL 3b (Figure 1B). All FL 3 tumors with a DLBL component consisted of (large or small) blasts exclusively and were, therefore, designated as FL 3b + DLBL. In these cases, no clear-cut evidence was obtained for follicular colonization or partial follicular involvement of pre-existing germinal centers by DLBL. Based on these findings, the cases were stratified into 2 groups, depending on the presence of centrocytes: group A consisted of FL with preserved maturation to centrocytes (FL 1, 2, and 3a), and group B comprised all cases without centrocytes (FL 3b and FL 3b + DLBL).
Cytomorphologic features of FL 3.
(A) FL 3a. Neoplastic infiltrate is composed of large noncleaved cells (centroblasts) and small to medium-sized, sometimes large, cleaved cells (centrocytes). (B) FL 3b. A population of small and large noncleaved cells (centroblasts) is seen, without an admixture of centrocytes. Original magnification, × 800. Stained with Giemsa.
Cytomorphologic features of FL 3.
(A) FL 3a. Neoplastic infiltrate is composed of large noncleaved cells (centroblasts) and small to medium-sized, sometimes large, cleaved cells (centrocytes). (B) FL 3b. A population of small and large noncleaved cells (centroblasts) is seen, without an admixture of centrocytes. Original magnification, × 800. Stained with Giemsa.
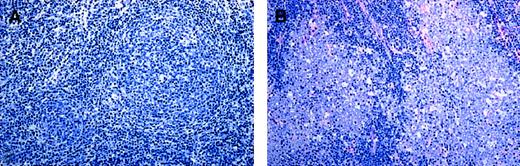
In group A the neoplastic follicles were poorly demarcated and seemed to merge with the interfollicular infiltrate, while follicles in group B lymphomas were often sharply demarcated from the surrounding interfollicular area (Figure 2A-B). Most cases of FL 3a (7 of 11), in spite of their high average content of centroblasts, contained some follicles with fewer than 15 blasts per HPF (usually between 5 and 15).
Structural features of FL 3.
(A) FL 3a. Note that the neoplastic follicular infiltrates are poorly demarcated to the surrounding interfollicular zone and seem to merge with the T-cell area. (B) In contrast, in FL 3b, follicles are sharply demarcated, and there is no merging with the interfollicular infiltrate. Original magnification, × 150. Stained with Giemsa.
Structural features of FL 3.
(A) FL 3a. Note that the neoplastic follicular infiltrates are poorly demarcated to the surrounding interfollicular zone and seem to merge with the T-cell area. (B) In contrast, in FL 3b, follicles are sharply demarcated, and there is no merging with the interfollicular infiltrate. Original magnification, × 150. Stained with Giemsa.
Mitotic and proliferation indices
As shown in Table 1, the mean number of mitotic figures and the mean number of nuclei labeled by the Ki67 antigen in group A correlated significantly with the subgroups as defined by cytologic grade (P < .001). No significant difference was found regarding mitotic or proliferation indices between FL 3a and group B; however, groups A and B were significantly different with respect to these items.
Proliferation and mitotic indices, CD10, Bcl-2, p53 expression and plasmacytoid differentiation in FL
| Morphology . | FL 1 . | FL 2 . | FL 3a . | P FL 1/2 vs FL 3a . | FL 3b . | FL 3b + DLBL . | FL 3b ± DLBL . | P FL 1-3a vs FL 3b ± DLBL . | P FL 3a vs FL 3b ± DLBL . |
|---|---|---|---|---|---|---|---|---|---|
| Proliferation median | 20 | 40 | 70 | < .0001 | 85 | 80 | 80 | < .0001 | < .11 |
| (range) | (10-50) | (20-70) | (40-80) | (40-90) | (50-100) | (40-100) | |||
| Mitoses median | 12 | 34 | 54 | < .0001 | 55 | 63 | 58 | < .0001 | < .14 |
| (range) | (3-48) | (10-80) | (22-80) | (20-146) | (31-124) | (20-146) | |||
| CD 10 (%) | 29/29 | 32/33 | 11/11 | < .67 | 3/4 | 5/12 | 8/16 | < .0001 | < .01 |
| (100) | (97) | (100) | (75) | (42) | (50) | ||||
| bcl-2 (%) | 29/29 | 33/33 | 8/11 | = .0001 | 2/4 | 9/12 | 11/16 | < .001 | < .83 |
| (100) | (100) | (73) | (50) | (75) | (69) | ||||
| p53 (%) | 0/29 | 1/33 | 1/11 | < .17 | 0/4 | 5/12 | 5/16 | = .0001 | < .18 |
| (0) | (3) | (9) | (0) | (42) | (31) | ||||
| CIg (%) | 1/29 | 1/33 | 0/11 | < .55 | 3/4 | 4/12 | 7/16 | = .0001 | < .01 |
| (3) | (3) | (0) | (75) | (33) | (44) |
| Morphology . | FL 1 . | FL 2 . | FL 3a . | P FL 1/2 vs FL 3a . | FL 3b . | FL 3b + DLBL . | FL 3b ± DLBL . | P FL 1-3a vs FL 3b ± DLBL . | P FL 3a vs FL 3b ± DLBL . |
|---|---|---|---|---|---|---|---|---|---|
| Proliferation median | 20 | 40 | 70 | < .0001 | 85 | 80 | 80 | < .0001 | < .11 |
| (range) | (10-50) | (20-70) | (40-80) | (40-90) | (50-100) | (40-100) | |||
| Mitoses median | 12 | 34 | 54 | < .0001 | 55 | 63 | 58 | < .0001 | < .14 |
| (range) | (3-48) | (10-80) | (22-80) | (20-146) | (31-124) | (20-146) | |||
| CD 10 (%) | 29/29 | 32/33 | 11/11 | < .67 | 3/4 | 5/12 | 8/16 | < .0001 | < .01 |
| (100) | (97) | (100) | (75) | (42) | (50) | ||||
| bcl-2 (%) | 29/29 | 33/33 | 8/11 | = .0001 | 2/4 | 9/12 | 11/16 | < .001 | < .83 |
| (100) | (100) | (73) | (50) | (75) | (69) | ||||
| p53 (%) | 0/29 | 1/33 | 1/11 | < .17 | 0/4 | 5/12 | 5/16 | = .0001 | < .18 |
| (0) | (3) | (9) | (0) | (42) | (31) | ||||
| CIg (%) | 1/29 | 1/33 | 0/11 | < .55 | 3/4 | 4/12 | 7/16 | = .0001 | < .01 |
| (3) | (3) | (0) | (75) | (33) | (44) |
Expression of CD10, p53, Bcl-2, and plasmacytoid differentiation
CD10 was expressed in 97% to 100% of cases in group A but only 50% of cases in group B (P < .0001). Two FL A (one FL grade 1 and one FL grade 2) displayed plasmacytoid differentiation, whereas monotypic cytoplasmic immunoglobulin light chain expression was present in 7 of 16 (44%) group B tumors (3 of 4 FL 3b and 4 of 12 FL 3b + DLBL) (P = .0001). P53 was overexpressed in 2 group A cases (one case each of FL grade 2 and grade 3a) but in 5 of 16 (31%) group B cases (all cases were FL 3b with a DLBL component;P = .0001). Bcl-2 was positive in 70 of 73 (96%) FL from group A and in 11 of 16 (69%) FL from group B (P < .001). In group B FL without or with a DLBL component (FL3 or FL 3 + DLBL, respectively), there was no statistically significant difference in any of these features. Among FL 3 + DLBL cases, expression of the immunohistochemical marker profile was concordant in the follicular and diffuse parts. Hence, follicular colonization of reactive germinal centers by divergent CD10 or Bcl-2 expression or by the revelation of particular structures of the meshwork of FDCs, as identified by CD23 staining, was not indicated. Because of the 100% positivity of FL grades 1 and 2 for Bcl-2 expression in the current series, there was also a significant difference between FL 1 and 2 and FL 3a (73% positive). FL 3a and FL 3b ± DLBL were statistically different with respect to the frequency of cases with CD10 expression and plasmacytoid differentiation. Respective values are detailed in Table 1.
Cytogenetic investigations
All cases included in the study displayed clonal chromosome aberrations. The mean number of aberrations was 4.1 in FL 1, 4.3 in FL 2, 6.5 in FL 3a, 8.0 in FL 3b, and 9.3 in FL 3b + DLBL (Table2). Comparing the number of aberrations between groups A and B, the latter harbored significantly more aberrations (both numerical and structural; P < 0.0001). Of interest, the number of structural chromosome alterations was significantly lower in FL 1 and 2 than in FL 3a.
Cytogenetic findings in follicular lymphomas
| Morphology (n > 8 of 89) . | FL 1 . | FL 2 . | FL 3a . | P FL1 and 2 vs FL 3a . | FL 3b . | FL 3b + DLBL . | FL 3b ± DLBL . | P FL 1-3a vs FL 3b ± DLBL . | P FL 3a vs FL 3b ± DLBL . |
|---|---|---|---|---|---|---|---|---|---|
| Total | 4.1 | 4.3 | 6.5 | < .06 (< .67) | 8.0 | 9.3 | 8.9 | < .0001 | < .06 (< .01) |
| Structural | 2.0 | 2.3 | 3.7 | < .01 (< .83) | 5.0 | 4.5 | 4.6 | < .0001 | < .19 (< .001) |
| Numerical | 2.0 | 1.9 | 2.7 | < .35 | 3.0 | 4.8 | 4.3 | < .001 | < .09 |
| t(14;18) (%) | 25/29 | 28/33 | 8/11 | < .30 | 0/4 | 2/12 | 2/16 | < .0001 | < .01 |
| (86) | (85) | (73) | (0) | (17) | (13) | ||||
| BP 3q27 (%) | 1/29 | 1/33 | 2/11 | < .05 (< .11) | 0/4 | 7/12 | 7/16 | < .0001 | < .17 |
| (3) | (3) | (18) | (0) | (58) | (44) | ||||
| + 1q (%) | 2/29 | 5/33 | 0/11 | < .24 | 3/4 | 4/12 | 7/16 | < .001 | < .02 (< .07) |
| (7) | (15) | (0) | (75) | (33) | (44) | ||||
| − 1p (%) | 1/29 | 2/33 | 4/11 | < .01 (< .45) | 1/4 | 2/12 | 3/16 | < .30 | < .31 |
| (3) | (6) | (36) | (25) | (17) | (19) | ||||
| + 5/5q (%) | 2/29 | 3/33 | 2/11 | < .30 (< .02) | 0/4 | 3/12 | 3/16 | < .30 | < .98 |
| (7) | (9) | (18) | (0) | (25) | (19) | ||||
| − 6q (%) | 7/29 | 5/33 | 4/11 | < .21 | 2/4 | 6/12 | 8/16 | < .03 | < .49 |
| (24) | (15) | (36) | (50) | (50) | (50) | ||||
| + 6p (%) | 3/29 | 4/33 | 1/11 | < .83 | 1/4 | 2/12 | 3/16 | < .40 | < .49 |
| (10) | (12) | (9) | (25) | (17) | (19) | ||||
| + 7 (%) | 3/29 | 11/33 | 1/11 | < .31 | 1/4 | 1/12 | 2/16 | < .46 | < .79 |
| (10) | (33) | (9) | (25) | (8) | (13) | ||||
| − 10q (%) | 3/29 | 4/33 | 1/11 | < .83 | 0/4 | 2/12 | 2/16 | < .57 | < .79 |
| (10) | (12) | (9) | (0) | (17) | (13) | ||||
| + 12/12q (%) | 4/29 | 7/33 | 3/11 | < .46 | 1/4 | 1/12 | 2/16 | < .53 | < .34 |
| (14) | (21) | (27) | (25) | (8) | (13) | ||||
| + 17q (%) | 3/29 | 1/33 | 1/11 | < .75 | 1/4 | 3/12 | 4/16 | < .03 (< .23) | < .30 |
| (10) | (3) | (9) | (25) | (25) | (25) | ||||
| − 17p (%) | 0/29 | 2/33 | 2/11 | < .05 (< .27) | 1/4 | 4/12 | 5/16 | < .01 | < .45 |
| (0) | (6) | (18) | (25) | (33) | (31) | ||||
| + 18/der(18) t(14;18)(%) | 5/29 | 4/33 | 2/11 | < .76 | 0/4 | 5/12 | 5/16 | < .13 | < .46 |
| (17) | (12) | (18) | (0) | (42) | (31) | ||||
| + X (%) | 6/29 | 3/33 | 2/11 | < .76 | 0/4 | 4/12 | 4/16 | < .34 | < .68 |
| (21) | (10) | (18) | (0) | (33) | (25) |
| Morphology (n > 8 of 89) . | FL 1 . | FL 2 . | FL 3a . | P FL1 and 2 vs FL 3a . | FL 3b . | FL 3b + DLBL . | FL 3b ± DLBL . | P FL 1-3a vs FL 3b ± DLBL . | P FL 3a vs FL 3b ± DLBL . |
|---|---|---|---|---|---|---|---|---|---|
| Total | 4.1 | 4.3 | 6.5 | < .06 (< .67) | 8.0 | 9.3 | 8.9 | < .0001 | < .06 (< .01) |
| Structural | 2.0 | 2.3 | 3.7 | < .01 (< .83) | 5.0 | 4.5 | 4.6 | < .0001 | < .19 (< .001) |
| Numerical | 2.0 | 1.9 | 2.7 | < .35 | 3.0 | 4.8 | 4.3 | < .001 | < .09 |
| t(14;18) (%) | 25/29 | 28/33 | 8/11 | < .30 | 0/4 | 2/12 | 2/16 | < .0001 | < .01 |
| (86) | (85) | (73) | (0) | (17) | (13) | ||||
| BP 3q27 (%) | 1/29 | 1/33 | 2/11 | < .05 (< .11) | 0/4 | 7/12 | 7/16 | < .0001 | < .17 |
| (3) | (3) | (18) | (0) | (58) | (44) | ||||
| + 1q (%) | 2/29 | 5/33 | 0/11 | < .24 | 3/4 | 4/12 | 7/16 | < .001 | < .02 (< .07) |
| (7) | (15) | (0) | (75) | (33) | (44) | ||||
| − 1p (%) | 1/29 | 2/33 | 4/11 | < .01 (< .45) | 1/4 | 2/12 | 3/16 | < .30 | < .31 |
| (3) | (6) | (36) | (25) | (17) | (19) | ||||
| + 5/5q (%) | 2/29 | 3/33 | 2/11 | < .30 (< .02) | 0/4 | 3/12 | 3/16 | < .30 | < .98 |
| (7) | (9) | (18) | (0) | (25) | (19) | ||||
| − 6q (%) | 7/29 | 5/33 | 4/11 | < .21 | 2/4 | 6/12 | 8/16 | < .03 | < .49 |
| (24) | (15) | (36) | (50) | (50) | (50) | ||||
| + 6p (%) | 3/29 | 4/33 | 1/11 | < .83 | 1/4 | 2/12 | 3/16 | < .40 | < .49 |
| (10) | (12) | (9) | (25) | (17) | (19) | ||||
| + 7 (%) | 3/29 | 11/33 | 1/11 | < .31 | 1/4 | 1/12 | 2/16 | < .46 | < .79 |
| (10) | (33) | (9) | (25) | (8) | (13) | ||||
| − 10q (%) | 3/29 | 4/33 | 1/11 | < .83 | 0/4 | 2/12 | 2/16 | < .57 | < .79 |
| (10) | (12) | (9) | (0) | (17) | (13) | ||||
| + 12/12q (%) | 4/29 | 7/33 | 3/11 | < .46 | 1/4 | 1/12 | 2/16 | < .53 | < .34 |
| (14) | (21) | (27) | (25) | (8) | (13) | ||||
| + 17q (%) | 3/29 | 1/33 | 1/11 | < .75 | 1/4 | 3/12 | 4/16 | < .03 (< .23) | < .30 |
| (10) | (3) | (9) | (25) | (25) | (25) | ||||
| − 17p (%) | 0/29 | 2/33 | 2/11 | < .05 (< .27) | 1/4 | 4/12 | 5/16 | < .01 | < .45 |
| (0) | (6) | (18) | (25) | (33) | (31) | ||||
| + 18/der(18) t(14;18)(%) | 5/29 | 4/33 | 2/11 | < .76 | 0/4 | 5/12 | 5/16 | < .13 | < .46 |
| (17) | (12) | (18) | (0) | (42) | (31) | ||||
| + X (%) | 6/29 | 3/33 | 2/11 | < .76 | 0/4 | 4/12 | 4/16 | < .34 | < .68 |
| (21) | (10) | (18) | (0) | (33) | (25) |
P values refer to all cases analyzed. Pvalues in brackets and italics, referring to untreated cases exclusively, are given only if significance levels changed. Mean values are given for total, structural, and numerical aberrations.
The cytogenetic hallmark of follicular lymphoma, the t(14;18)(q32;q21) chromosome translocation, was detected in 61 of 73 (84%) FL cases in group A but in only 2 of 16 (13%) FL cases in group B (0 of 4 FL 3b and 2 of 12 [17%] FL 3b + DLBL). This difference is highly significant (P < .0001). In 6 lymphomas (4 FL 1 and 2 FL 2), t(14;18) was encountered as the sole aberration.
The only other chromosomal breakpoint recurrently involved in reciprocal balanced aberrations was in band 3q27 (the site of theBCL6 gene), including t(3;14)(q27;q32). Aberrations involving 3q27 were seen in one case in FL 1, one case in FL 2, 2 cases in FL 3a, 0 of 4 cases in FL 3b, and 7 of 12 (58%) cases in FL 3b + DLBL. Again, the difference between FL with preserved maturation to centrocytes (group A, 4 of 73) and FL without a centrocyte component (group B, 7 of 16) is statistically significant (P < .0001). Of note, the presence of a 3q27 marker chromosome was the only genetic feature that was of statistical relevance in the differentiation of FL 3b with (7 of 12, 58%) or without (0 of 4) a DLBL component. The total number of breaks at 14q32 was also different (P < .01) between FL grades 1, 2, and 3a (63 of 73) and FL 3b ± DLBL (9 of 16).
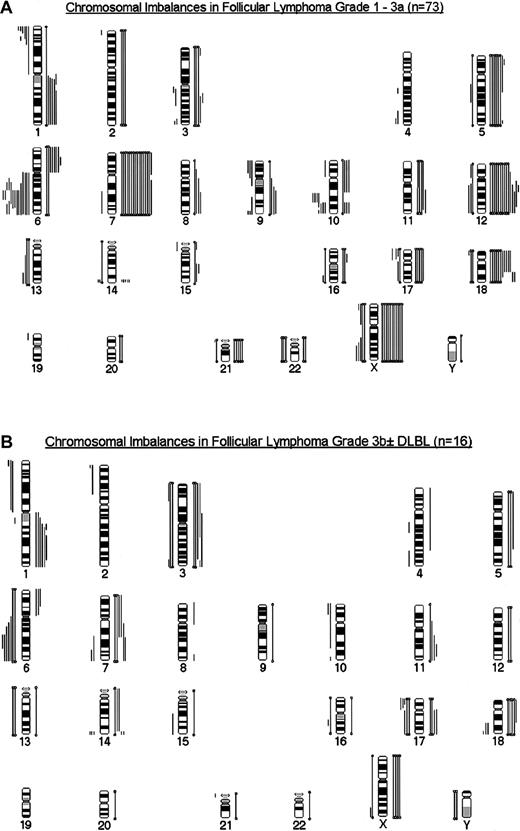
Secondary chromosome aberrations were detected in 80 tumors. Six FLs exclusively harbored t(14;18), and 3 t(14;18)-negative FL group A cases equally displayed only one chromosomal aberration (+X,+6, del(10)(q24)). Several recurring numerical and structural chromosomal aberrations were detected (Figure3A,B). Those leading to genetic imbalances (gains and losses of material) in more than 9 (10%) patients are depicted in Table 2, and their frequencies are given in the different grades. As can be seen from this table, the frequency of most of these aberrations was not significantly different in the various grades of group A cases, especially with regard to the most frequent chromosomal gains, +7, +12/12q, and +X, and to deletions in 6q. For gains of chromosomes 5/5q and 12/12q, a steady increase could be observed with grade in FL 1 to 3a. Structural chromosome aberrations and deletions in the short arms of chromosomes 1 and 17 were significantly rarer in FL 1 and 2 than in FL 3a. When all features were compared for cases without treatment before biopsy, +5/5q was the only marker that differentiated between FL1 and 2 versus FL 3a (Table 2). When cases in FL 1 to 3a were grouped according to proliferation indices equaling 40% and greater than 40%, deletions in 1p were significantly more frequent in the latter group (P < .01). Structural and total chromosome aberrations, deletions in 1p and 17p, and gains of chromosomes 5/5q and 7 were significantly more numerous in the group with mitotic indices greater than 40 per 10 HPF than in cases with a mitotic index equaling 40 per 10 HPF. When FL group A and group B cases were compared, overrepresentation of 1q and 17q and deletions in 6q and 17p were significantly more frequent in FL 3b ± DLBL.
Summary of chromosomal imbalances.
FL grade 3a (A) and FL 3b ± DLBL (B). Lines on the left of the ideogram depict chromosomal losses, whereas lines on the right symbolize chromosomal gains. Closed bars represent monosomies and trisomies.
Summary of chromosomal imbalances.
FL grade 3a (A) and FL 3b ± DLBL (B). Lines on the left of the ideogram depict chromosomal losses, whereas lines on the right symbolize chromosomal gains. Closed bars represent monosomies and trisomies.
Clinical features
For clinical correlation analyses, only data from 62 untreated patients were evaluated. Median follow-up time was 44 months (range, 8-124 months). The only clinical parameter that was different between cases classified as FL 1 or 2 and FL 3a was the number of cases with International Prognostic Index (IPI) scores of 3 (18% and 67%, respectively; P < .02). Bone marrow involvement was more frequent in FL 3a (67%) than in FL 3b ± DLBL (20%;P < .04). By univariate analysis, the only clinical features of predictive value for OS in all patients were age (P = .01) and elevated lactate dehydrogenase (LDH) level (P < .001). For patients in group A, age greater than 60 years and high IPI scores were poor prognostic markers. FFS was predicted by IPI (all cases, P = .01; group A,P < .01) and elevated LDH level (all cases,P < .001; group A, P < .01).
In the biologically homogeneous cases in group A, neither grade nor mitotic (more than 40 mitoses/10 HPF) or proliferation (more than 40%) indices were predictive of OS. Elevated proliferation index greater than 30% was of borderline statistical importance in FFS (FL 1-3a,P = .09; all cases, P = .05).
FL grading did not affect outcome. Distinctions between FL 3a and FL 3b ± DLBL did not predict OS, nor did distinctions between groups A and B. Moreover, there was no significant statistical difference in OS or FFS when FL 1 and 2 were compared with FL 3a and 3b ± DLBL.
Number of structural chromosome abnormalities (3 or more), however, was found to be correlated with prognosis in FL 1 to 3a (P = .05) and in all FL groups (P = .02). Only one single chromosome aberration predicted survival. The presence of a supernumerary chromosome 18 or of a +der(18)t(14;18) was associated with poor prognosis among FL 1 to 3a (P = .03) and in all FL groups (P < .01). The presence or absence of t(14;18), deletions in 1p or 6q, and gains of chromosomes 5/5q, 7, 12 or X, did not influence survival in either group. Parameters statistically associated with adverse outcome in multiparametric regression analyses were elevated LDH level (P < .0001), age greater than 60 years (P < .01), and +18/der(18)t(14;18) (P < .05) in all groups and elevated LDH level (P < .01) and +18/der(18)t(14;18) (P < .01) in FL 1 to 3a.
Discussion
Follicular lymphoma is regarded as a distinct entity by virtue of its characteristic cellular composition of follicle center cells (centroblasts and centrocytes), uniform immunophenotype (CD10+), and common cytogenetic background displaying the t(14;18)(q32;q21) chromosome translocation in most cases.11 In the Kiel classification,2 3however, lymphomas with follicular growth patterns were placed in different categories and were classified as centroblastic–centrocytic or as follicular centroblastic, the latter diagnosis indicating high-grade neoplasia.
Recently, evidence has accumulated that follicular lymphoma grade 3, as defined in the REAL and WHO classifications, differs from FL grades 1 and 2 clinically4,12,13 and biologically. FL grade 3 has been reported to express the Bcl-2 protein in only 50% of patients.14,15 The CD10 membrane glycoprotein is found in 70% to 100% of patients with FL 1 and 2, but in only 20% of patients with FL 3.14 The t(14;18) translocation was detected by banding analyses in 73% to 93% of patients with FL 1 and 2, but it has been less frequently (59%-67%) demonstrated in patients with FL 3.16 17
Reviewing the cases of FL included in this study, we found that FL 3, on morphologic grounds, seemed to be a heterogeneous disease group. Twenty-seven of our 89 cases were classified as FL grade 3 (15 lymphomas) or as FL grade 3 + DLBL (12 cases). In 11 of 15 FL 3, centrocytes still formed part of the neoplastic infiltrate. In contrast, 4 FL grade 3 and all 12 FL 3 + DLBL cases were exclusively composed of (basophilic) blasts, thus fulfilling the criteria for follicular centroblastic lymphoma as defined in the Kiel classification.2 We concluded, therefore, that FL grade 3 might comprise at least 2 diseases. Eleven cases showing preserved maturation to centrocytes seemed more closely related to FL 1 and 2 tumors (FL grade 3a, as described in the new WHO classification1), whereas 16 FL without centrocytes constituted a follicular, or partly follicular, variant of diffuse large B-cell lymphoma (FL 3b ± DLBL).
This subdivision was further substantiated by the different antigenic profile and, in particular, by the different cytogenetic characteristics of FL 3a and FL 3b ± DLBL. FL 3a was CD10+ in all cases, failed to show plasmacytoid differentiation, and harbored only one case with p53 overexpression. In contrast, FL 3b ± DLBL expressed CD10 in only 50%, showed plasmacytoid differentiation in 7 of 16 (44%), and overexpressed p53 in 5 of 16 (31%) of tumors. Bcl-2 expression was decreased in FL 3a and FL 3b ± DLBL (73% and 69%, respectively) in comparison with FL grades 1 and 2 (100%).
The most striking difference encountered between the 2 types of FL grade 3 was the high frequency of t(14;18) in FL 3a (73%); only 2 of 16 (13%) FL 3b tumors (both with a DLBL component) harbored the translocation. Interestingly, all 9 grade 1 and 2 cases negative for t(14;18) were Bcl-2 positive, corroborating previous findings,18 whereas 2 cases of FL 3a negative for the translocation equally failed to express Bcl-2. The possibility of cryptic BCL2 gene rearrangements, however, in FL without cytogenetic t(14;18) was recently pointed out by Vaandrager et al,19 and a similar mechanism for Bcl-2 expression in spite of negativity for t(14;18) cannot be ruled out in our study.
In contrast to the low frequency of the t(14;18) translocation in FL 3b ± DLBL, 7 of 16 (44%) cases harbored breakpoints involving 3q27, the site of the BCL6 gene. This finding, implicating a possible deregulation of BCL6, might not be entirely surprising given the high frequency of 3q27/BCL6 alterations in DLBL20-22 and the importance of BCL6 in germinal center formation and function.23 It is striking that all 3q27-rearranged FL 3b tumors harbored a DLBL component, whereas no tumor with an exclusively follicular growth pattern was 3q27 positive. Based on our data, however, purely follicular FL 3b tumors are rare neoplasms, and answering whether their negativity for 3q27 and 18q21 (BCL6 and BCL2) alterations points to a particular transformation pathway will have to await the analysis of more cases. On the other hand, the high prevalence of 3q27 alterations in FL 3b + DLBL may point to follicular colonization of reactive germinal centers by DLBL. In the 12 cases in our series, however, no clear-cut evidence for that phenomenon was obtained by morphologic or immunohistochemical criteria.
Primary chromosome abnormalities, like the t(14;18) translocation and the 3q27/BCL6 rearrangements, are believed to be strongly associated with distinct tumor entities24; hence, their different frequencies in the current series of 2 types of FL 3 point to a substantially different transformation pathway and, therefore, to separate entities. Secondary chromosome aberrations that occur in addition to the primary cytogenetic alterations are believed to be related to progression, rather than to initiation, of the disease.24 In follicular lymphoma, t(14;18), leading to the overexpression of the BCL2 gene product, seems to rescue the cells from apoptosis,25 thus permitting tumor cells to acquire secondary chromosome alterations because of their prolonged lifespan.26 Therefore, additional chromosome aberrations have been correlated with grade and prognosis in FL,16,17,27-30 reflecting the concept that t(14;18) primarily leads to a highly differentiated (centrocyte-rich) neoplasm. Secondary alterations characterize, then, certain “dedifferentiated” stages, the morphologic equivalents of which are increased blast content and higher mitotic and proliferation indices. The frequency and nature of the secondary chromosome alterations in our FL series (Table 2) are in good agreement with those reported in the literature.24 31-33 Interestingly, in the biologically homogeneous group A cases that display preserved maturation to centrocytes, CD10 reactivity, and high frequency of t(14;18), the distribution of most single aberrations was not associated with grade. The total number of aberrations, and especially of structural aberrations, and additional copies of chromosomes 5 and 12 increased with grade. Only 3 single aberrations were unevenly distributed between FL 1 and 2 and FL 3a—deletions in 1p, deletions in 17p, and breakpoints involving 3q27. All 3 aberrations occurred in cases with low or intermediate blast content as well (Table 2).
Comparing the presence of secondary chromosomal alterations in groups A and B, several were more frequent in the latter group, again indicating a different biology for group B lymphomas. Their nature and frequency in the subgroup of FL without maturation to centrocytes is similar to that described for diffuse large B-cell lymphomas.33-35
The distribution of primary and secondary chromosomal aberrations in FL, therefore, strongly supports our concept that FL 1 to 3a and FL 3b ± DLBL represent different biologic entities and that the latter represent a follicular, or a partly follicular, variant of DLBL and do not belong to the spectrum of follicle center cell lymphomas in the strict sense. Moreover, the negativity of most FL 3b ± DLBL for the t(14;18) chromosome translocation strongly rules out the concept that these tumors represent a transformed stage of follicle center cell lymphomas.
The striking underrepresentation of t(14;18)16,17 and of Bcl-2 and CD10 expression14,15,36,37 in FL 3 documented in the literature, therefore, obviously is not an inherent feature of a particular biologic subgroup of FL. It rather results from the heterogeneity of cases classified as FL 3, comprising true follicle center cell lymphomas (FL 3a) and follicular variants of DLBL. This biologic heterogeneity of FL 3 may also help to explain the inconsistent clinical findings and therapeutic recommendations in the literature regarding the proper treatment of the so-called large-cell variant of FL.4-6,38 The results of many studies indicate, indeed, that FL 3 lymphomas pursue a more aggressive disease course but that they may be cured in some cases with aggressive therapy, similar to the situation in DLBL.6 39
We were also able to confirm the high impact of clinical parameters on prognoses for the biologically homogeneous subgroup of FL 1 to 3a tumors.40 Interestingly, we failed to demonstrate an importance of grading or of increasing proliferation index–mitotic index in assessing OS relative risk, though an elevated proliferation index greater than 30% seemed to adversely influence FFS. The importance of (cyto)genetic parameters in assessing tumor grade or biologic risk in FL has long been debated (reviewed in Knutsen26). In our cases of FL 1 to 3a, only one single abnormality, +18/+der(18)t(14;18), negatively influenced survival. In the series of Tilly et al,30 the occurrence of breaks in 6q, 17p, and the (elevated) total number of breakages were strong negative prognostic indicators. Because fluorescence in situ hybridization has proved to be more sensitive in the detection of certain aberrations than classical cytogenetic analysis,41-43 final assessment of the prognostic impact of single chromosome alterations will require the testing of proposed risk aberrations in large, controlled clinical studies with this technique. Even in the current small series of FL 1 to 3a, however, we arrived at results similar to those of Tilly et al,30namely that the number of chromosomal alterations in a given case may be an important factor in assessing prognosis in FL. This has also been demonstrated in solid tumors, possibly reflecting the grade of karyotypic instability.44
We thank H. Brückner, A. Trumpfheller, M. Reichert, S. Roth, P. Stempfle, and K. Heintz for their expert technical assistance. We also thank E. Schmitt for artful photographic work. We are indebted to our clinical colleagues for providing clinical data, especially to R. Kuse and R. Sonnen (Hamburg) and to U. Gunzer (Würzburg).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
German Ott, Pathologisches Institut der Universität, Josef-Schneider- Strasse 2, 97080 Würzburg, Germany; e-mail:path042@mail.uni-wuerzburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal