Imatinib mesylate (STI571) is a promising new treatment for chronic myelogenous leukemia (CML). The effect of imatinib mesylate on primitive malignant progenitors in CML has not been evaluated, and it is not clear whether suppression of progenitor growth represents inhibition of increased proliferation, induction of apoptosis, or both. We demonstrated here that in vitro exposure to concentrations of imatinib mesylate usually achieved in patients (1-2 μM) for 96 hours inhibited BCR/ABL-positive primitive progenitors (6-week long-term culture–initiating cells [LTCICs]) as well as committed progenitors (colony-forming cells [CFCs]). No suppression of normal LTCICs and significantly less suppression of normal CFCs were observed. A higher concentration of imatinib mesylate (5 μM) did not significantly increase suppression of CML or normal LTCICs but did increase suppression of CML CFCs, and to a lesser extent, normal CFCs. Analysis of cell division using the fluorescent dye carboxyfluorescein diacetate succinimidyl ester indicated that imatinib mesylate (1-2 μM) inhibits cycling of CML primitive (CD34+CD38−) and committed (CD34+CD38+) progenitors to a much greater extent than normal cells. Conversely, treatment with 1 to 2 μM imatinib mesylate did not significantly increase the percentage of cells undergoing apoptosis. Although a higher concentration of imatinib mesylate (5 μM) led to an increase in apoptosis of CML cells, apoptosis also increased in normal samples. In summary, at clinically relevant concentrations, imatinib mesylate selectively suppresses CML primitive progenitors by reversing abnormally increased proliferation but does not significantly increase apoptosis. These results suggest that inhibition of Bcr-Abl tyrosine kinase by imatinib mesylate restores normal hematopoiesis by removing the proliferative advantage of CML progenitors but that elimination of all CML progenitors may not occur.
Introduction
Chronic myelogenous leukemia (CML) is a hematopoietic stem cell malignant disease1 that arises from a reciprocal translocation between chromosomes 9 and 22.2It is characterized by a massive expansion of myeloid progenitors as well as more differentiated cells originating from the malignant clone. The disease progresses from an initial chronic phase through an accelerated phase followed by an inevitable acute leukemia or blast crisis as the malignant cells lose their ability to differentiate. The translocation (9;22)(q34;q11) fuses a truncated BCR gene (chromosome 9) to sequences upstream of the second exon ofABL (chromosome 22). The resulting fusion gene encodes a protein tyrosine kinase, Bcr-Abl, with more elevated and disregulated activity compared with c-abl. Expression of Bcr-Abl in mice was found to induce a CML-like myeloproliferative disorder,3-7indicating that Bcr-Abl plays a key role in leukemic transformation. Additional studies have shown that the tyrosine kinase activity is critical to the transforming ability of Bcr-Abl,7 8 making it an attractive target for drug development.
Imatinib mesylate (STI571; formerly CGP571418B; Gleevec; Novartis Pharmaceuticals, East Hanover, NJ) is a 2-phenylaminopyrimidine derivative developed as a potent inhibitor of the Abl protein tyrosine kinases (v-Abl, Bcr-Abl, and c-Abl).9,10 It also has activity against the platelet-derived growth-factor receptor (PDGF-R),10 c-Kit,11 ARG,12 and their fusion proteins, Tel-Abl and Tel-PDGF-R,13 but does not affect other kinases.
Phase I and phase II clinical trials of imatinib mesylate in the treatment of chronic-phase CML showed that it is well tolerated, with few adverse effects.14 Complete hematologic responses and cytogenetic responses, including complete cytogenetic responses, were reported in a significant proportion of patients. Activity against more advanced accelerated and blast-crisis phases of CML was also observed.15 Imatinib mesylate is currently approved for the treatment of patients with chronic-phase CML in whom interferon therapy has failed and for accelerated-phase and blast-crisis disease. However, the durability of responses to imatinib mesylate, the agent's impact on long-term survival and normal hematopoiesis, and its role in the treatment of CML compared with other modalities remain unclear.
In vitro studies have shown that imatinib mesylate inhibits growth of cell lines expressing Bcr-Abl.10,13,16,17 Additionally, the numbers of colony-forming cells (CFCs) in peripheral blood or bone marrow from patients with CML have been found to be reduced, with minimal inhibition of normal cells.10,17 One study showed that imatinib mesylate inhibited the enhanced replating ability of granulocyte-macrophage colony-forming units (CFU-GMs) from patients with chronic-phase CML.18 However, these studies primarily addressed the effect of imatinib mesylate on mature or committed hematopoietic progenitors; the effects of this agent on the more primitive cells in which the disease arises are not well understood. Short exposure to high concentrations of imatinib mesylate (100 μM for 48 hours or 10 μM for 7 days) was found in one study to suppress growth of long-term culture–initiating cells (LTCICs).19 However, this study did not address whether clinically relevant doses of imatinib mesylate suppress primitive progenitors.
It is important to determine the mechanism by which imatinib mesylate acts to restore normal hematopoiesis in patients with chronic-phase CML, since this may help in predicting the depth of response to imatinib mesylate, aid in designing improved therapeutic interventions, and suggest mechanisms of resistance. The mechanism by which the initial BCR/ABL-positive clone is able to overtake hematopoiesis is thought to be enhanced proliferation.20,21 Inhibition of apoptosis was also reported to play a role in expansion and preservation of the leukemic clone,22,23 but this was not confirmed in other studies24,25 and its role remains unclear. In vitro studies in cell lines as well as primary cells have shown that imatinib mesylate inhibits proliferation.10,13,16,17 Other studies suggested that imatinib mesylate induces apoptosis in Bcr-Abl–dependent cell lines.26 27 However, the effect of exposure to imatinib mesylate on proliferation or apoptosis in primary cells has not been described. Because cell lines are inherently different from primary cells, this issue must be addressed directly in primary cells from samples from patients with CML. It also needs to be clarified whether these effects extend to primitive progenitors or are limited to more committed progenitor cells because of their different growth characteristics. If the primary effect of Bcr-Abl kinase inhibition by imatinib mesylate in primary primitive progenitors is to reduce abnormally increased proliferation, this will imply that imatinib mesylate exposure may not by itself completely eliminate primitive malignant progenitors.
Here, we examined the effect of imatinib mesylate on primitive and committed progenitor cells from patients with CML and healthy donors. Cells were exposed to imatinib mesylate for 1 to 4 days and the effects on LTCICs and CFCs were measured. In addition to examining the effect of imatinib mesylate treatment on CML cells relative the effect in untreated controls, a comparison with normal treated cells addresses the possibility of non–Bcr-Abl–specific effects through inhibition of c-Kit, PDGF-R, or other unidentified targets. To assess how imatinib mesylate restores normal hematopoiesis, we investigated whether a reduction in primitive progenitor growth induced by exposure to the agent reflects inhibition of proliferation, induction of apoptosis, or both.
Patients, materials, and methods
Patients
Heparin-treated bone marrow samples were obtained from 13 patients with CML and 4 healthy donors who were enrolled in this study after informed consent was obtained by using guidelines approved by the Institutional Review Board of the City of Hope National Medical Center. The 9 men and 4 women with CML ranged in age from 18 to 69 years and had either chronic-phase (9 patients) or accelerated-phase (4 patients) disease. Time since diagnosis ranged from 1 to 25 months for patients with chronic-phase disease and 10 months to 6.5 years for patients with accelerated-phase disease. Two patients had received no previous treatment, and 11 had been treated with hydroxyurea. Four patients had also received interferon but had been off treatment for at least 1 month at the time of the study. One patient had received treatment with anagrelide.
Selection of CD34+ progenitors
Bone marrow mononuclear cells (BMMNCs) were isolated by means of Ficoll-Hypaque density-gradient centrifugation (specific gravity, 1.077; Sigma Diagnostics, St Louis, MO) for 30 minutes at 400g. CD34+ cells were selected from BMMNCs by using immunomagnetic column separation (Miltenyi Biotech, Auburn, CA).
Drug treatment of cells
CD34+ cells were cultured in multiwell tissue-culture plates (Falcon) in serum-free medium (SFM; StemPro; Gibco BRL, Gaithersburg, MD) supplemented with growth factors at concentrations similar to that found in serum-conditioned medium (granulocyte-macrophage colony-stimulating factor [GM-CSF], 200 pg/mL; granulocyte colony-stimulating factor [G-CSF], 1 ng/mL; stem cell factor [SCF], 200 pg/mL; leukemia inhibitory factor [LIF], 50 pg/mL; macrophage inflammatory protein α [MIP-1α], 200 pg/mL; and interleukin 6 [IL-6], 1 ng/mL)28-30 and containing 0 to 10 μM imatinib mesylate. Cells were cultured for 24 to 96 hours at 37°C in a humidified atmosphere with 5% carbon dioxide, after which they were harvested, washed with Iscoves modified Dulbecco medium (IMDM; Gibco, Grand Island, NY), and assayed for progenitor numbers, apoptosis, or proliferation.
Progenitor assays
CFCs.
CD34+ cells were plated in semisolid methylcellulose progenitor culture for 14 to 18 days and assessed for the presence of colonies of CFU-GMs and erythroid burst-forming units as described previously.31
Limiting-dilution assay of primitive progenitors (LTCICs).
Limiting-dilution assays of CD34+ cells were performed by plating cells on M2-10B4 feeders in 96-well plates as described previously.32 At the end of the 6-week culture period, 50% of the medium was removed and wells were overlaid with CFC-growth–supporting medium. After 14 days, wells were scored as positive or negative for the presence of CFCs. The frequency of LTCICs was calculated with L-Calc software (StemCell Technologies, Vancouver, BC) on the basis of the reciprocal of the concentration of test cells that yielded 37% negative wells.
Apoptosis assay
Cells treated with imatinib mesylate for 24 to 96 hours were labeled with annexin V conjugated with fluorescein isothiocyanate (FITC; BD-PharMingen, San Diego, CA) and Via-Probe 7-aminoactinomycin D (7-AAD; BD-PharMingen). Briefly, cells were washed once in phosphate-buffered saline and once in 1 × binding buffer (BD-PharMingen), and then 5 μL each of annexin V–FITC and 7-AAD was added to the cells. Cells were incubated at room temperature for 15 minutes, after which 300 μL 1 × binding buffer was added and cells were analyzed by flow cytometry. Apoptotic cells were defined as annexin V–FITC positive and 7-AAD negative.
Proliferation assay
CD34+-selected cells were suspended in 10 mL IMDM, and 5- (and 6-) carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) was added to obtain a concentration of 1.25 μM. Cells were incubated at 37°C in the dark for 10 minutes, with occasional mixing. Further dye uptake was stopped by the addition of 2 mL cold fetal-calf serum followed by centrifugation at 500g for 4 minutes. The cell pellet was resuspended to obtain a concentration of 250 × 103 cells/mL in SFM supplemented with growth factors (GM-CSF, 200 pg/mL; G-CSF, 1 ng/mL; SCF, 200 pg/mL; LIF, 50 pg/mL; MIP-1α, 200 pg/mL; and IL-6, 1 ng/mL); transferred to 6-well tissue-culture plates; and incubated at 37°C overnight to allow excess unbound dye to leak out of the cells. Cells were labeled with CD34-allophycocyanin and CD38-phycoerythrin antibodies (Becton Dickinson, San Jose, CA). CD34+CD38dim and CD34+CD38+ subsets were obtained by fluorescence-activated cell-sorter scanning (FACS). These subsets were further selected for CFSE fluorescence by using a narrow gate (40-channel width on a 1024-channel log amplifier; MoFlo; Cytomation, Fort Collins, CO). Cell subsets selected by FACS were cultured for 96 hours as described above in the presence of 0 to 5 μM imatinib mesylate. At the end of the culture period, proliferation was analyzed by flow cytometry (FACSCalibur; Becton Dickinson). The percentage of cells in each generation was determined by using ModFit software (Verity, Topsham, ME), with the position of the parent generation set on the basis of the fluorescence profile of an aliquot of cells treated with Colcemid (0.1 μg/mL) immediately after sorting.
Fluorescent in situ hybridization analysis
Cells were labeled and analyzed for the presence of theBCR/ABL translocation by using the LSI dual-labeledBCR/ABL DNA probe (Vysis, Downers Grove, IL) as described previously.32
Statistical analysis
Data obtained from multiple experiments were reported as the mean ± 1 SEM. Significance levels were determined by Student t test analysis. Values for the concentration that inhibits 50% (IC50) were determined by fitting a 1-phase exponential decay curve to the CFC and LTCIC data by using GraphPad Prism software (GraphPad, San Diego, CA).
Results
Treatment of CML cells with imatinib mesylate is associated with a dose-dependent decrease in the number of primitive progenitors
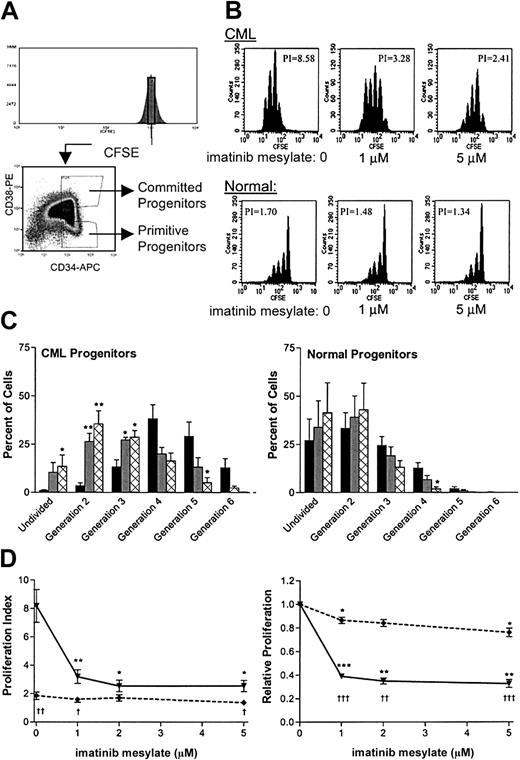
The effect of imatinib mesylate on progenitor growth was evaluated by in vitro exposure of CML CD34+ cells to imatinib mesylate for 24 to 96 hours under physiologic growth-factor conditions followed by enumeration of both primitive (LTCICs) and committed (CFCs) progenitors (Figure 1). Following exposure to imatinib mesylate, cells were washed to remove the drug, and progenitor assays were performed in the absence of further drug exposure. The use of this preincubation procedure allowed us to evaluate the effects of imatinib mesylate directly on progenitor cells in the absence of effects on differentiating cells generated during the progenitor assay and also to later correlate the effects of identical exposures to imatinib mesylate on proliferation and apoptosis. The number of progenitors at the end of the preincubation period would be expected to reflect both increases due to self-renewing cell divisions and decreases associated with differentiation and apoptosis.
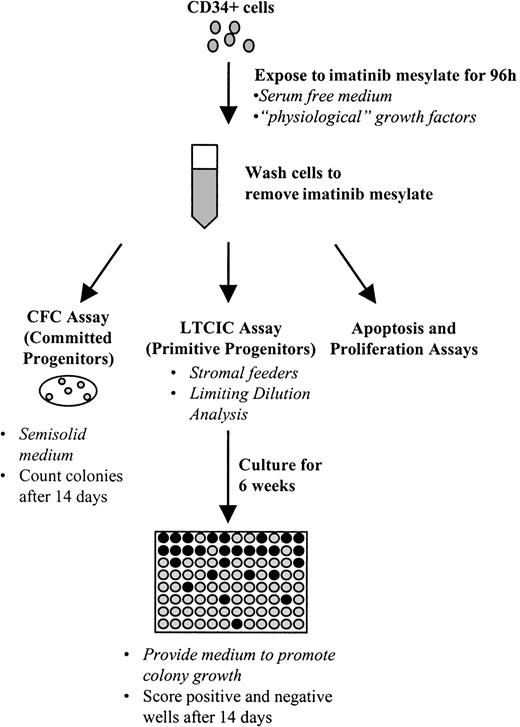
Experimental procedure used to assay the effect of imatinib mesylate on progenitor cells.
The basic experimental approach is outlined here. CD34+cells were exposed to imatinib mesylate for 96 hours in serum-free medium containing growth factors. Cells were then plated in assays for primitive progenitors (LTCICs) or committed progenitors (CFCs), or were analyzed in apoptosis or proliferation assays.
Experimental procedure used to assay the effect of imatinib mesylate on progenitor cells.
The basic experimental approach is outlined here. CD34+cells were exposed to imatinib mesylate for 96 hours in serum-free medium containing growth factors. Cells were then plated in assays for primitive progenitors (LTCICs) or committed progenitors (CFCs), or were analyzed in apoptosis or proliferation assays.
The median frequency of CFCs in the absence of imatinib mesylate was higher for CML CD34+ cells (median, 219/1000 cells) than for normal CD34+ cells (122/1000 cells) after 24 hours of incubation and increased to 288/1000 cells after 96 hours of incubation. Normal CFC numbers did not increase during the same period (going from 122 to 111/1000 cells). In contrast, the frequency of CML LTCICs in the absence of imatinib mesylate decreased during the incubation period from 10.3 to 5.4 CD34+ cells/1000. Normal LTCICs also decreased during the incubation period (from 11.0 to 4.6/10 000).
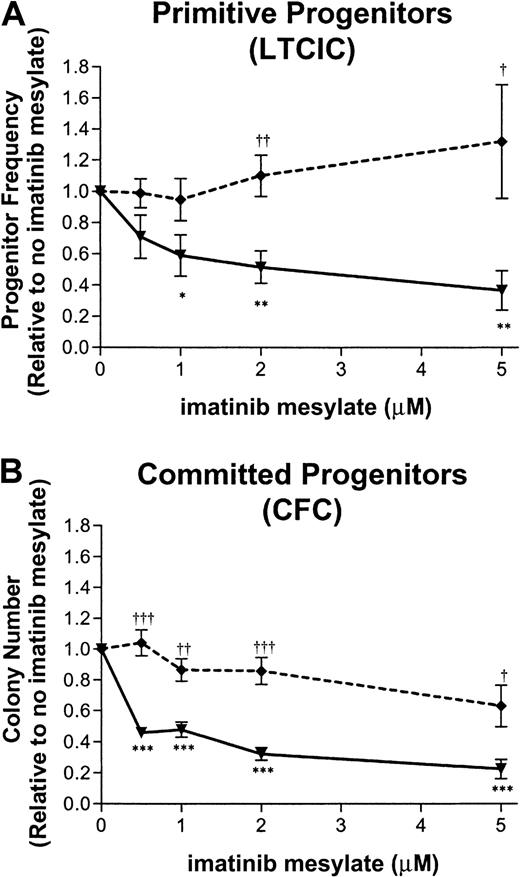
The effect of imatinib mesylate on primitive progenitors is shown in Figure 2A. Exposure to 1 μM imatinib mesylate for 96 hours suppressed CML LTCICs to a level 57% ± 12% (n = 5) of that measured for cells not exposed to the drug. At a higher concentration of imatinib mesylate (5 μM), CML LTCICs showed an increase in suppression (35% ± 12%; n = 5). In contrast, normal LTCICs were not suppressed after exposure to 1 μM (93% ± 13%; n = 4) or 5 μM imatinib mesylate (132% ± 36%; n = 4). For CML LTCICs, the IC50 was 1.7 μM imatinib mesylate.
Inhibition of primitive and committed progenitor growth following exposure to imatinib mesylate.
CD34+ cells from patients with CML or healthy donors were exposed to imatinib mesylate at the concentrations indicated for 96 hours. Subsequently, cells were assayed for primitive (A) and committed (B) progenitors. The mean ± SEM, graphed for CML (—▾—) and normal (- - ♦ - -) samples, is based on replicate experiments (CML, n = 5; normal, n = 4). Concentrations of imatinib mesylate at which CML progenitors were significantly suppressed compared with no exposure to imatinib mesylate are indicated by asterisks below the curve (3 asterisks, P < .001; 2 asterisks, P < .01; and 1 asterisk, P < .05). Concentrations of imatinib mesylate at which CML progenitor frequency was significantly different from normal progenitor frequency are indicated by daggers above the curve (3 daggers, P < .001; 2 daggers,P < .01; and 1 dagger, P < .05). (A) LTCIC frequency was calculated in limiting-dilution assays. The progenitor frequency normalized to the frequency of progenitors in the absence of imatinib mesylate is shown for each concentration of the drug. LTCIC frequency (mean ± SEM) in the absence of imatinib mesylate was 15 ± 10/1000 input CD34+ cells for CML samples and 7 ± 3/1000 cells for normal samples. (B) CFC frequency is plotted for each concentration of imatinib mesylate, normalized to the colony number obtained in the absence of imatinib mesylate. CFC frequency (mean ± SEM) in the absence of imatinib mesylate was 366 ± 87/1000 input CD34+ cells for CML samples and 101 ± 29/1000 cells for normal samples.
Inhibition of primitive and committed progenitor growth following exposure to imatinib mesylate.
CD34+ cells from patients with CML or healthy donors were exposed to imatinib mesylate at the concentrations indicated for 96 hours. Subsequently, cells were assayed for primitive (A) and committed (B) progenitors. The mean ± SEM, graphed for CML (—▾—) and normal (- - ♦ - -) samples, is based on replicate experiments (CML, n = 5; normal, n = 4). Concentrations of imatinib mesylate at which CML progenitors were significantly suppressed compared with no exposure to imatinib mesylate are indicated by asterisks below the curve (3 asterisks, P < .001; 2 asterisks, P < .01; and 1 asterisk, P < .05). Concentrations of imatinib mesylate at which CML progenitor frequency was significantly different from normal progenitor frequency are indicated by daggers above the curve (3 daggers, P < .001; 2 daggers,P < .01; and 1 dagger, P < .05). (A) LTCIC frequency was calculated in limiting-dilution assays. The progenitor frequency normalized to the frequency of progenitors in the absence of imatinib mesylate is shown for each concentration of the drug. LTCIC frequency (mean ± SEM) in the absence of imatinib mesylate was 15 ± 10/1000 input CD34+ cells for CML samples and 7 ± 3/1000 cells for normal samples. (B) CFC frequency is plotted for each concentration of imatinib mesylate, normalized to the colony number obtained in the absence of imatinib mesylate. CFC frequency (mean ± SEM) in the absence of imatinib mesylate was 366 ± 87/1000 input CD34+ cells for CML samples and 101 ± 29/1000 cells for normal samples.
Similarly, the number of committed progenitors (CFCs) decreased in CML cells in a dose-dependent manner following treatment with imatinib mesylate (Figure 2B). Exposure to 1 μM imatinib mesylate for 96 hours suppressed CML CFCs to a level 48% ± 5% (n = 5) of that for untreated cells, whereas normal CFCs showed minor suppression (87% ± 7%; n = 4). CML CFCs were further suppressed when the imatinib mesylate concentration was increased to 5 μM (22% ± 6%; n = 5), resulting in an IC50 of 0.6 μM. However, normal CFCs were also suppressed by 5 μM imatinib mesylate, though to a lesser extent (63% ± 13%; n = 4); the IC50 was 7.6 μM, suggesting that non–Bcr-Abl–dependent mechanisms may contribute to the increased CFC suppression at higher concentrations of imatinib mesylate.
Varying the time that CD34+ cells were exposed to imatinib mesylate (24 to 96 hours) showed that the effect of imatinib mesylate was enhanced by prolonged exposure (Figure3). The addition of imatinib mesylate led to a decrease in CFC frequency for CML CD34+ cells, reversing the increase observed in the absence of imatinib mesylate. At all concentrations analyzed, there was significantly more suppression of CML CFCs after a 96-hour exposure to imatinib mesylate than after a 24-hour exposure (P = .0005 for 1 μM,P = .0003 for 2 μM, and P = 0.0032 for 5 μM).
Inhibition of CML progenitors increases with longer exposure to imatinib mesylate.
CFC frequency for CML CD34+ cells, normalized to frequency for cells not exposed to imatinib mesylate, is plotted as a function of exposure time. Data graphed are for 1 μM (- - ♦ - -]), 2 μM (—● - -), and 5 μM (—▾—) imatinib mesylate and shown as the mean ± SEM for replicate experiments (24 hours, n = 7; and 48-96 hours, n = 5). Progenitor frequency is significantly suppressed as exposure time increased from 24 to 96 hours (3 asterisks, P < .001; 2 asterisks,P < .01).
Inhibition of CML progenitors increases with longer exposure to imatinib mesylate.
CFC frequency for CML CD34+ cells, normalized to frequency for cells not exposed to imatinib mesylate, is plotted as a function of exposure time. Data graphed are for 1 μM (- - ♦ - -]), 2 μM (—● - -), and 5 μM (—▾—) imatinib mesylate and shown as the mean ± SEM for replicate experiments (24 hours, n = 7; and 48-96 hours, n = 5). Progenitor frequency is significantly suppressed as exposure time increased from 24 to 96 hours (3 asterisks, P < .001; 2 asterisks,P < .01).
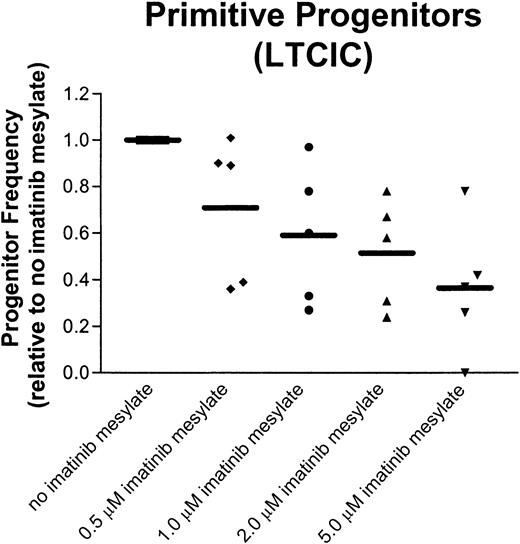
There was considerable variability in CFC and LTCIC inhibition by imatinib mesylate among samples from different patients with CML (Figure 4). Because this variability could reflect intersample differences in the number ofBCR/ABL-negative progenitors, which would not be expected to be suppressed, cells were harvested following colony-formation assays and analyzed for the presence of BCR/ABL by FISH. With the exception of one sample (discussed below), in the absence of imatinib mesylate, LTCICs and CFCs were highly BCR/ABL positive after 96 hours of incubation (42%-89%; mean, 78.4%).
CML primitive progenitors show a broad range of responsiveness to imatinib mesylate.
CML CD34+ cells exposed to imatinib mesylate for 96 hours were assayed for LTCIC frequency by limiting-dilution analysis. The progenitor frequency, normalized to the frequency of progenitors in the absence of imatinib mesylate, is shown for each sample at the indicated concentrations of the drug. The mean is indicated by a solid bar.
CML primitive progenitors show a broad range of responsiveness to imatinib mesylate.
CML CD34+ cells exposed to imatinib mesylate for 96 hours were assayed for LTCIC frequency by limiting-dilution analysis. The progenitor frequency, normalized to the frequency of progenitors in the absence of imatinib mesylate, is shown for each sample at the indicated concentrations of the drug. The mean is indicated by a solid bar.
The percentage of BCR/ABL-positive cells did not decrease significantly on treatment with imatinib mesylate (Figure5). Although there was a suppression ofBCR/ABL-positive progenitor numbers (calculated as the progenitor number times the percentage of cells that wereBCR/ABL positive on FISH analysis), the failure to increase the percentage of BCR/ABL-negative cells might indicate that additional stimuli may be required to allow efficient outgrowth of normal progenitors. The variability among samples did not correlate with differences in the stage of the disease or prior treatment.
Imatinib mesylate suppresses growth of
BCR/ABL-positive LTCICs and CFCs. Total (▪) andBCR/ABL-positive (░) progenitor frequencies (LTCICs and CFCs) for CML CD34+ cells exposed to imatinib mesylate for 96 hours are shown. The frequency of BCR/ABL-positive progenitors equals the total number of progenitors times the percentage of cells that were BCR/ABL positive on FISH analysis of pooled colonies. The data plotted are normalized to the number of total progenitors observed in the absence of imatinib mesylate and are shown as the mean ± SEM.
Imatinib mesylate suppresses growth of
BCR/ABL-positive LTCICs and CFCs. Total (▪) andBCR/ABL-positive (░) progenitor frequencies (LTCICs and CFCs) for CML CD34+ cells exposed to imatinib mesylate for 96 hours are shown. The frequency of BCR/ABL-positive progenitors equals the total number of progenitors times the percentage of cells that were BCR/ABL positive on FISH analysis of pooled colonies. The data plotted are normalized to the number of total progenitors observed in the absence of imatinib mesylate and are shown as the mean ± SEM.
Imatinib mesylate treatment is associated with decreased proliferation of CML primitive and committed progenitors
We next investigated whether inhibition of primitive progenitor growth by imatinib mesylate reflected inhibition of proliferation or induction of apoptosis. Cell division was analyzed by using the fluorescent tracking dye CFSE. Cells labeled with CFSE were sorted to provide a narrow band of CFSE fluorescence to enhance definition of generations and then further subdivided into populations of primitive progenitors (CD34+CD38dim) or committed progenitors (CD34+CD38+; Figure6A). The sorted cells were grown with various concentrations of imatinib mesylate (0 to 5 μM) under physiologic growth-factor conditions for 96 hours and then analyzed for cell division. Representative data are shown in Figure 6B, and the percentages of cells in each generation, as determined with ModFit software, are summarized in Figure 6C. Exposure to imatinib mesylate resulted in an increase in the fraction of undivided cells and a reduction in the number of cell divisions undergone by CML primitive progenitors during 4 days of culture. There was a significant increase in undivided cells with exposure to 5 μM imatinib mesylate (P = .047). In addition, the number of CML primitive progenitor cells undergoing only a single cell division increased significantly in response to imatinib mesylate (P = .002 at 1 μM and P = .003 at 5 μM).
Proliferation of CML primitive progenitors is suppressed in cells exposed to imatinib mesylate.
Cell division in the presence of imatinib mesylate was measured for CML and normal progenitors by analysis using the fluorescent tracking dye CFSE. (A) Immunomagnetically purified CD34+ cells were uniformly labeled with CFSE and sorted for a narrow band (40 channels) of fluorescence (upper panel). The labeled cells were also sorted for CD34 and CD38 expression to yield subsets of primitive (CD34+CD38dim) and committed (CD34+CD38+) progenitors. (B) After a 96-hour exposure to imatinib mesylate (0, 1, or 5 μM), cells were analyzed by FACS for CFSE fluorescence. Representative data are shown for CML and normal primitive progenitors. The calculated PI for each plot is indicated. (C) The percentage of cells in each generation, as determined by computer fitting of the data, is summarized for CML and normal primitive progenitors. Data are shown as the mean ± SEM results from 4 independent experiments (n = 3 for CML progenitors exposed to 5 μM imatinib mesylate). Data shown are for cells not exposed to imatinib mesylate (▪), cells exposed to 1 μM imatinib mesylate (░), and cells exposed to 5 μM imatinib mesylate (⊠). Significant changes in the percentage of cells in any generation in response to imatinib mesylate exposure are indicated (2 asterisks, P < .01; and 1 asterisk,P < 0.05). (D) The PI for CML (—▾—) and normal (- - ♦ - -) samples is shown as a function of the imatinib mesylate concentration. Data shown in the left panel are the mean PI ± SEM values for primitive progenitors (n = 4). The same data after normalization to the PI in the absence of imatinib mesylate are shown in the right panel. Significant changes in the PI in response to imatinib mesylate exposure are indicated (3 asterisks,P < .001; 2 asterisks, P < .01; and 1 asterisk,P < .05). Concentrations of imatinib mesylate at which CML results differed significantly from normal results are indicated with daggers below the curve (3 daggers, P < .001; 2 daggers, P < .01; and 1 dagger,P < 0.05).
Proliferation of CML primitive progenitors is suppressed in cells exposed to imatinib mesylate.
Cell division in the presence of imatinib mesylate was measured for CML and normal progenitors by analysis using the fluorescent tracking dye CFSE. (A) Immunomagnetically purified CD34+ cells were uniformly labeled with CFSE and sorted for a narrow band (40 channels) of fluorescence (upper panel). The labeled cells were also sorted for CD34 and CD38 expression to yield subsets of primitive (CD34+CD38dim) and committed (CD34+CD38+) progenitors. (B) After a 96-hour exposure to imatinib mesylate (0, 1, or 5 μM), cells were analyzed by FACS for CFSE fluorescence. Representative data are shown for CML and normal primitive progenitors. The calculated PI for each plot is indicated. (C) The percentage of cells in each generation, as determined by computer fitting of the data, is summarized for CML and normal primitive progenitors. Data are shown as the mean ± SEM results from 4 independent experiments (n = 3 for CML progenitors exposed to 5 μM imatinib mesylate). Data shown are for cells not exposed to imatinib mesylate (▪), cells exposed to 1 μM imatinib mesylate (░), and cells exposed to 5 μM imatinib mesylate (⊠). Significant changes in the percentage of cells in any generation in response to imatinib mesylate exposure are indicated (2 asterisks, P < .01; and 1 asterisk,P < 0.05). (D) The PI for CML (—▾—) and normal (- - ♦ - -) samples is shown as a function of the imatinib mesylate concentration. Data shown in the left panel are the mean PI ± SEM values for primitive progenitors (n = 4). The same data after normalization to the PI in the absence of imatinib mesylate are shown in the right panel. Significant changes in the PI in response to imatinib mesylate exposure are indicated (3 asterisks,P < .001; 2 asterisks, P < .01; and 1 asterisk,P < .05). Concentrations of imatinib mesylate at which CML results differed significantly from normal results are indicated with daggers below the curve (3 daggers, P < .001; 2 daggers, P < .01; and 1 dagger,P < 0.05).
A proliferation index (PI), which represents the sum of the cells in all generations divided by the computed number of original parent cells theoretically present at the start of the experiment, can be generated by ModFit software. This single index simplifies comparison between samples and conditions. The results indicated that imatinib mesylate inhibits division of CML primitive and committed progenitor cells to a much greater extent than in normal cells. Data on proliferation of primitive progenitors exposed to imatinib mesylate are summarized in Figure 6D. Untreated CML primitive progenitors were significantly more proliferative than untreated normal primitive progenitors (P = .006), reflecting the presence of fewer quiescent cells and an increased number of cell divisions. Exposure to 1 μM imatinib mesylate significantly reduced the PI for CML primitive progenitors (P = .006). At 2 μM imatinib mesylate, the PI of CML primitive progenitors was reduced further, such that they no longer showed significantly enhanced proliferation compared with normal primitive progenitors (P = .227). When the data were normalized by comparison with the frequency of progenitors in samples not exposed to imatinib mesylate, the PI was significantly reduced for CML progenitors at all concentrations of imatinib mesylate assessed (P < .0001 at 1 μM, P < .0015 at 2 μM, and P < .0024 at 5 μM). This inhibition was significantly greater for CML than for normal primitive progenitors (P < .0001 at 1 μM, P < .0011 at 2 μM, and P < .0008 at 5 μM). The normalized PI for committed progenitors also indicated that there was a significant inhibition of proliferation in CML progenitors exposed to imatinib mesylate (P = .0052 at 1 μM, P < .0019 at 2 μM, and P < .0004 at 5 μM). These results suggest that the inhibition of cell division is an important mechanism in the suppression of both primitive and committed CML progenitors, suppressing the proliferative advantage present in these cells.
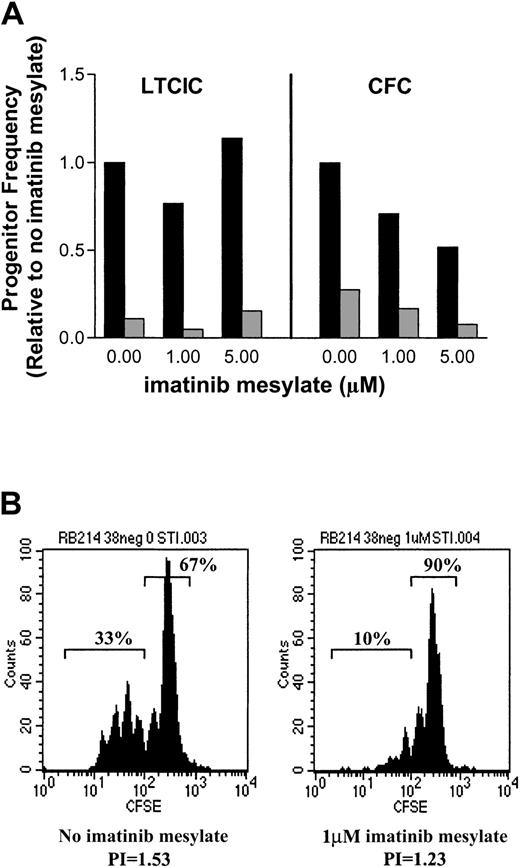
One CML patient sample that showed almost no suppression of LTCICs and only minimal suppression of CFCs (Figure7A) was found on FISH analysis to have a very low percentage of BCR/ABL-positive cells. In this sample, the fraction of BCR/ABL-positive CFCs decreased with exposure to imatinib mesylate. CFCs without such exposure were 27.5%BCR/ABL positive, 23.5% at 1 μM imatinib mesylate and 15% at 5 μM imatinib mesylate. However, this decrease was not observed for LTCICs. The minimal progenitor suppression might represent the presence of a large proportion of nonresponsive normal cells. A mixed cell population was also apparent in the results of the CFSE-labeling experiments (Figure 7B). In the absence of imatinib mesylate, there were 2 distinct populations of cells. A large peak of undivided cells (67% undergoing 0-1 cell division) was readily apparent, as was a second population of highly proliferative cells that had undergone 2 to 5 cell divisions (33%). On exposure to 1 μM imatinib mesylate, proliferation was decreased and the cells were more uniformly quiescent (90% undergoing 0-1 cell division).
A CML sample that showed minimal response to imatinib mesylate was found to have a very high percentage of
BCR/AB-negative cells. (A) The frequency of total (▪) and BCR/ABL-positive (░) LTCIC and CFC progenitors is shown relative to results in cells not exposed to imatinib mesylate. (B) CFSE analysis of primitive progenitors after 96 hours in the absence of imatinib mesylate (left panel) revealed a mixture of proliferative cells (33%) and quiescent cells (67%). Exposure to 1 μM imatinib mesylate increased the percentage of quiescent cells (90%; right panel). The PI is shown below each plot.
A CML sample that showed minimal response to imatinib mesylate was found to have a very high percentage of
BCR/AB-negative cells. (A) The frequency of total (▪) and BCR/ABL-positive (░) LTCIC and CFC progenitors is shown relative to results in cells not exposed to imatinib mesylate. (B) CFSE analysis of primitive progenitors after 96 hours in the absence of imatinib mesylate (left panel) revealed a mixture of proliferative cells (33%) and quiescent cells (67%). Exposure to 1 μM imatinib mesylate increased the percentage of quiescent cells (90%; right panel). The PI is shown below each plot.
Effects of imatinib mesylate treatment on apoptosis of CML progenitors
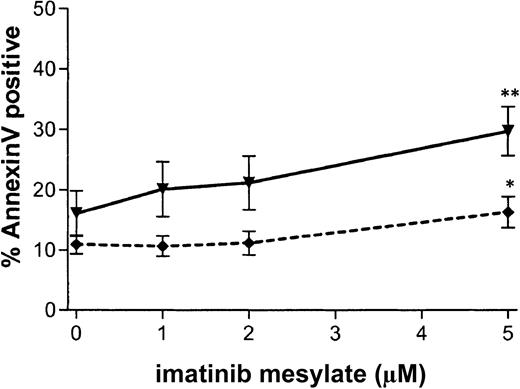
To assess the effect of imatinib mesylate on apoptosis, CD34+ cells were grown with various concentrations of imatinib mesylate (0 to 5 μM) under physiologic growth-factor conditions for 96 hours, labeled with annexin V–FITC and 7-AAD, and then analyzed by flow cytometry for cells positively labeled by annexin V but negative for 7-AAD. Results are summarized in Figure8. Treatment with 1 μM imatinib mesylate did not significantly increase apoptosis in CML CD34+ cells compared with no such treatment (P = .12). When 5 μM imatinib mesylate was used, a significant increase in apoptosis of CML cells was observed (from 16.1% ± 3.8% to 29.8% ± 4.1%; n = 6;P = .007). However, apoptosis was also significantly increased in normal samples at this concentration (from 10.9% ± 1.6% to 16.4% ± 2.6%; n = 3; P = .046). Under no conditions was apoptosis of CML cells significantly greater than that of normal cells, thus suggesting that non–Bcr-Abl–dependent mechanisms may contribute to the increased apoptosis at higher concentrations of imatinib mesylate. For 2 patient samples, there was an increase in apoptosis at 1 μM imatinib mesylate. This may correlate with increased sensitivity to imatinib mesylate, since these samples also showed a high degree of suppression of CFCs and LTCICs with exposure to the agent.
Apoptosis is not specifically induced in CML CD34+ cells in response to imatinib mesylate exposure.
CML and normal CD34+ cells were exposed to imatinib mesylate (0-5 μM) for 96 hours. Apoptosis was analyzed by flow cytometry as the percentage of cells positively labeled by annexin V–FITC and negative for 7-AAD. The percentage of apoptotic cells is plotted as a function of the concentration of imatinib mesylate for CML (—▾—) and normal (- - ♦ - -) samples. Data shown are the mean ± SEM values for replicate experiments (CML, n = 6; and normal, n = 3). Significant increases in the percentage of apoptotic cells on exposure to imatinib mesylate are indicated with asterisks (2 asterisks, P < .01; and 1 asterisk,P < .05).
Apoptosis is not specifically induced in CML CD34+ cells in response to imatinib mesylate exposure.
CML and normal CD34+ cells were exposed to imatinib mesylate (0-5 μM) for 96 hours. Apoptosis was analyzed by flow cytometry as the percentage of cells positively labeled by annexin V–FITC and negative for 7-AAD. The percentage of apoptotic cells is plotted as a function of the concentration of imatinib mesylate for CML (—▾—) and normal (- - ♦ - -) samples. Data shown are the mean ± SEM values for replicate experiments (CML, n = 6; and normal, n = 3). Significant increases in the percentage of apoptotic cells on exposure to imatinib mesylate are indicated with asterisks (2 asterisks, P < .01; and 1 asterisk,P < .05).
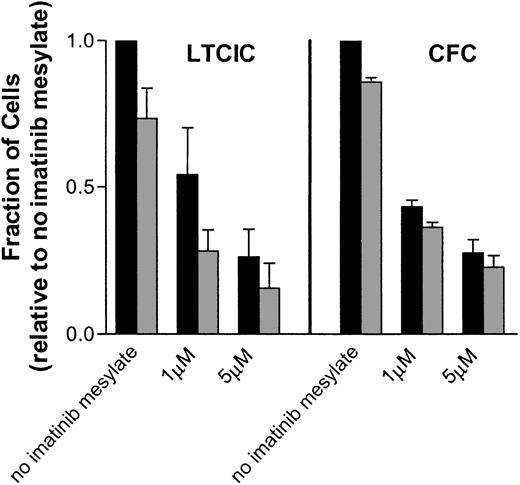
The major mechanism of suppression of CML progenitors by imatinib mesylate is decreased proliferation
The loss of both committed and primitive progenitors (Figure 2) appears to primarily reflect a decrease in proliferation. Table1 shows a summary of the data from each assay for CML cells exposed to 1 μM imatinib mesylate compared with those not exposed to imatinib mesylate. The first row shows the mean percentage decrease in progenitor frequency for primitive progenitors (LTCICs) or committed progenitors (CFCs). The second row indicates the percentage decrease in cell division measured by the proliferation assay based on CFSE labeling. Results for primitive progenitors reflect data from the CD34+CD38dim population, whereas the committed progenitor data are from the CD34+CD38+ population. The percentage decrease in viability (bottom row) is based on data for all CD34+cells and is therefore the same in each column. These data show that the major mechanism associated with the decrease in both primitive and committed progenitors is a decrease in proliferation, not an increase in apoptosis.
Suppression of frequency of chronic myelogenous leukemia progenitors compared with reduction in proliferation and viability
| Assay . | Primitive progenitors (% decrease) . | Committed progenitors (% decrease) . |
|---|---|---|
| Progenitor frequency | 43 ± 12 | 52 ± 5 |
| Proliferation inhibition | 61 ± 2 | 34 ± 5 |
| Viability decrease | 5 ± 3 | 5 ± 3 |
| Assay . | Primitive progenitors (% decrease) . | Committed progenitors (% decrease) . |
|---|---|---|
| Progenitor frequency | 43 ± 12 | 52 ± 5 |
| Proliferation inhibition | 61 ± 2 | 34 ± 5 |
| Viability decrease | 5 ± 3 | 5 ± 3 |
Values are the mean ± SEM percentage decrease in the different variables for progenitor cells exposed to 1 μM imatinib mesylate for 96 hours compared with cells with a similar incubation but without imatinib mesylate.
Discussion
We evaluated the effect of imatinib mesylate on primitive progenitor cells from patients with CML. Previous studies have shown that imatinib mesylate effectively suppressesBCR/ABL-positive committed progenitor cells.10 17 Here, we showed that this effect extends to primitive progenitors as well. Importantly, suppression was shown to occur under physiologic growth-factor conditions and at clinically relevant concentrations of imatinib mesylate. The amount of suppression observed was dose-dependent, with an average IC50 of 0.6 μM for CML CFCs and 1.7 μM for CML LTCICs exposed to the drug for 96 hours. Therefore, LTCICs appear to be somewhat less responsive to imatinib mesylate than are CFCs. Although suppression was observed with an exposure to imatinib mesylate as short as 24 hours, the amount of suppression increased with longer exposure to the drug. Under the most stringent conditions (5 μM imatinib mesylate for 96 hours), CML LTCICs decreased by 63% and CML CFCs by 78%. However, exposure for this length of time did not completely eliminate malignant CFCs or LTCICs. Under these same conditions, progenitors from healthy donors were not suppressed, except at the highest concentrations, and then only at the level of CFCs and not LTCICs.
We analyzed the mechanisms underlying the growth suppression. CFSE assays confirmed that CML primitive and committed progenitors had increased proliferation compared with their normal counterparts, including both increased cell division and a reduced number of quiescent cells, particularly for primitive progenitor cells. Although the increased proliferation was quite apparent in the CFSE assay, it did not translate into an increased frequency of LTCICs. This can be attributed to the observation that CML progenitors have increased differentiation and decreased self-renewal compared with normal progenitors.32-34 Imatinib mesylate suppressed proliferation of primitive and committed CML progenitors. It largely reversed the proliferative advantage of primitive progenitors, decreasing cell divisions and increasing the proportion of undivided cells. Because cell division may be associated with loss of primitive progenitor capacity, the decrease in proliferation by imatinib mesylate may in fact reduce the loss of primitive progenitors through this mechanism and contribute to the trend toward the increased number of normal LTCICs observed following exposure to imatinib mesylate. This could also explain why the suppression of LTCIC frequency was less than the suppression of CD34+CD38− cell proliferation in the CFSE assays.
Although there is evidence from studies of BCR/ABL-positive cell lines that apoptosis is induced by treatment with imatinib mesylate,26 27 we did not consistently observe an increase in apoptosis in primary CD34+ cells at clinically achieved concentrations of the agent. Increased apoptosis was observed in 2 samples that also had the greatest sensitivity to inhibition of proliferation. However, the percentage of apoptotic cells never exceeded 32% in any sample for cells exposed to imatinib mesylate at a concentration below 5 μM, suggesting that increased apoptosis was not the major contributor to suppression of progenitor growth. Although apoptosis was induced when cells were exposed to 5 μM imatinib mesylate, increased apoptosis was also observed in normal samples, though to a lesser extent than in CML samples, suggesting that non–Bcr-Abl–dependent mechanisms may contribute to this process.
Our findings are consistent with a hypothetical model in which Bcr-Abl tyrosine kinase activity results in enhanced proliferation of primitive and committed progenitors derived from the malignant clone, leading to a growth advantage over their normal counterparts, and in which exposure to imatinib mesylate, by reducing the abnormally increased proliferation of BCR/ABL-positive progenitors, removes their growth advantage. The effect of imatinib mesylate on primitive progenitor growth must reflect a balance of its effects on proliferation, survival, and self-renewal. Here, we showed that the predominant effect of imatinib mesylate is to inhibit proliferation. These results are consistent with those of a study by Marley et al18 in which a colony-replating assay was used to show that imatinib mesylate suppresses progenitor cell proliferation. In addition, Marley et al were unable to detect an increase in apoptosis associated with imatinib mesylate treatment.
Although we did not observe a decrease in proliferation to a level below that of normal progenitors under the conditions of our assay, an increase in the time of exposure to imatinib mesylate beyond the 96 hours we examined would be expected to increase suppression of CML progenitors further. Consistent with this idea, we observed increased progenitor suppression after 96 hours of exposure to imatinib mesylate compared with 24 or 48 hours. Another factor leading to preferential loss of CML progenitors may be their decreased self-renewal capacity.32-34 Inhibition of progenitor proliferation in combination with decreased self-renewal may be sufficient to preferentially inhibit growth of malignant compared with normal progenitors during prolonged exposure to imatinib mesylate. This would also be consistent with the observation that more than 3 months of treatment is usually required for cytogenetic responses to imatinib mesylate treatment in patients with CML.
An important implication of our studies is that in the absence of a significant apoptotic effect, malignant primitive progenitors may persist and not be completely eliminated by treatment with imatinib mesylate. Studies to determine the effects of long-term exposure to imatinib mesylate on residual malignant progenitors are under way. It should be noted that although we did not observe a consistent induction of apoptosis in our assays, it is possible that apoptosis may play a role in the elimination of progenitors in some patients. In the future, it will be important to explore the possibility that, in addition to the direct effects on progenitor growth described here, imatinib mesylate may indirectly affect progenitor growth by correcting abnormalities in responsiveness to microenvironmental influences such as ligands for adhesion30,35-37 and chemokine receptors.38-40
Our studies suggest that short-term exposure to imatinib mesylate ex vivo may not be sufficient to eliminate malignant progenitors. This indicates that use of imatinib mesylate alone may not adequately deplete malignant stem cells from CML autografts. Although apoptosis was not induced at low concentrations of imatinib mesylate, there was an increase in apoptosis when the agent was used at a concentration of 5 μM. The use of additional apoptosis-inducing agents may increase the elimination of BCR/ABL-positive cells and enhance the efficiency of imatinib mesylate as a purging agent.
The range of responsiveness under the conditions studied was quite broad (residual CFC values ranged from 2% to 52% and LTCIC values from 0% to 114%). Our data do not indicate a strong association between response or lack of response to imatinib mesylate and stage of disease, time since diagnosis, or prior treatment. Only in a single sample (Figure 7) did the presence of a low percentage ofBCR/ABL-positive cells (< 30%) correlate with decreased inhibition of progenitor growth. This sample contained a mixture of cells that were sensitive to imatinib mesylate and cells that were not responsive to the agent. Correlative studies to determine whether differences in the responsiveness of CML progenitors to imatinib mesylate in vitro correspond to in vivo responses are under way. In addition, correlation of the effect of imatinib mesylate with Bcr-Abl kinase activity and downstream signaling pathways may provide information about why progenitors from some samples are more responsive than others.
To understand further the effect of imatinib mesylate on primary CML progenitors, it will be important to examine downstream signaling pathways affected by inhibition of Bcr-Abl tyrosine kinase activity. Decreased quiescence of CML progenitors may be related to activation of Ras, Raf, and mitogen-activated protein kinase, leading to activation of c-Myc and c-Fos and subsequent increases in transcription.41 Enhanced cell-cycle progression from G1 to S may be related to increased activated cyclin D complexes42 or decreases in p27KIP.43-46The role of these signaling pathways in the response to imatinib mesylate will be the focus of future studies.
In summary, we found that imatinib mesylate suppresses growth of CML but not normal primitive progenitors. At clinically relevant concentrations, imatinib mesylate reversed the abnormally increased proliferation that characterizes CML primitive progenitors but did not significantly increase apoptosis. These results suggest that inhibition of Bcr-Abl tyrosine kinase activity by imatinib mesylate restores normal hematopoiesis in patients with CML by removing the proliferative advantage of CML progenitors but that it may not eliminate all CML primitive progenitors. In future studies, we will investigate the signaling mechanisms underlying the effect of imatinib mesylate on primary primitive progenitor cells and correlate the results with clinical response.
We thank Novartis Pharmaceuticals, East Hanover, NJ, for providing imatinib mesylate for these studies. We acknowledge the excellent technical support of Lucy Brown and Claudio Spalla, Analytical Cytometry Core, who performed all the cell sorting; and we thank Kewei Cui, Allen Lin, Paula Goldfarb, Alison Ahlers, Jeanine Stevenson, and the physicians and staff in the Division of Hematology and Bone Marrow Transplantation for assistance with patient samples.
Supported in part by Public Health Services grant CA74455 from the National Cancer Institute, American Cancer Society grant RPG-99-202-01-LBC, and The Leukemia and Lymphoma Society Translational Research Grant 6468 to R.B. and by Public Health Services grant CA33572 to M.L.S.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ravi Bhatia, Division of Hematology and Bone Marrow Transplantation, City of Hope National Medical Center, Duarte, CA 91010; e-mail: rbhatia@coh.org.

![Fig. 3. Inhibition of CML progenitors increases with longer exposure to imatinib mesylate. / CFC frequency for CML CD34+ cells, normalized to frequency for cells not exposed to imatinib mesylate, is plotted as a function of exposure time. Data graphed are for 1 μM (- - ♦ - -]), 2 μM (—● - -), and 5 μM (—▾—) imatinib mesylate and shown as the mean ± SEM for replicate experiments (24 hours, n = 7; and 48-96 hours, n = 5). Progenitor frequency is significantly suppressed as exposure time increased from 24 to 96 hours (3 asterisks, P < .001; 2 asterisks,P < .01).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/10/10.1182_blood.v99.10.3792/6/m_h81022549003.jpeg?Expires=1769101304&Signature=su-d3UzhA-iAYShDtbb2uHpU7AsiLPtvP~r5GN0RHyFnoqRRXxYO6nzQsEkq~qaBDt4ujs-1hRWbaaBhshym1vQ4UEQJQDjrW2ViWc4rNHM3iqLgzWgOYTiO0Q0Z7Yt4XMUH1ZghMiivXEoPE2U~wdxwEqTa8sJCb404CtYKhL5CJu8fvCnjDwtxWZqeC1wzuRT4A8JGni1uqTqvzLZrleKZ2OL4RccD7OpXW7ScX7DwJf59Na3wqd5vMgmTG~TQJMMBt-fmZ9uDNEFN1rIbFR-xwGRbHrMJUFyEyxMm7j3P6XVq8SCdiCL64JqghAU0WYY1N83xnIjwwfNTV1nPxA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal