Treatment of patients with human immunodeficiency virus (HIV) protease inhibitors such as ritonavir can result in increases in CD4+ T-cell counts that are independent of a reduction in HIV-1 viral load. This lack of correlation between the 2 has led to the identification of additional effects of ritonavir that potentially alter HIV disease pathogenesis. Our previous studies indicated that ritonavir directly affects immune cell activation, proliferation, and susceptibility to apoptosis. We show here that ritonavir inhibited the activation and proliferation of primary endothelial cells and decreased the production of tumor necrosis factor α (TNF-α) interleukin 6 (IL-6), IL-8, and vascular endothelial growth factor, factors that all contribute to tumor neovascularization and to the development of Kaposi sarcoma (KS) lesions. Ritonavir also suppressed the expression of vascular cell adhesion molecule 1, intercellular adhesion molecule 1, and E-selectin, which correlated with a functional decrease in leukocyte adhesion. Transcriptional activation of nuclear factor-κB, as induced by the KS-promoting factor TNF-α, the HIV-1 Tat protein, or the human herpesvirus 8 protein ORF74, was inhibited by ritonavir. KS-derived cell lines underwent apoptosis in vitro after treatment with ritonavir at concentrations that are obtained in clinical therapy (3-15 μM). In a KS mouse xenotransplantation model, ritonavir inhibited tumor formation and progression by KS-derived cells. Taken together, these data suggest that ritonavir has antineoplastic effects that are independent from its ability to inhibit the HIV protease.
Introduction
Protease inhibitors (PIs) such as ritonavir have been successfully used in the clinical treatment of human immunodeficiency virus 1 (HIV-1) infection, with patients exhibiting a marked decrease in HIV viral load and a subsequent increase in CD4+ T-cell counts. The extent of the clinical benefits achieved with HIV PI therapy thus far exceeds that of any other kind of antiretroviral therapy.1-4 However, clinical studies in some HIV patients treated with PIs have yielded unexpected results. Increases in CD4+ T-cell counts have sometimes been noted even though viral loads remained persistently high due to the presence of PI-resistant viral strains.5,6 In past studies, we have demonstrated that the HIV PI ritonavir improves immune cell viability by decreasing cell susceptibility to apoptosis in HIV-free in vitro systems.7-9 These observations provided some insight into a possible explanation for the dissociation of CD4+ T-cell counts from HIV viral loads in patients treated with ritonavir. Evidence of alternative effects by ritonavir on cellular proteases, such as the cysteine proteases cathepsin D and E, was presented in the drug's original description.10 More recently, ritonavir has been shown to inhibit the chymotrypsinlike activity of the 20S proteasome,11 12 although concentrations necessary to demonstrate the in vitro effect exceeded the achievable therapeutic drug levels.
Kaposi sarcoma (KS), a neoplasm of endothelial cell origin, is the most common malignancy associated with HIV infection.21Clinical treatments for the acquired immunodeficiency syndrome (AIDS)–related malignancy KS have generally revolved around aggressive chemotherapy and highly active antiretroviral therapies in the belief that decreasing the HIV viral load would eliminate a main contributory factor.2,13,14 The HIV PI ritonavir appeared to be particularly effective against KS.15-18 Similar to the improvement sometimes seen in CD4+ T-cell counts in the absence of reduction in viral loads, amelioration of KS lesions can occur rapidly without a concomitant reduction in HIV-1 titers.16-18 With these findings in mind, we suspected that ritonavir might have direct inhibitory effects on KS development, independent of its effects on the HIV protease.
Inflammatory cytokines, chemokines, and angiogenic factors cooperate in the induction of endothelial cell activation and are important for tumor neovascularization and KS development.19-21Specifically, interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), IL-6, and IL-8 are increased in patients with all forms of KS and in individuals at high risk for KS.22-24 These cytokines induce endothelial cells to acquire the phenotypic and functional features of KS spindle-shaped cells. Histologically, KS lesions are characterized by spindle-shaped KS cells of endothelial cell origin and a large immune cell infiltrate composed of monocytes, T cells, and macrophages. Production and release of basic fibroblast growth factor and vascular endothelial growth factor (VEGF) by these cells leads to angiogenesis, vascular permeability, and edema, which are also typical histologic features of KS.25 KS spindle cells express high levels of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and endothelial leukocyte adhesion molecule 1 (ELAM-1 or E-selectin), which enhance the infiltration and binding of immune cells within the lesions.26 27
These observations suggest that cytokines and growth factors produced by activated immune cells and endothelial cells predispose an individual to KS development and progression by providing the initial signals required for KS lesion formation via autocrine and paracrine mechanisms. In addition, human herpesvirus 8 (HHV-8) has been shown to be a causal factor for KS development and appears to contribute to the inflammatory and angiogenic microenvironment.25,28,29 The Tat protein of HIV-1, shown to be a contributing factor for KS growth in vitro, also deregulates the expression of inflammatory and angiogenic factors.30 31
In this study, we investigated whether ritonavir directly affects angiogenesis-promoting factors and KS by assessing endothelial cell and KS cell survival, activation, and proliferation. We also studied the effects of ritonavir on tumor formation by KS cells in a mouse xenotransplantation model. We evaluated how the HIV PI alters the expression of proinflammatory cytokines, growth factors, and adhesion molecules that may contribute to the development of KS. In addition, because nuclear factor-κB (NF-κB) is a critical transcription factor required for regulated expression of genes involved in inflammation and immune response,32-34 we analyzed the effect of ritonavir on induction of NF-κB transcriptional activity by TNF-α, the HIV-1 Tat protein, and the HHV-8 protein ORF74, all factors that are relevant to KS pathogenesis.
Materials and methods
Cell cultures
Human umbilical vein endothelial cells (HUVECs) were obtained from Cell Systems (Kirkland, WA). Cells were maintained and expanded in Cell Systems' “complete” medium in a 5% CO2humidified incubator and used for experiments between passages 4 and 5. KSIMM cells were obtained from Dr Adriana Albini36and maintained in RPMI (Life Technologies, Gaithersburg, MD) with 10% heat-inactivated fetal bovine serum (FBS; Life Technologies) in 5% CO2. Peripheral blood mononuclear cells (PBMCs) were isolated by automated Ficoll/Hypaque density-gradient centrifugation using a CS-3000 Plus Blood Cell Separator (Fenwal Division, Baxter Healthcare, Deerfield, IL).
Monocytes were isolated from PBMCs using counterflow-centrifugal elutriation using a Beckman JE-5.0 rotor and a type A chamber (Beckman Instruments, Palo Alto, CA). Purity of the separated monocyte fraction was approximately 95% as determined by cytomorphology in Pappenheim stain and approximately 96% as determined by the expression of CD14 antigen (LeuM3; Becton Dickinson, Mountain View, CA) and measured by flow cytometry. Elutriated monocytes were cultured in medium (Cellgrow 40-101-LV, Mediatech, Herndon, VA) at a density of 3 × 106 cells/mL. Stimulation with lipopolysaccharide (LPS; Sigma Chemicals, St Louis, MO) was used in some experiments for inducing NF-κB activity.
The PBMCs were cultured in RPMI-1640, containing 10% FCS, antibiotics, and IL-2 (Life Technologies) at an initial concentration of 6 × 105 cells/mL. Inhibition by ritonavir of PBMC activation as induced by anti-CD3 monoclonal antibodies (no. 30110D, 0.5 μg/mL, Pharmingen, San Diego, CA) was measured over time as indicated. Recombinant TNF-α (Biosource, Camarillo, CA) was freshly resuspended in sterile water and added to the culture media. Cell viability was measured by trypan blue exclusion and a standardized automated colorimetric assay (WST-1 assay, Boehringer Mannheim, Mannheim, Germany). Cell assays were performed routinely in triplicate cultures for each treatment condition unless stated otherwise. Supernatants from cell cultures were collected and centrifuged at 12 000 rpm for 15 minutes to remove all cell debris and then assayed by enzyme-linked immunosorbent assay (ELISA) for cytokine content using plates precoated with the appropriate antibodies (R & D Systems, Minneapolis, MN). Samples were diluted accordingly to obtain readings within the quasi-linear range of the standard curves. All samples were run in duplicate. Statistical analyses for comparing data between treatment groups were performed. Data illustrated represent means with SD unless stated differently in legends to the figures.
Caspase assays
To assess the indirect effects of ritonavir on caspase-3 activity in cell cultures, HUVECs (passage 5) and KSIMM were incubated for 8 hours with different concentrations of ritonavir ranging from 0 to 50 μmol/L. Cells were harvested and caspase-3 activity was determined in cytosolic preparations using a fluorogenic assay, accordingly to the manufacturer's instructions (BD Pharmingen, San Diego, CA). Samples were normalized by protein content and cleavage of the synthetic fluorogenic tetrapeptide Ac-DEVD-AMC was measured with a microplate spectrofluorometer (Victor-2, Wallac, Gaithersburg, MD).
To determine the direct effects of ritonavir on activated caspases-1, -3, and -8, PBMCs were stimulated with anti-CD3 monoclonal antibody (mAb) and subsequently primed to apoptosis using CD95-agonistic mAbs (clone CH-11; 0.5 mg/L; Kamyia, San Francisco, CA). Cell lysates were prepared as the source for activated caspases. Specific inhibitors of caspases (zVAD-FMK for caspase-1, DEVD-FMK for caspase-3, and IETD-FMK for caspase-8, Enzyme System Products, Livermore, CA) and respective specific substrates (WEHD-AMC, DEVD-AMC, and IETD-AMC; Enzyme System Products) were used to evaluate the effect of ritonavir on the enzymatic reactions.
Adhesion assays
A shear force assay, as previously described,40 was adapted to measure adhesion of leukocytes to endothelial cells. HUVECs (passage 5) were grown to form a monolayer in 96-well plates. Wells were treated with indicated drugs for 2 hours and subsequently activated with TNF-α (5 ng/mL) for 4 hours. Jurkat, HL60, and U937 cells (American Type Culture Collection, Rockville, MD) were labeled with Calcein AM (0.1 μM; Molecular Probes, Eugene, OR) for 30 minutes in separate reactions, and then allowed to adhere to HUVECs for 2 hours. Nonadherent cells were removed by minimal shear force in 0.85% NaCl solution. Fluorescence, which is proportional to cell number, was measured using a Victor-2 fluorometer (Wallac). Standard curves with known numbers of cells were generated to ensure a linear relation between fluorescence and cell number. SDs were generated from 3 separate wells in 4 experiments performed with Jurkat cells and from 8 separate wells for each treatment condition in experiments with HL60 and U937 cells.
Flow cytometry analysis
For quantitative and qualitative determination of cell surface adhesion molecules, HUVEC cultures (passage 5) were treated with TNF (10 ng/mL) in presence or absence of 20 μmol/L ritonavir for 4 hours. Cells were detached with cell dissociation solution, containing 5 mmol/L EDTA (Life Technologies). Aliquots of 0.5 × 106cells were stained with phycoerythrin (PE)–conjugated antibodies for VCAM-1, ICAM-1, and E-selectin, as well as with appropriate isotype control antibodies (Pharmingen) and then fixed in 0.5 mL 2% paraformaldehyde. Samples were analyzed using a Becton Dickinson FACSCalibur flow cytometer and Cell Quest Analysis software.
Tumor studies in mice
Male immunodeficient beige-nude-xid (BNX) mice (obtained from Jackson Laboratory, Bar Harbor, ME) at the age of 6 to 10 weeks (with no more than a 2-week spread in a single experiment) were used for tumor formation and inhibition experiments with in vivo–selected KSIMM cells. Animals were housed in microisolator cages under sterile conditions and observed for at least 1 week to ensure proper health before study initiation. Lighting, temperature, and humidity were controlled centrally and recorded daily. Animals were injected with 7 × 106 KS cells (suspended in 300 μL phosphate-buffered saline [PBS]) subcutaneously into the posterior flanks. Treatment was started approximately 6 days after cell injections, when the tumors became visible and palpable. Randomized groups of mice (n = 10) were treated with ritonavir (30 mg/kg) or PBS by intraperitoneal injections daily for 15 days. Tumor growth and progression were monitored by biweekly measurements of tumors with calipers.
Assays for detection of NF-κB activation
Transfections and luciferase reporter gene assays.
To assess drug effects on NF-κB expression, transient transfection and reporter gene expression assays were used. The NF-κB reporter construct expresses the firefly luciferase gene controlled by a synthetic promoter containing 5 tandem binding sites for NF-κB (Stratagene, La Jolla, CA). KSIMM cells were transfected with Fugene (Boeringher-Mannheim, Indianapolis, IN) using 0.5 μg NF-κB luciferase reporter gene construct and 0.5 μg HIV Tat75 or HHV-8 ORF74 constructs,45 or control plasmids without NF-κB–dependent luciferase expression. A cytomegalovirus (CMV)β–galactosidase expression vector (Stratagene, 0.2 μg/transfection) was used as an internal control for the transfection efficiency, which was routinely about 35% in KSIMM cells. After 20 hours of transfection, cells were treated with ritonavir (Abbott Laboratories, North Chicago, IL) at indicated concentrations and, when appropriate, TNF (5 ng/mL) was added. Cells were lysed (cell lysis buffer; Promega, Madison, WI) 4 hours after treatments and protein concentrations were normalized by BCA assay (Pierce Biochemicals, Rockford, IL) for luciferase measurements (luciferase assay kit, Promega).
Electrophoretic mobility shift assays.
Electrophoretic mobility shift assays (EMSAs) were performed with nuclear extracts from elutriated human monocytes to measure effects of ritonavir on NF-κB activation. Monocytes were treated with different concentrations of the drug and activated with LPS (50 ng/mL) for 15 minutes. Cells were centrifuged at 4°C at 1200 rpm for 10 minutes, washed in ice-cold PBS, suspended in 100 μL sucrose buffer (0.32 M sucrose, 3 mM CaCl2, 2 mM Mg[OAc]2, 0.1 mM EDTA, pH 8, 10 mM Tris.HCl pH 7.9, 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride [PMSF], 0.5% NP-40, 1 mM NaF, 1 mM Na3VO4, 0.01 mg/mL aprotinin, 0.01 mg/mL leupeptin, 0.01 mg/mL pepstatin A) per 107 cells and incubated on ice. After centrifugation at 600g for 5 minutes at 4°C, supernatants were removed and the nuclear pellets washed in sucrose buffer lacking NP-40. Nuclear pellets were lysed in 50 μL Dignam buffer (20 mM Tris.Cl pH 7.9, 1.5 mM MgCl2, 420 mM NaCl, 2 mM EDTA pH 8, 1 mM PMSF, 1 mM NaF, 1 mM Na3VO4, 0.01 mg/mL aprotinin, 0.01 mg/mL leupeptin, 0.01 mg/mL pepstatin A, and 50 mM β-glycerol phosphate). Lysed pellets were incubated on ice for 20 minutes and centrifuged at 9000g. Supernatants were collected and stored at −80°C. Oligonucleotide probes containing NF-κB binding sites were labeled with α32P-dCTP. Protein concentrations of each sample were determined by BCA assay. Then 4 to 5 μg nuclear protein was incubated on ice in DNA binding buffer (10 mM Hepes pH 7.9, 50 mM KCl, 0.2 mM EDTA, 2.5 mM DTT, 10% glycerol, 0.05% NP-40), 2 to 3 μg poly(dI)-(dC), and labeled NF-κB probe (4 × 104 cpm). Samples were electrophoresed on a 4% nondenaturing acrylamide gel and the gel dried and autoradiographed.
Immunoblot analysis.
To measure the effect of ritonavir on activation-induced degradation of IκBα proteins, KSIMM cells were treated with TNF-α (5 ng/mL) and ritonavir (20 μmol/L) for 5.5, 10, 15, and 20 minutes. Cells were washed 2 times with ice-cold PBS and lysed in PBS-RIPA lysis buffer containing 1% NP-40, 0.1% sodium deoxycholate, 0.5% sodium dodecyl sulfate (SDS), 0.01 mg/mL aprotonin, 0.1 mg/mL PMSF, and 0.1 mM sodium orthovanadate. Cell debris was removed by centrifugation at 12 000 rpm for 15 minutes at 4°C. Cell lysates containing 50 μg protein were boiled for 90 seconds in an equal volume of sample buffer containing 2% SDS, 10 mM DTT, 60 mM Tris-HCl (pH, 6.8), and 0.01% bromphenol blue. Samples were fractionated by polyacrylamide gel electrophoresis on a 4% to 20% gradient gel, transferred to a nitrocellulose membrane, and analyzed by Western blot with an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech, Piscataway, NJ). IκBα proteins were detected with rabbit anti-IκBα 1-317 polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Blots were stripped and probed with anti-β actin rabbit polyclonal antibodies (Sigma).
Results
Ritonavir reduces the proliferation of primary endothelial cells and induces apoptosis in KS cells in vitro
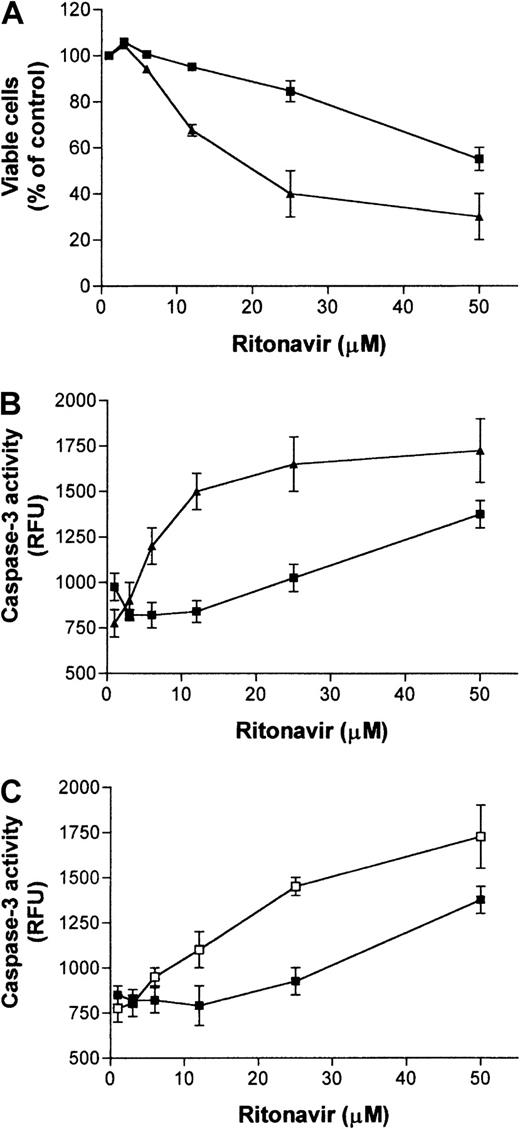
Because we had previously observed that ritonavir inhibited the activation, proliferation, and apoptotic cell death of primary human immune cells, but induced cell death in immortalized cell lines,35 we compared effects of ritonavir treatment on the KS cell line KSIMM36 and on primary HUVECs. Cells were treated for 24 hours with different concentrations of ritonavir (range, 0-50 μmol/L) and viability was assessed by a standardized WST-1 metabolic assay and correlated to trypan blue exclusion staining. The number of viable primary endothelial cells (HUVECs) counted was approximately 25% lower at the highest concentration (50 μmol/L) after 24 hours of treatment when compared with untreated cultures (Figure 1A). Because no significant cell death was detectable in these cultures, this decrease suggests that ritonavir inhibits HUVEC proliferation. In contrast, the number of viable KSIMM cells fell by about 60%, most likely a due to both an inhibition of cell proliferation and increased cell death.
Viability and apoptosis in KSIMM and HUVEC cultures treated with ritonavir.
Both adherent and nonadherent cells from cell cultures of KSIMM (triangles) or HUVECs (squares), treated with ritonavir at indicated concentrations for 40 hours, were harvested to determine the percentage of viable cells when compared to untreated cultures (A). SD of data points illustrated was less than 10% of the mean values as obtained from 3 different cultures per treatment concentration. Increased cell death in KSIMM cultures (▴), compared to HUVECs (▪), correlated with increased caspase-3 activity. Ritonavir-treated HUVECs differed in cell death from KSIMM (P = .015). Wilcoxon rank-sum tests were performed for all comparisons of the means. (B) Cultures were treated with ritonavir at indicated concentrations for 8 hours and enzymatic activity of caspase-3 was determined in protein extracts from cell lysates using a specific fluorogenic assay. Values represent means ± SD of triplicate determinations in relative fluorescence units (RFU). Caspase-3 values for treated HUVECs (▪) differed from KSIMM (▴; P = .015). Wilcoxon rank-sum tests were performed for all comparisons of the means. Activation of HUVECs with TNF-α increases caspase-3 activity in response to ritonavir treatment (C). Cultures were preactivated with TNF (10 ng/mL) and after 2 hours ritonavir was applied to cultures at indicated concentrations. Caspase-3 activity in activated HUVECs was measured in cell extracts after 6 hours (■) and compared with HUVECs that were not activated by TNF-α (▪). Values represent means ± SD of triplicate determinations. Caspase-3 activity in TNF-α–stimulated HUVECs differed from nonstimulated HUVECs (P = .045). Wilcoxon rank-sum tests were performed for all comparisons of the means. Four independent experiments with similar results were performed.
Viability and apoptosis in KSIMM and HUVEC cultures treated with ritonavir.
Both adherent and nonadherent cells from cell cultures of KSIMM (triangles) or HUVECs (squares), treated with ritonavir at indicated concentrations for 40 hours, were harvested to determine the percentage of viable cells when compared to untreated cultures (A). SD of data points illustrated was less than 10% of the mean values as obtained from 3 different cultures per treatment concentration. Increased cell death in KSIMM cultures (▴), compared to HUVECs (▪), correlated with increased caspase-3 activity. Ritonavir-treated HUVECs differed in cell death from KSIMM (P = .015). Wilcoxon rank-sum tests were performed for all comparisons of the means. (B) Cultures were treated with ritonavir at indicated concentrations for 8 hours and enzymatic activity of caspase-3 was determined in protein extracts from cell lysates using a specific fluorogenic assay. Values represent means ± SD of triplicate determinations in relative fluorescence units (RFU). Caspase-3 values for treated HUVECs (▪) differed from KSIMM (▴; P = .015). Wilcoxon rank-sum tests were performed for all comparisons of the means. Activation of HUVECs with TNF-α increases caspase-3 activity in response to ritonavir treatment (C). Cultures were preactivated with TNF (10 ng/mL) and after 2 hours ritonavir was applied to cultures at indicated concentrations. Caspase-3 activity in activated HUVECs was measured in cell extracts after 6 hours (■) and compared with HUVECs that were not activated by TNF-α (▪). Values represent means ± SD of triplicate determinations. Caspase-3 activity in TNF-α–stimulated HUVECs differed from nonstimulated HUVECs (P = .045). Wilcoxon rank-sum tests were performed for all comparisons of the means. Four independent experiments with similar results were performed.
To determine if the cell death induced by ritonavir was due to increased apoptosis, we analyzed caspase-3 activation. This cysteine protease, which is activated during the early stages of apoptosis,37 cleaves and activates other caspases in a cascade leading to apoptosis. In a concentration window between 6 and 25 μmol/L, caspase-3 activity was increased in KSIMM but not in the nonproliferating HUVEC cultures (Figure 1B). These studies suggest that tumorigenic KS cells are more susceptible to cell death mediated by ritonavir than are their primary cell counterparts.
Because previous studies showed that rapidly proliferating cells are more susceptible to apoptosis than are slowly proliferating, quiescent, or differentiated cells,38 we determined whether we could alter the susceptibility of HUVECs to apoptosis by inducing activation with TNF-α. Treatment with the cytokine increased the proliferation rate, and subsequently, these cells became more susceptible to ritonavir-mediated apoptosis, suggesting that TNF-α and ritonavir can cooperate in inducing apoptosis in activated HUVECs (Figure 1C).
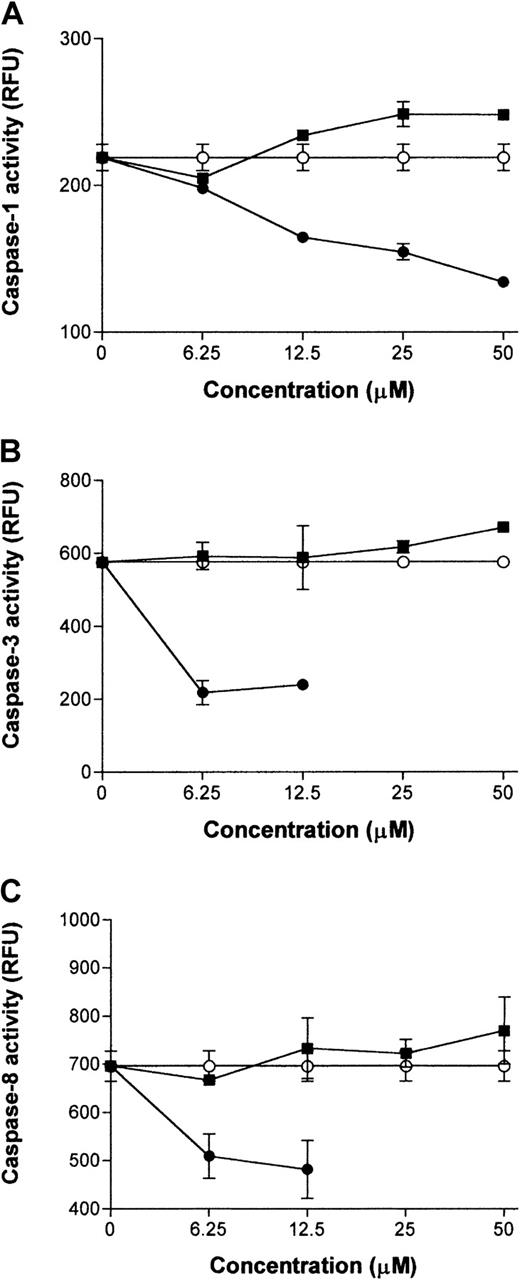
In previous experiments, we reported that treatment of PBMC cultures with ritonavir resulted in decreased caspase-1 expression and lower caspase-3 activity.8 9 In the context of differential effects on endothelial cells, we were interested to know if ritonavir alters the enzymatic activity of caspases in a direct manner. Activation of caspases was induced in PBMC cultures by stimulation with an anti-CD3 mAb and a CD95 agonistic mAb CH11. Cell lysates of these cultures served as the source for activated caspase-1, -3, and -8, and caspase-specific fluorogenic substrates and inhibitors were used to measure the effect of ritonavir in enzymatic reactions. As depicted in Figure 2, ritonavir has no direct inhibitory effects on the cleavage of the tetrapeptide substrates that are specific for each respective caspase-1, -3, and -8. Thus, the drug alters cellular events prior to the induction of caspases, when affecting cell activation and apoptosis pathways.
Ritonavir does not directly inhibit caspase-1, -3, and -8 activity.
Protein extracts prepared from activated PBMCs, triggered to undergo apoptosis by CD95 agonistic mAb CH11 (0.5 mg/mL), were assayed for activity of caspase-1 (A), caspase-3 (B), and caspase-8 (C) using specific fluorogenic substrates. Ritonavir (▪) was added to the enzymatic reactions at the concentrations indicated and substrate conversion was recorded at the saturation points of the reactions. Because ritonavir did not inhibit these caspases directly, the assays were validated using specific inhibitors (●) for the respective caspases as controls. Uninhibited reactions are depicted as open circles (○). Values shown represent means of triplicate determinations ± SD. For caspase-1, the ritonavir-treated group did not differ significantly from the control group, but differed from the inhibitor group (P = .0019). P values for differences between inhibitor and ritonavir-treated groups for caspase-3 and caspase-8 were .011 and 019, respectively. The ritonavir-treated group did not differ significantly from the control group for these as well. Wilcoxon rank-sum tests were performed for all comparisons of the means. Results from one experiment are shown of 3 performed using cells from 3 different donors, with similar results.
Ritonavir does not directly inhibit caspase-1, -3, and -8 activity.
Protein extracts prepared from activated PBMCs, triggered to undergo apoptosis by CD95 agonistic mAb CH11 (0.5 mg/mL), were assayed for activity of caspase-1 (A), caspase-3 (B), and caspase-8 (C) using specific fluorogenic substrates. Ritonavir (▪) was added to the enzymatic reactions at the concentrations indicated and substrate conversion was recorded at the saturation points of the reactions. Because ritonavir did not inhibit these caspases directly, the assays were validated using specific inhibitors (●) for the respective caspases as controls. Uninhibited reactions are depicted as open circles (○). Values shown represent means of triplicate determinations ± SD. For caspase-1, the ritonavir-treated group did not differ significantly from the control group, but differed from the inhibitor group (P = .0019). P values for differences between inhibitor and ritonavir-treated groups for caspase-3 and caspase-8 were .011 and 019, respectively. The ritonavir-treated group did not differ significantly from the control group for these as well. Wilcoxon rank-sum tests were performed for all comparisons of the means. Results from one experiment are shown of 3 performed using cells from 3 different donors, with similar results.
Ritonavir inhibits the production of KS-promoting inflammatory cytokines and growth factors
To investigate how the inhibition of cell activation, proliferation, and the induction of apoptosis by the drug affected the expression of different inflammatory cytokines, we treated HUVEC cultures with ritonavir and measured the released cytokines by ELISA. After 48 hours, the level of IL-8 decreased in a dose-dependent manner from 172 ± 17 pg/mL (untreated) to 48 ± 10 pg/mL (20 μmol/L) in cultures that were kept at 70% to 90% cell confluence (data not shown). Decreases in TNF-α, IL-6 (38%), and VEGF (30%) in HUVEC culture supernatants after 24 hours of treatment (20 μmol/L) were only detectable by ELISA when cells were grown at the highest density possible without inducing significant cell death. We were not able to determine changes in other cytokines and growth factors such as IL-1α, IL-1β, IL-12, granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), and INF-γ levels in these cultures (data not shown). As mentioned previously, KS lesions are composed of a mixed cellularity that includes the predominant KS spindle cells and a large immune cell infiltrate. PBMCs interact closely with KS spindle cells and endothelial cells within KS lesions. Because PBMCs are believed to be important sources of inflammatory factors that promote KS pathogenesis, we tested whether ritonavir could inhibit the induction of cytokine expression following activation through the T-cell receptor by anti-CD3 antibodies. The production of INF-γ, TNF-α, IL-1β, and IL-6 was decreased in a dose- and time-dependent manner (Figure3) without significant toxicity to cells, because the inhibition was reversible after removal of the drug from cultures (data not shown).
Dose- and time-dependent inhibition of inflammatory cytokines by ritonavir.
Human PBMCs were stimulated with anti-CD3 mAbs and treated with the indicated concentrations of ritonavir (30 μmol/L [♦], 10 μmol/L [▴], 3 μmol/L [▪], and untreated control [○]). Supernatants were collected at indicated time points, cleared, and assayed for cytokines by ELISA, (A) IFN-γ, (B) TNF-α, (C) IL-1β, and (D) IL-6. Results from one representative experiment are shown of 4 performed with similar data. Data points illustrated represent means of duplicate determinations, SD ≤ 12%.
Dose- and time-dependent inhibition of inflammatory cytokines by ritonavir.
Human PBMCs were stimulated with anti-CD3 mAbs and treated with the indicated concentrations of ritonavir (30 μmol/L [♦], 10 μmol/L [▴], 3 μmol/L [▪], and untreated control [○]). Supernatants were collected at indicated time points, cleared, and assayed for cytokines by ELISA, (A) IFN-γ, (B) TNF-α, (C) IL-1β, and (D) IL-6. Results from one representative experiment are shown of 4 performed with similar data. Data points illustrated represent means of duplicate determinations, SD ≤ 12%.
Ritonavir inhibits cell adhesion and expression of cell adhesion molecules
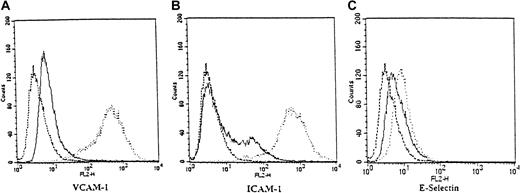
Leukocyte adhesion to endothelial cells is an early step in inflammation and is believed to play an important role in the promotion of KS lesions. The adhesion of leukocytes to endothelial cells is mediated by endothelial cell adhesion molecules (ECAMs) such as ICAM-1, VCAM-1, and E-selectin. Expression of these molecules is highly dependent on NF-κB and is induced by proinflammatory stimuli such as TNF-α.39 We investigated by flow cytometry the effects of ritonavir treatment on the expression of these ECAMs in HUVECs, stimulated with a low dose of TNF-α (5 ng/mL). After 4 hours of treatment with ritonavir (20 μmol/L) the expression of VCAM-1, ICAM-1, and E-selectin induced by TNF-α was inhibited (Figure4). This effect of ritonavir was reversible, because 24 hours after the initial dose, the differences in ECAM expression between treated and untreated cells declined markedly (data not shown).
Inhibition of cell adhesion molecule expression by ritonavir.
Flow cytometry (FACS) histograms of adhesion molecule expression were obtained from endothelial cells (HUVECs) that were treated in cultures (about 80% confluent) with TNF-α (5 ng/mL) or with TNF-α and ritonavir (20 μmol/L) for 4 hours. Aliquots of detached cells were stained with PE-conjugated antibodies for VCAM-1 (A), ICAM-1 (B), and E-selectin (C), as well as isotype-matched controls. Expression of adhesion molecules on TNF-α– and ritonavir-treated cells (solid line) was compared with TNF-α–stimulated cells (dotted line) and untreated cells (dashed line) as depicted in histogram overlays. Two independent experiments with similar results were performed.
Inhibition of cell adhesion molecule expression by ritonavir.
Flow cytometry (FACS) histograms of adhesion molecule expression were obtained from endothelial cells (HUVECs) that were treated in cultures (about 80% confluent) with TNF-α (5 ng/mL) or with TNF-α and ritonavir (20 μmol/L) for 4 hours. Aliquots of detached cells were stained with PE-conjugated antibodies for VCAM-1 (A), ICAM-1 (B), and E-selectin (C), as well as isotype-matched controls. Expression of adhesion molecules on TNF-α– and ritonavir-treated cells (solid line) was compared with TNF-α–stimulated cells (dotted line) and untreated cells (dashed line) as depicted in histogram overlays. Two independent experiments with similar results were performed.
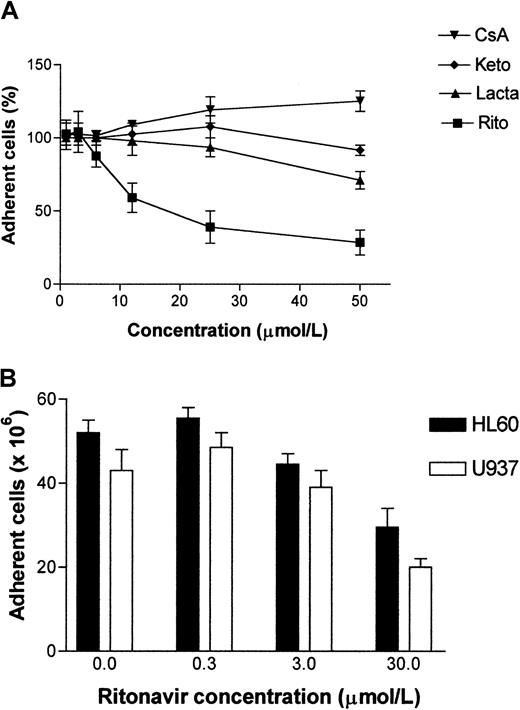
To determine if this decrease in adhesion molecule expression had functional consequences, we carried out static binding assays in which leukocyte cell adhesion to activated endothelial cell monolayers was measured. Human T-lymphoid Jurkat cells, and monocytoid cell lines U937 and HL60 were fluorescently labeled with Calcein-AM and allowed to adhere to endothelial cells. Nonadherent cells were removed by limited shear force.40 Fluorescence of remaining cells was measured and the cell number determined using a standard curve of labeled cells. As controls, cyclosporin A (calcineurin inhibitor), ketokonazole (cytochrome P450 inhibitor), and lactacystin (proteasome inhibitor) were included at comparable concentrations. The inhibition of leukocyte adhesion to endothelial cells by ritonavir was dose dependent (Figure 5), with less than 60% of adherent cells remaining at concentrations higher than 30 μmol/L.
Dose-dependent inhibition of leukocyte adhesion to endothelial cells.
Confluent monolayer cultures of HUVECs were treated with indicated drugs at different concentrations for 2 hours before labeled adhesion-indicator cells were added. Percentages of adherent Jurkat cells (A) were calculated in 4 independent experiments as average of triplicate determinations for each treatment condition when compared to untreated cultures after 16 hours of adhesion. Data points in panel A represent mean percentage ± SD of adherent Jurkat cells from the 4 experiments. CsA indicates cyclosporin A; keto, ketoconazole; lacta, lactacystin; rito, ritonavir. The treatment groups differed from each other P = .0018. Kruskal-Wallis test was performed for analysis of the means. Inhibition of adhesion of HL60 and U937 cells to HUVECs by ritonavir (B) was determined in 8 wells per treatment condition. All adherent cell HUVEC monolayers were activated with TNF-α (5 ng/mL) and subsequently treated with ritonavir at indicated concentrations for 4 hours. Calcein AM-labeled HL60 and U937 cells were dispensed on top of the HUVECs in fresh culture media and allowed to adhere for 30 minutes. Cells that remained adherent after exposure to minimal shear force were then read as fluorescence intensity and cell numbers were calculated based on standard curves for each cell type (mean values and SD are illustrated). Adhesion of U937 and HL60 in the treated samples differed from the untreated samples (P = .010 and P = .015, respectively). Wilcoxon rank-sum tests were performed for all comparisons of the means.
Dose-dependent inhibition of leukocyte adhesion to endothelial cells.
Confluent monolayer cultures of HUVECs were treated with indicated drugs at different concentrations for 2 hours before labeled adhesion-indicator cells were added. Percentages of adherent Jurkat cells (A) were calculated in 4 independent experiments as average of triplicate determinations for each treatment condition when compared to untreated cultures after 16 hours of adhesion. Data points in panel A represent mean percentage ± SD of adherent Jurkat cells from the 4 experiments. CsA indicates cyclosporin A; keto, ketoconazole; lacta, lactacystin; rito, ritonavir. The treatment groups differed from each other P = .0018. Kruskal-Wallis test was performed for analysis of the means. Inhibition of adhesion of HL60 and U937 cells to HUVECs by ritonavir (B) was determined in 8 wells per treatment condition. All adherent cell HUVEC monolayers were activated with TNF-α (5 ng/mL) and subsequently treated with ritonavir at indicated concentrations for 4 hours. Calcein AM-labeled HL60 and U937 cells were dispensed on top of the HUVECs in fresh culture media and allowed to adhere for 30 minutes. Cells that remained adherent after exposure to minimal shear force were then read as fluorescence intensity and cell numbers were calculated based on standard curves for each cell type (mean values and SD are illustrated). Adhesion of U937 and HL60 in the treated samples differed from the untreated samples (P = .010 and P = .015, respectively). Wilcoxon rank-sum tests were performed for all comparisons of the means.
Ritonavir inhibits KS tumor growth in a mouse model
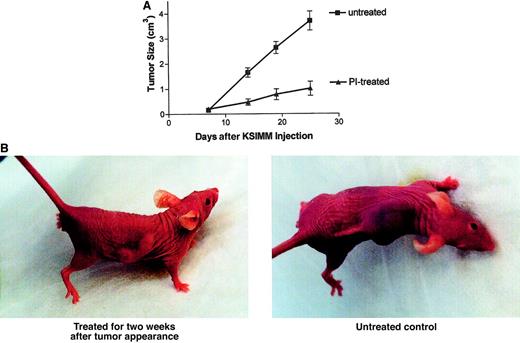
To determine if the in vitro effects on cells and tumor-promoting factors would translate into in vivo effects, a KS tumor xenotransplantation mouse model was used. The KSIMM cell line was adapted by in vivo selection and repeated passages to form tumors in immunodeficient BNX mice. Mice (n = 10/group) were inoculated with KSIMM cells subcutaneously (7 × 106 cells/mouse). After visible tumor formation (5-7 days), mice were given intraperitoneal injections of ritonavir, 30 mg/kg per day, for 15 days. The control group received PBS. The growth was monitored by measuring the tumor size. Treatment of mice with ritonavir inhibited KSIMM tumor development and growth significantly (Figure6A). Figure 6B contains photographs that show an example of a treated and untreated tumor in BNX mice. No adverse effect of the treatment was noted on mice in a control group without tumors during a follow-up period of 3 months.
Inhibition of KS tumor growth by ritonavir.
(A) KSIMM cells (7 × 106cells/mouse) were xenotransplanted into BNX mice (n = 10/group). After visible tumor formation, mice were given intraperitoneal injections of ritonavir, 30 mg/kg per day, for 15 days. The control group received PBS. Tumor growth and progression were monitored by biweekly measurements of tumors with calipers. The treated group differed from the untreated group (P = .001). Wilcoxon rank-sum tests were performed for all comparisons of the means. (B) Photographs depicting a treated versus untreated mouse. The treated mouse was treated with ritonavir for 2 weeks following tumor appearance. The untreated mouse, an age-matched control, received PBS for 2 weeks after tumor appearance.
Inhibition of KS tumor growth by ritonavir.
(A) KSIMM cells (7 × 106cells/mouse) were xenotransplanted into BNX mice (n = 10/group). After visible tumor formation, mice were given intraperitoneal injections of ritonavir, 30 mg/kg per day, for 15 days. The control group received PBS. Tumor growth and progression were monitored by biweekly measurements of tumors with calipers. The treated group differed from the untreated group (P = .001). Wilcoxon rank-sum tests were performed for all comparisons of the means. (B) Photographs depicting a treated versus untreated mouse. The treated mouse was treated with ritonavir for 2 weeks following tumor appearance. The untreated mouse, an age-matched control, received PBS for 2 weeks after tumor appearance.
Ritonavir inhibits NF-κB transcriptional activity
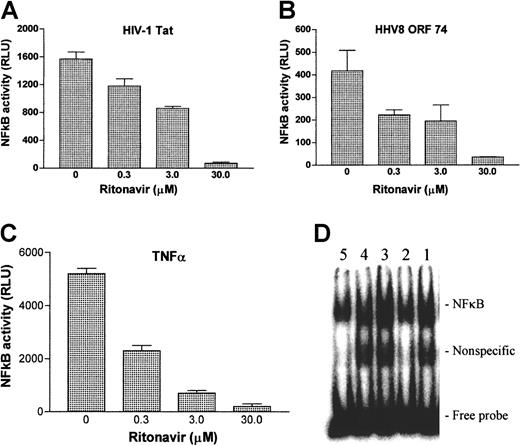
To better understand the mechanism of the antitumorigenic action of ritonavir, we determined the effect of ritonavir on NF-κB activity as induced by the inflammatory cytokine TNF-α and by the viral proteins HIV-1 Tat and HHV-8 ORF74, which are all believed to contribute to the pathogenesis of KS.41-45 NF-κB transcriptional activity was determined by luciferase-reporter transient transfection assays, using a promoter construct with consensus NF-κB binding sites. Ritonavir inhibited the induction of NF-κB transcriptional activity by Tat, ORF74, and TNF-α in a dose-dependent manner at concentrations ranging from 0.3 to 30 μmol/L (Figure 7A-C). To determine whether the effect of ritonavir was specific for NF-κB activity, we tested 2 control luciferase expression vectors that lack responsive elements, but have constitutive expression of luciferase from a CMV and SV40 promoter. We found no differences in luciferase readings (data not shown) between the treated and untreated samples. EMSAs were used to confirm the validity of reporter expression assays. NF-κB translocation, measured in nuclear extracts from activated primary human monocyte cultures, indicated a dose-dependent inhibition by ritonavir of TNF-induced activation (Figure 7D).
Inhibition of NF-κB activity by ritonavir.
KSIMM cells were transfected with a NF-κB luciferase reporter construct and an expression vector encoding HIV-Tat protein (pCV1-Tat) (A) or HHV8 ORF74 (pSG5-ORF74) (B). Twenty hours after transfection, cells were treated with indicated amounts of ritonavir and luciferase activity was measured 6 hours later. (C) Luciferase readings derived from cells that were activated with TNF and treated with ritonavir simultaneously. Values shown are averages derived from 3 independent cultures, with SDs represented by the error bars. These experiments were repeated twice with similar results. Autoradiography (D) of EMSAs for NF-κB activation. Monocytes were treated with indicated concentrations of ritonavir and activated with TNF-α (10 ng/mL) for 20 minutes. Double-stranded NF-κB consensus oligonucleotide and nuclear protein extracts reacted for 30 minutes and products were separated in 4% polyacrylamide gel electrophoresis. Lane 1: activated, untreated control; lane 2: not activated, untreated control; lane 3, activated, treated with 1 μmol/mL ritonavir; lane 4: activated, treated with 5 μmol/mL; lane 5: treated with 25 μmol/mL.
Inhibition of NF-κB activity by ritonavir.
KSIMM cells were transfected with a NF-κB luciferase reporter construct and an expression vector encoding HIV-Tat protein (pCV1-Tat) (A) or HHV8 ORF74 (pSG5-ORF74) (B). Twenty hours after transfection, cells were treated with indicated amounts of ritonavir and luciferase activity was measured 6 hours later. (C) Luciferase readings derived from cells that were activated with TNF and treated with ritonavir simultaneously. Values shown are averages derived from 3 independent cultures, with SDs represented by the error bars. These experiments were repeated twice with similar results. Autoradiography (D) of EMSAs for NF-κB activation. Monocytes were treated with indicated concentrations of ritonavir and activated with TNF-α (10 ng/mL) for 20 minutes. Double-stranded NF-κB consensus oligonucleotide and nuclear protein extracts reacted for 30 minutes and products were separated in 4% polyacrylamide gel electrophoresis. Lane 1: activated, untreated control; lane 2: not activated, untreated control; lane 3, activated, treated with 1 μmol/mL ritonavir; lane 4: activated, treated with 5 μmol/mL; lane 5: treated with 25 μmol/mL.
To further understand the mechanism of the observed NF-κB inhibition, we looked at the degradation of IκBα in KSIMM cells. NF-κB is sequestered in an inactive form in the cytoplasm through binding to IκB proteins. Stimulation of cells by viral genes or exogenous stimuli such as TNF-α leads to the rapid phosphorylation by the IκB kinase (IKK) complex, ubiquitination, and proteolytic degradation of IκB, which frees NF-κB to translocate to the nucleus and activate the transcription of its target genes.46 Consistent with previously reported results,11 ritonavir-treatment of KSIMM cells inhibited the degradation of IκBα, which was induced by TNF-α (data not shown).
Discussion
Clinical use of HIV PIs such as ritonavir in the prevention and treatment of HIV-associated KS has led to a drastic decline of this malignancy in the patient population with access to these drugs. Past studies have described the direct effects of ritonavir on immune cells, effects that are independent of HIV protease inhibition.9,35,75 Inhibition of cell activation correlated with a lower susceptibility to apoptosis in primary cells.8 9 In our present work, we demonstrate that ritonavir treatment directly inhibits KS-promoting factors and the growth of tumors from KS-derived cells xenotransplanted into immunodeficient mice. This is independent of any effects of ritonavir on HIV-1 or HHV-8, because KSIMM cells are not infected with either virus. Although this model of KS may not accurately represent KS lesions in patients, these mouse tumors are composed of KS cells that behave similarly and are dependent on the same cytokines and growth factors as KS spindle cells within lesions. To substantiate this unexpected finding, possible reasons for the antitumor effects were investigated and confirmed at the cellular and molecular biologic levels.
Ritonavir decreased the production of the NF-κB–dependent cytokines IL-6, IL-8, and TNF-α by HUVECs, all of which may contribute significantly to the angiogenesis, inflammation, and immune cell infiltration seen in KS lesions.21,29,47 IL-6 has been implicated in angiogenesis and induces expression of the proangiogenic growth factor VEGF in endothelial cells.48,49 We found reduction by ritonavir of VEGF in endothelial cell cultures. Furthermore, ritonavir inhibited the expression of INF-γ, TNF-α, IL-1β, and IL-6 in PBMCs. These inflammatory cytokines are produced by infiltrating leukocytes in KS lesions and are believed to perpetuate KS lesion development.20 50
Ritonavir treatment of endothelial cells inhibited ICAM-1, VCAM-1, and E-selectin expression–suppressed leukocyte adhesion to these cells. Adherence of HIV-infected or HHV-8–infected monocytes to activated endothelial cells is thought to be an important contributor to KS lesion development.27 KS lesions are a complex mixture of different cell types, including the characteristic spindle cells, but also fibroblasts, microvascular endothelial cells, dendritic cells, and a prominent infiltrate of extravasated erythrocytes and lymphocytes.25,29 During tissue inflammation, endothelial cells become adhesive for circulating blood cells and support their transmigration into inflamed tissue. This process is influenced by NF-κB–dependent adhesion molecules such as VCAM-1, ICAM-1, and E-selectin, which have been shown to also play a role in angiogenesis and the formation of tumor metastases.39 51
Primary endothelial cells responded differently than KS cell lines to ritonavir treatment in vitro at a concentration range comparable to that achieved clinically. Ritonavir has been directly linked to inhibition of the cell cycle at the G1/S checkpoint.10,12 Possibly, the drug causes apoptosis in rapidly dividing cells due to conflicting signals, similar to the induction of apoptosis by c-myc in serum-deprived fibroblasts.52,53 For example, a decrease in NF-κB activity in cells treated with ritonavir, in combination with other persistent factors promoting KS cell growth, that are not themselves affected by ritonavir, may lead to apoptosis. This could be the reason that primary endothelial cells become more susceptible to ritonavir-mediated apoptosis when activated by TNF-α. Studies demonstrating that inhibition of NF-κB potentiates TNF-α–induced apoptosis support this concept.54 55
Ritonavir inhibited NF-κB activation by HIV-1 Tat, HHV-8 ORF74, and TNF-α. These proteins are highly relevant to the development of AIDS-KS as well as other HIV-related malignancies.29,56The HIV-1 viral protein Tat was shown to be a powerful inducer of NF-κB transcriptional activity in lymphoid cells.57,58The ORF74 molecule activates NF-κB45and plays a key role in the promotion of angiogenesis in KS lesions.41,59TNF-α, which is elevated in HIV infection, activates NF-κB and has been shown to promote the growth of KS lesions.26 60 In confirmation of NF-κB inhibition experiments, our data indicate that treatment of KS cells with ritonavir blocked the TNF-α–induced degradation of IκBα.
The exact role of NF-κB deregulation in the development and progression of KS is yet to be determined. Generally, NF-κB is known to promote cell activation, proliferation, and oncogenesis.61,62 Chromosomal amplification, overexpression, and the rearrangement of genes encoding Rel/NF-κB factors have been noted in many human hematologic malignancies and solid tumors,63 and resistance to apoptosis by many tumors treated with chemotherapy is mediated by NF-κB alterations.54,55 Inhibition of NF-κB has been shown to sensitize chemoresistant and radioresistant tumors to the apoptotic potential of therapeutic compounds such as TNF-α.54,55 64 It is possible that ritonavir sensitizes KS tumors to therapeutic agents commonly used in the treatment of KS through inhibition of NF-κB.
Recent in vitro studies have shown ritonavir to be an inhibitor of the chymotrypsinlike activity of the 20S proteasome.11 It is tempting to base a mechanistic explanation for the action of ritonavir on KS on these findings, because the proteasome represents the cell's major nonlysosomal tool to rapidly degrade and process proteins by adenosine triphosphate/ubiquitin-dependent proteolysis. Proteins involved in cell cycle progression and survival, such as p53, cyclins, the cyclin-dependent kinase inhibitors p21 and p27, bax, and bcl-2,65-69 are substrates of the proteasome. One of the best known pathways regulated by the proteasome is that of NF-κB. IκB is degraded by the proteasome after ubiquitination, thereby allowing NF-κB to translocate to the nucleus and activate transcription.70 The proteasome also regulates the posttranslational processing of NF-κB into its active form.70 Recent studies have shown that experimental proteasome inhibitors may represent a novel class of potent and effective antitumor agents.38,71 72 One might therefore speculate that ritonavir inhibits the transactivation potential of NF-κB induced by activators such as TNF-α, Tat, and ORF74 through inhibition of the cellular 20S proteasome, which could subsequently result in the decreases that we observed in expression of NF-κB–dependent adhesion molecules and cytokines. Unfortunately, however, this concept is not entirely supported by our data derived from in vitro cell culture experiments. When proteasome activity was measured in cell lysates from ritonavir-treated cells over a period of 24 hours, no inhibition of chymotrypsinlike activity was observed (data not shown). Because the system was validated and standardized with known proteasome inhibitors (lactacystin, MG132), the lack of effect in culture on proteasomal activity by ritonavir stands in contrast to its significant anti-inflammatory effects. Thus, it is possible that inhibition of NF-κB activation by ritonavir is linked to additional pathways other than proteasome inhibition.
Deregulation of the cellular protease network has been shown to be responsible for aggressive clinical behavior in several human malignancies.73,74 One key group of proteases involved in invasion and metastases includes urokinase plasminogen activator, cathepsins B, D, and L, and matrix metalloproteinases.74These proteases catalyze the degradation of the interstitial matrix and basement membrane, and enhanced activity can be used as prognostic marker for rapid progression in various cancers. It is currently not known whether ritonavir affects any of these proteases or whether the drug targets unknown cellular aspartyl proteases.
In this paper, we demonstrate antineoplastic features of the HIV PI, ritonavir, effective against KS both in vitro and in vivo. These findings may help to explain why ritonavir therapy can result in the clinical improvement of AIDS-KS without a concomitant decrease in HIV viral load. It would be interesting to evaluate the drug's effect in patients with non–AIDS-related KS. Our results justify further studies to evaluate the utility of ritonavir for other tumors in addition to chemotherapy and radiation, which are often hindered in their effectiveness by resistance due to deregulated NF-κB.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Frank F. Weichold, Morgan State University, 1700 E Coldspring La, Spencer Hall, Rm G12, Baltimore, MD 21251; e-mail:fweichol@morgan.edu.

![Fig. 3. Dose- and time-dependent inhibition of inflammatory cytokines by ritonavir. / Human PBMCs were stimulated with anti-CD3 mAbs and treated with the indicated concentrations of ritonavir (30 μmol/L [♦], 10 μmol/L [▴], 3 μmol/L [▪], and untreated control [○]). Supernatants were collected at indicated time points, cleared, and assayed for cytokines by ELISA, (A) IFN-γ, (B) TNF-α, (C) IL-1β, and (D) IL-6. Results from one representative experiment are shown of 4 performed with similar data. Data points illustrated represent means of duplicate determinations, SD ≤ 12%.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/10/10.1182_blood.v99.10.3771/6/m_h81022573003.jpeg?Expires=1769087957&Signature=wx8bybq3Bk3h9zsrUAMtL6IDne13UEXO8Fo1mpVzpFtRjjk3fc33zQ-4MfvgIBxa37Vt07S1Do7kskqOqszYX0Mx5m27L0THERIpM~H3EAGm70EAW4h7GQG502Ft59HnXANjeOrWWt54JIUy4-CxV~dM6JZukkqs~~4CVuS3rJGgFsSSaXGzwpXCbKbtop9Rvpy5jhwwQb5N8-aL3f4MPLsg0QrZwiR1xQDcIMbEIa5tDtE-1q8yqBSKdwAc6ugM1kAB8cE-4MFw4EnCaaYxO0iDL6HVzIaEjMupUFkry2mQ3RPnT7j8y9ryl1QPLiydBj9hDq5PQ84PS9lVYtSQtQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal