Overexpression of the breast cancer resistance protein (BCRP) efflux pump in human cancer cell lines results in resistance to a variety of cytostatic agents. The aim of this study was to analyze BCRP protein expression and activity in acute myeloid leukemia (AML) samples and to determine whether it is up-regulated due to clonal selection at relapse/refractory disease. BCRP protein expression was measured flow cytometrically with the monoclonal antibodies BXP-34 and BXP-21 in 20 paired samples of de novo and relapsed/refractory AML. BXP-34/immunoglobulin G1 ratios were observed of 1.6 ± 0.5 (mean ± SD, range 0.8-2.7) and BXP-21/immunoglobulin G2a ratios of 4.9 ± 3.0 (range 1.1-14.5) in the patient samples versus 9.8 ± 6.8 and 6.5 ± 2.4, respectively, in the MCF-7 cell line. BCRP activity was determined flow cytometrically by measuring mitoxantrone accumulation in absence and presence of the inhibitor fumitremorgin C. Mitoxantrone accumulation, expressed as mean fluorescence intensity (MFI), varied between 44 and 761 MFI (227 ± 146 MFI) and correlated inversely with BCRP expression (r = −0.58, P < .001). Addition of fumitremorgin C showed a small increase in mitoxantrone accumulation (11 ± 29 MFI, n = 40) apart from the effect of PSC833 and MK-571. No consistent up-regulation of BCRP expression or activity was observed at relapse/refractory disease; some cases showed an increase and other cases a decrease at relapse. Relatively high BCRP expression correlated with immature immunophenotype, as determined by expression of the surface marker CD34 (r = 0.54, P = .001). In conclusion, this study shows that BCRP protein is expressed at low but variable levels in AML, especially in immature CD34+cells. BCRP was not consistently up-regulated in relapsed/refractory AML.
Introduction
The multidrug resistance proteins P-glycoprotein (P-gp)1 and multidrug resistance protein 1 (MRP1),2,3 which are members of the ATP-binding cassette (ABC) family of transport proteins, have been extensively studied. They are both described to be poor prognostic factors in the treatment of acute myeloid leukemia (AML).4-7 An additional member of the ABC transport family is the breast cancer resistance protein (BCRP), also known as MXR, ABCP, or ABCG2, a 655–amino acid protein encoded by the BCRP gene located on chromosome 4q22.8,9 Overexpression of BCRP has been observed in human cancer cell lines selected for resistance with mitoxantrone,10,11 doxorubicin plus verapamil,12 and topotecan.13 Transfection and overexpression of BCRP in the human breast cancer cell line MCF-7 resulted in resistance to the cytostatic agents mitoxantrone, daunorubicin, and topotecan.8 Substrate specificities of BCRP, P-gp, and MRP1 are distinct but overlapping. Daunorubicin and mitoxantrone are transported by BCRP, P-gp, and MRP1,6,14whereas verapamil is especially transported by P-gp, calcein is effluxed only by MRP1, and lysotracker green was reported as a substrate for BCRP.14 Reversal of BCRP-mediated, but not of P-gp– or MRP-mediated multidrug resistance has been described with fumitremorgin C (FTC), which was effective in reversing resistance to mitoxantrone, doxorubicin, and topotecan in multidrug-selected cell lines.15
AML patients are treated with intensive chemotherapy regimens, including daunorubicin, idarubicin, and mitoxantrone. Despite these intensive regimens, a considerable number of AML patients show resistance to these drugs and do not obtain complete remission or demonstrate a relapse.16-19 Recently, BCRP messenger RNA (mRNA) expression has been found to be expressed at variable levels in blast cells from de novo AML patients.20 So far no data have been published concerning the functional activity of BCRP in AML. In a previous study we have reported that clonal selection of P-gp– or MRP-expressing cells does not play a role at relapse in AML; the observed changes in P-gp and MRP activity in relapse versus primary AML samples were correlated with changes in maturation stage as determined by immune phenotyping.21 In the present study we were interested in whether clonal selection of BCRP-expressing cells might occur in AML at relapse. Therefore, we determined the expression of BCRP protein with flow cytometry using the recently described BXP-34 and BXP-21 monoclonal antibodies22,23 in 20 paired samples of de novo and relapsed AML. The functional activity of BCRP was determined by measuring the capacity to extrude mitoxantrone in the absence or presence of the BCRP inhibitor FTC in cell lines and samples from patients with AML by means of flow cytometry. Because it has been described that BCRP expression in normal hematopoietic cells is restricted to the most primitive cells,23-25 we were interested in a possible correlation between BCRP expression and primitive immunophenotype in AML. Therefore, we determined the expression of the immature CD34+/CD38− and CD34+/CD33− subpopulations and the more mature CD34+/CD33+ and CD34−/CD33+ subpopulations of AML cells and correlated the expression of these subpopulations with BCRP protein expression.
Patients, materials, and methods
Cell lines
The drug-sensitive human breast cancer cell line MCF-7, its mitoxantrone-resistant counterpart MCF-7 MR,11 which overexpresses BCRP but not P-gp or MRP1, and its doxorubicin-selected subline MCF-7 Dox40,11 which overexpresses P-gp, were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS). The MCF-7 MR and the MCF-7 Dox40 cell lines were cultured in the presence of 80 nM mitoxantrone and 400 nM doxorubicin, respectively. Cells were cultured in drug-free medium at least 7 days prior to the analyses.
Patients
After informed consent, bone marrow aspirates or peripheral blood were collected from AML patients at the time of diagnosis and relapse or refractory disease, between July 1992 and November 2000 at the University Hospital Groningen. Patients were classified at presentation of the disease according to the French-American-British (FAB) classification. Leukemic blasts were enriched by Ficoll-Isopaque (Nycomed, Oslo, Norway) density gradient centrifugation; cryopreserved in RPMI 1640 medium (BioWhittaker, Brussels, Belgium) supplemented with 10% FCS (Hyclone, Logan, UT) and 10% dimethyl sulfoxide (Merck, Amsterdam, The Netherlands); and stored at −196°C. Paired cases, which were available at the time of diagnosis as well as at relapse, were selected. Upon analysis, AML blasts were thawed, treated with deoxyribonuclease (Boehringer Mannheim, Almere, The Netherlands), washed with RPMI 1640 medium, and incubated for 30 minutes in RPMI 1640 medium supplemented with 10% FCS at 37°C, 5% CO2. If more than 10% T cells were present, as determined by the percentage of CD3+ cells, T lymphocytes were depleted by 2-aminoethylisothiouronium bromide–treated sheep red blood cell rosetting. T-cell sheep red blood cell rosettes were removed by Ficoll-Isopaque density gradient centrifugation. May-Grünwald/Giemsa staining was performed to determine the percentage of blast cells in the AML cells, and in all cases the percentage of blast cells was more than 90%.
Normal bone marrow samples
Normal bone marrow samples were obtained after informed consent from 3 patients who underwent cardiac surgery. Samples were cryopreserved, thawed, and T cells were depleted as described above. Viability of the cells was determined by trypan blue exclusion.
Flow cytometric detection of BCRP protein expression
The BCRP protein expression of the tumor cell lines and AML blasts was measured with the BXP-34 and BXP-21 monoclonal antibodies, which recognize internal epitopes of the BCRP protein. Cells (0.5 × 106) were incubated for 10 minutes at room temperature with fluorescence-activated cell sorter (FACS) lysing solution (Becton Dickinson Immunocytometry Systems, Erembodegem, Belgium) to permeabilize the cell membranes. Then the cells were incubated for 15 minutes at room temperature with 1% goat serum in phosphate-buffered saline (140 mM NaCl, 9.0 mM Na2HPO4.2H2O, 1.3 mM NaH2PO4.2H2O, pH 7.4) containing 2% bovine serum albumin. Thereafter, the cells were incubated for 60 minutes at room temperature with 10 μL BXP-34 or BXP-21 mouse antibodies obtained from hybridoma supernatants or with 10 μL (0.5 μg) immunoglobulin G1 (IgG1) or IgG2a isotype control. Cells were washed with phosphate-buffered saline/bovine serum albumin and incubated for 20 minutes with phycoerythrin-conjugated rabbit antimouse F(ab′)2 fragments. Phycoerythrin fluorescence was measured on a FACSCalibur flow cytometer. BCRP protein expressions were expressed as adjusted for IgG1 or IgG2a control, ie, the ratio of BXP-34/IgG1 or BXP-21/IgG2a antibody fluorescence.
Flow cytometric detection of mitoxantrone accumulation
The ability of tumor cell lines and AML blasts to extrude mitoxantrone in the absence or presence of the BCRP inhibitor FTC,15 the P-gp inhibitor PSC833 (provided by Novartis Pharma, Basel, Switzerland), the leukotriene D4 receptor antagonist and MRP inhibitor MK-571,28 or combinations of these inhibitors was measured in a flow cytometric assay.29Cells (0.5 × 106) were preincubated with the inhibitors for 20 minutes at 37°C, 5% CO2, in the following combinations: RPMI 1640 medium alone (0.5 mL), RPMI 1640 medium plus FTC (10 μM),29 the maximum nontoxic doses of PSC833 (2 μg/mL) and MK-571 (20 μM),30 FTC (10 μM) plus PSC833 (2 μg/mL), FTC (10 μM) plus MK-571 (20 μM), PSC833 (2 μg/mL) plus MK-571 (20 μM) (if sufficient cells were available), or FTC (10 μM) plus PSC833 (2 μg/mL) plus MK-571 (20 μM). Thereafter, mitoxantrone (10 μM) was added and the cells were incubated for 60 minutes at 37°C, 5% CO2. Cells were washed with ice-cold RPMI 1640 medium. Fluorescence of mitoxantrone was analyzed with a FACSCalibur flow cytometer (Becton Dickson Medical Systems, Sharon, MA) equipped with an argon laser. The blast population was gated by forward and side scatter characteristics. The mitoxantrone fluorescence of 5000 gated events was logarithmically measured at a laser excitation wavelength of 635 nm through a 670 nm bandpass filter. Mitoxantrone accumulation was expressed as mean fluorescence intensity (MFI). The effects of the inhibitors were expressed as a shift of MFI of the mitoxantrone accumulation.
Flow cytometric detection of P-gp and MRP activity
Functional activity of the P-gp and MRP transporter proteins was demonstrated as described previously.29 In short, to analyze P-gp activity we used rhodamine 123 (Rh123) (Sigma Chemical, Bornem, Belgium) in combination with the P-gp inhibitor PSC833. To determine MRP (MRP1 and homlogs MRP2 and MRP3) activity, the compound carboxyfluorescein (CF) was used in combination with the MRP inhibitor MK-571. Rh123 and CF fluorescence of 5000 events was measured with a FACSCalibur flow cytometer. The efflux-blocking factors of the inhibitors were expressed as MFI in inhibitor-blocked cells divided by the MFI in unblocked cells.
Immunophenotype
To determine the maturation stage of the AML blasts, phenotyping was performed using monoclonal antibodies against CD34 in combination with CD38 or CD33 or IgG isotype–matched controls21(Becton Dickinson, Mountain View, CA). A total of 0.5 × 106 cells were incubated with 5 μL fluorescein isothiocyanate– or phycoerythrin-labeled mouse monoclonal antibodies for 20 minutes at 4°C, washed with RPMI 1640 medium, and analyzed with a FACSCalibur flow cytometer. The percentages of the following subclasses of cells were determined: CD34+, CD33+, the immature subpopulations of CD34+/CD38− and CD34+/CD33− blasts, and the more mature subclasses of CD34+/CD33+ and CD34−/CD33+ blasts.
Statistical analysis
The parametric Student t test and the Mann-WhitneyU and Wilcoxon nonparametric tests were used to calculate significant differences, and correlations were calculated using the Spearman bivariate nonparametric correlation test. Data were expressed as mean ± SD. P values below .05 were considered significant.
Results
Cell lines
BCRP protein expression in cell lines.
First, to test whether the expression of BCRP protein could be measured by flow cytometry, we studied BCRP protein expression in the drug-sensitive and BCRP low-expressing cell line MCF-7 in the mitoxantrone-resistant and BCRP-overexpressing cell line MCF-7 MR and in the P-gp–overexpressing cell line MCF-7 Dox40. BCRP protein expression was measured using the monoclonal antibodies BXP-34 and BXP-21 in conjunction with IgG isotype controls. A BXP-34/IgG1 ratio of 9.8 ± 6.8 (mean ± SD, n = 3) (Table1) was observed in the MCF-7 cell line versus a high expression of 50.6 ± 8.1 (n = 3,P < .05) in the MCF-7 MR cell line; the BXP-21/IgG2a ratio was 6.5 ± 2.4 (n = 4) in the MCF-7 cell line versus 11.3 ± 0.6 (n = 4, P < .05) in the resistant MCF-7 MR cell line. The P-gp–overexpressing MCF-7 Dox40 cell line showed a low level of BCRP expression: a BXP-34/IgG1 ratio of 7.6 ± 0.6 (n = 3) and a BXP-21/IgG2a ratio of 2.3 ± 0.3 (n = 3).
BCRP protein expression in MCF-7 cell lines
| . | BXP-34/IgG1 . | BXP21/IgG2a . |
|---|---|---|
| MCF-7 S | 9.8 ± 6.8 | 6.5 ± 2.4 |
| MCF-7 MR | 50.6* ± 8.1 | 11.3* ± 0.6 |
| MCF-7 Dox40 | 7.6 ± 0.6 | 2.3 ± 0.3 |
| . | BXP-34/IgG1 . | BXP21/IgG2a . |
|---|---|---|
| MCF-7 S | 9.8 ± 6.8 | 6.5 ± 2.4 |
| MCF-7 MR | 50.6* ± 8.1 | 11.3* ± 0.6 |
| MCF-7 Dox40 | 7.6 ± 0.6 | 2.3 ± 0.3 |
The results are expressed as MFI ratios and represent mean ± SD of at least 3 experiments.
P < .05 compared with MCF-7.
Mitoxantrone accumulation in cell lines.
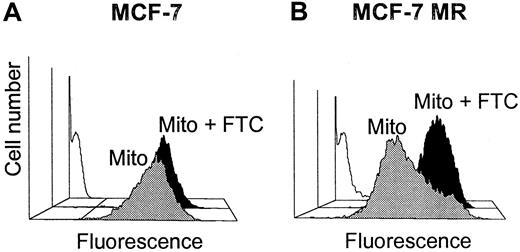
Then, to determine whether the BCRP protein expression was reflected by the capacity to extrude mitoxantrone, the accumulation of mitoxantrone was measured in the cell lines. The accumulation, expressed as MFI, after exposure to mitoxantrone (10 μM) was 211 ± 15 (n = 4) in the MCF-7 cell line versus 93 ± 36 (n = 3, P < .05) in the MCF-7 MR and 109 ± 41 in the MCF-7 Dox40 (n = 3,P < .05) cell lines. This reflects a high capacity of the MCF-7 MR and MCF-7 Dox40 cell lines to extrude mitoxantrone (Table2 and Figure1). To check whether the efflux of mitoxantrone in the MCF-7 MR cell line was BCRP-mediated, the BCRP inhibitor FTC (10 μM) was added. In the MCF-7 cell line, the presence of FTC caused a change in mitoxantrone accumulation from 211 ± 15 MFI to 298 ± 14 MFI, representing a shift of 86 ± 14 MFI (n = 4). In the MCF-7 MR cell line, the addition of FTC caused a larger change in mitoxantrone accumulation from 93 ± 36 MFI to 341 ± 89 MFI (a shift of 254 ± 54, P < .05), which indicated that the high capacity of the MCF-7 MR cell line to transport mitoxantrone was mediated by BCRP (Table 2 and Figure 1). In the P-gp–overexpressing cell line, MCF-7 Dox40 FTC had a limited effect; a shift of 22 ± 22 MFI was observed. Next, the effect of the P-gp inhibitor PSC833 (2 μg/mL) was determined on the accumulation of mitoxantrone. The addition of PSC833 caused low mitoxantrone accumulation shifts of 52 ± 6 MFI (n = 3) in the MCF-7 cell line and 23 ± 9 MFI (n = 3) in the MCF-7 MR cell line, in contrast to a high shift of 242 ± 93 MFI (n = 3, P < .05) in the P-gp–overexpressing MCF-7 Dox40 cell line. This corresponded with the P-gp activities in these cell lines, as determined by measuring the Rh123 efflux-blocking capacity of PSC833. Low Rh123 efflux-blocking factors by PSC833 of 1.2 ± 0.3 and 1.1 ± 0.2 (n = 3) were observed in the MCF-7 and MCF-7 MR cell lines, respectively, versus a high factor of 20.3 ± 2.0 in MCF-7 Dox40 cell line (n = 3, P < .05). MK-571, an inhibitor of MRP1, showed a shift of 89 ± 14 MFI (n = 4) in the MCF-7, 54 ± 16 MFI (n = 3) in the MCF-7 MR, and 36 ± 39 MFI (n = 3) in the MCF-7 Dox40 cell lines. MRP activity, as determined by the CF efflux-blocking factor of MK-571, was 3.3 ± 0.3 in the MCF-7, 4.6 ± 0.9 in the MCF-7 MR, and 7.6 ± 0.9 in the MCF-7 Dox40 cell lines (n = 3) (Table 2).
Mitoxantrone accumulation studies in MCF-7 and MCF-7 MR cell lines
| . | Mitoxantrone accumulation, MFI . | FTC shift, MFI . | PSC833 shift, MFI . | P-gp activity . | MK-571 shift, MFI . | MRP activity . |
|---|---|---|---|---|---|---|
| MCF-7 | 211 ± 15 | 86 ± 14 | 52 ± 6 | 1.2 ± 0.3 | 89 ± 14 | 3.3 ± 0.3 |
| MCF-7 MR | 93* ± 36 | 254* ± 54 | 23 ± 9 | 1.1 ± 0.2 | 54 ± 16 | 4.6 ± 0.9 |
| MCF-7 Dox40 | 109* ± 41 | 22* ± 22 | 242† ± 93 | 20.3† ± 2.0 | 36 ± 39 | 7.6 ± 0.9 |
| . | Mitoxantrone accumulation, MFI . | FTC shift, MFI . | PSC833 shift, MFI . | P-gp activity . | MK-571 shift, MFI . | MRP activity . |
|---|---|---|---|---|---|---|
| MCF-7 | 211 ± 15 | 86 ± 14 | 52 ± 6 | 1.2 ± 0.3 | 89 ± 14 | 3.3 ± 0.3 |
| MCF-7 MR | 93* ± 36 | 254* ± 54 | 23 ± 9 | 1.1 ± 0.2 | 54 ± 16 | 4.6 ± 0.9 |
| MCF-7 Dox40 | 109* ± 41 | 22* ± 22 | 242† ± 93 | 20.3† ± 2.0 | 36 ± 39 | 7.6 ± 0.9 |
The results represent mean ± SD of at least 3 experiments. The concentrations used are as follows: mitoxantrone, 10 μM; FTC, 10 μM; PSC833, 2 μg/mL; and MK-571, 20 μM. P-gp activity is expressed as PSC833 efflux-blocking factor of Rh123. MRP activity is expressed as MK-571 efflux-blocking factor of CF.
P < .05 compared with MCF-7.
P < .05 compared with MCF-7 and MCF-7 MR.
Mitoxantrone accumulation.
Accumulation was measured flow cytometrically in MCF-7 (A) and MCF-7 MR (B) cell lines after 60 minutes of incubation without (blank) or with mitoxantrone (10 μM) in the presence (black) or absence (gray) of 10 μM FTC.
Mitoxantrone accumulation.
Accumulation was measured flow cytometrically in MCF-7 (A) and MCF-7 MR (B) cell lines after 60 minutes of incubation without (blank) or with mitoxantrone (10 μM) in the presence (black) or absence (gray) of 10 μM FTC.
Patients
Patient characteristics.
Paired bone marrow or peripheral blood samples were collected from 20 AML patients. The patients were treated with intensive chemotherapy regimens according to the protocol of the Hovon 4/4A or Hovon 29,26 27 which included the chemotherapeutic agents cytosine arabinoside (Ara-C, 200 mg/m2, intravenously, days 1-7), daunorubicin (45 mg/m2, intravenously, days 1-3), or idarubicin (12 mg/m2, intravenously, days 5-7) in the induction cycle of chemotherapy and Ara-C (2 mg/m2, intravenously, days 1-6) plus amsacrine (120 mg/m2, intravenously, days 4-6) in the second induction cycle. Patients with FAB classification M3 received all-trans-retinoic acid. After the 2 induction cycles, patients were to receive a third cycle consisting of mitoxantrone (10 mg/m2, intravenously, days 1-5) plus etoposide (100 mg/m2, intravenously, days 1-5) or receive an autograft transplantation after conditioning treatment with busulfan (4 mg/kg, orally, days 7-4 prior to transplantation) and cyclophosphamide (60 mg/kg, intravenously, days 3-2 prior to transplantation). After treatment 15 AML patients reached complete remission, 4 patients reached partial remission defined as less than 25% of AML blasts in the bone marrow smear, and 1 patient was refractory to the treatment. Patient characteristics are described in Table 3.
Characteristics of AML patients
| Patient no. . | FAB classification . | Treatment . | Remission . |
|---|---|---|---|
| 1 | M2 | Ara-C, ida, amsa, asct | CR |
| 2 | M2 | Ara-C, ida, amsa, etop, mito | PR |
| 3 | M5 | Ara-C, ida, amsa, etop, mito | CR |
| 4 | M0 | Ara-C, ida, amsa, asct | CR |
| 5 | M5 | Ara-C, ida, amsa, etop, mito | CR |
| 6 | M1 | Ara-C, ida, amsa, asct | NR |
| 7 | M5 | Ara-C, dauno, etop, mito | CR |
| 8 | M3 | Ara-C, ida, amsa, etop, mito | CR |
| 9 | M2 | Ara-C, ida, amsa, asct | PR |
| 10 | M4 | Ara-C, ida, amsa, etop, mito | CR |
| 11 | M1 | Ara-C, ida, etop, mito | CR |
| 12 | M2 | Ara-C, ida, amsa, asct | CR |
| 13 | M2 | Ara-C, dauno, amsa, etop, mito | CR |
| 14 | M2 | Ara-C, ida, amsa, asct | CR |
| 15 | M4 | Ara-C, ida, amsa, etop, mito | CR |
| 16 | M2 | Ara-C, dauno, amsa, etop, mito | PR |
| 17 | M2 | Ara-C, etop, mito | CR |
| 18 | M4 | Ara-C, dauno, asct | CR |
| 19 | M2 | Ara-C, dauno, amsa, asct | CR |
| 20 | M0 | Ara-C, dauno, amsa, asct | PR |
| Patient no. . | FAB classification . | Treatment . | Remission . |
|---|---|---|---|
| 1 | M2 | Ara-C, ida, amsa, asct | CR |
| 2 | M2 | Ara-C, ida, amsa, etop, mito | PR |
| 3 | M5 | Ara-C, ida, amsa, etop, mito | CR |
| 4 | M0 | Ara-C, ida, amsa, asct | CR |
| 5 | M5 | Ara-C, ida, amsa, etop, mito | CR |
| 6 | M1 | Ara-C, ida, amsa, asct | NR |
| 7 | M5 | Ara-C, dauno, etop, mito | CR |
| 8 | M3 | Ara-C, ida, amsa, etop, mito | CR |
| 9 | M2 | Ara-C, ida, amsa, asct | PR |
| 10 | M4 | Ara-C, ida, amsa, etop, mito | CR |
| 11 | M1 | Ara-C, ida, etop, mito | CR |
| 12 | M2 | Ara-C, ida, amsa, asct | CR |
| 13 | M2 | Ara-C, dauno, amsa, etop, mito | CR |
| 14 | M2 | Ara-C, ida, amsa, asct | CR |
| 15 | M4 | Ara-C, ida, amsa, etop, mito | CR |
| 16 | M2 | Ara-C, dauno, amsa, etop, mito | PR |
| 17 | M2 | Ara-C, etop, mito | CR |
| 18 | M4 | Ara-C, dauno, asct | CR |
| 19 | M2 | Ara-C, dauno, amsa, asct | CR |
| 20 | M0 | Ara-C, dauno, amsa, asct | PR |
Ida indicates idarubicin; amsa, amsacrine; asct, autologous stem cell transplantation, including conditioning regimen with busulfan and cyclophosphamide; etop, etoposide; mito, mitoxantrone; dauno, daunorubicin; CR, complete remission; PR, partial remission; and NR, no response.
BCRP protein expression in patient samples.
To study whether BCRP is expressed at the protein level in AML cells and whether it is up-regulated at relapse or refractory disease, we determined BCRP protein expression in the AML patient samples. In 38 of the 40 AML samples, a sufficient cell number was obtained to measure BCRP protein expression. The BXP-34/IgG1 ratio varied between 0.8 and 2.7 (1.6 ± 0.5, mean ± SD, n = 38) in the group of AML patients. The BXP-34/IgG1 ratios in all AML samples were lower than the observed ratios in the MCF-7 (9.8 ± 6.8) cell line. BCRP expression in the patient samples could be measured more sensitively with the BXP-21 monoclonal antibody; BXP-21/IgG2a ratios varied between 1.1 and 14.5 (4.9 ± 3.0, n = 38) in the whole group of AML samples versus ratios of 6.5 ± 2.4 in the MCF-7 and 11.3 ± 0.6 in the MCF-7 MR cell lines; 27 (73%) of the 37 samples showed BXP-21 levels below the level of the MCF-7 cell line, 9 (24%) patient samples showed BXP-21 levels between the MCF-7 and MCF-7 MR cell lines, and 1 (3%) patient sample showed a higher BXP-21/IgG2a ratio than the MCF-7 MR cell line. A correlation between BXP-34/IgG1 and BXP-21/IgG2a ratios was observed (r = 0.48, P = .003, n = 37) in the whole group of AML samples. The normal bone marrow mononuclear cells showed a BXP-34/IgG1 ratio of 1.7 ± 0.2 (n = 3), which was not significantly different from the patient samples, and a BXP-21/IgG2a ratio of 1.3 ± 0.1 (n = 3), which was lower than the patient samples (P < .05). Because the measurement of BCRP expression with BXP-21 appeared to be more sensitive than BXP-34 in the AML samples, these data were used in the following statistical analyses.
Although the overall expression of BCRP protein was slightly higher at relapse versus primary samples, no significant up-regulation of BCRP protein expression was observed at relapse (5.3 ± 3.3, n = 19) versus primary disease (4.4 ± 2.7, P = .4, n = 18). However, increases or decreases of BCRP protein expression at relapse could be observed in individual cases; BCRP expression was higher in 6 relapses and lower in 7 relapsed cases, whereas in 5 cases BCRP expression had not changed at relapse. Table4 shows the data of the individual patient samples.
BCRP protein expression and mitoxantrone accumulation in the individual AML patients
| Patient no. . | BXP-21/IgG2a ratio . | Mitoxantrone, MFI . | ||
|---|---|---|---|---|
| Primary . | Relapse . | Primary . | Relapse . | |
| 1 | 1.57 | 1.70 | 761 | 302 |
| 2 | 4.44 | 6.56 | 139 | 120 |
| 3 | 1.97 | 6.98 | 445 | 115 |
| 4 | 4.83 | 4.91 | 111 | 107 |
| 5 | 2.74 | 2.15 | 270 | 419 |
| 6 | 4.57 | 7.51 | 70 | 44 |
| 7 | 2.85 | 1.98 | 165 | 177 |
| 8 | 1.82 | 4.05 | 390 | 399 |
| 9 | 8.77 | 9.24 | 96 | 89 |
| 10 | 10.06 | 9.00 | 155 | 144 |
| 11 | 3.88 | 2.94 | 179 | 202 |
| 12 | 3.93 | 4.29 | 238 | 322 |
| 13 | 4.42 | 3.52 | 488 | 269 |
| 14 | 8.29 | 14.49 | 222 | 275 |
| 15 | 1.08 | 1.25 | 422 | 292 |
| 16 | 3.33 | 7.96 | 354 | 184 |
| 17 | nd | nd | 202 | 211 |
| 18 | 2.34 | 4.63 | 109 | 115 |
| 19 | 7.68 | 2.85 | 102 | 126 |
| 20 | 5.88 | 5.14 | 127 | 107 |
| Patient no. . | BXP-21/IgG2a ratio . | Mitoxantrone, MFI . | ||
|---|---|---|---|---|
| Primary . | Relapse . | Primary . | Relapse . | |
| 1 | 1.57 | 1.70 | 761 | 302 |
| 2 | 4.44 | 6.56 | 139 | 120 |
| 3 | 1.97 | 6.98 | 445 | 115 |
| 4 | 4.83 | 4.91 | 111 | 107 |
| 5 | 2.74 | 2.15 | 270 | 419 |
| 6 | 4.57 | 7.51 | 70 | 44 |
| 7 | 2.85 | 1.98 | 165 | 177 |
| 8 | 1.82 | 4.05 | 390 | 399 |
| 9 | 8.77 | 9.24 | 96 | 89 |
| 10 | 10.06 | 9.00 | 155 | 144 |
| 11 | 3.88 | 2.94 | 179 | 202 |
| 12 | 3.93 | 4.29 | 238 | 322 |
| 13 | 4.42 | 3.52 | 488 | 269 |
| 14 | 8.29 | 14.49 | 222 | 275 |
| 15 | 1.08 | 1.25 | 422 | 292 |
| 16 | 3.33 | 7.96 | 354 | 184 |
| 17 | nd | nd | 202 | 211 |
| 18 | 2.34 | 4.63 | 109 | 115 |
| 19 | 7.68 | 2.85 | 102 | 126 |
| 20 | 5.88 | 5.14 | 127 | 107 |
The results are given of BXP-21/IgG2a ratios and mitoxantrone accumulations of the individual patients at diagnosis of the AML and at relapse.
No effect of the particular chemotherapeutic agent used could be observed on the BCRP protein expression at relapse or refractory disease of the patients treated with daunorubicin (4.3 ± 2.1, n = 6), idarubicin (5.7 ± 3.8, n = 13), or mitoxantrone plus etoposide (4.6 ± 2.8, n = 11) versus de novo AML (4.4 ± 2.0, P = .75; 4.1 ± 3.0,P = .09; and 3.6 ± 2.6, P = .76; respectively).
The expression of BCRP protein correlated with the functional expression of MRP, as determined in a previous study21(r = 0.43, P = .007, n = 38), but not with the functional expression of P-gp (P = .12).
Mitoxantrone accumulation in patient samples.
The next purpose was to study whether a high BCRP protein expression was reflected by a high BCRP activity, resulting in a low mitoxantrone accumulation, and whether the BCRP activity was increased in relapsed or refractory AML. Therefore, the intracellular accumulation of mitoxantrone was determined after 60 minutes of exposure (10 μM) in the absence and presence of the BCRP inhibitor FTC (10 μM). In addition, the capacity of P-gp and MRP to extrude mitoxantrone was measured by incubating with mitoxantrone plus the inhibitors PSC833 (2 μg/mL) and MK-571 (20 μM). Furthermore, combinations of the 3 inhibitors were added to check whether BCRP, P-gp, and MRP showed an additive effect in extruding mitoxantrone. The intracellular accumulation of mitoxantrone in the absence of the inhibitors varied widely between the whole group of AML samples (mean 227 ± 146 MFI, range 44-761, n = 40), whereas the normal bone marrow samples showed a higher mitoxantrone accumulation of 695 ± 153 MFI, (P < .05, n = 3). In 17 patient samples the mitoxantrone accumulation was higher than the mitoxantrone level of the MCF-7 cell line (211 ± 15 MFI); in 20 patient samples the mitoxantrone level was between the levels of the MCF-7 and MCF-7 MR (93 ± 36 MFI) cell lines, and in 3 patient samples the mitoxantrone accumulation was even lower than the MCF-7 MR cell line.
The mitoxantrone accumulation in the whole group of relapsed samples was slightly lower (201 ± 107 MFI, n = 20) versus the whole group of primary samples (252 ± 176 MFI, n = 20), but the difference was not significant (P = .39). In 5 individual patient cases (patient nos. 1, 3, 13, 15, and 16) the accumulation of mitoxantrone was lower at relapse versus de novo AML, in 3 relapses (nos. 5, 12, and 14) the mitoxantrone accumulation was higher than in the primary samples, and in 12 cases no major differences (< 50 MFI) were found in mitoxantrone level between primary and relapsed or refractory samples (individual cases are shown in Table 4).
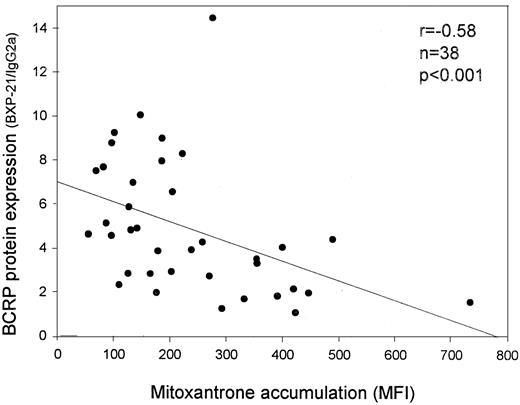
A strong correlation was observed between the intracellular mitoxantrone level and the expression of BCRP protein as measured with BXP-34 and BXP-21; patients with the highest expression of BCRP protein showed the lowest level of mitoxantrone accumulation (r = −0.37, P = .025 for BXP-34 andr = −0.58, P < .001 for BXP-21, n = 38) (Figure 2). In addition, a correlation existed between the capacity to extrude mitoxantrone and the functional activities of P-gp (r = −0.56, P < .001, n = 40) and MRP (r = −0.58, P < .001, n = 40), which were determined in these samples in the previous study,21 indicating that P-gp and MRP also extrude mitoxantrone in these patient samples. When the capacity of P-gp to extrude mitoxantrone was inhibited by PSC833, the correlation between high BCRP protein expression and low mitoxantrone accumulation remained present (r = −0.33, P = .048 for BXP-34 andr = −0.56, P < .001 for BXP-21, n = 38). In addition, when the capacity of MRP to extrude mitoxantrone was inhibited by MK-571, the correlation between high BCRP protein expression and low mitoxantrone accumulation was maintained (r = −0.37, P = .023 for BXP-34 andr = −0.48, P = .003 for BXP-21, n = 38). When both P-gp and MRP activity were inhibited using the combination of PSC833 and MK-571, which could be performed in 18 patient samples, the correlation between mitoxantrone accumulation and BCRP expression, as measured with BXP-34, was even stronger (r = −0.57,P = .018, n = 18). However, this correlation was not observed between mitoxantrone accumulation and BXP-21 in this small group of patient samples (r = −0.36,P = .14, n = 18).
Correlation between BCRP protein expression and mitoxantrone accumulation in AML patient samples.
BCRP expression was measured with the BXP-21 monoclonal antibody, and mitoxantrone accumulation was measured after 60 minutes of incubation with mitoxantrone (10 μM).
Correlation between BCRP protein expression and mitoxantrone accumulation in AML patient samples.
BCRP expression was measured with the BXP-21 monoclonal antibody, and mitoxantrone accumulation was measured after 60 minutes of incubation with mitoxantrone (10 μM).
The addition of the BCRP inhibitor FTC (10 μM) showed a small increase of the mitoxantrone accumulation in the patient samples (11 ± 29 MFI). The addition of PSC833 alone showed an increase of 54 ± 56 MFI, and the addition of MK-571 alone showed an increase of 32 ± 55 MFI. The addition of FTC to the combination of PSC833 and MK-571 caused an increase of the shift from 80 ± 64 MFI to 98 ± 69 MFI (n = 18), which indicates that BCRP has a low capacity to transport mitoxantrone independently of P-gp and MRP.
Immunophenotype.
Because it has been described that the expression of P-gp and MRP are correlated with the expression of CD34,31 and with the early maturation stage, as determined by immune phenotyping,21 we were interested if BCRP protein was also especially expressed at the early stage in AML cells. Therefore, we determined the percentages of the immature (CD34+/CD38− and CD34+/CD33−) and more mature (CD34+/CD33+ and CD34−/CD33+) subclasses of cells. The percentages of cells in the immunophenotypic subclasses were correlated with BCRP protein expression and mitoxantrone accumulation. The results are shown in Table 5. Indeed, a high expression of BCRP protein, as measured with BXP-21, was correlated with a high percentage of CD34+ cells in the whole group of AML patients (r = 0.54, P = .001, n = 38), which was predominantly due to a correlation between the percentage of the immature CD34+/CD33− subclass of the CD34+ cells and BCRP expression (r = 0.40,P = .02, n = 38). A low BCRP protein expression was observed in the cases with a high percentage of the more mature CD34−/CD33+ blasts (r = −0.56,P < .001, n = 38).
Correlations between immune phenotype, BCRP expression, and mitoxantrone accumulation
The results represent correlation coefficients between the percentages of the different immune phenotypic subclasses and BCRP protein expression, expressed as BXP-21/IgG2a ratios, and mitoxantrone accumulation, expressed as MFI. P values are presented in parentheses.
Significant correlations.
These results were underlined by a correlation between a low mitoxantrone accumulation and a high percentage of CD34+cells (r = −0.35, P = .03, n = 40), especially the immature CD34+/CD38− subset of cells (r = −0.60, P < .001, n = 38). In addition, a correlation was found between a high mitoxantrone accumulation and a high percentage of CD33+ blasts (r = 0.45, P = .005, n = 38), which mainly consisted of the mature CD34−/CD33+ cells (r = 0.61, P = .001, n = 38). Furthermore, these correlations became even stronger when the capacity of P-gp and MRP to extrude mitoxantrone was inhibited with PSC833 and MK-571 (Table5), indicating that the correlation between BCRP activity and immature immunophenotype is stronger than the correlations of P-gp and MRP activities with immature immunophenotype.
In summary, a relatively high BCRP activity and protein expression were observed in AML cells with an immature immunophenotype, whereas a low BCRP activity and protein expression were found in AML cells with a mature immunophenotype.
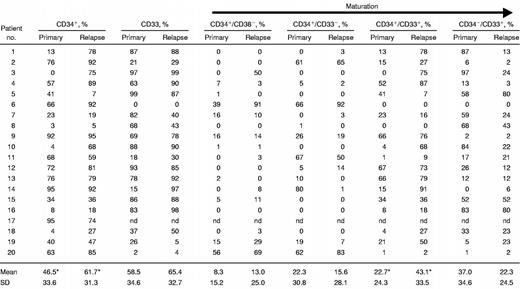
In addition, increases and decreases in the percentages of the immunophenotypic subclasses at relapse/refractory disease versus primary AML were analyzed (Table 6). The mean percentage of CD34+ cells in the whole group of AML samples was 54.1% ± 32.9% (n = 40). The percentage of CD34+ cells at relapse (61.7% ± 31.3%, n = 20) was increased versus de novo AML (46.5% ± 33.6%, P = .03, n = 20). The increase in CD34+ population was predominantly caused by an increase of the more mature CD34+/CD33+ subpopulation at relapse (43.1% ± 33.5%, n = 19) versus diagnosis (22.7% ± 24.3%,P = .004, n = 15). No difference was observed between primary and relapsed samples in the percentages of the other subclasses of cells.
Phenotype of the AML blasts at diagnosis and at relapse or refractory disease
nd indicates not done.
Significant difference between primary and relapsed/refractory samples.
Treatment with idarubicin or daunorubicin was not correlated with an increase of any of the specific subsets of AML cells. The AML patients who were not treated with mitoxantrone and etoposide but received an autograft transplantation showed a higher percentage of CD34+ cells (78.0% ± 22.3%) and of CD33+cells (60.8% ± 36.8%) at relapse as compared with the primary AML (55.8% ± 31.7%, P = .01, n = 9 for CD34 and 43.6% ± 35.6%, P = .04, n = 9 for CD33). This was predominantly caused by an increase in the CD34+/CD33+ subfraction at relapse (53.8% ± 35.8%) versus the primary AML (26.6% ± 27.5%,P = .01, n = 9).
Discussion
The present study demonstrates for the first time that BCRP protein expression can be analyzed by flow cytometry in AML samples. Thus far, BCRP expression in AML samples has been studied at the mRNA level20,32 or at protein level with immunohistochemistry.22 In the latter study using the monoclonal antibody BXP-34, we observed no BCRP protein expression in the MCF-7 cell line and in AML samples.22 The flow cytometric assay presented in the current study appeared to be more sensitive, because distinct levels of BCRP expression were observed in the MCF-7 cell line and the AML patient samples.
In the flow cytometric assay BXP-21 was more sensitive than BXP-34 for patient samples, whereas in the cell lines the reverse was noticed. This difference was predominantly due to high values of the IgG2a isotype control in the cell lines. A low correlation was observed between BXP-21 and BXP-34 expression (r = 0.48), which can be explained by the low and therefore insensitive BXP-34 expression levels.
BCRP protein expression correlated strongly with mitoxantrone accumulation, with patients with relatively high BCRP protein expression showing a low mitoxantrone accumulation. When P-gp and MRP activity were inhibited with PSC833 or MK-571 alone, the correlation with BCRP expression, as determined with the monoclonal antibodies BXP-34 and BXP-21, remained. When both P-gp and MRP activity were inhibited simultaneously with the combination of PSC833 and MK-571, the correlation between mitoxantrone accumulation and BCRP expression remained in the case of the BXP-34 monoclonal antibody. The specific epitopes of the BCRP protein that are recognized by BXP-34 and BXP-21, respectively, have not yet been identified. In the present study as well as in an additional study (unpublished data), we observed a better correlation between BCRP activity and expression as measured with BXP-34 versus the BXP-21 monoclonal antibody in clinical samples. We therefore hypothesize that BXP-21, although it specifically binds to BCRP and shows no cross-reactivity with other proteins,22might recognize the BCRP protein only in a not functionally active state in these clinical samples.
The reversing substance FTC appeared to increase effectively the mitoxantrone accumulation in the cell lines. A small increase of the mitoxantrone accumulation was observed upon addition of FTC in the patient samples. Moreover, the addition of FTC to the combination of PSC833 and MK-571 caused an additional increase in mitoxantrone accumulation, which confirms that BCRP transports mitoxantrone in addition to P-gp and MRP. PSC833 had a more distinct effect on the accumulation of mitoxantrone than MK-571 and FTC. Therefore, P-gp seems to play the most important role in extruding mitoxantrone in the AML patient samples. These findings emphasize that the resistance mediated by drug efflux pumps is a complex phenomenon and might provide a plausible explanation for the disappointing results of the clinical use of a specific inhibitor of only one transporter protein.33,34 Moreover, it has been demonstrated that the inhibition or absence of one transporter protein might force or induce the activity of additional transporters,35 36 which may further limit the effectivity of the selective inhibition of transporter proteins.
For P-gp and MRP1 it has been observed that selected cell lines acquire much higher levels of P-gp and MRP1 expression and function than clinical samples.30 The P-gp and MRP1 expression and function in the clinical AML samples appeared, however, to correlate with drug resistance4,6 7; BCRP expression might follow the same pattern.
BCRP protein expression was not consistently up-regulated at relapse or refractory disease. In some cases BCRP expression was higher and in other cases lower at relapse, which was related to the immunophenotype of the AML cells. A strong positive correlation was observed between BCRP protein expression and immature phenotype, reflected by CD34 positivity, whereas a strong negative correlation was found between BCRP protein expression and a more mature phenotype (CD34−/CD33+). Interestingly, the correlation between BCRP expression and maturation stage has been described recently for normal hematopoietic cells. BCRP expression has been observed especially in the most primitive and quiescent hematopoietic progenitors,23-25 as has also been found for P-gp and MRP,21,31 suggesting a role for the transporter proteins in maintaining a primitive phenotype in hematopoietic cells, as has been previously described for an ABC transporter in Dictyosteliumcells.37 Alternatively, it has been described that P-gp plays a role in protecting cells from apoptosis,38 39which also might be the case in stem cells or primitive AML cells with high expressions of P-gp as well as BCRP.
A recently presented study reported an up-regulation of BCRP mRNA expression at relapse in a group of 20 paired samples of primary and relapsed or refractory AML in which a 1.7-fold increase of mRNA expression was observed.32 The present study did not confirm these data with BCRP protein expression. Because both studies present the results of a small and selected group of 20 paired AML samples, this might explain the discrepant results.
An increased percentage of CD34+ cells was detected at relapse or refractory disease versus de novo AML, mainly consisting of the CD34+/CD33+ subpopulation of cells, which is consistent with findings in other studies describing increased CD34+ 40 and CD33+ 40,41 populations at relapse in AML.
In summary, the present study shows that BCRP protein is expressed in AML blasts at variable levels. Furthermore, BCRP in AML blasts, although at a low level, seems capable of actively transporting mitoxantrone, a substrate used in the treatment of AML, and therefore appears to be an additional transporter in AML. BCRP expression and reversal of BCRP-mediated transport should be studied in a larger group of AML patients in the future to determine the effect of BCRP expression and activity on clinical outcome. BCRP expression, as also observed for P-gp and MRP, was not consistently up-regulated in all relapsed/refractory AML cases but was up-regulated in some cases and decreased in other cases and appeared to be correlated with immature immunophenotype.
Supported by a grant of the Foundation of Pediatric Oncology Groningen (SKOG 99-01).
Submitted August 7, 2001; accepted January 9, 2002.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
E. G. E. de Vries, Division of Medical Oncology, Dept of Internal Medicine, University Hospital Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands; e-mail:e.g.e.de.vries@int.azg.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal