The origin of T cells after highly active antiretroviral therapy (HAART) in patients infected with human immunodeficiency virus 1 (HIV-1) is now under discussion. The possibility of renewed lymphopoiesis in aged thymuses is still controversial. In this work we combine the analysis of naı̈ve T cells, T-cell receptor excision circles (TRECs), and computed tomography scanning of thymic tissue to further assess whether the thymus is involved in immune reconstitution. Fifteen antiretroviral-naı̈ve HIV-1–infected patients were evaluated during 48 weeks of HAART. At baseline, significant correlation was present among age and both thymic volume and TRECs, and between naı̈ve T cells and TRECs. After starting HAART, there was a significant increase at week 12 in naı̈ve CD4+and CD8+ T cells, TRECs, and thymic volume. The initial net increases in naı̈ve T cells and TREC counts were significantly correlated. Changes in thymic volume and TRECs were also indirectly related; splitting the population into 2 groups of high and low baseline TREC levels, only the group with low TREC levels had significant increases in both TRECs and thymic volume. Thus, the increase in thymic volume might be functional, in response to depleted TREC levels. Taken together, our data strongly suggest a thymic role in immune reconstitution, at least in patients with depleted baseline TREC levels.
Introduction
Infection by human immunodeficiency virus 1 (HIV-1) is characterized by a progressive depletion of not only naı̈ve and memory CD4+ T-cell subsets, but also the naı̈ve subset of CD8+ T cells.1,2 These alterations can be partially corrected after inhibition of HIV-1 replication by highly active antiretroviral therapy (HAART), although the mechanism for which T-cell counts increase thereafter is still controversial. In fact, more than one mechanism might be involved. Soon after starting HAART, a rapid increase in peripheral blood CD4+ T-cell counts probably reflects a redistribution from lymph node–sequestered memory T cells,3,4 which ends after a few weeks. On the other hand, naı̈ve CD4+ and CD8+ T-cell increases are slower but maintained for a longer period.5
An especially interesting issue is whether the thymus contributes to the repopulation of naı̈ve T cells or whether they are derived from other sources. In the setting of pediatric HIV-1 infection, naı̈ve T-cell repopulation after antiretroviral treatment is early6 and correlated with enlarged thymic volumes.7 However, the thymic role in immune reconstitution of adults remains a controversial issue, due to the accepted thymic involution in adulthood. Thymic evaluation by computed tomography (CT) scans has shown a higher initial increase in naı̈ve T-cell counts in patients with abundant thymic tissue after 48 weeks of HAART.8 However, early increases in naı̈ve T cells after starting HAART have been also reported in some thymectomized HIV-1–infected patients.9 In addition, the determination of thymic output by quantification of T-cell receptor excision circles (TRECs) has been recently described. TRECs are byproducts of T-cell receptor (TCR) gene rearrangements, generated during lymphocyte maturation in the thymus.10,11They are stable, not duplicated during mitosis, and rapidly diluted in proliferating T-cell subpopulations. It has been shown that TREC levels are increased after HAART in adult patients,10,11suggesting a renewed thymic function. However, the decrease in T-cell turnover after HAART12 13 may be an alternative explanation for the TREC levels increases.
Thus, the objective of this work was to further study whether immune reconstitution after HAART includes the generation of recent thymic emigrants (RTEs). In this way, we have combined the quantification of thymic volumes (by CT), TREC levels, and naı̈ve T cells, in HIV-1–infected patients following 48 weeks of HAART.
Patients, materials, and methods
Patients
From August 1998, all consecutive, antiretroviral-naı̈ve HIV-1–infected patients starting HAART were candidates for study. HAART was defined as 2 nucleoside reverse transcriptase inhibitors plus a protease or a nonnucleoside reverse transcriptase inhibitor or both. By December 2000, 26 patients had agreed to participate and 15 had reached 48 weeks of follow-up. All patients reported therapy compliance of 95% or higher. Patients were evaluated at baseline and at weeks 12, 24, 36, and 48 of treatment. At each visit, CD4+, CD8+, and CD3+ T-cell counts and HIV-1 RNA levels were determined in fresh samples. Peripheral blood mononuclear cells (PBMCs) were isolated and kept frozen in liquid nitrogen until further analysis of TRECs and naı̈ve T-cell subsets. Patients underwent a thoracic CT for the measurement of the thymic volume at baseline and at weeks 12, 24, and 48 of treatment, after written informed consent was obtained. Six CT determinations from the 60 initially programmed were lost (10%), belonging to 5 patients. This study was approved by the Ethical Committee of the hospital.
CT
Mediastinal CT scans were performed with a modified method as previously described.14 Briefly, CT scans were obtained on a 3000 GE Sytec scanner, using 5-mm thick contiguous sections at 5-mm intervals. Measurements were always performed by the same radiologist, who worked with coded samples. For the determination of the thymic volume, the first and last slices containing thymic tissue were identified. Then, thymic tissue in all the slices between the first and the last was carefully delimited to exclude mediastinal fat infiltration, based on the different density observed (higher density for soft tissues and lower density for surrounding fat). The software (CT Sytec software, 2.02 version) then integrated all the defined thymic areas across the slices (being the first and last slice volumes automatically halved to account for partial volume averaging) and gave the calculated thymic volume in cubic centimeters. Additionally, thymic tissue was scored on a 0 to 5 scale to distinguish fat infiltration within the parenchyma, similarly to a previously described index (0 for only fat, and 5 for only thymic tissue15).
Flow cytometry
Cells were thawed and an aliquot assessed for percentages of naı̈ve T-cell subsets (CD4+CD45RA+CD45RO− and CD8+CD45RA+CD11alow) as previously described.6 Absolute counts were calculated by multiplying their representation in frozen samples by the absolute CD4+and CD8+ T-cell subset counts obtained from fresh blood samples.
Quantification of TREC levels in PBMCs
One proposed molecular marker for determination of RTEs are the TRECs generated during thymocyte maturation. A polymerase chain reaction (PCR)–based method for quantifying δRec-ΨJα TRECs has been described by Douek et al.10 We have adapted this PCR to a real-time PCR setting, using a LightCycler (Roche Molecular Biochemicals, Mannheim, Germany), for quantification of both the characteristic signal-joint sequences harbored by TRECs and genomic β-globin gene sequences (to normalize by DNA content).
The TREC levels in each PBMC sample were determined using an external standard curve made with DNA from a unique pediatric thymic sample for the entire study. A postnatal thymus sample was obtained during corrective cardiac surgery of an HIV-uninfected 2-year-old patient, after approbation by the Ethical Committee of the hospital. A single-cell suspension of thymocytes was obtained by mechanical disruption and Fycoll-Hypaque centrifugation. Previously, the 376–base pair amplification product of the signal-joint sequence was obtained from that thymic sample using PCR conditions described below, and cloned into the pGEM T-EASY vector (Promega, Madison, WI). Identity of the cloned PCR product was confirmed by sequencing. Using known concentrations of the cloned fragment, the number of TREC molecules in the reference thymic sample was calculated as 62 500 TREC/106 cells.
All follow-up samples from each individual were simultaneously measured in duplicate PCR reactions. All samples available were PCR positives for TRECs, with a distribution between 100 and 11 000 TREC/ 106 PBMCs.
In addition to the TREC/106 PBMCs, the absolute counts of TREC/μL were derived for each sample. Because all the TRECs in PBMCs are included inside the CD3+ subpopulation,11the TREC/106 PBMC proportion was corrected by the percentage of CD3+ cells found by flow cytometry in the PBMCs, yielding the number of TREC/106 CD3+cells. This proportion was then multiplied by absolute CD3+cell counts obtained in fresh blood by flow cytometry.
The amplified signal-joint TREC fragment was detected by fluorescence resonance energy transfer between 2 adjacent hybridization probes. Jurkat E6-1 DNA was used as a negative control. PCR reactions were performed in a volume of 20 μL, with 400 nmol/L of each primer (5′-AAA GAG GGC AGC CCT CTC CAA GGC, 5′-AGG CTG ATC TTG TCT GAC ATT TGC TCC G), 100 nmol/L of each detection probe (5′-AGG GAT GTG GCA TCA CCT TTG TTG ACA labeled on its 3′ end with fluorescein, 5′-GGC ACC CCT CTG TTC CCC ACA GGA, labeled with the acceptor fluorophore LightCycler RED 640 at its 5′ end), and 100 to 400 ng DNA. LightCycler DNA Master Hybridization Probes buffer was used (Roche Molecular Biochemicals) with a final Mg++ concentration of 3.5 mM. After an initial denaturation step at 95°C for 120 seconds, amplification was performed by 45 cycles of denaturation (95°C for 0 seconds), annealing (63°C for 40 seconds), and extension (72°C for 40 seconds).
To normalize by genomic DNA concentration, primers GH20 and PC04 for the human β-globin gene were used.16 PCR reactions were performed in a volume of 20 μL, with 400 nmol/L of each primer, 3.5 mmol/L Mg++, 10 to 40 ng DNA, and Lightcycler DNA SYBR buffer. After an initial denaturation step at 95°C for 60 seconds, amplification was performed by 35 cycles of denaturation (95°C for 0 seconds), annealing (60°C for 15 seconds), and extension (72°C for 15 seconds). The amplification product was detected using SYBR Green I dye, which strongly increases its fluorescence after binding to double-stranded DNA.
Quantification of HIV-1 RNA
Plasma HIV-1 RNA copies were measured by a quantitative PCR (HIV Monitor Test kit version 1.5, Roche Molecular System, Hoffman-La Roche, Basel, Switzerland) according to the manufacturer's instructions. This assay has a detection limit of 50 HIV-1 RNA copies/mL.
Statistical analysis
Results are expressed as mean ± SEM. The Spearman correlation coefficient analysis was used to assess the correlation between variables. The Wilcoxon signed rank test was used to compare follow-up values versus baseline and the Mann-Whitney U test was used for differences among groups of patients. Statistical analyses were performed using the SPSS software package (Statistical Package for the Social Sciences 8.0, Chicago, IL).
Results
Characteristics of the patients at baseline
Fifteen patients were included in the study. The mean age of the patients was 33.5 years (range, 25-60 years), and 3 of them were women. Mean CD4+ T-cell count was 347 cells/μL (range, 8-804 cells/μL; 4 patients had fewer than 200 CD4+ T cells/μL). The mean plasma HIV-1 RNA level was 4.1 log10copies/mL (range, 3.0-5.6 log10 copies/mL).
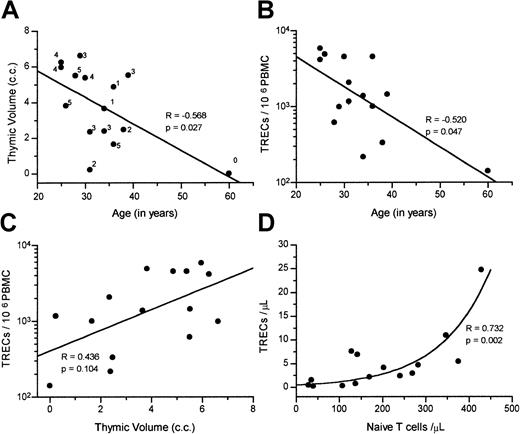
At baseline, the thymic function–related markers were significantly interrelated. Figure 1A shows the inverse correlation between age and thymic volume. Note that patients with high thymic tissue scores were also younger. TREC/106 PBMC values were inversely correlated with age (Figure 1B) and directly correlated with thymic volume (Figure 1C). TREC levels were very low among the 3 patients with acquired immunodeficiency syndrome (between 140 and 333 TREC/106 PBMCs). Finally, a significant correlation was present between the number of TRECs and both the naı̈ve CD4+ (R = 0.696,P = .004) and naı̈ve CD8+ T-cell counts (R = 0.568, P = .027) at baseline. Naı̈ve T-cell counts were then combined, and Figure 1D shows the significant relationship between the total naı̈ve T-cells count (CD4+ plus CD8+) and TREC counts.
Correlation among thymic function–related markers at baseline.
Correlations were found at baseline between (A) age with thymic volume (thymic tissue score is also shown); (B) age with TREC/106PBMCs; (C) thymic volume and TREC/106 PBMCs; and (D) absolute counts per microliter of naı̈ve T cells (CD4+ plus CD8+) and TRECs. The Spearman correlation coefficient and P values are indicated within each figure.
Correlation among thymic function–related markers at baseline.
Correlations were found at baseline between (A) age with thymic volume (thymic tissue score is also shown); (B) age with TREC/106PBMCs; (C) thymic volume and TREC/106 PBMCs; and (D) absolute counts per microliter of naı̈ve T cells (CD4+ plus CD8+) and TRECs. The Spearman correlation coefficient and P values are indicated within each figure.
Patients' response to HAART
The mean change from baseline in plasma HIV-1 RNA at week 12 was −2.53 log10 copies/mL. From week 12 to the end of follow-up, viral load levels were generally below 50 copies/mL. CD4+ T-cell counts were increased from 347 ± 66 cells/μL to 489 ± 73 cells/μL at week 48. The CD4+ T-cell increase versus baseline was significant from week 12 of treatment (Wilcoxon signed rank test,P = .005). There were no significant changes in mean CD8+ T-cell counts during the follow-up period.
Changes in thymic function–related markers after HAART
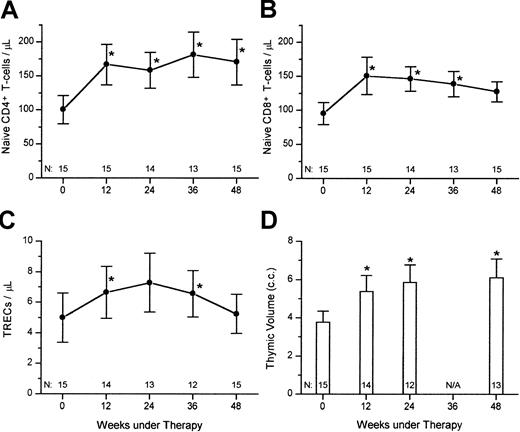
For kinetics studies, TRECs were expressed as absolute counts per microliter rather than a proportion in PBMCs. After starting HAART, there was a significant increase in naı̈ve CD4+ T cells/μL, naı̈ve CD8+ T cells/μL, TREC/μL, and thymic volumes for the overall population (Figure2). All these thymic function–related markers were already significantly increased versus baseline within 12 weeks (Wilcoxon signed rank test, P < .05). Thymic scores of radiographic density were not significantly changed between baseline and the whole study period (data not shown).
Response of thymic function–related markers to HAART.
Shown are the mean (± SEM) of (A) naı̈ve CD4+ T cells/μL; (B) naı̈ve CD8+ T cells/μL; (C) TRECs/μL, and thymic volume, for 15 HIV-1–infected subjects starting HAART (* Wilcoxon signed rank test, P < .05).
Response of thymic function–related markers to HAART.
Shown are the mean (± SEM) of (A) naı̈ve CD4+ T cells/μL; (B) naı̈ve CD8+ T cells/μL; (C) TRECs/μL, and thymic volume, for 15 HIV-1–infected subjects starting HAART (* Wilcoxon signed rank test, P < .05).
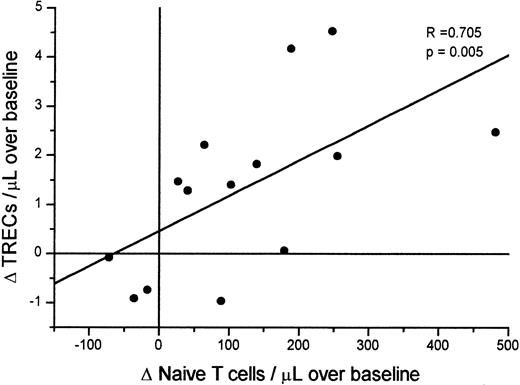
The analysis of these simultaneous increases showed a direct relationship between early changes in naı̈ve T cells and TRECs. The change at week 12 (versus baseline) in TREC counts was significantly correlated with changes in both the naı̈ve CD4+ (R = 0.582, P = .029, Spearman test) and the naı̈ve CD8+ T-cell counts (R = 0.582, P = .029). Figure3 shows the correlation between changes in TRECs and the combined CD4+ plus CD8+naı̈ve T-cell counts. No more significant correlation was present at 24, 36, or 48 weeks between changes in TRECs and naı̈ve T cells.
Correlation between change in naı̈ve T-cell counts/μL and TREC counts/μL after initiation of HAART.
Δ corresponds to the differences in naı̈ve T-cell counts and of TREC/μL between baseline and week 12 of HAART. Correlation was tested by the Spearman test. No significant correlations were present in the differences between baseline and week 24, 36, or 48.
Correlation between change in naı̈ve T-cell counts/μL and TREC counts/μL after initiation of HAART.
Δ corresponds to the differences in naı̈ve T-cell counts and of TREC/μL between baseline and week 12 of HAART. Correlation was tested by the Spearman test. No significant correlations were present in the differences between baseline and week 24, 36, or 48.
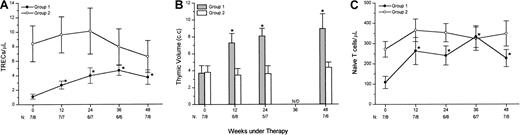
Something that caught our attention was the more evident increase in TREC counts after HAART in patients with low baseline TREC levels. Thus, our 15 patients were split into 2 groups, according to the baseline median TREC count (2.92 TREC/μL), for further analysis. Patients with TREC counts at baseline below the median were designated group 1 (n = 7), and patients above or equal to the median were designated group 2 (n = 8). There was no significant difference in age (Mann-Whitney U test, P = .104) between the groups. Baseline CD4+ T-cell counts were significantly lower in group 1 (208 ± 89 cells/μL) than in group 2 (469 ± 75 cells/μL, Mann-Whitney U test, P = .049).
After starting HAART, only the group 1 patients had significant increases in TREC/μL counts versus baseline (Figure4A, Wilcoxon signed rank test,P < .05). Thus, no more significant differences in TREC counts were present between the groups at weeks 36 and 48 (Mann WhitneyU test). In the same way, the slopes of the regression line fitting the patients' TREC/μL counts during follow-up were higher for group 1 (+4.85 × 10−2/d) than for group 2 (−3.0 × 10−2/d, Mann-Whitney U test,P = .011).
Relations between baseline TREC counts and response to HAART in thymic function–related variables.
Fifteen HIV-1–infected patients receiving HAART were split into 2 groups of below (group 1) or equal or above (group 2) the median TREC counts at baseline and analyzed for changes in TREC/μL (A), thymic volume (B), and CD4+ plus CD8+ naı̈ve T-cell counts (C). Only the group 1 patients had significant increases versus baseline in these markers (*P < .05, Wilcoxon signed rank test). The number of patients in group 1 and group 2 at each time point is shown at the bottom of each panel.
Relations between baseline TREC counts and response to HAART in thymic function–related variables.
Fifteen HIV-1–infected patients receiving HAART were split into 2 groups of below (group 1) or equal or above (group 2) the median TREC counts at baseline and analyzed for changes in TREC/μL (A), thymic volume (B), and CD4+ plus CD8+ naı̈ve T-cell counts (C). Only the group 1 patients had significant increases versus baseline in these markers (*P < .05, Wilcoxon signed rank test). The number of patients in group 1 and group 2 at each time point is shown at the bottom of each panel.
At baseline, no significant differences between the groups were found for thymic volumes (Mann-Whitney U test,P = .908). However, thymic volumes were significantly increased after HAART in group 1 (Figure 4B) but not in group 2. In this way, thymic volume was significantly higher in group 1 than in group 2 at week 12 (P = .013), week 24 (P = .006), and very near to statistical significance at week 48 (P = .057).
Naı̈ve CD4+ and CD8+ T-cell counts were significantly lower in group 1 (47.9 ± 20.9 cells/μL and 60.5 ± 15.1 cells/μL, respectively) than in group 2 (146.4 ± 25.8 cells/μL and 125.7 ± 22.4 cells/μL, respectively) at baseline (Mann-Whitney U test,P = .015 and P = .037, for naı̈ve CD4+ and CD8+ T-cell counts, respectively). After HAART, CD4+ plus CD8+naı̈ve T-cell counts were increased in both groups, and no more significant differences were present between them at any treatment point. The increases in total naı̈ve T-cell counts were significant versus baseline from week 12 for group 1 (Figure 4C, Wilcoxon signed rank test, P < .05), and were very near to statistical significance for group 2 (Wilcoxon signed rank test,P = .050 at weeks 12 and 24 versus baseline).
Discussion
To study the role of the thymus during immune reconstitution in HIV-1–infected adult patients, we have analyzed changes in naı̈ve T cells, TREC levels, and thymic volume in a group of antiretroviral naı̈ve patients receiving 48 weeks of HAART. The main finding of this study was the significant increase in both TREC levels and thymic volumes after starting HAART. These increases were particularly evident among the patients with low TREC counts at baseline. As far as we know, this is the first study to combine thymic imaging, TREC levels, and naı̈ve T-cell analysis to analyze thymic functionality. Taken together, our data strongly suggest the possibility of a new production of RTE after inhibition of HIV-1 replication, in a functional response of the thymus to a state of low TREC levels.
It has been previously demonstrated that HAART induces an increase in the proportion of TRECs in both PBMCs and sorted CD4+ or CD8+ T cells from HIV-1–infected patients.10,11 This fact has been related to enhanced thymic function after HAART because the only known pathway for TREC production in humans is thymocyte differentiation. However, the proportion of TRECs in naı̈ve T cells is the result of a complex equilibrium between thymic output, cell death, and cell proliferation. In this way, it has been shown that cell proliferation is the leading force depleting TREC levels after HIV-1 infection.17 Thus, TREC increases have been also related to the concomitant reduction in T-cell proliferation and activation after HAART.12 13 In this work, we have found significant increases in both proportions (data not shown) and absolute TREC counts after HAART. This increase in absolute number needs an active output of TRECs to peripheral blood, rather than a simple reduction in T-cell division rates or the elimination of TREC-negative, activated T cells (which can alter TREC proportions). Although we cannot exclude a redistribution of lymph node–sequestered naı̈ve T cells as the source for TREC influx in peripheral blood, a simultaneous increase in thymic volume should suggest that new TRECs are being generated and exported as RTE cells by the thymus.
In this work, we also show a significant increase in patients' thymic volumes after starting HAART. The possibility of increasing thymic tissue in the setting of HIV-1 infection had been previously suggested by CT scans in antiretroviral-naı̈ve, HIV-1–infected adults.15 However, common limitations of thymic imaging techniques are the true delimitation of thymic and mediastinal fat tissues and the inability to differentiate between active thymopoiesis and lymphocytic infiltration.18 Thus, other thymic function markers should be additionally addressed when assessing renewed lymphopoiesis in aged thymus. In this way, the relationships between thymic volume and thymic function have been supported by the described significant association between changes in thymic volume and number of CD4+ naı̈ve T cells after HAART in children.7 In adults, this relationship has been hampered by the absence of reports assessing thymic size dynamic after HAART, although a relationship between amount of thymic tissue after 48 weeks of HAART and the early changes in naı̈ve T cells has been shown.8
As far as we know, the thymic measurement technique used in this work has not previously been used in other HIV-1–infected patients. Our detailed delineation of thymic shadows along several thin sections allowed us to accurately determine the thymic volume, minimizing possible inclusion of mediastinal fat. Moreover, we have found relations between TREC levels and thymic volume; at baseline, a direct relation between TREC/106 PBMCs and thymic volume was present. In addition, we have found that when the patients were divided into 2 groups with low or high baseline TREC counts, only the group with low TRECs had significant increases in both TRECs and thymic volumes. Taken together, these simultaneous increases in TREC levels and thymic volumes after HAART may be easily interpreted as a functional increase in thymic volume, in response to depleted TREC levels, suggesting a real production of RTEs after HAART.
Another key issue in the evaluation of thymic involvement in the increase in naı̈ve T-cell subsets after HAART is the quantification of thymopoiesis versus peripheral expansion of mature T cells. In healthy individuals, naı̈ve T cells are resting cells, switching to a memory phenotype when activated to proliferate.19 In HIV-1 infection, however, an increased rate in T-cell proliferation is shown for all T-cell subsets, including naı̈ve T cells.12,20 A homeostatic peripheral expansion of naı̈ve T cells seems to be possible in HIV-1 infection, being an alternative source of naı̈ve T cells. Peripheral expansion of naı̈ve T cells will be in agreement with the significantly lower TREC proportion per naı̈ve T cells in HIV-1–infected versus uninfected subjects, with the exponential relationship between remaining TREC and naı̈ve T-cell counts previous to HAART shown in this work, with the reported higher increase in naı̈ve than in TREC counts after HAART11 (and this work), and with the lack of correlation between changes in TREC and naı̈ve T-cell counts after 24, 36, or 48 weeks of HAART (this work). To assess how many naı̈ve T cells are from thymic origin, an important issue is to extrapolate RTE production from TREC increases. The number of TRECs per RTEs is unknown, although it should be similar to children's peripheral blood and cord blood T cells (approximately 1 TREC for each 10-20 naı̈ve T cells,10,11 21 and data not shown). According to these values, the patients with the lower TREC counts at baseline, who had evidence of thymic reactivation after HAART, showed a significant increase at week 12 of +1.5 TREC/μL (equivalent to 15-30 RTE/μL). This suggests that RTEs might be the 10% to 20% of the naı̈ve T-cell increase (+155 naı̈ve T cells/μL). At week 24, the net increases were 2.76 TREC/μL and 121 naı̈ve T cells/μL (20%-40%). These data suggest that thymic function seems to be recuperated early after control of viral replication, although additional pathways of naı̈ve T-cell repopulation are also present, such as the peripheral expansion of RTEs or mature preexisting naı̈ve T cells.
In conclusion, in this work we have found evidence supporting a functional increase in thymic size after HAART, engaged in an early generation of new RTEs in adult patients. The generation of RTEs was more evident in patients showing depleted TREC levels prior to antiretroviral therapy.
We would like to thank the individuals who participated in this study, as well as M. Olivera and A. Gayoso for excellent technical assistance.
Supported by research grants from “Fondo de Investigaciones Sanitarias” (FIS 00/0521), Fondo para la Investigación y Prevención del SIDA en España (FIPSE 2132/00, integrated by Ministerio de Sanidad y Consumo, Abbott Laboratories, Boehringer Ingelgeim, Bristol Myers Squibb, GlaxoSmithKline, Merck Sharp and Dohme, and Roche), and by the kindly support of Fundación Wellcome España.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Manuel Leal, Department Medicina Interna, Hospital U. Virgen del Rocı́o, CP 41013, Av Manuel Siurot s/n, Seville, Spain; e-mail: mleal@cica.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal