Decay-accelerating factor (DAF) and CD59 are 2 glycosylphosphatidylinositol-anchored membrane proteins that inhibit complement activation at the C3 and C5b-9 step, respectively. CD59 is considered critical for protecting erythrocytes from spontaneous complement attack, as deficiency of CD59 or CD59/DAF, but not of DAF alone, on human erythrocytes renders them sensitive to complement lysis in paroxysmal nocturnal hemoglobinuria syndrome. To evaluate the relative roles of CD59 and DAF in vivo, we have generated and studied a CD59 knockout and a CD59/DAF double-knockout mouse. CD59-deficient and CD59/DAF–double-deficient mouse erythrocytes were highly sensitive to antibody-induced complement lysis in vitro, yet neither CD59 knockout nor CD59/DAF double-knockout mouse developed spontaneous hemolytic anemia. Consistent with the latter observation, erythrocytes from the 2 strains of mutant mice were shown to have a normal lifespan in vivo. In contrast, mouse erythrocytes deficient in complement receptor 1 (CR1)–related gene y (Crry), a membrane C3 inhibitor with DAF and membrane cofactor protein activities, were rapidly eliminated from the circulation by a complement-dependent mechanism. Compared with DAF-deficient erythrocytes, Crry-deficient erythrocytes incurred higher levels of spontaneous C3 deposition in vivo. These findings demonstrate that CD59 and DAF are not indispensable on murine erythrocytes. Rather, effective C3 regulation on the cell surface, provided by Crry rather than DAF, is necessary for mouse erythrocytes to resist spontaneous complement attack. Our results raise the possibility that proper control of C3 activation may also be critical on human erythrocytes, where CR1 but not DAF could be the principal regulator of spontaneous C3 activation.
Introduction
Complement is a form of natural immunity that plays an important role in host defense.1 Activation of the complement system must be carefully controlled on autologous cells to avoid self injury. This is particularly important for cells in the vascular space, such as erythrocytes and endothelial cells, that have permanent contact with the plasma complement system. Control of complement activation on autologous cells is provided by complement regulatory proteins in the fluid phase and on the cell membrane.2-4 In humans, 2 critical membrane complement regulatory proteins are CD59 and decay-accelerating factor3(DAF, CD55).5-7 Both proteins attach to the cell membrane via a glycosylphosphatidylinositol (GPI) anchor. DAF inhibits C3 cleavage by accelerating the decay of C3 convertases5,6whereas CD59 inhibits C5b-9 (membrane attack complex [MAC]) formation by preventing the binding of C9 to the nascent C5b-8 complex.7,8 CD59 and DAF have been extensively studied in the context of the hemolytic anemia syndrome, paroxysmal nocturnal hemoglobinuria (PNH).9-12 Defects in GPI-anchor biosynthesis in hematopoietic stem cells, resulting in the absence of all GPI-anchored proteins including CD59 and DAF from affected blood cells, are the underlying cause of PNH.13 CD59 deficiency is considered more critical for the increased sensitivity of blood cells to complement injury in PNH, as selective CD59 but not DAF deficiency, due to germline mutation of the respective gene, caused a PNH-like syndrome in a patient.14 15
To assess the relative roles of CD59 and DAF in vivo in protecting erythrocytes from spontaneous complement attack, we have generated, by gene targeting, a mouse that is deficient in the CD59a gene. In the mouse, 2 CD59 genes have been identified.16,17 One CD59 gene (CD59a) is expressed broadly in various mouse tissues including vascular and blood cells, whereas the second gene (CD59b) is expressed only in the mouse testis.16,17 We then crossed the CD59a gene knockout mouse with our previously generated DAF knockout mouse18 and derived a CD59a/DAF double-knockout mouse. In this report, we describe the analysis of erythrocyte sensitivity to spontaneous and induced complement activation in CD59a- and CD59a/DAF–deficient mice, as well as in mice deficient in DAF and complement receptor 1 (CR1)–related gene y (Crry). Despite increased sensitivity to antibody-induced complement lysis in vitro, CD59a- and CD59a/DAF–deficient mouse erythrocytes were resistant to spontaneous complement attack in vivo. In contrast, mouse erythrocytes deficient in Crry, which is a membrane C3 convertase inhibitor with both DAF and membrane cofactor protein activities,19 20 were rapidly eliminated from the circulation in a complement-dependent manner. These results demonstrate that effective C3 regulation on the cell surface, provided by Crry rather than DAF, is required for mouse erythrocytes to resist spontaneous complement attack. They also raise the possibility that a similar requirement may exist on human erythrocytes, where proper control of spontaneous C3 activation could be provided by CR1 rather than DAF.
Materials and methods
Gene targeting of the CD59a gene
A mouse genomic library (129/Sv strain) was screened for CD59 genes as described.17 A phage clone with an approximately 17-kilobase (kb) insert was identified and found to contain the first 3 exons of the CD59a gene (Figure 1A). To construct a targeting vector, a 2.2 kb XbaI-EcoRI restriction fragment containing the third exon of CD59a was ligated into the pPNT vector downstream of the neomycin (NEO) gene cassette.21 A 7-kb fragment containing the first exon of CD59a and corresponding to a 5′NotI- EcoRI restriction fragment of the phage insert (Figure 1A) was ligated into the pBluescript vector (Stratagene, La Jolla, CA) at NotI and EcoRI sites. This fragment was then subcloned into the pPNT vector at NotI andXhoI sites upstream of the NEO gene cassette (Figure 1A). The targeting vector was linearized by NotI digestion and transfected into TL-1 embryonic stem (ES) cells (kindly provided by Dr P. Laboski, University of Pennsylvania). ES cell culture, DNA transfection, and selection of gene-targeted ES cell clones were carried out as described in previous studies.18Drug-resistant ES cells were screened by Southern blot analysis of genomic DNAs after HindIII digestion with a 282–base pair (bp) probe specific to CD59a exon 4 (nucleotides 248 to 529)16 (Figure 1A). Additional screening to confirm proper targeting was performed with a NEO probe as well as byBamHI digestion of the DNAs. Positive ES cell clones were expanded and used for blastocyst microinjection to produce chimeric and eventually knockout mice by following standard procedures.22 Age-matched (8 to 12 weeks) male wild-type and CD59a knockout littermates from heterozygous mating were used in all experiments.
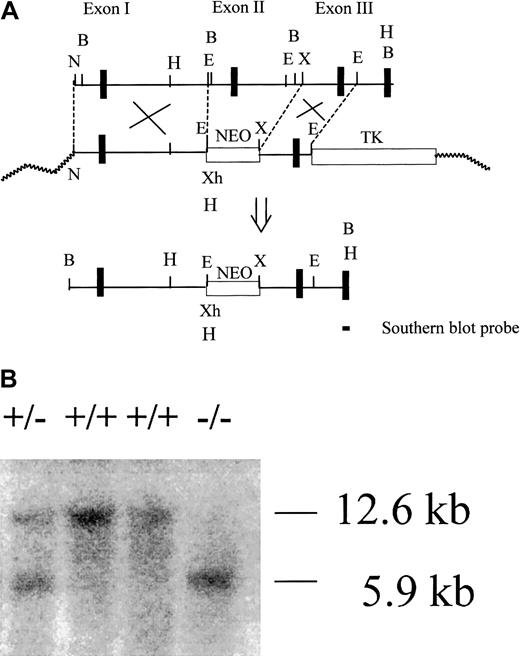
Gene targeting strategy for mouse CD59a.
(A) Partial restriction map of the CD59a gene fragment and construction of the targeting vector. N, NotI; X, XbaI; E,EcoRI; B, BamHI; Xh, XhoI, H,HindIII. After correct targeting, a 7.6-kb fragment containing CD59a exon 2 should be deleted. Exons are represented by thick vertical bars. (B) Representative Southern blot analysis of wild-type (+/+), heterozygous (+/−) and knockout (−/−) mouse genomic DNA after HindIII digestion. The probe used was a 282-bp cDNA fragment specific to CD59a exon 4. The targeted allele was identified by the 5.9-kb band while the wild-type allele was indicated by the 12.6-kb band.
Gene targeting strategy for mouse CD59a.
(A) Partial restriction map of the CD59a gene fragment and construction of the targeting vector. N, NotI; X, XbaI; E,EcoRI; B, BamHI; Xh, XhoI, H,HindIII. After correct targeting, a 7.6-kb fragment containing CD59a exon 2 should be deleted. Exons are represented by thick vertical bars. (B) Representative Southern blot analysis of wild-type (+/+), heterozygous (+/−) and knockout (−/−) mouse genomic DNA after HindIII digestion. The probe used was a 282-bp cDNA fragment specific to CD59a exon 4. The targeted allele was identified by the 5.9-kb band while the wild-type allele was indicated by the 12.6-kb band.
Generation of CD59a/DAF, DAF/C3, and Crry/C3 double-knockout mice
CD59a/DAF and DAF/C3 double-knockout mice were generated by cross-breeding DAF knockout18 mice with CD59a knockout and a strain of C3 knockout mice23 (kindly provided by Dr John Lambris, University of Pennsylvania, Philadelphia). Screening of double-knockout mice was carried out by fluorescence-activated cell sorter (FACS) analysis of DAF and CD59a on erythrocytes, and by reverse transcriptase–polymerase chain reaction (RT-PCR) of the C3 locus.23 Crry/C3 double-knockout mice were obtained as described previously.24 All double-knockout mice were on a mixed 129/C57BL/6 background.
Northern and RT-PCR analysis
Northern blot analysis of CD59a and CD59b messenger RNA (mRNA) was carried out as previously described.17 RT-PCR was performed with the following primers: Ex1, 5′-AGCACAGTCACTGGCGAT-3′ (upstream, specific to CD59a exon 1); Ex2, 5′-ATGAGAGCTCAGAGGGGA-3′ (upstream, specific to CD59a exon 2); Ex3, 5′-CTGTTAGCCTCACATGCTACC-3′ (upstream, specific to CD59a exon 3); Ex4, 5′-GTTAAACTGGCAGCATCTGAA-3′ (downstream, specific to CD59a exon 4); cd59b-1, 5′-CTGTTAGCCTCACATGCTACC-3′ (upstream, nucleotides 158 to 178),17 cd59b-2, 5′-TCAATGAGGAAGTTTCTGCG-3′ (downstream, specific to CD59b nucleotides 408 to 427).17
FACS analysis
A monoclonal rat antimouse CD59a25 was provided by Dr Paul Morgan (University of Wales, Cardiff, United Kingdom); a monoclonal hamster antimouse DAF26 was provided by Dr Noriko Okada (Nagoya City University, Japan); a polyclonal rabbit antimouse Crry was provided by Dr Michael Holers (University of Colorado Health Sciences Center, Denver). Fluorescein isothiocyanate (FITC)–conjugated donkey antirat immunoglobulin G (IgG), F(ab′)2, was obtained from Jackson ImmunoResearch Laboratories (West Grove, PA); phycoerythrin (PE)–conjugated goat antihamster IgG was obtained from Pharmingen (San Diego, CA); and PE-conjugated goat antirabbit IgG was obtained from Sigma (St Louis, MO). Total blood cells, obtained by tail vein bleeding, were washed 3 times in phosphate-buffered saline (PBS) and resuspended in PBS at 2 × 107 cells per milliliter. After staining with primary and secondary antibodies, cells were analyzed by FACScan (Becton Dickinson, San Jose, CA).
Plasma and urine hemoglobin measurement and in vivo cobra venom factor experiment
Blood (100 or 200 μL) samples were collected into tubes containing 5 or 10 μL 0.5 M EDTA to prevent clotting. Plasma was prepared from total blood by centrifugation at 5000 rpm for 10 minutes at 4°C in a microcentrifuge. Urine samples were collected by housing mice in individual metabolic cages for 24 hours. To determine plasma hemoglobin concentration, freshly prepared plasma samples were diluted 1:10 in 0.942 M Na2CO3 and measured at 380 nm, 415 nm, and 450 nm on a UV/VIS spectrophotometer. The hemoglobin concentration (cHb) in grams per liter for each diluted sample was calculated by means of the following formula: cHb = 1.65 × A415−0.93 × A380−0.73 × A450.27Urine hemoglobin levels were estimated by measuring the optical density at 414 nm (OD414) of the collected samples in 96 wells with the use of an enzyme-linked immunosorbent assay (ELISA) plate reader.
To determine the effect of cobra venom factor (CVF) treatment on intravascular hemolysis, mice were injected intraperitoneally with 10 μg CVF (from Naja naja kaouthia [Sigma] dissolved in 200 μL saline).28 Blood (100 μL) was collected before and 1 hour after CVF treatment, and plasma was prepared as described above. The OD414 of the plasma samples was determined in a 96-well plate by means of an ELISA plate reader. CVF-induced systemic complement activation was confirmed by ELISA analysis of C3 cleavage products by means of a C3b/C3d–specific rat antimouse C3 monoclonal antibody (kindly provided by Dr John Lambris, University of Pennsylvania).
Erythrocyte and reticulocyte counts and determination of blood hemoglobin concentration
Blood (50 μL) was collected from tail vein bleeding into tubes containing 2.5 μL 0.5 M EDTA. Total erythrocyte number and blood hemoglobin concentration were determined on a Mascot Multispecies Hematology Systems analyzer (CDC Technologies, Oxford, CT). The percentage of immature erythrocytes (reticulocytes) was determined by FACS analysis by means of a Thiazole Orange–based kit (Retic-Count) from Becton Dickinson.29
Hemolysis and C3 deposition assays in vitro
Antibody-induced complement hemolysis assays were carried out as described previously.18 Erythrocytes were first sensitized with a monoclonal mouse erythrocyte autoantibody 34-3C (1:50 or 1:200 dilution of a 10 mg/mL stock solution). The hybridoma for 34-3C, an erythrocyte-reactive IgG2a murine autoantibody, was originally derived from the autoimmune New Zealand black mice30,31and was kindly provided by Dr Raphael Clynes (The Rockefeller University, New York, NY). The 34-3C was purified from ammonium sulfate–precipitated concentrated tissue culture supernatant followed by protein A-G affinity chromatography. Antibody-sensitized cells were treated with serum complement at 37°C for 30 minutes. Normal mouse, human, and rat sera were obtained from Sigma. C8-depleted human serum was obtained from Quidel (San Diego, CA). Human serum was used at 1:40 dilution, and rat serum was used at 1:20 dilution. Because mouse serum has low lytic activity in vitro owing to the high tendency of spontaneous C3 cleavage and consumption,32 human C5b-7 sites were first assembled on the surface of mouse erythrocytes by using C8-depleted human serum. After washing, cells bearing human C5b-7 sites were exposed to mouse serum to provide the source of C8 and C9 and to develop the lytic reaction. Assays of antibody-induced mouse C3 deposition were carried out as described previously18 with the use of monoclonal antibody 34-3C (1:200) and mouse serum (1:5 dilution), and a FITC-conjugated goat antimouse C3 (ICN, Orangeburg, NY) for detection by FACS.
Assessment of erythrocyte survival in vivo
To compare the lifespans of mouse erythrocytes in vivo, cells were biotinylated in vitro and reintroduced into either complement-sufficient (autologous or C57BL/6 mice from Jackson Laboratory) or C3-deficient mice through the tail vein. Biotinylation of erythrocytes was carried out by following the procedure of Suzuki and Dale.33 Blood samples were collected at 5 minutes after erythrocyte infusion and then at various indicated time points thereafter. Collected erythrocytes were stained with R-PE–conjugated streptavidin (Molecular Probes, Eugene, OR), and the percentage of biotinylated cells was determined for each time point. In some experiments, they were also doubly stained for C3 deposition with a FITC-conjugated goat antimouse C3 (ICN).
Results
Generation of CD59a-deficient mice
Mice deficient in CD59a were generated by homologous recombination in ES cells. The CD59a gene was targeted by a replacement vector that substituted a NEO gene cassette for a 7.6-kb fragment containing exon 2 in the CD59a gene (Figure1A). Because exon 2 encodes the protein translation initiation codon (ATG) and the N-terminal signal peptide of CD59a,16 17 no CD59a protein synthesis should occur in the knockout mouse. Correctly targeted alleles in ES cells and mice were identified by HindIII digestion of cellular or tail DNA and Southern hybridization with a complementary DNA (cDNA) probe specific to exon 4 of CD59a (Figure 1A). In this screening, the wild-type allele should give a 12.6-kb band, while the targeted allele should give a 5.9-kb band owing to deletion of the 7.6-kb fragment and the introduction from subcloning of a new HindIII site 5′ of the NEO gene cassette (Figure 1A). As expected, only the 12.6-kb band was detected in wild-type ES cells and mice (Figure 1B) while both the 12.6-kb band and a 5.9-kb band were detected in targeted ES cells (data not shown) and heterozygous mice (Figure 1B). In homozygous CD59a knockout mice, only the 5.9-kb band was detected (Figure 1B).
From 2 electroporation experiments, 4 CD59a-targeted ES clones were identified. Injection of clone no. 66 to C57BL/6 blastocysts resulted in 3 high-percentage male chimeras, all of which subsequently transmitted the mutation through germ line. Intercross between CD59a heterozygous knockout mice produced CD59a knockout mice at the expected Mendelian ratio (of the initial 71 pups screened, 16 were wild-type, 20 were knockout and 35 were heterozygous). To confirm that CD59a gene expression was disrupted in the knockout mice, Northern blot analysis was carried out. As shown in Figure2Ai, there were 2 distinct CD59a mRNA species in each of the tissues examined. In the kidney and heart the larger band predominated, whereas in the testis the smaller band predominated. These mRNA species may result from the use of different polyadenylation sites in exon 4 of the CD59a gene.16 17Figure 2Ai shows that CD59a mRNAs were detected in the wild-type but not in the knockout mouse heart and kidney (lanes 1-4, panel Ai). Interestingly, in the knockout mouse testis, CD59a mRNAs were still detected (lane 6, Figure 2Ai). However, the mRNA species were slightly smaller in the knockout testis than in the wild-type testis (this was particularly evident for the lower band of CD59a mRNA) (lanes 5-6, Figure 2Ai). In contrast, expression of the testis-specific CD59b gene was not affected in the CD59a knockout mice (Figure 2Aii).
Confirmation of successful inactivation of the CD59a gene.
(A) Northern blot analysis of CD59a (panel Ai) and CD59b (panel Aii) mRNAs from wild-type (lanes 1,3,5) and knockout (lanes 2,4,6) mouse heart (lanes 1-2), kidney (lanes 3-4), and testis (lanes 5-6). Equal RNA loading is indicated in panel Aiii. The membrane was first hybridized with a 370-bp CD59a cDNA fragment17 (panel Ai), and then stripped and rehybridized with a CD59b-specific probe (panel Aii). Positions of the 18S and 28S ribosomal RNA bands in Ai and Aii are indicated by two horizontal lines. (B) RT-PCR analysis of RNAs from wild-type (WT) and knockout (KO) mouse testis and heart. Primer combinations for CD59a mRNA were as indicated, and 2 primers, cd59b-1 and cd59b-2, were used for CD59b mRNA (primer abbreviations and sequences appear in “Materials and methods”). (C) FACS analysis of CD59a expression on wild-type, heterozygous, and knockout mouse erythrocytes. Wild-type cells stained only with secondary antibody are represented by shaded area.
Confirmation of successful inactivation of the CD59a gene.
(A) Northern blot analysis of CD59a (panel Ai) and CD59b (panel Aii) mRNAs from wild-type (lanes 1,3,5) and knockout (lanes 2,4,6) mouse heart (lanes 1-2), kidney (lanes 3-4), and testis (lanes 5-6). Equal RNA loading is indicated in panel Aiii. The membrane was first hybridized with a 370-bp CD59a cDNA fragment17 (panel Ai), and then stripped and rehybridized with a CD59b-specific probe (panel Aii). Positions of the 18S and 28S ribosomal RNA bands in Ai and Aii are indicated by two horizontal lines. (B) RT-PCR analysis of RNAs from wild-type (WT) and knockout (KO) mouse testis and heart. Primer combinations for CD59a mRNA were as indicated, and 2 primers, cd59b-1 and cd59b-2, were used for CD59b mRNA (primer abbreviations and sequences appear in “Materials and methods”). (C) FACS analysis of CD59a expression on wild-type, heterozygous, and knockout mouse erythrocytes. Wild-type cells stained only with secondary antibody are represented by shaded area.
To determine if the anomalous CD59a mRNA species detected in the knockout mouse testis represented a truncated form of CD59a mRNA, RT-PCR analysis of wild-type and knockout mouse heart and testis RNAs was performed. As shown in Figure 2B, when primers specific to CD59a exons 2 and 4 were used, a 273-bp PCR product was amplified from the wild-type, but not the knockout, mouse testis and heart. This result was consistent with a successful deletion of CD59a exon 2, as expected from the targeting strategy (Figure 1A). When primers specific to exons 3 and 4 were used, equal amounts of a 212-bp PCR product were detected in the wild-type and knockout mouse testis, but the signal intensity was greatly reduced in the knockout mouse heart. When primers specific to exons 1 and 4 were used, a 360-bp product was amplified from the wild-type mouse testis and heart, but a shortened, 270-bp product was detected in the knockout mouse tissues. This shortened PCR product was again present in less abundance in the knockout mouse heart than in the knockout mouse testis. Collectively, these results suggested that exon 2 in CD59a had been successfully deleted. They also indicated that a truncated form of CD59a mRNA (lacking exon 2) was still transcribed and remained stable in the mutant mouse testis, but only a residual amount of such a message was present in the mutant mouse kidney and heart (ie, detectible by RT-PCR but not by Northern blot analysis). Consistent with the result of Northern blot analysis, when a pair of CD59b-specific primers were used, a 270-bp product was detected in equal abundance in the wild-type and knockout mouse testis, but no signal was detected in the mouse heart of either genotype (Figure 2B).
To confirm that the synthesis of CD59a protein had been disrupted in the mutant mouse, FACS analysis of wild-type, heterozygous, and knockout mouse erythrocytes was carried out with the use of a monoclonal rat anti-CD59a antibody. Figure 2C shows that CD59a was highly expressed on the wild-type mouse erythrocytes but was completely absent on the knockout mouse erythrocytes. Erythrocytes from heterozygous CD59a knockout mouse had an approximately 50% reduction in CD59a expression, suggesting that there was gene dosage effect in CD59a mRNA transcription.
CD59a-deficient mouse erythrocytes were more susceptible than wild-type cells to antibody-induced autologous and heterologous MAC lysis in vitro
To determine if CD59a-deficient mouse erythrocytes were more susceptible to MAC attack, antibody-sensitized wild-type and knockout mouse cells were subjected to a complement-mediated lytic assay in vitro. Figure 3A shows that, over a range of mouse serum dilutions, CD59a-deficient mouse erythrocytes were significantly more sensitive than wild-type cells to lytic attack by the MAC assembled from human C5b-7 and mouse C8/C9. Neither C8-deficient human serum alone nor mouse serum alone caused any appreciable lysis under similar experimental conditions (data not shown). This result thus confirmed the activity of CD59a in regulating autologous C8/C9 binding to C5b-7 complexes on the cell surface. In separate experiments, the relative sensitivity of wild-type and CD59a-deficient mouse erythrocytes to human and rat complements was determined and compared with that of DAF- and Crry-deficient cells. This experiment revealed an interesting variation in the order of sensitivity of the 3 mutant cell types to different heterologous complements. As shown in Figure 3, CD59a-deficient mouse erythrocytes were much more sensitive than DAF-deficient cells to rat complement lysis (Figure 3B), but were significantly less sensitive than DAF-deficient cells to human complement lysis (Figure 3C). Notably, mouse Crry appeared to be a poor regulator of rat and human complement. Crry-deficient erythrocytes were not sensitive to rat complement lysis (Figure 3B) and were significantly less sensitive than either DAF-deficient or CD59a-deficient erythrocytes to human complement (Figure 3C).
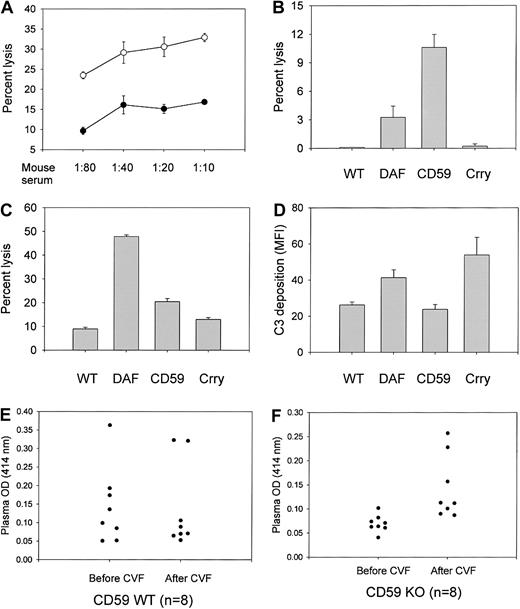
Increased susceptibility of CD59a-deficient mouse erythrocytes to induced complement lysis.
(A) Antibody-induced complement lysis of wild-type (filled circle; n = 3 mice) and CD59a knockout (open circle; n = 3) mouse erythrocytes by MAC assembled from human C5b-7 and mouse C8/C9. Antibody-sensitized cells were sequentially exposed to C8-depleted human serum (1:20) and mouse serum at the indicated dilutions.P < .05 for all serum dilutions by Student ttest. (B) Antibody-induced lysis by rat complement (1:20) of wild-type (WT), DAF knockout (DAF), CD59a knockout (CD59), and Crry/C3 knockout (Crry) mouse erythrocytes (n = 4 mice for each group). DAF and CD59a knockout cells were significantly more sensitive than wild-type cells (P < .05 andP < .001, respectively, by Student t test). Crry/C3 knockout erythrocytes were not more sensitive than wild-type cells. (C) Antibody-induced lysis by human complement (1:40) of wild-type and various knockout mouse erythrocytes (n = 4 mice for each group). All knockout erythrocytes were more sensitive than wild-type cells to human complement lysis (P < .001 for DAF and CD59a knockout;P < .01 for Crry/C3 knockout; Student t test). (D) Antibody-induced mouse C3 deposition on wild-type and various knockout mouse erythrocytes (n = 8 mice for each group). CD59a knockout mouse erythrocytes had levels of C3 deposition similar to those of wild-type mouse erythrocytes (P = .45, Student t test). Both DAF knockout and Crry/C3 knockout mouse erythrocytes had more C3 deposition than the wild-type cells (P < .01 for DAF knockout and P < .05 for Crry/C3 knockout mice, Student t test). C3 deposition on DAF knockout and Crry/C3 knockout mouse erythrocytes was not significantly different (P = .26, Student t test). (E) (F) CD59a-deficient mouse erythrocytes were more susceptible to CVF-induced autologous complement lysis. Injection of CVF (10 μg per mouse) caused systemic complement activation (data not shown) and increased plasma hemoglobin levels (measured by OD414) in knockout mice (panel F; n = 8 mice; P < .01, Student t test), but not in wild-type mice (panel E; n = 8 mice; P = .9, Student t test). Plasma samples were taken immediately before and 1 hour after CVF injections. Data presented are representative of at least 3 independent experiments.
Increased susceptibility of CD59a-deficient mouse erythrocytes to induced complement lysis.
(A) Antibody-induced complement lysis of wild-type (filled circle; n = 3 mice) and CD59a knockout (open circle; n = 3) mouse erythrocytes by MAC assembled from human C5b-7 and mouse C8/C9. Antibody-sensitized cells were sequentially exposed to C8-depleted human serum (1:20) and mouse serum at the indicated dilutions.P < .05 for all serum dilutions by Student ttest. (B) Antibody-induced lysis by rat complement (1:20) of wild-type (WT), DAF knockout (DAF), CD59a knockout (CD59), and Crry/C3 knockout (Crry) mouse erythrocytes (n = 4 mice for each group). DAF and CD59a knockout cells were significantly more sensitive than wild-type cells (P < .05 andP < .001, respectively, by Student t test). Crry/C3 knockout erythrocytes were not more sensitive than wild-type cells. (C) Antibody-induced lysis by human complement (1:40) of wild-type and various knockout mouse erythrocytes (n = 4 mice for each group). All knockout erythrocytes were more sensitive than wild-type cells to human complement lysis (P < .001 for DAF and CD59a knockout;P < .01 for Crry/C3 knockout; Student t test). (D) Antibody-induced mouse C3 deposition on wild-type and various knockout mouse erythrocytes (n = 8 mice for each group). CD59a knockout mouse erythrocytes had levels of C3 deposition similar to those of wild-type mouse erythrocytes (P = .45, Student t test). Both DAF knockout and Crry/C3 knockout mouse erythrocytes had more C3 deposition than the wild-type cells (P < .01 for DAF knockout and P < .05 for Crry/C3 knockout mice, Student t test). C3 deposition on DAF knockout and Crry/C3 knockout mouse erythrocytes was not significantly different (P = .26, Student t test). (E) (F) CD59a-deficient mouse erythrocytes were more susceptible to CVF-induced autologous complement lysis. Injection of CVF (10 μg per mouse) caused systemic complement activation (data not shown) and increased plasma hemoglobin levels (measured by OD414) in knockout mice (panel F; n = 8 mice; P < .01, Student t test), but not in wild-type mice (panel E; n = 8 mice; P = .9, Student t test). Plasma samples were taken immediately before and 1 hour after CVF injections. Data presented are representative of at least 3 independent experiments.
To ascertain that the increased sensitivity of CD59a knockout mouse erythrocytes to complement lysis was not due to alteration of other membrane complement regulators, the expression of Crry and DAF on wild-type and CD59a-deficient mouse erythrocytes was measured by FACS analysis. No difference between wild-type and CD59a knockout mice in erythrocyte Crry and DAF levels was observed (mean fluorescence intensity for Crry, 1.900 ± 0.132 for wild-type and 1.873 ± 0.133 for knockout, n = 8; mean fluorescence intensity for DAF, 2.430 ± 0.096 for wild-type and 2.490 ± 0.152 for knockout, n = 8). We next determined if CD59a deficiency affected antibody-induced C3 deposition on erythrocytes. Levels of C3 deposited onto antibody-sensitized wild-type, CD59a-deficient, DAF-deficient, and Crry-deficient mouse erythrocytes were measured by FACS. Figure 3D shows that CD59a-deficient erythrocytes incurred no more C3 deposition than did the wild-type cells. In contrast, both DAF-deficient and Crry-deficient erythrocytes had significantly increased C3 deposition compared with wild-type cells. It is notable that, unlike hemolytic assays with rat and human complement (Figure 3B-C), Crry-deficient cells appeared to be the most sensitive in this assay (Figure 3D), suggesting that murine Crry is an important C3 regulator of autologous complement.
CD59a-deficient mouse erythrocytes were more susceptible to autologous complement lysis in vivo during systemic complement activation induced by CVF
To determine if CD59a-deficient mouse erythrocytes were more susceptible to complement lysis in vivo under conditions of abnormal complement activation, systemic complement activation was induced by CVF injection. CVF from Naja naja is a known activator of mouse C3 and C5,34 and extensive fluid-phase C3 activation after CVF treatment in our study was confirmed by ELISA detection of C3 cleavage products (data not shown). Figure 3E-F shows that 1 hour after CVF injection, plasma hemoglobin levels, as measured by OD414, were significantly elevated in the CD59 knockout cohort, whereas no significant changes were observed in the similarly treated wild-type cohort.
Synergy between CD59a and DAF in the protection of mouse erythrocytes from antibody-induced autologous and heterologous MAC lysis
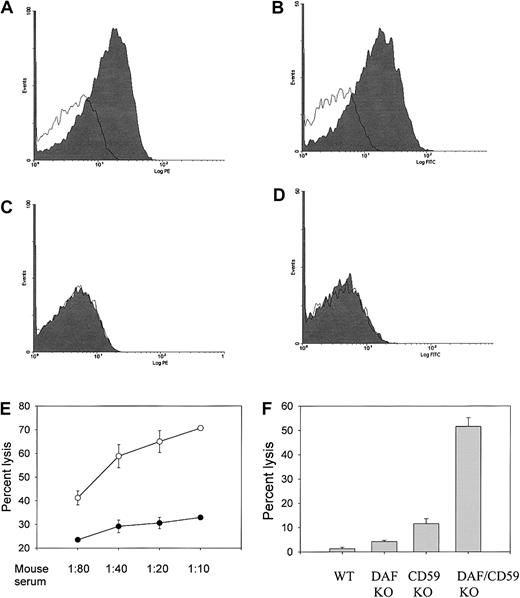
Despite the observed sensitivity of their erythrocytes to lysis during forced complement activation, CD59a knockout mice grew and lived normally with no evident signs of hemolytic anemia. Plasma and urine hemoglobin levels, as measured by OD414, were not significantly elevated (data not shown). To evaluate whether spontaneous complement-mediated intravascular hemolysis might result from a combined CD59 and DAF deficiency, as in the human PNH syndrome,12 we crossed the CD59a knockout mouse with our previously generated GPI-DAF knockout mouse18 and derived a CD59a/DAF double-knockout mouse. Figure4A-D demonstrates that neither CD59a nor DAF is expressed on the erythrocytes of the double-knockout mouse. In an antibody-induced complement lysis assay in vitro, CD59a/DAF double-knockout erythrocytes were shown to have markedly increased sensitivity to autologous and heterologous MAC attack (Figure 4E-F). For example, in the experiment shown in Figure 4F, percentage of lysis by rat complement of wild-type, DAF-deficient, CD59a-deficient, and CD59a/DAF–double-deficient erythrocytes was 1.35%, 4.22%, 11.59%, and 51.59%, respectively. Thus, the protective effect of DAF and CD59a on mouse erythrocytes in this antibody-induced complement lysis test is synergistic rather than simply additive.
Synergistic protection of mouse erythrocytes by CD59a and DAF from induced complement activation and lysis.
(A-D) FACS analysis showing that CD59a (panels A,C) and DAF (panels B,D) are not present on the double-knockout mouse erythrocytes (panels C-D) but are present on wild-type mouse erythrocytes (panels A-B). Open areas indicate control staining with secondary antibodies only. (E) CD59a/DAF double-knockout mouse erythrocytes (open circles) were much more sensitive than CD59a knockout erythrocytes (filled circles) to antibody-induced complement lysis by MAC assembled from human C5b-7 and mouse C8/C9 (n = 3 mice for each group; P < .01 for all serum dilutions, Student t test). Experimental conditions were the same as in Figure 3A. (F) CD59a/DAF double-knockout mouse erythrocytes were much more sensitive than either DAF knockout or CD59a knockout mouse erythrocytes to antibody-induced complement lysis by rat complement (n = 4 for each group; P < .001 between double-knockout and DAF or CD59a knockout mice, Studentt test). Experimental conditions were the same as in Figure 3B. Lysis data are representative of 3 different experiments.
Synergistic protection of mouse erythrocytes by CD59a and DAF from induced complement activation and lysis.
(A-D) FACS analysis showing that CD59a (panels A,C) and DAF (panels B,D) are not present on the double-knockout mouse erythrocytes (panels C-D) but are present on wild-type mouse erythrocytes (panels A-B). Open areas indicate control staining with secondary antibodies only. (E) CD59a/DAF double-knockout mouse erythrocytes (open circles) were much more sensitive than CD59a knockout erythrocytes (filled circles) to antibody-induced complement lysis by MAC assembled from human C5b-7 and mouse C8/C9 (n = 3 mice for each group; P < .01 for all serum dilutions, Student t test). Experimental conditions were the same as in Figure 3A. (F) CD59a/DAF double-knockout mouse erythrocytes were much more sensitive than either DAF knockout or CD59a knockout mouse erythrocytes to antibody-induced complement lysis by rat complement (n = 4 for each group; P < .001 between double-knockout and DAF or CD59a knockout mice, Studentt test). Experimental conditions were the same as in Figure 3B. Lysis data are representative of 3 different experiments.
CD59a and DAF are not indispensable for homeostatic protection of murine erythrocytes from spontaneous complement attack
It is notable that despite the markedly increased sensitivity of CD59a/DAF–double-deficient erythrocytes to antibody-induced complement lysis in vitro, CD59a/DAF double-knockout mice were likewise quite normal, with no obvious signs of spontaneous hemolytic anemia as judged by total peripheral erythrocyte counts and blood hemoglobin concentrations (Figure 5A). Free hemoglobin concentrations in freshly prepared plasma samples were likewise not significantly elevated in the 2 knockout mouse strains: cHb for wild-type, 0.99296 ± 0.24824 mM (1.6 ± 0.4 g/L); CD59a knockout, 1.67562 ± 0.43442 mM (2.7 ± 0.7 g/L); CD59/DAF double knockout, 0.86884 ± 0.18618 mM (1.4 ± 0.3 g/L) (n = 10 for each group). Although a small percentage of CD59a knockout mice appeared to have elevated reticulocyte counts in one experiment (data not shown), the average reticulocyte count of the knockout mice (n = 16) was not significantly elevated in this experiment or in a second experiment in which CD59a/DAF double-knockout mice were also studied (wild-type, 10.6% ± 1.04%, n = 11; CD59a knockout, 10.2% ± 0.45%, n = 20; CD59a/DAF double knockout, 10.6% ± 0.55%, n = 13).
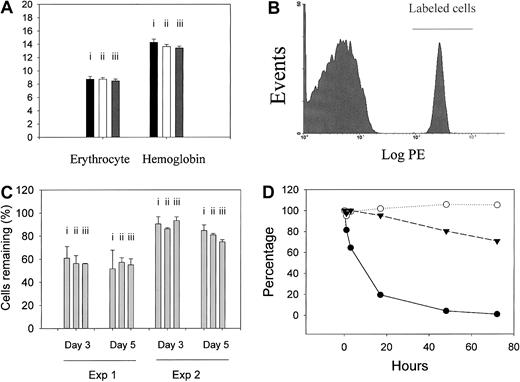
Normal in vivo survival of CD59a-deficient and CD59a/DAF–double-deficient but not Crry-deficient mouse erythrocytes.
(A) Erythrocyte counts (× 1012/L) and blood hemoglobin concentrations (g/dL) were not significantly different between wild-type (i, n = 5 mice), CD59a knockout (ii, n = 5 mice), and CD59a/DAF double-knockout mice (iii, n = 5 mice). (B) Biotinylated erythrocytes could be easily distinguished from unlabeled cells by FACS analysis (example shown is erythrocytes taken from a C57BL/6 mouse that received biotinylated wild-type erythrocytes 12 hours earlier). (C) Percentage of biotinylated erythrocytes remaining at day 3 and 5 after reinfusion. There was no significant difference between wild-type and CD59a knockout or CD59a/DAF double-knockout mice. Experiment 1: cells were taken from wild-type (i, n = 3 mice); CD59a knockout (ii, n = 4 mice), or CD59a/DAF double-knockout mice (iii, n = 4 mice) and, after labeling, were reinfused into the same donor mice. Experiment 2: cells were taken from wild-type (i, n = 3 mice), CD59a knockout (ii, n = 3 mice), or CD59a/DAF double-knockout mice (iii, n = 3 mice) and, after labeling, were transfused into male C57BL/6 mice. Percentage of labeled cells detected in Experiment 1 at days 3 and 5 were generally lower. This may reflect increased hematopoiesis stimulated by the initial blood drawing. (D) Erythrocytes from Crry-deficient mice (filled circles, obtained from Crry/C3 double-knockout mice) but not from Crry-sufficient littermate controls (Crry wild-type but C3-deficient, filled triangles) were rapidly eliminated after transfusion into wild-type recipients. In contrast, Crry-deficient erythrocytes (obtained from Crry/C3 double-knockout mice) were stable when transfused into C3 knockout recipient mice (open circles). Average values from 2 mice in each group are shown. The difference between the open circle and filled triangle curves is not considered significant as typically between 80% and 100% of labeled cells remain when cells are assayed at day 3. In panels C and D, blood samples were taken at 5 minutes after labeled cells were intravenously infused, and the percentage of labeled cells at this time point was regarded as 100% for later reference. Data shown are representative of at least 3 different experiments.
Normal in vivo survival of CD59a-deficient and CD59a/DAF–double-deficient but not Crry-deficient mouse erythrocytes.
(A) Erythrocyte counts (× 1012/L) and blood hemoglobin concentrations (g/dL) were not significantly different between wild-type (i, n = 5 mice), CD59a knockout (ii, n = 5 mice), and CD59a/DAF double-knockout mice (iii, n = 5 mice). (B) Biotinylated erythrocytes could be easily distinguished from unlabeled cells by FACS analysis (example shown is erythrocytes taken from a C57BL/6 mouse that received biotinylated wild-type erythrocytes 12 hours earlier). (C) Percentage of biotinylated erythrocytes remaining at day 3 and 5 after reinfusion. There was no significant difference between wild-type and CD59a knockout or CD59a/DAF double-knockout mice. Experiment 1: cells were taken from wild-type (i, n = 3 mice); CD59a knockout (ii, n = 4 mice), or CD59a/DAF double-knockout mice (iii, n = 4 mice) and, after labeling, were reinfused into the same donor mice. Experiment 2: cells were taken from wild-type (i, n = 3 mice), CD59a knockout (ii, n = 3 mice), or CD59a/DAF double-knockout mice (iii, n = 3 mice) and, after labeling, were transfused into male C57BL/6 mice. Percentage of labeled cells detected in Experiment 1 at days 3 and 5 were generally lower. This may reflect increased hematopoiesis stimulated by the initial blood drawing. (D) Erythrocytes from Crry-deficient mice (filled circles, obtained from Crry/C3 double-knockout mice) but not from Crry-sufficient littermate controls (Crry wild-type but C3-deficient, filled triangles) were rapidly eliminated after transfusion into wild-type recipients. In contrast, Crry-deficient erythrocytes (obtained from Crry/C3 double-knockout mice) were stable when transfused into C3 knockout recipient mice (open circles). Average values from 2 mice in each group are shown. The difference between the open circle and filled triangle curves is not considered significant as typically between 80% and 100% of labeled cells remain when cells are assayed at day 3. In panels C and D, blood samples were taken at 5 minutes after labeled cells were intravenously infused, and the percentage of labeled cells at this time point was regarded as 100% for later reference. Data shown are representative of at least 3 different experiments.
To further investigate if CD59a knockout and CD59a/DAF double-knockout mouse erythrocytes are more susceptible to spontaneous complement destruction in vivo, erythrocytes were biotinylated in vitro and infused into mice. This procedure allowed the biotinylated cells to be monitored for their in vivo lifespans. Figure 5B shows that biotinylated erythrocytes could be easily distinguished from unlabeled cells by FACS analysis after staining with PE-conjugated streptavidin. In the first experiment, erythrocytes (from 150 μL blood) were labeled in vitro and reinfused back to the same mice (autologous transfusion). Percentages of biotinylated cells detected in blood samples taken at 5 minutes were similar in the 3 groups of mice (5.37% ± 0.34% for wild-type, n = 3; 5.93% ± 0.38% for CD59a knockout, n = 4; 5.28% ± 0.44% for CD59a/DAF double knockout, n = 4). Although the percentage of biotinylated erythrocytes generally decreased with time as might be expected, CD59a knockout and CD59a/DAF double-knockout cells did not undergo accelerated elimination from the circulation when monitored from 5 minutes to 5 days after infusion (Figure 5C, experiment 1). Similar results were obtained when biotinylated cells (from 200 μL blood) were transfused heterologously into wild-type C57BL/6 mice (Figure5C, experiment 2). However, in this experiment (experiment 2, Figure5C), the percentage of biotinylated cells recovered at 5 minutes from the double-knockout mouse group appeared to be lower (6.20% ± 0.55% for wild-type, 6.47% ± 0.26% for CD59a knockout, 4.90% ± 0.25% for CD59a/DAF double knockout; n = 3 for all groups). This may represent a procedural variation or an indication that CD59a/DAF double-knockout erythrocytes are more susceptible to natural antibody-mediated transfusion reaction occurring in the first 5 minutes. Nevertheless, as shown in Figure 5C, the kinetics of elimination of the double-knockout cells from 5 minutes to 5 days was not significantly different from that of the wild-type cells.
Crry is critical for homeostatic protection of murine erythrocytes from spontaneous complement attack
In stark contrast with CD59a- and CD59a/DAF–deficient erythrocytes, when mouse erythrocytes deficient in Crry were transfused into wild-type mice, they were eliminated rapidly, 80% by day 1 and completely by day 2 (filled circles, Figure 5D). This rapid elimination is unlikely to be due to blood group differences (transfusion reaction), as erythrocytes from Crry wild-type littermate controls with similar genetic makeups (C3-deficient, 129/C57BL/6 mixed background) did not meet the same fate (filled triangles, Figure 5D). Furthermore, the rapid elimination of Crry-deficient erythrocytes was dependent on an intact host complement system since such cells were protected from removal when transfused to C3-deficient mice (open circles, Figure 5D). Thus, Crry, but not CD59a and DAF, is indispensable for murine erythrocyte protection in vivo from spontaneous complement destruction.
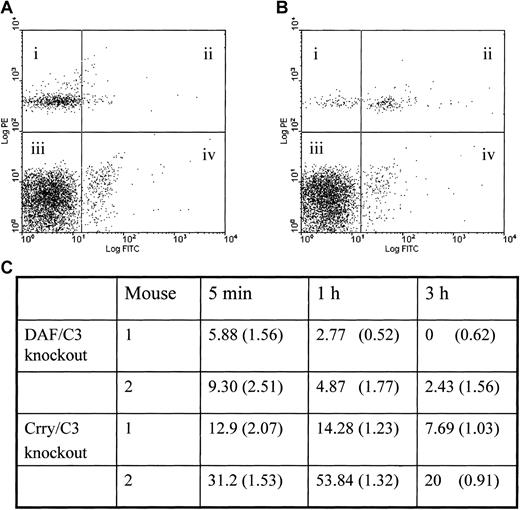
To confirm that Crry-deficient mouse erythrocytes were more sensitive to spontaneous complement attack, we transfused erythrocytes obtained from Crry/C3 or DAF/C3 double-knockout mice into wild-type mice and measured surface C3 deposition at various time points. Figure6 shows that at 5 minutes, 1 hour, and 3 hours after the transfusion procedure, the percentage of transfused cells that were C3+ was significantly higher in the remaining Crry-deficient cells than in the DAF-deficient cells. This result suggested that on erythrocytes Crry plays a more important role than DAF as a regulator of spontaneous complement activation.
Spontaneous C3 deposition in Crry-deficient and DAF-deficient mouse erythrocytes.
Crry-deficient mouse erythrocytes incur higher levels of spontaneous C3 deposition than DAF-deficient erythrocytes in vivo. Erythrocytes obtained from Crry/C3 knockout or DAF/C3 knockout mice were biotinylated and transfused into C57BL/6 wild-type recipients. Blood samples were taken at 5 minutes, 1 hour, and 3 hours, and erythrocytes were analyzed by 2-colored FACS, with PE staining for the transfused biotinylated knockout cells (quadrants i and ii) and FITC for C3 (quadrants ii and iv). Examples of the 2-colored FACS analysis are given in panels A and B (for DAF/C3 knockout and Crry/C3 knockout erythrocytes, respectively); examples were cells taken at 1 hour after transfusion. Percentages of the transfused knockout cells that were C3+ are calculated by the formula ii/(i+ii) × 100 and are given in panel C. The values for Crry/C3 knockout cells are significantly higher than those for DAF/C3 knockout cells (P < .05, Student t test). Percentages of the endogenous erythrocytes of the recipient mice that were C3+ are calculated by the formula iv/(iii+iv) × 100 and are given in brackets in panel C. No significant difference in such values is observed between mice that received Crry-deficient erythrocytes and mice that received DAF-deficient erythrocytes, suggesting that the difference observed with the transfused cells was specific and not an experimental artifact.
Spontaneous C3 deposition in Crry-deficient and DAF-deficient mouse erythrocytes.
Crry-deficient mouse erythrocytes incur higher levels of spontaneous C3 deposition than DAF-deficient erythrocytes in vivo. Erythrocytes obtained from Crry/C3 knockout or DAF/C3 knockout mice were biotinylated and transfused into C57BL/6 wild-type recipients. Blood samples were taken at 5 minutes, 1 hour, and 3 hours, and erythrocytes were analyzed by 2-colored FACS, with PE staining for the transfused biotinylated knockout cells (quadrants i and ii) and FITC for C3 (quadrants ii and iv). Examples of the 2-colored FACS analysis are given in panels A and B (for DAF/C3 knockout and Crry/C3 knockout erythrocytes, respectively); examples were cells taken at 1 hour after transfusion. Percentages of the transfused knockout cells that were C3+ are calculated by the formula ii/(i+ii) × 100 and are given in panel C. The values for Crry/C3 knockout cells are significantly higher than those for DAF/C3 knockout cells (P < .05, Student t test). Percentages of the endogenous erythrocytes of the recipient mice that were C3+ are calculated by the formula iv/(iii+iv) × 100 and are given in brackets in panel C. No significant difference in such values is observed between mice that received Crry-deficient erythrocytes and mice that received DAF-deficient erythrocytes, suggesting that the difference observed with the transfused cells was specific and not an experimental artifact.
Discussion
In this study, we have generated a CD59a knockout and a CD59a/DAF double-knockout mouse and analyzed their erythrocyte sensitivity to spontaneous and induced complement-mediated lysis in vitro and in vivo. CD59 is a GPI-anchored membrane protein that inhibits the formation of the terminal C5b-9 lytic complex.7,8,35 It is considered a critical regulator of spontaneous complement-mediated cellular damage on erythrocytes, as deficiency of CD59 or CD59 and DAF, but not DAF alone, on human blood cells caused erythrocyte sensitivity to complement injury, which is characteristic of PNH.12,14,15 36 However, because only one case of selective CD59 deficiency is known in humans, the evidence supporting a critical role of CD59 and not of C3 convertase inhibitors in protecting erythrocytes from spontaneous complement attack remains circumstantial.
To provide an in vivo animal model for studying the role of CD59 relative to C3 convertase inhibitors in homeostatic protection of erythrocytes and other host cells, we have generated a CD59a knockout mouse by gene targeting. Two CD59 genes have been identified in the mouse.16,17 In this study, we have chosen to inactivate the CD59a gene because it is broadly expressed and is the apparent functional homolog of human CD59 in various mouse tissues except the testis where the second CD59 gene (CD59b) is specifically expressed.17 Successful disruption of the CD59a gene was confirmed by Northern and RT-PCR analysis, and its lack of expression on erythrocytes was established by FACS. The expression of CD59b in the testis was unaffected in the CD59a knockout mouse, and no compensatory induction of CD59b in other tissues was observed. In vitro and in vivo experiments demonstrated that CD59a-deficient mouse erythrocytes were significantly more susceptible to autologous and heterologous MAC lysis under conditions of induced complement activation by antibodies or CVF (Figure 3). It is of interest to note the differential sensitivities of CD59a-deficient, DAF-deficient, and Crry-deficient mouse erythrocytes to rat and human complement lysis. Among the 3 types of regulator-deficient mouse erythrocytes, CD59a-deficient cells were the most sensitive to rat complement lysis (Figure 3B), whereas DAF-deficient erythrocytes were the most sensitive to human complement lysis (Figure 3C). Thus, depending on the source of the complement, either a MAC inhibitor (CD59a) or a C3 convertase inhibitor (DAF) could provide more effective protection against C5b-9–mediated cellular damage. Interestingly, Crry-deficient mouse erythrocytes were not sensitive to rat complement lysis (Figure 3B) and were only slightly more sensitive to human complement lysis than the wild-type cells (Figure 3B-C). These results suggest that while the regulatory functions of C3 convertase and MAC inhibitors are not totally species restrictive, their relative activities and effectiveness do vary according to the source of the heterologous complement.37-40 This may reflect the relative degree of structural divergence of the regulators in question as well as of their target complement components among the different species.
Despite the increased sensitivity of their erythrocytes to induced complement lysis, CD59a knockout mice were phenotypically normal and did not develop spontaneous hemolytic anemia. They had normal erythrocyte counts and blood hemoglobin concentrations. Increased presence of free hemoglobin in fresh plasma and urine, as might be expected if ongoing intravascular hemolysis occurs, was not detected in the mutant mice. These findings contrast with the PNH-like phenotype of a human CD59-deficient patient36,41 and suggest that CD59a does not play a critical role in homeostatic protection of murine erythrocytes. It should be noted that evidence for the occurrence of mild intravascular hemolysis has been described for an independently generated CD59a knockout mouse line.42 Similarly to our CD59a knockout mouse, however, this mouse also had normal erythrocyte counts and blood hemoglobin concentrations and was not anemic.42 It is possible that background genes significantly affect total plasma complement activity, potentially explaining the difference between the 2 strains with regard to this mild spontaneous phenotype. Indeed, the mild intravascular hemolysis reported by Holt et al42 was male mice–specific, which was attributed to a higher plasma complement activity in the male mice.42 Regardless of that, both our study and that of Holt et al42 support the conclusion that CD59a plays a significant role in preventing induced complement injury on mouse erythrocytes but is not a critical regulator of spontaneous complement attack on these cells.
One potential explanation for the resistance of CD59a-deficient mouse erythrocytes to spontaneous complement lysis is that there is tighter regulation at the C3 activation step. To help address this possibility, we produced a CD59a/DAF double-knockout mouse and studied erythrocyte sensitivity to spontaneous complement attack in this mouse. As a comparison, we also investigated the sensitivity of Crry-deficient mouse erythrocytes to spontaneous complement attack in vivo. Crry is a rodent-restricted transmembrane C3 convertase inhibitor that is related to CR1 and has both DAF and membrane cofactor protein activities.19 20 In antibody-induced complement activation assays in vitro, CD59a/DAF double-deficient mouse erythrocytes were shown to be much more susceptible than either DAF-deficient or CD59a-deficient cells to autologous and heterologous MAC attack (Figure4). Interestingly, the double-knockout mice likewise did not develop spontaneous hemolytic anemia. They had normal erythrocyte counts and displayed no signs of ongoing intravascular hemolysis. Furthermore, transfusion experiments confirmed that both CD59a-deficient and CD59a/DAF double-deficient erythrocytes had normal lifespans in vivo (Figure 5C).
In striking contrast to CD59a-deficient and CD59a/DAF double-deficient erythrocytes, Crry-deficient mouse erythrocytes were rapidly eliminated from the circulation in a complement-dependent manner (Figure 5D). Whether this involved intravascular or complement receptor–mediated extravascular hemolysis of the transferred cells remains to be determined. This finding suggests that Crry plays a more prominent role than DAF on murine erythrocytes in regulating spontaneous complement activation. This conclusion was supported by result of FACS analysis, which showed more C3 deposition on transfused Crry-deficient erythrocytes than on DAF-deficient erythrocytes at several initial time points after transfusion (Figure 6). Previous in vitro experiments, using recombinant soluble murine Crry-immunoglobulin and DAF-immunoglobulin fusion proteins, also indicated that when functioning as fluid phase inhibitors, Crry is more active than DAF as an alternative pathway complement regulator.43
In summary, our results suggest that while CD59a and DAF protect mouse erythrocytes from damage caused by abnormally activated complement, protection of murine erythrocytes from spontaneous complement attack is provided mainly by the C3 convertase inhibitor Crry. How can we reconcile this finding with the observation in humans that deficiency of the C3 convertase inhibitor DAF alone is inconsequential, whereas deficiency of CD59 caused a PNH-like blood cell sensitivity to complement? It is possible that the membrane structure of mouse erythrocytes is sufficiently different from that of human erythrocytes (eg, in the abundance and composition of sialic acids), with the result that the relative importance of C3 versus C5b-9 regulation is not the same in the 2 species. Alternatively, efficient regulation at the C3 step, by Crry in the mouse and by a C3 convertase regulator other than DAF in humans, is important in both species. In addition to DAF, CR1 is another C3 convertase inhibitor expressed on human erythrocytes. Like Crry, CR1 is a transmembrane protein and has both DAF and membrane cofactor protein activities. Although CR1 is a much larger molecule than mouse Crry (with 30 and 5 short consensus repeats for CR1 and Crry, respectively) and is thought to function mainly as an extrinsic complement regulator on neighboring cells,44 45 little is known about its potential relevance in the regulation of spontaneous complement activation on human erythrocytes, and as far as we know, no human CR1 deficiency has ever been described. In this regard, it should be noted that the rapid elimination of Crry-deficient mouse erythrocytes indicates a much more severe phenotype than the intravascular hemolysis associated with PNH. Complete CR1 deficiency in humans may therefore not be viable if CR1 plays a similarly critical role on human erythrocytes.
We thank Mr Luis Garcia for technical help. We also thank Dr Paul Morgan for the antimouse CD59a antibody, Dr Noriko Okada for the antimouse DAF antibody, and Dr Michael Holers for the antimouse Crry antibody. We are grateful to Dr John Lambris for the help in ELISA assays of complement activation in CVF-treated mouse sera and for providing the C3 knockout mice.
Supported by an Established Investigator Award from the American Heart Association and by National Institutes of Health grant AI 44970.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
W.-C. Song, Center for Experimental Therapeutics, University of Pennsylvania School of Medicine, 1351 BRBII/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail:song@spirit.gcrc.upenn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal