Microglial cells and astrocytes are capable of processing and presenting antigens for efficient activation of T cells. However, the antigen-presenting function and role of cerebrovascular endothelial cells (CVEs) in central nervous system inflammatory responses remain controversial. We compared the expression of necessary accessory molecules and the functional antigen-presenting capacity of cloned SJL/J CVEs and primary astrocytes in response to the pro-inflammatory cytokines interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α). Astrocytes and CVEs up-regulated major histocompatibility complex (MHC) class II, and primarily B7-1 as opposed to B7-2, in response to IFN-γ. TNF-α inhibited the IFN-γ–induced up-regulation of MHC class II on CVEs correlating to a decrease in the mRNA for the class II transactivator (CIITA), whereas CIITA expression in astrocytes was unaffected. Unlike astrocytes, CVEs did not elicit significant MHC class II-restricted T-cell responses. Furthermore, we have found that CVE monolayers are altered following T-cell contact, implicating CVE/T-cell contact in the breakdown of the blood–brain barrier during neuro-inflammatory responses.
Introduction
The brain has been considered an immune-privileged site in the presence of an intact blood–brain barrier (BBB). The BBB consists of tight junctions between cerebrovascular endothelial cells (CVEs), and it serves as a physical barrier limiting T-cell and antibody passage into the central nervous system (CNS).1Few unactivated T cells are capable of extravasating through the BBB, regardless of their antigen specificity, but low numbers of T cells can be found in the CNS of healthy humans and rats, so there appears to be a role for T-cell surveillance of the CNS in the absence of inflammation. Under these conditions, the low expression of major histocompatibility complex (MHC) class II and T-cell costimulation/adhesion molecules on CNS-resident cells renders the CNS an unsuitable site for T-cell priming. However, during an inflammatory immune response, the BBB is disrupted and all T cells and mononuclear cells have the ability to traffic into the brain and spinal cord and to recognize target antigens within the CNS.2-4 Under these conditions, potential antigen-presenting cells (APCs) residing in the CNS are likely to play a major role in T-cell responses and may contribute to the propagation or regulation of inflammatory immune responses in the CNS, as they do in multiple sclerosis (MS).
The BBB is damaged by demyelinating diseases such as MS and its animal model, experimental autoimmune encephalomyelitis (EAE). MS and EAE are characterized by CNS infiltration of myelin protein-specific Th1 cells, pro-inflammatory cytokine production of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), mononuclear cell accumulation, and destruction of myelin.5,6 CNS-resident cells, particularly microglia, pericytes, astrocytes, and CVEs, have the potential to affect the immune response by serving as APCs in the target organ. To serve as an APC, a cell must be able to express the requisite molecules to deliver the 2 signals necessary to activate a T cell.7Signal 1 is the antigen-specific signal delivered by antigen in MHC class II binding to the T-cell receptor (TCR). Signal 2, the costimulatory signal, is potently delivered by the B7 family of molecules binding to CD28 on the surface of the T cell.8Other costimulatory/adhesion molecules (ie, CD40, CD152, CD106) can deliver or enhance signal 2.9-11Professional APCs, such as B lymphocytes, macrophages, and dendritic cells, have been reported to constitutively express MHC class II and B7-1/2. Nonprofessional APCs must up-regulate MHC class II and B7 family members in response to an inflammatory stimulus. The class II transactivator (CIITA), which is absent in the human disease bare-lymphocyte syndrome,12 has been found to be essential for the inducible and constitutive expression of MHC class II,13,14 and it is the master switch for MHC class II expression. Moreover, the CIITA has a significant effect on the expression of other proteins associated with class II antigen processing and presentation, including H-2M and invariant chain (Ii).15 CIITA promoters III and IV contain IFN-γ response elements that confer IFN-γ–inducible CIITA expression and, in turn, MHC class II expression.16 17
Astrocytes, the major glial cell type of the CNS, are known to play a major role in maintaining the BBB.18,19 They provide nutritive functions and encapsulate inflammatory lesions, and they are likely to influence immune responses in the CNS. Our laboratory has reported that astrocytes exposed to the pro-inflammatory cytokine, IFN-γ, up-regulated MHC class II and B7 costimulatory molecules and were capable of activating naive transgenic T cells and memory Th1 lines derived from these cells.20 Moreover, we have found that T-cell stimulation with IFN-γ–treated SJL/J astrocytes is sufficient to activate T cells specific for the immunodominant encephalitogenic epitope of proteolipid protein (PLP139-151) for efficient transfer of EAE.21 There has been controversy regarding the purity of astrocytes from primary cultures, but Soos et al17 demonstrated that astrocyte clones are capable of processing and presenting protein antigen to activate T cells, albeit relatively inefficiently, when compared with microglial cells.22 We and others have demonstrated that microglial cell lines are fully capable of up-regulating MHC class II and B7 molecules to activate antigen-specific Th1 cells to proliferate and secrete pro-inflammatory cytokines.23 These observations suggest that astrocytes and microglia may actively participate in promoting immune responses in the CNS.
Little is known, however, concerning the role that CVEs may play in CNS immune responses. It has been demonstrated that IFN-γ–treated endothelial cells, including those comprising the CNS microvasculature, can express many of the cell surface molecules, including MHC class II molecules, required for T-cell activation.24,25 Moreover, the ability of CVEs to up-regulate the expression of MHC class II molecules appears to correlate with susceptibility to EAE in mouse and rat species,26,27 despite evidence that MHC compatibility between CVEs and encephalitogenic T cells is not required for the induction of EAE.28-33 It has been hypothesized that the disparate results reported to date may be attributed to the level of contamination of primary endothelial cell cultures with so-called professional APCs.34 To circumvent the possibility of contamination, we used stable, cloned SJL/J CVE lines to unambiguously evaluate their constitutive and pro-inflammatory cytokine-induced antigen presentation capacity. CVEs up-regulated MHC class II correlating to the activity of the class II transactivator protein. Interestingly, TNF-α inhibited the IFN-γ–induced up-regulation of CIITA-dependent molecules on CVEs, but not on astrocytes, by down-regulating the induction of CIITA mRNA. In addition, B7-1 and VCAM, but not B7-2, CD40, or ICAM-1, were expressed on IFN-γ–treated CVEs, whereas IFN-γ–treated astrocytes up-regulated B7-1, ICAM-1, and VCAM. In turn, astrocytes, but not CVEs, are capable of eliciting MHC class II-restricted T-cell responses. Instead, CVE morphology changes following CD4+ T-cell interaction, suggesting that T cells are capable of inducing changes in the cellular structure of the BBB.
Materials and methods
Mice
SJL/J 15-17d pregnant female mice and 5- to 6-week-old female mice were purchased from Harlan Labs, (Bethesda, MD). Mice were housed in Northwestern University's animal facility. One- to 3-day-old neonates were used for the isolation of astroglial cells.
Media
CVE and astroglial cultures were maintained in Dulbecco modified Eagle medium–Ham F12 (1:1) (DMEM-F12) (Sigma, St Louis, MO) supplemented with 10% fetal calf serum and adjusted to a final concentration of 6 g/L glucose, 2.4 g/L NaHCO3, 0.37 g/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (DMEM-F12). Media and fetal bovine serum were free of endotoxin contamination.
Cerebrovascular endothelial cell isolation
The procedure for isolating and deriving clonal populations of CVEs has been published elsewhere.27 Briefly, brains were removed from 2- to 4-week-old SJL/J mice, and the meninges and macroscopic pial vessels were removed by dissection. Brains were transferred to a second Petri dish, and the tissue was minced. Fragments were washed and resuspended in phosphate-buffered saline with 0.1% bovine serum albumin. The suspension was placed in a Wheaton homogenizer with 2 to 4 strokes of the B pestle to ensure the proper degree of homogenization to free the microvessels. The homogenate was passed by gravity through a succession of Nitex filters (Tetko, Switzerland) with 149-μm, 74-μm, and 20-μm pores. The filters were washed, and the 74-μm and 20-μm filters were placed in 50-mL conical tubes containing a 0.1% solution of collagenase–dispase (Boehringer, Indianapolis, IN) at 37°C and were digested through manual shaking for 3 hours. Then the filters were discarded. Digested material was centrifuged at 15g for 5 minutes, and the pellets were resuspended in 40 mL complete growth medium containing 20 μg/mL endothelial cell growth supplement (Collaborative Research, Bedford, MA), 50 μg/mL heparin (Elkins-Sinn, Cherry Hill, NJ), and 10% fetal bovine serum (FBS). Microvessel suspension was plated (1 mL suspension per 5 cm2) on plastic-ware coated with collagen type IV (Sigma) at 1 mg/mL and human serum fibronectin (Boehringer) at 10 μg/mL and was incubated at 37°C with 5% CO2. Daily microscopic observations were performed, and the culture medium was changed every 2 to 3 days.
Selective mobilization of CVE was accomplished through the use of porcine pancreatin (Sigma) during early passages. Most, if not all, muscle cells and many of the astrocytes and pericytes remained attached to the coated plates. Cells were cloned by limiting dilution onto coated 16-well glass chamber slides (Costar, Cambridge, MA). After 2 weeks of culture, wells with sufficient growth were passaged into 6-well plates (Costar) and then into 25 cm2 flasks (Costar). Three different fluorescent staining techniques were used to ensure that an endothelial cell phenotype was maintained. Cells were marked with antibodies to factor VIII-related antigen (abV ImmuneResponse, Derry, NH), antibodies to angiotensin-converting enzyme (gift of Dr R. Auerbach, University of Wisconsin), and acetylated low-density lipoprotein (Biomedical Technologies, Stoughton, MA). Cloned cells remained diploid and retained their differentiation markers for at least 20 passages. All experiments were performed with cells before passage 20.
Astroglial cell isolation
Tissue culture flasks were coated from 3 hours to overnight with 10 μg/mL poly-D-lysine (Sigma) and were rinsed with balanced salt solution supplemented with 3% fetal bovine serum (BSS-3%) before the addition of isolated cells. Brains were removed from 1- to 3-day-old neonatal mice, hindbrains were dissected away, and meninges were removed. Left and right hemispheres were transferred to a nylon mesh bag and gently dissociated. Cells in suspension were passed through #60 and #100 stainless steel screens (Sigma) to remove large pieces of debris and tissue. Cells were pelleted, resuspended in DMEM-F12 complete medium, and seeded in the poly-D-lysine–coated tissue culture flasks and were incubated at 37°C, 7.5% CO2. Fresh medium was added every 3 to 4 days. After 12 to 14 days, microglia and oligodendrocytes were removed from the astroglial bed layer by shaking the flasks on an orbital shaker for 1 hour at 100 rpm and for 24 hours at 300 rpm. Astroglial cells that adhered to the flask were trypsin treated and replated to ensure lack of microglial cell contamination.
Purity of naive and IFN-γ–treated astrocytes was determined by intracellular staining with antibodies to glial fibrillary acidic protein (GFAP). Cells were adhered overnight to poly-D-lysine–coated coverslips. Cells were fixed for 10 minutes at room temperature with 2% paraformaldehyde (Sigma), rinsed with PBS, and permeabilized with PBS–0.2% fish skin gelatin (FSG; Sigma)–0.5% saponin for 20 minutes. Cells were rinsed with PBS, and nonspecific binding was blocked with PBS–0.2% FSG for 30 minutes, then incubated with primary anti-GFAP (1:200; DAKO, Carpinteria, CA) for 1 hour at 37°C. Cells were rinsed with PBS–0.2% FSG and were incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin (1:200; Calbiochem, La Jolla, CA) for 1 hour at 37°C. Cells were washed with PBS–FSG, mounted on glass slides, and examined under a fluorescence microscope. Astrocyte cultures were greater than 98% GFAP+.
Cell surface staining and analysis
CVEs and astrocytes were incubated in media alone, mouse recombinant IFN-γ (rIFN-γ) (100 U/mL; R&D Systems, Minneapolis, MN), mouse rTNF-α (500 U/mL; R&D Systems), or both cytokines at 37°C, 7.5% CO2 for 48 hours. Cells were washed in the flasks with cold PBS and then gently removed using cell scrapers. Cells were washed and resuspended in 2.4 G2 supernatant and were incubated for 30 minutes at 4°C with anti I-As-biotin (MKS4), anti–ICAM-1–phycoerythrin (PE) (3E2) and anti–VCAM-1-biotin (429) (BD PharMingen, San Diego, CA), anti–B7-1-PE (16-10A1), anti–B7-2-PE (GL1), anti–CD40-PE (HM40-3), anti-CD95 (clone Jo2), anti-CD95L (clone Kay 10), or the appropriate isotype-matched controls. Cells were washed in isotonic-buffered saline, and biotinylated antibodies were detected by subsequent staining with streptavidin-PE (BD PharMingen) for 20 minutes in the dark at 4°C. Cells were then washed and resuspended in 200 μL isotonic-buffered saline. Data collection and analysis were performed on a Becton Dickinson FACSCalibur fluorescence-activated cell sorter using CellQuest software. Nonspecific background staining was determined by incubating samples with isotype-matched control monoclonal antibodies. Mean fluorescence shifts were determined by subtracting the mean fluorescence intensity of isotype controls from the mean fluorescence intensity of the specific stain.
RT-PCR analyses
CVEs and astrocytes were incubated in media alone, rIFN-γ, rTNF-α, or both cytokines for 48 hours. Cells were scraped from the flasks and washed with PBS. Total RNA was isolated from the extract using an RNeasy Total RNA Kit (Qiagen, Germany). 1-2 μg of total RNA was used to make total cDNA using a Clontech cDNA kit (Clontech Laboratories, Palo Alto, CA) per reaction and PCR was performed using approximately 1/5 of total cDNA and Taq DNA polymerase (Qiagen GmbH, Hilden, Germany). Primers for CIITA (sense, 5′-CAA GTC CCT GAA GGA TGT GGA-3′; anti-sense, 5′-ACG TCC ATC ACC CGG AGG GAC-3′) and actin (sense, 5′-GTG GGC CGC TCT AGG CAC CAA; anti-sense, 5′-CTC TTT GAT GTC ACG CAC GAT TTC) were synthesized at Northwestern University's Biotechnology Center. Polymerase chain reaction (PCR) products were visualized by ethidium bromide agarose (2%) gel electrophoresis.
Isolation of Thy1.2+ T cells
Spleens were harvested from syngeneic SJL/J or allogeneic A/J mice and were mashed through 100 mesh screens into single-cell suspensions. Red blood cells were lysed with 2 mL/spleen Tris NH4Cl, and remaining live cells were counted. Cells were labeled with 10 μL anti-CD90.2 conjugated to microbeads per 107 total cells for 15 minutes on ice. After thorough washing, labeled cells were collected using the AutoMACS (Miltenyi Biotec, Auburn, CA) possel program.
Mixed lymphocyte cultures
Astrocytes were removed from tissue culture flasks using trypsin, washed, resuspended in DMEM-F12 complete medium, and cultured in poly-D-lysine–treated 96-well flat-bottom tissue culture plates (Becton Dickinson Labware) (2 × 104/well) in the presence or absence of rIFN-γ (100 U/mL), TNF-α (500 U/mL), or both and were incubated for 48 hours before the assay. CVEs were cultured in T75 flasks in the presence or absence of rIFN-γ (100 U/mL), TNF-α (500 U/mL), or both for 48 hours before the assay. CVEs were trypsinized, washed, and plated (5 × 104/well) in 96-well flat-bottom tissue culture plates (Corning). Immediately preceding the addition of T cells and antigen, the CVEs or astrocytes were irradiated with 30 Gy, then gently, but extensively, washed with BSS-3% FCS to remove residual cytokine. Cells (4 × 105 CD90.2+) from A/J spleens were added to the cultures. Irradiated (30 Gy) SJL/J splenocytes (4 × 105/well) were used as a control allogeneic APC population in separate wells. Proliferation assays were carried out in DMEM–10% FCS supplemented with 40 μM indomethacin (Sigma) and 2 mM aminoguanidine (Sigma). Culture wells were pulsed with 0.037 MBq/well 3[H]-TdR (ICN Radiochemicals, Irvine, CA) for the final 24 hours of the 72-hour incubation period.3[H]-TdR uptake was detected using a top-count microplate scintillation counter (Packard Instruments, Meriden, CT), and results are expressed as the mean of triplicate cultures ± SEM.
Long-term T-cell lines
Long-term PLP139-151 Th1 lines and Th2 lines were established from the lymph nodes of SJL/J mice, primed 10 days prior with 100 μg peptide in complete Freund adjuvant supplemented with 200 μgMycobacterium tuberculosis H37Ra. Every 3 to 4 weeks, live T cells were isolated on Ficoll-Histopaque (Amersham Pharmacia Biotech AB, Uppsala, Sweden) by centrifugation at 1200 rpm at room temperature for 15 minutes and were propagated by in vitro stimulation of 1 × 106 T cells with 5 × 106 irradiated syngeneic splenic APCs and 25 μM appropriate peptide for 96 hours. Th2 lines were skewed by repeated stimulations in the presence of 10 μg/mL anti IL-12 (R&D), 10 μg/mL anti IFN-γ (R&D), and 0.1 ng/mL recombinant IL-4 (R&D). All stimulation assays were performed in DMEM-10:DMEM (Sigma) supplemented with 10% FBS (Sigma), 2 × 10−3 M L-glutamine (GIBCO BRL, Grand Island, NY), 100 U/mL penicillin (GIBCO BRL), 100 μg/mL streptomycin (GIBCO BRL), 5 × 10−5 M 2-mercaptoethanol, and 0.1 mM nonessential amino acids. After stimulation, T cells were rested in DMEM-10 supplemented with 2 U/mL recombinant IL-2 (Boehringer Mannheim Biochemicals, Indianapolis, IN). All antigen presentation assays using T-cell lines were conducted 14 to 30 days after stimulation.
Antigen presentation assays
Astrocytes were removed from tissue culture flasks using trypsin, washed, resuspended in DMEM-F12 complete medium, and cultured in poly-D-lysine–treated 96-well flat bottom tissue culture plates (Becton Dickinson Labware, Bedford, MA) (1 × 104/well) in the presence or absence of rIFN-γ (100 U/mL), TNF-α (500 U/mL), or both and were incubated for 48 hours before the assay. CVEs were cultured in T75 flasks in the presence or absence of rIFN-γ (100 U/mL), TNF-α (500 U/mL), or both for 48 hours before the assay. CVEs were trypsinized, washed, and plated (1 × 104/well) in 96-well flat-bottom tissue culture plates (Corning, NY). Immediately preceding the addition of T cells and antigen, the CVEs or astrocytes were irradiated with 30 Gy, then gently, but extensively, washed with BSS-3% to remove residual cytokine. Myelin-specific T cells (5 × 104) or CD90.2+ T cells (4 × 105) were added to the 96-well plates in the presence of peptide or staphylococcal enterotoxin B (SEB). Irradiated (30 Gy) syngeneic splenocytes (4 × 105/well) were used as a control APC population in separate wells. Proliferation assays were carried out in DMEM-10 supplemented with 40 μM indomethacin (Sigma) and 2 mM aminoguanidine (Sigma). Culture wells were pulsed with 0.037 MBq/well 3[H]-TdR (ICN Radiochemicals, Irvine, CA) for the final 24 hours of the 72-hour incubation period.3[H]-TdR uptake was detected using a top-count microplate scintillation counter (Packard Instruments, Meriden, CT), and results are expressed as the mean of triplicate cultures ± SEM.
T-cell hybridoma stimulation
PLP139-151–specific T-cell hybridomas were generated. Briefly, lymph node T cells from previously primed mice were restimulated in vitro with PLP139-151 and were fused with BW5147 fusion partners using polyethylene glycol (Sigma). Successful fusions were selected in DMEM-10 containing HAT (Sigma). Long-term T-cell hybridomas were cultured in DMEM-10, and T-cell receptor engagement was measured through IL-2 production as determined by enzyme-linked immunosorbent assay (ELISA; Endogen, Cambridge, MA). CVEs were cultured in T75 flasks in the presence or absence of rIFN-γ (100 U/mL), TNF-α (500 U/mL), or both for 48 hours before assay. CVEs were trypsinized, washed and plated (1 × 104/well) in 96-well flat-bottom tissue culture plates (Corning). Immediately preceding the addition of T-cell hybridomas and antigen, the CVEs were gently, but extensively, washed with BSS-3% FCS to remove residual cytokine. PLP139-151-specific T cell hybridomas (5 × 104) were added to the 96-well plates in the presence or absence of peptide. SJL/J splenocytes (4 × 105/well) were used as a control APC population in separate wells. Hybridoma assays were carried out in DMEM-10. After 24 hours, culture supernatants were collected and IL-2 levels were determined using an ELISA Minikit (Endogen). Data are represented as average absorbance of triplicate cultures at 490 nm ± SD.
Coculture analysis
CVE clones were allowed to grow to confluence in a 48-well tissue culture plate or chamber slide. T cells (2.5 × 105) ± peptide were added to the monolayers overnight. Pictures were taken using a Nikon N90 AF 35-mm camera and were scanned into Photoshop using Polaroid Insight. For fluorescence analysis of live versus dead cells, chamber slides were washed in PBS, stained with propidium iodide (Molecular Probes, Eugene, OR), 1:2000 in PBS, washed in PBS again, stained with DAPI (Sigma), 1:25 000 in PBS, washed in PBS again, dried, and cover-slipped using Vectashield mounting medium (Vector, Burlingame, CA). Slides were examined, and images were acquired by epifluorescence using the SPOT RT camera (Diagnostic Instruments, Sterling Heights, MI) and Metamorph Imaging Software (Universal Imaging, Downingtown, PA).
Results
TNF-α inhibits IFN-γ–induced MHC class II up-regulation on SJL/J CVEs, but not on astrocytes
We have previously demonstrated that astrocytes from EAE-susceptible SJL/J mice up-regulate MHC class II and B7-1 upon incubation with IFN-γ in vitro.21 However, the inflammatory environment of the CNS during immune-mediated demyelinating diseases and infection is characterized by the expression of multiple cytokines, including the major pro-inflammatory cytokines, IFN-γ and TNF-α.35 TNF-α has been suggested to play a necessary role in the development of EAE.36-38 Thus, we first sought to determine whether MHC class II expression on BBB cells was differentially affected by incubation with IFN-γ or TNF-α alone or with both cytokines in combination.
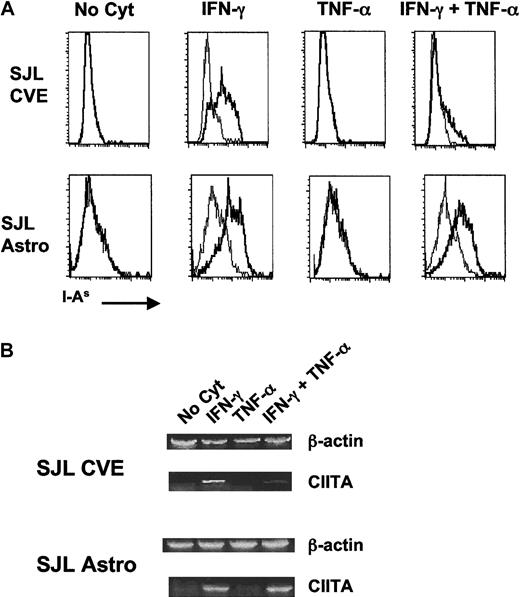
As shown in Figure 1A, neither astrocytes nor CVEs constitutively expressed MHC class II, but both cell types up-regulated MHC class II when incubated with IFN-γ alone. Incubation with TNF-α alone did not result in the up-regulation of surface MHC class II on either cell population. However, co-incubation of CVEs with TNF-α and IFN-γ resulted in a dramatic diminution of cell-surface MHC class II in comparison with the level of expression with IFN-γ alone. Interestingly, this effect was not seen in astrocyte cultures in which the level of MHC class II on the surface was comparable in both culture conditions. The decrease in surface expression of MHC class II on CVEs was TNF-α dose-dependent and was not caused by changes in cell viability (data not shown) or by a general decrease in cell surface markers because surface expression of the costimulatory–adhesion molecules B7-1 and ICAM-1 (Figure2) was unaffected or increased upon incubation of CVEs with IFN-γ and TNF-α. Interestingly, these data demonstrate that the expression of MHC class II, a central molecule in antigen presentation, is differentially regulated in the different cell types comprising the BBB.
Comparison of expression of MHC class II (A) and CIITA (B) on cytokine-treated SJL/J CVEs and astrocytes.
Cloned SJL/J CVEs or astrocytes were cultured with no cytokine, 100 U/mL IFN-γ, 500 U/mL TNF-α, or both cytokines for 48 hours. Cells were analyzed for relative expression of I-As by (A) flow cytometry and (B) CIITA mRNA by RT-PCR. (A) Histogram plots of the flow cytometric staining are shown plotted as the number of positive cells versus fluorescence intensity with I-As staining in bold. Isotype control staining is plotted in the fine line. (B) RNA was isolated from CVE and astrocyte cultures, and PCR samples were prepared as described in “Materials and methods.” Data shown are scanned images of ethidium bromide-stained 2% agarose gels.
Comparison of expression of MHC class II (A) and CIITA (B) on cytokine-treated SJL/J CVEs and astrocytes.
Cloned SJL/J CVEs or astrocytes were cultured with no cytokine, 100 U/mL IFN-γ, 500 U/mL TNF-α, or both cytokines for 48 hours. Cells were analyzed for relative expression of I-As by (A) flow cytometry and (B) CIITA mRNA by RT-PCR. (A) Histogram plots of the flow cytometric staining are shown plotted as the number of positive cells versus fluorescence intensity with I-As staining in bold. Isotype control staining is plotted in the fine line. (B) RNA was isolated from CVE and astrocyte cultures, and PCR samples were prepared as described in “Materials and methods.” Data shown are scanned images of ethidium bromide-stained 2% agarose gels.
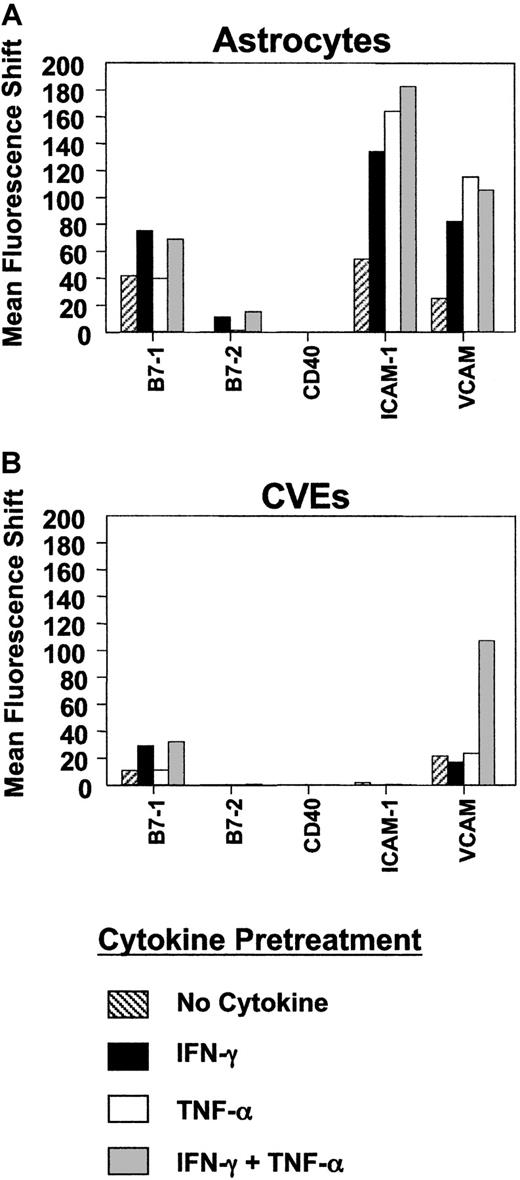
Comparison of costimulatory and adhesion molecule expression on SJL/J astrocytes and CVEs.
SJL/J astrocytes or CVEs were cultured with no cytokine, 100 U/mL IFN-γ, 500 U/mL TNF-α, or both cytokines for 48 hours. Cells were analyzed for relative expression of B7-1, B7-2, CD40, ICAM-1, and VCAM-1 using flow cytometric analysis. Data are plotted as mean fluorescence shifts (mean fluorescence intensity of the marker − mean fluorescence intensity of the isotype control) for each marker on cells following cytokine pretreatment.
Comparison of costimulatory and adhesion molecule expression on SJL/J astrocytes and CVEs.
SJL/J astrocytes or CVEs were cultured with no cytokine, 100 U/mL IFN-γ, 500 U/mL TNF-α, or both cytokines for 48 hours. Cells were analyzed for relative expression of B7-1, B7-2, CD40, ICAM-1, and VCAM-1 using flow cytometric analysis. Data are plotted as mean fluorescence shifts (mean fluorescence intensity of the marker − mean fluorescence intensity of the isotype control) for each marker on cells following cytokine pretreatment.
Differential expression of MHC class II on APCs is regulated by the CIITA molecule.13,14,39 To better understand the mechanism underlying the differential effects of TNF-α on SJL/J CVEs and astrocytes, we investigated the level of transcription of CIITA mRNA in these 2 cell populations after cytokine coculture. The primary cytokine that controls the expression of CIITA is IFN-γ.14 However, studies have demonstrated that TGF-β plays a role in down-regulating this protein at the promoter level16,40 and that different tissues might use differential regulatory pathways for CIITA with respect to various cytokines.41 42 Semiquantitative reverse transcription (RT)-PCR was performed on RNA samples derived from CVEs and astrocytes incubated with media alone, IFN-γ, TNF-α, or both cytokines together. Similar to the expression of MHC class II on the cell surface, the amount of CIITA mRNA expressed by CVEs was significantly decreased in cultures incubated with both cytokines compared to IFN-γ alone (Figure 1B). The amount of CIITA mRNA in astrocytes, however, was not diminished in cultures exposed to the combination of IFN-γ and TNF-α. Therefore, this is the first report demonstrating that the CIITA is differentially regulated in these 2 CNS cell types in response to different pro-inflammatory cytokines.
Limited expression of costimulatory/adhesion molecules by SJL/J CVEs compared to SJL/J astrocytes
So-called professional APCs express MHC class II and numerous costimulatory and adhesion molecules. One factor that might influence the ability of SJL/J CVEs to serve as APCs is their ability to express costimulatory or adhesion molecules that are critical for T-cell activation. Therefore, we examined constitutive and cytokine-induced expression of various costimulatory molecules on SJL/J CVEs compared to that of SJL/J astrocytes using flow cytometry. B7-1 and B7-2 molecules expressed on APCs interact with CD28 expressed on T cells to induce critical second signals necessary for antigen-specific T-cell activation.8 In SJL/J astrocytes, for example, blocking either B7-1 or B7-2 can diminish the magnitude of T-cell proliferation in vitro, though B7-1 is functionally dominant.21 Previous experiments examining the ability of endothelial cells to express B7 costimulatory molecules have yielded disparate results.43-45 We found that B7-1 was constitutively expressed at a low level on the surfaces of the cloned CVEs, and this level was unaffected by incubation with TNF-α (Figure 2). However, B7-1 expression was significantly increased upon stimulation with IFN-γ, and the level was further enhanced by incubation with IFN-γ and TNF-α in combination. B7-2 was not constitutively expressed, nor was it detected by flow cytometric analysis on SJL/J CVEs stimulated with IFN-γ or TNF-α. Similar results were observed on determination of B7-1 and B7-2 mRNA levels measured by semiquantitative PCR (data not shown). The B7-1–dominant expression pattern on SJL CVEs was similar to what we observed on examination of SJL/J astrocytes before and after activation with Th1 cytokines (Figure 2). Interestingly, CD40, another important costimulatory molecule,46 was neither constitutively nor inducibly expressed on CVEs or astrocytes by the various cytokines. VCAM-1 and ICAM-1 have also been shown to decrease the activation threshold of T cells.47-49 VCAM-1 was expressed constitutively on the SJL CVE clones and was further up-regulated by the combined effects of IFN-γ and TNF-α, whereas ICAM-1 was not expressed on CVEs under any cytokine conditions tested. In contrast, VCAM-1 and ICAM-1 were both constitutively expressed on SJL/J astrocytes, and the expression of each adhesion molecule was significantly up-regulated with Th1 cytokine activation. Therefore, SJL/J CVEs inducibly express B7-1, which normally can act alone as a potent costimulatory molecule for the induction of Th1 proliferation,50 but they do not express the full-repertoire of costimulatory/adhesion molecules normally seen in professional APCs. In contrast, astrocytes express a more complete repertoire with constitutive B7-1, VCAM-1, and ICAM-1 expression and inducible expression of B7-2.
SJL astrocytes, but not CVEs, elicit MHC class II–restricted T-cell responses
I-As has been detected on IFN-γ–stimulated CVEs by flow cytometry and RT-PCR (Figure 1 and data not shown). To measure the functional expression of I-As on the surfaces of SJL/J astrocytes and CVEs, mixed lymphocyte cultures were set up using these CNS-resident APC populations as stimulators and T cells from A/J (H-2k) mice as responders. Using this assay, functional I-As expression on the surfaces of different stimulator cell populations can be detected as a function of allogeneic T-cell proliferation. Astrocytes and CVEs were pretreated with media alone or with media containing IFN-γ, TNF-α, or both cytokines. As seen in Figure 3A, bulk SJL splenocytes elicited a vigorous alloresponse, indicating that the A/J T cells recognized the presence of allogeneic I-As–bearing cells. Untreated astrocytes did not elicit a significant alloresponse, suggesting that these cells expressed an insufficient level of I-As. All cytokine-pretreated groups of astrocytes elicited significant allogeneic T-cell responses, confirming the presence of MHC class II on their surfaces (Figure 3A). Interestingly, the T-cell proliferative responses correlated closely with the levels of I-As detected by flow cytometry (Figures 1A, 3A). In contrast, none of the CVE stimulators elicited significant allogeneic T-cell responses (Figure 3A), suggesting that CVEs are not competent APCs or stimulators, despite their surface expression of MHC class II.
Comparison of MHC class II-restricted T-cell responses elicited by cytokine-activated SJL/J astrocytes and CVEs.
SJL/J astrocytes or SJL/J CVEs were incubated for 48 hours with no cytokine, IFN-γ (100 U/mL), TNF-α (500 U/mL), or both cytokines. (A) Cells were washed, harvested, irradiated (30 Gy), and plated (104/well) before the addition of 5 × 104Thy 1.2+ allogeneic (A/J) T cells. (B) Cells were washed, harvested, irradiated (30 Gy), and plated (104/well) before the addition of 5 × 104 Thy 1.2+ syngeneic (SJL/J) T cells and 10μg/mL SEB. Irradiated syngeneic splenocytes (4 × 105/well) were used as a control APC population in separate wells in both experiments. T-cell proliferative responses were measured by 3[H]-thymidine incorporation. Results are expressed as mean cpm (× 10−3) ± SEM of triplicate wells. Stimulation indices are noted beside each bar.
Comparison of MHC class II-restricted T-cell responses elicited by cytokine-activated SJL/J astrocytes and CVEs.
SJL/J astrocytes or SJL/J CVEs were incubated for 48 hours with no cytokine, IFN-γ (100 U/mL), TNF-α (500 U/mL), or both cytokines. (A) Cells were washed, harvested, irradiated (30 Gy), and plated (104/well) before the addition of 5 × 104Thy 1.2+ allogeneic (A/J) T cells. (B) Cells were washed, harvested, irradiated (30 Gy), and plated (104/well) before the addition of 5 × 104 Thy 1.2+ syngeneic (SJL/J) T cells and 10μg/mL SEB. Irradiated syngeneic splenocytes (4 × 105/well) were used as a control APC population in separate wells in both experiments. T-cell proliferative responses were measured by 3[H]-thymidine incorporation. Results are expressed as mean cpm (× 10−3) ± SEM of triplicate wells. Stimulation indices are noted beside each bar.
Because we have been unable to detect functional MHC class II expression on the surfaces of CVEs as determined by allogeneic T-cell proliferation (Figure 3A), the relative abilities of astrocytes and CVEs to present the superantigen SEB to stimulate syngeneic T cells was tested. Superantigen presentation was chosen because SEB binds to MHC class II outside the peptide-binding groove and also to Vβ3, 7, 8, and 17 chains of the TCR to activate T cells expressing these Vβ chains. In this assay, astrocytes and CVEs were stimulated with media alone or with media containing IFN-γ, TNF-α, or both cytokines for 48 hours. After 48 hours, these cells were harvested, washed, and cultured with syngeneic purified splenic T cells and 1 μg SEB. Bulk splenocytes from SJL/J mice were capable of presenting SEB and eliciting a vigorous T-cell response with a stimulation index (SI) of 159 (Figure 3B). T cells cultured alone with SEB had virtually no response, indicating that the T-cell population was not contaminated with any MHC class II-bearing cells. Astrocytes cultured with media alone or with TNF-α expressed little MHC class II (Figure 1A) and elicited low, albeit significant (SI, 3), T-cell responses to SEB (Figure 3B). Astrocytes stimulated with IFN-γ or the combination of IFN-γ + TNF-α up-regulated class II expression (Figure 1A) and, in turn, presented SEBs eliciting strong T-cell responses (SI, 117 and 89, respectively) (Figure 3B). CVEs cultured with media alone or with TNF-α expressed no detectable MHC class II (Figure 1A) and did not present SEB to activate T cells (Figure 3B). However, CVEs stimulated with IFN-γ did up-regulate the surface expression of MHC class II, as seen by flow cytometry (Figure 1A), and, consequently, were capable of presenting SEB to elicit a significant response (SI, 3.8) from the T cells (Figure 3B), indicating the presence of a low level of functional class II expression on these cells. Similarly, CVEs pretreated with IFN-γ and TNF-α moderately up-regulated their expression of class II, enabling them to present SEB and to elicit a small response from the T cells (SI, 2.6) (Figure 3B). Finally, the measurement of MHC class II expression on CVEs and astrocytes by functional assay correlated with the data found using flow cytometry. However, CVEs appeared to be weak APCs when compared with astrocytes and professional splenic APCs.
CVEs do not activate myelin epitope-specific Th1 and Th2 lines
To better understand the role CVEs may play during an immune response within the CNS, we investigated whether these cells were capable of presenting antigen for the activation of Th1 cells involved in CNS demyelination, as we have previously reported for astrocytes.21 Purified cultures of CVE clones were incubated with media alone or media containing IFN-γ, TNF-α, or both cytokines for 48 hours. The cells were then cultured with PLP139-151, the immunodominant encephalitogenic epitope on proteolipid protein in SJL/J mice, and a peptide-specific Th1 line. SJL/J CVEs could not stimulate significant proliferation of the PLP139-151–specific T-cell line, even after pre-incubation with IFN-γ alone, a condition that leads to significant up-regulation of MHC class II (Figures 1, 4A). Albeit low, it is notable that CVEs pretreated with IFN-γ did elicit Th1 proliferation to a greater degree than the other 3 treatment conditions (SI of 2.5 compared with SIs of 0.8, 0.6; 1.0 μM) (Figure 4A). To verify this lack of responsiveness to CVE antigen presentation, the experiment was repeated numerous times. Varying culture conditions, including the concentration of peptide (1 μM to 100 μM) and numbers of CVE clones in culture (1 × 103/well to 1 × 105/well), yielded consistently negative results. CVEs failed to activate Th1 lines specific for either PLP178-191 or PLP56-70 (data not shown). Additionally, supernatants were collected from all the cultures to measure cytokine production by ELISA, but none of the CVE + Th1 cultures produced cytokines (data not shown). Thus, despite the expression of significant levels of MHC class II on the surfaces of CVEs, these cells were unable to serve as efficient APCs to activate Th1 lines to proliferate or produce cytokines.
Comparison of the antigen-presenting capabilities of cytokine-treated SJL/J astrocytes and CVEs for Th1 and Th2 cells.
SJL/J CVEs were incubated for 48 hours with no cytokine, IFN-γ (100 U/mL), TNF-α (500 U/mL), or both cytokines. Cells were washed, harvested, and irradiated (30 Gy) before the addition of 5 × 104 PLP139-151–specific Th1 cells (A), Th2 cells (B), or T-cell hybridomas (C) along with antigen. Irradiated syngeneic splenocytes (4 × 105/well) were used as a control APC population in separate wells. (A, B) T-cell proliferative responses were measured by 3[H]-thymidine incorporation. Results are expressed as mean cpm (× 10−3) ± SEM of triplicate wells using 104 CVEs per well and the indicated concentration of PLP139-151. Stimulation indices are noted beside each bar. (C) T-cell hybridoma responses were measured by collecting culture supernatants after 24 hours and determining IL-2 levels by ELISA. Data are expressed as average absorbance of triplicate wells at 450 nm ± SD.
Comparison of the antigen-presenting capabilities of cytokine-treated SJL/J astrocytes and CVEs for Th1 and Th2 cells.
SJL/J CVEs were incubated for 48 hours with no cytokine, IFN-γ (100 U/mL), TNF-α (500 U/mL), or both cytokines. Cells were washed, harvested, and irradiated (30 Gy) before the addition of 5 × 104 PLP139-151–specific Th1 cells (A), Th2 cells (B), or T-cell hybridomas (C) along with antigen. Irradiated syngeneic splenocytes (4 × 105/well) were used as a control APC population in separate wells. (A, B) T-cell proliferative responses were measured by 3[H]-thymidine incorporation. Results are expressed as mean cpm (× 10−3) ± SEM of triplicate wells using 104 CVEs per well and the indicated concentration of PLP139-151. Stimulation indices are noted beside each bar. (C) T-cell hybridoma responses were measured by collecting culture supernatants after 24 hours and determining IL-2 levels by ELISA. Data are expressed as average absorbance of triplicate wells at 450 nm ± SD.
Fabry et al29 reported the activation of Th2 but not of Th1 cells as a consequence of antigen presentation by primary BALB/c brain microvessel endothelial cells. Based on that report, the relative abilities of cytokine-stimulated SJL/J CVE clones to activate PLP139-151–specific Th2 cells were tested (Figure 4B). Similar to the Th1 lines, CVE clones were not capable of activating Th2 cells to proliferate (Figure 4B) or produce IL-5 and IL-10 (data not shown). In contrast to the earlier report, we find that CVEs are not capable of serving as APCs for the activation of myelin-specific Th1 or Th2 cells.
CVE clones are not capable of activating T-cell hybridomas to secrete IL-2
One possible explanation for the failure of CVEs to activate Th1 and Th2 cells is the low level of costimulatory molecule expression on CVEs compared to that on astrocytes and professional APCs (Figure 2). Therefore, we asked whether CVEs could activate costimulation-independent PLP139-151–specific T-cell hybridomas. Following treatment with media alone or with IFN-γ, TNF-α, or both cytokines, CVE clones were unable to activate T-cell hybridomas to produce IL-2 (Figure 4C), but irradiated bulk splenocytes efficiently activated these hybridomas to produce IL-2 in an antigen-dependent manner (Figure 4C). Again, these experiments led to the same conclusion: despite the expression of MHC class II, CVE clones are poor APCs.
T cells damage CVE and astrocyte monolayers
CVEs do not appear to serve as competent APCs in vitro despite their expression of surface molecules necessary for antigen presentation. Although CVEs may not activate T cells in the CNS, they are involved in T cell entry into the CNS. Previous reports have indicated that encephalitogenic and nonencephalitogenic CD4+ T cells can cause damage to Lewis rat CVE monolayers,51 suggesting that EAE induction may involve T-cell–mediated damage to CNS vasculature.
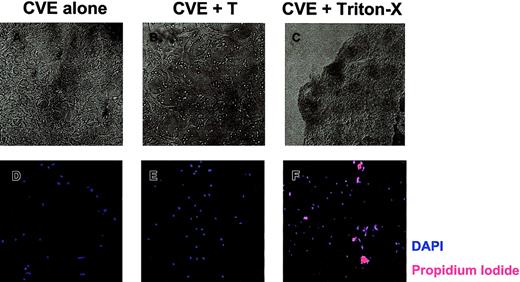
To determine whether SJL/J CVEs are susceptible to T-cell–mediated damage, CVE monolayers were cultured with PLP139-151–specific T cells and were examined 18 hours later. Figure5A displays healthy, confluent CVEs that were cultured alone. After coculture with T cells overnight, there was clear evidence that the CVE monolayer had been damaged, as indicated by fewer, more constricted cell processes (Figure 5B). These data are representative of 5 experiments with a variety of myelin peptide-specific T-cell lines cultured in the presence and absence of antigen. Consequently, rested T cells used in antigen presentation assays (Figure 4) were capable of damaging CVEs, which may, in part, explain why CVEs cannot activate T cells as measured by proliferation. Using phase-contrast microscopy, we observed morphologic changes associated with apoptosis in CVEs. To determine whether CVEs actually undergo apoptosis as a result of T-cell interactions, live versus dead cells were examined. After culturing CVEs in the presence or absence of T cells overnight, the monolayers were washed and stained with DAPI and propidium iodide (PI) to analyze total versus dead cells, respectively. When CVEs were cultured alone, no PI-positive cells were detected, indicating that all the cells in the culture were alive (Figure 5D). Surprisingly, no PI-positive cells were found in CVE monolayers following overnight coculture with T cells (Figure 5E). As a positive control, CVEs treated with Triton-X stained positive for PI (Figure 5F). In summary, T cells induce morphologic changes in CVE monolayers without causing apoptosis, as indicated by the lack of PI staining.
T-cell–induced damage to CVE monolayers.
CVEs were cultured 18 to 20 hours alone (A, D), with PLP139-151–specific Th1 cells and no antigen (B, E), or with 0.02% Triton X-100 (C, F). (A-C) Representative pictures were taken using a Nikon N90 AF 35-mm camera and scanned into Adobe Photoshop using Polaroid Insight for analysis (original magnification, 200×). (D-F) Monolayers were then washed and stained with DAPI (blue) and PI (red) to quantitate the total number of cells and the number of dead cells, respectively. Slides were examined and images were acquired through epifluorescence using the SPOT RT camera, and they were analyzed with Metamorph Imaging Software.
T-cell–induced damage to CVE monolayers.
CVEs were cultured 18 to 20 hours alone (A, D), with PLP139-151–specific Th1 cells and no antigen (B, E), or with 0.02% Triton X-100 (C, F). (A-C) Representative pictures were taken using a Nikon N90 AF 35-mm camera and scanned into Adobe Photoshop using Polaroid Insight for analysis (original magnification, 200×). (D-F) Monolayers were then washed and stained with DAPI (blue) and PI (red) to quantitate the total number of cells and the number of dead cells, respectively. Slides were examined and images were acquired through epifluorescence using the SPOT RT camera, and they were analyzed with Metamorph Imaging Software.
Discussion
CNS-resident cells may play an important role in the inflammation seen in autoimmune demyelinating diseases such as MS and EAE, during either disease initiation or disease progression. Microglial cells and astrocytes can activate T cells following exposure to pro-inflammatory cytokines, such as IFN-γ and TNF-α, that are expressed in the diseased CNS35,52,53; thus, they may play a role in presenting endogenous myelin epitopes, leading to the phenomenon of epitope spreading.54 However, the role of CVEs as potential APCs in these inflammatory processes is not fully understood. Although CVEs can clearly up-regulate the expression of surface MHC class II, their capacity to stimulate CD4+ T cells is uncertain. Additionally, the mechanisms that govern the expression of MHC class II and other molecules on the CVE cell surface required for T-cell activation remain unclear.
Our first goal was to better understand the regulation of MHC class II on the CVE surface. Interestingly, the ability of CVEs to up-regulate MHC class II on the cell surface in response to IFN-γ in mice is strain dependent and may correspond to susceptibility to EAE.26,27 Welsh et al27 have reported the up-regulation of MHC class II on CVEs from EAE-susceptible SJL/J mice, but not on CVEs from EAE-resistant BALB/c mice. However, other cytokines, including TNF-α, may also play a role in the regulation of MHC class II on endothelial cells. TNF-α is produced by CNS-resident cells in EAE,35,52 along with infiltrating mononuclear cells, and may be of great importance in the pathogenesis of this disease.36-38,55 We found that TNF-α treatment did not up-regulate MHC class II expression of cultured CVEs. Rather, TNF-α significantly reduced the surface expression of MHC class II when added to cultures in combination with IFN-γ (Figure 1), as found in previous studies,56 an effect that has been reported to be dependent on the maturation and differentiation level of the cells tested.57 We have extended this observation by showing that this regulation is correlated with the expression of the CIITA as evidenced by TNF-α–induced down-regulation of CIITA mRNA (Figure1B). This response pattern is different from our findings (Figures1B)21 and those of others58 using astrocytes, in which there is either no effect or an increase in surface MHC class II when TNF-α is added to cultures in combination with IFN-γ. There is precedent, however, for cytokines other than TNF-α to have an inhibitory role in the regulation of the CIITA. It has been shown that TGF-β has a similar down-regulatory effect on IFN-γ–induced MHC class II and CIITA expression in astrocytes and other cells.16,40 59 TGF-β–mediated inhibition also appears to be cell-type specific because the pattern did not hold when other cell lines were tested. Our observations in combination with these suggest that the regulation of the CIITA and its associated molecules may be more complicated than has previously been demonstrated. In fact, the regulation of the CIITA may be cell specific and dependent on the immune regulatory function of the cell.
To serve as efficient APCs to CD4+ T cells, CVEs must be able to up-regulate MHC class II and costimulatory molecules. Using flow cytometry, we compared surface adhesion and costimulatory molecule expression on cytokine-stimulated CVEs and astrocytes. We found that CVEs constitutively expressed low levels of B7-1 and VCAM-1 on their surfaces. Following IFN-γ or IFN-γ + TNF-α stimulation, CVEs up-regulated B7-1 and VCAM-1, but not B7-2, CD40, or ICAM-1 (Figure 2). Therefore, IFN-γ–activated CVEs expressed significant levels of MHC class II and the B7-1 costimulatory molecule. On the other hand, astrocytes constitutively expressed the costimulatory molecule, B7-1, and the adhesion molecules, ICAM-1 and VCAM-1. Following cytokine activation, astrocytes up-regulated B7-1, B7-2, ICAM-1, and VCAM-1 (Figure 2). Therefore, pro-inflammatory cytokine-stimulated astrocytes expressed MHC class II, B7 costimulation, and the adhesion molecules ICAM-1 and VCAM-1. When mean fluorescence shifts were compared, astrocytes expressed costimulatory and adhesion molecules at much higher levels than CVEs (Figure 2).
Various groups have shown that endothelial cells treated with IFN-γ can activate T-cell responses,29,31,60 with one report suggesting that CVEs are capable of serving as APCs for the adoptive transfer of EAE.33 Alternatively, an equal number of investigations have shown that CVEs are not competent APCs and that they may even induce T-cell unresponsiveness.28,32,61 It has been hypothesized that the disparate results of these studies may relate to the level of contamination of the primary endothelial cell cultures with professional APCs, such as smooth muscle cells or pericytes.34 To obviate this problem, we usedcloned SJL/J CVEs, and the data unequivocally show that CVEs do not function as competent APCs. In contrast, other CNS-resident cells (ie, astrocytes and microglia) can serve as efficient APCs in a pro-inflammatory environment (Figure 3).20,21,23Strikingly, astrocyte APC function closely correlates with their MHC class II expression (Figures 1, 3). Despite the expression of MHC class II, CVEs do not elicit robust MHC class II-restricted T-cell responses (Figures 1, 3, 4). IFN-γ–treated CVEs did not activate allogeneic T cells, suggesting that the level of MHC class II on the surface is insufficient to stimulate even polyclonal T-cell responses (Figure 3A). CVEs can present the superantigen, SEB, inducing T-cell proliferation, but the T-cell response to CVE presentation was significantly reduced compared to professional and nonprofessional APCs (Figure 3B). In contrast to our results, Rott et al62 previously reported that astrocytes were not capable of presenting SEB for T-cell proliferation. However, in their studies, SJL macrophages were also incapable of eliciting an SEB response, suggesting that the antigen concentration or the responder T-cell population was insufficient to measure SEB-induced proliferation. CVEs are unable to elicit MHC class II-dependent responses despite IFN-γ–inducible class II expression that is comparable to astrocytes (Figure 1A). It is unlikely that costimulation influences allo-antigen and superantigen reactivity, but reports have demonstrated that a cell's peptide repertoire can influence allogeneic T-cell responses and superantigen binding.63,64 Invariant chain and DM activity determine the peptide repertoire and, in turn, the cell's ability to present superantigen and elicit allogeneic responses.65Therefore, it remains possible that CVEs have altered invariant chain and DM activity that influence their peptide repertoire, affecting their ability to elicit alloreactivity and to bind superantigen. We went on to clearly demonstrate that cloned CVEs cannot activate myelin peptide-specific Th1 or Th2 lines to proliferate (Figure 4) or to produce cytokines (data not shown). Our cloned population of CVEs expresses minimal levels of costimulatory and adhesion molecules (Figure 2) that may be necessary for sufficient T-cell receptor engagement and T-cell activation. Therefore, costimulation-independent T-cell hybridomas were tested, but CVEs were still unable to function as competent APCs (Figure 4). Altogether, our results clearly demonstrate that CVEs, unlike astrocytes and microglia, are not competent APCs and are unlikely to serve as CNS-resident APCs in vivo.
Previous reports have indicated that encephalitogenic and nonencephalitogenic CD4+ T cells can cause damage to Lewis rat CVE monolayers,51 suggesting that EAE induction may involve T-cell–mediated damage to CNS vasculature. On close analysis, CVEs did not look particularly healthy following in vitro culture with T cells (Figure 5B). Further analysis revealed that CVEs undergo morphologic changes after coculture with myelin peptide-specific T cells. Myelin peptide-specific T cells do not appear to induce apoptosis of CVEs, but the CVE monolayer is significantly changed by T-cell interactions. CVEs express high levels of Fas (CD95), but blockade of Fas–FasL interactions by inclusion of rFas-Fc in T-cell–CVE cultures did not inhibit the development of T-cell–induced changes in CVE monolayer architecture (data not shown). Experiments are under way to determine the mechanism(s) underlying T-cell–induced damage to the CVE monolayers. It is possible that CD4+ T cells activated in vivo by neuroantigen–CFA may interact with and damage endothelial cells of the BBB to allow inflammatory cells to nonspecifically enter the CNS. Others have demonstrated that CD4+ T cells can have cytotoxic function.66-68In particular, Sedjwick et al68 demonstrated that encephalitogenic and nonencephalitogenic CD4+ T-cell lines cause antigen-specific damage to Lewis rat brain microvascular endothelial cells. Furthermore, they found that rats developed hemorrhage in the spinal cord and brain following the transfer of MBP-activated splenocytes. These data suggest that CNS endothelial cells are primary targets of activated T cells.
In conclusion, the observations outlined in this paper have led us to conclude that CVEs are not inducible APCs. Instead, we speculate that T-cell–CVE interactions damage the BBB, facilitating T-cell entry into the CNS during neuro-inflammation.
We thank S. Mark Tompkins, PhD, for his discussions and critical review of this manuscript.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2001-12-0229.
Supported in part by United States Public Health Service National Institutes of Health grants NS-26543 and NS-30871. K.B.G. was funded by NIH Training Grant IT32AB0759301. A.M.G. was supported by NIH Training Grant AI-07476.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen D. Miller, Department of Microbiology-Immunology, Northwestern University Medical School, 303 East Chicago Ave, Chicago, IL 60611; e-mail:s-d-miller@northwestern.edu.

![Fig. 3. Comparison of MHC class II-restricted T-cell responses elicited by cytokine-activated SJL/J astrocytes and CVEs. / SJL/J astrocytes or SJL/J CVEs were incubated for 48 hours with no cytokine, IFN-γ (100 U/mL), TNF-α (500 U/mL), or both cytokines. (A) Cells were washed, harvested, irradiated (30 Gy), and plated (104/well) before the addition of 5 × 104Thy 1.2+ allogeneic (A/J) T cells. (B) Cells were washed, harvested, irradiated (30 Gy), and plated (104/well) before the addition of 5 × 104 Thy 1.2+ syngeneic (SJL/J) T cells and 10μg/mL SEB. Irradiated syngeneic splenocytes (4 × 105/well) were used as a control APC population in separate wells in both experiments. T-cell proliferative responses were measured by 3[H]-thymidine incorporation. Results are expressed as mean cpm (× 10−3) ± SEM of triplicate wells. Stimulation indices are noted beside each bar.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/10/10.1182_blood-2001-12-0229/6/m_h81022557003.jpeg?Expires=1767987327&Signature=z9l5dXTubW6pO~8cLjnIeGrmdBDHIqdruh-NfE-a9sQ36T3LgQW6fZJ4d9OYBN6dey1w3jd~E5dT-C0INQfn8Uimw1XUFQ5aj62YjYmxFKlZURL4SnISB~bFtr3H2OQ8ayAJ7GUj8L2N4I5yhwPpikBENE1Nd7Mdr-HtE~fZ8QF2CW5g3bRmGCpmJdwQ6mzvCyUKeCSvla9UMH21KgRwLox1CN89Obq6s2t3QZl0rNdgiQWTL7baElTZpUpp5iX-HCslPll0r2muBq1jj3NSXnH6JmiVNYzwdhBIIi~28HklhtQfL5ur-puWrIqEUWGqMSWHyusqH90SIsjnPDRGRA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Comparison of the antigen-presenting capabilities of cytokine-treated SJL/J astrocytes and CVEs for Th1 and Th2 cells. / SJL/J CVEs were incubated for 48 hours with no cytokine, IFN-γ (100 U/mL), TNF-α (500 U/mL), or both cytokines. Cells were washed, harvested, and irradiated (30 Gy) before the addition of 5 × 104 PLP139-151–specific Th1 cells (A), Th2 cells (B), or T-cell hybridomas (C) along with antigen. Irradiated syngeneic splenocytes (4 × 105/well) were used as a control APC population in separate wells. (A, B) T-cell proliferative responses were measured by 3[H]-thymidine incorporation. Results are expressed as mean cpm (× 10−3) ± SEM of triplicate wells using 104 CVEs per well and the indicated concentration of PLP139-151. Stimulation indices are noted beside each bar. (C) T-cell hybridoma responses were measured by collecting culture supernatants after 24 hours and determining IL-2 levels by ELISA. Data are expressed as average absorbance of triplicate wells at 450 nm ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/10/10.1182_blood-2001-12-0229/6/m_h81022557004.jpeg?Expires=1767987327&Signature=wmEFi0ZrOsw-~28XWfD5XlNhiVzkwcRnfun1D7Gzds4DLWMwLVYfy7IaGHNJz9UVvbhUKwK3D5JAkg3TJJqFVmxyZPRn3WEeIWDXNyd9-Am2Ub6pnmBz~ZmAtgW-nhINwCJeJVSN0Sj9zCP16bVQd34o0KHlEvphrbWHwS5sFPHjdKaOwsB1MUCb7Y~WOmCPeLlECovOKqJp2ggTGzEib~So51IkRu9AKaOkdQ3p6Ufj90ATCVQehwyKmcIofpJEeiuhzLzK0C90tA4w6UWutFIW9HdHgCCtgUSNuoAUtkskZ7A7bucRRN4hPXWP2aUdP4hdQbmAH99X~Mb6V1xRow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal