To examine the role of the fibrinogen γ chain in the assembly and secretion of this multichain protein, we synthesized a series of fibrinogen variants with truncated γ chains, terminating between residues γ379 and the C-terminus, γ411. The variant fibrinogens were synthesized from altered γ-chain complementary DNAs in cultured Chinese hamster ovary cells. Immunoassays of the culture media demonstrated that only those variants with γ chain longer than 386 residues were secreted and that the concentration of fibrinogen decreased with the length of the γ chain, from 1.4 μg/mL for normal fibrinogen to 0.39 μg/mL for γ 387 fibrinogen. Immunoassays of cell lysates showed that all variant γ chains were synthesized, although the levels varied significantly. For variants longer than 386 residues, levels decreased with length but remained near normal. In contrast, expression of the 4 variants with 386 residues or less was about 20-fold reduced. Quantitative reverse transcription–polymerase chain reaction demonstrated that the γ-chain messenger RNA level was independent from chain length. Western blot analyses showed that lysates expressing variants with 387 residues or more contained species comparable to the known intermediates in fibrinogen assembly, including half-molecules. For shorter variants, these intermediates were not evident. We conclude that residues near the C-terminus of the γ chain are essential for fibrinogen assembly, and more specifically, that γ387 is critical. We propose that the loss of residue γ387 destabilized the structure of γ chain, preventing assembly of αγ and βγ dimers, essential intermediates in the assembly of normal fibrinogen.
Introduction
Fibrinogen is a 340-kd plasma glycoprotein consisting of 2 copies of 3 polypeptide chains, Aα, Bβ, and γ, linked by an extensive network of 29 intrachain and interchain disulfide bonds.1,2 A separate gene encodes each polypeptide chain. The 3 chains are synthesized, assembled into the 6-chain molecule, and secreted from hepatocytes into the plasma. Studies of fibrinogen expressed from the endogenous genes in human hepatocytes or from transfected complementary DNAs (cDNAs) in baby hamster kidney (BHK) cells have shown that assembly occurs through specific intermediates.3 4 These intermediates, αγ dimers, βγ dimers, and αβγ half-molecules, all include the γ chain.
Normal fibrinogen levels are 2 to 4 mg/mL in plasma. Hypofibrinogenemia or afibrinogenemia, defined as reduced or immeasurable levels of fibrinogen in plasma, can be hereditary. Although the first case of afibrinogenemia was reported in 1920,5 the genetic basis of these abnormalities was first demonstrated in 1999.6Since that time, 26 different mutations have been identified in cases of afibrinogenemia or hypofibrinogenemia.7-19 These mutations were found in all 3 genes, and include missense, nonsense, and frameshift mutations; splice-site abnormalities; and large deletions. Thus, fibrinogen deficiencies are associated with a variety of genetic changes, although to date none of these arises from reduced gene expression induced by mutations within promoter elements. We recently reported the hypofibrinogen Matsumoto IV was associated with the missense mutation γ153 Cys→Arg.7 We found that expression of this variant fibrinogen in Chinese hamster ovary (CHO) cells was defective, demonstrating that secretion of the variant was reduced relative to secretion of normal fibrinogen. This finding suggested that the tertiary structure of the γ chain is important for normal assembly and secretion of fibrinogen, at least in cultured cells.
Considering our studies with Matsumoto IV fibrinogen7together with the studies of fibrinogen assembly,3 4 we hypothesized that the C-terminal region of the γ chain (residues 143-411), which forms a single globular domain, has a critical role in fibrinogen secretion. To explore this hypothesis, we synthesized a series of γ-chain mutants truncated between residue γ379 and the C-terminus, γ411. Our results demonstrated that residues from γ387Ile to the C-terminus are essential for assembly and secretion of fibrinogen from cultured cells.
Materials and methods
Construction of mutant expression vectors
The fibrinogen γ-chain expression vector, pMLP-γ,20 was altered by oligonucleotide-directed mutagenesis using the Transformer Site-Directed Mutagenesis kit (Clontech Laboratories, Palo Alto, CA) and nine 5′-phosphorylated mutagenesis primers (Table 1) and a 5′-phosphorylated selection primer (5′-TCTAGGGCCCAGGCTTGTTTGC), which deleted a unique HindIII site in the vector.21Plasmid DNA was prepared from ampicillin-resistant colonies and the complete γ-chain cDNA of plasmids lacking HindIII sites were sequenced using a BigDye Terminator Cycle Sequencing Ready Reaction Kit, and ABI Prism 310 Genetic Analyzer (Applied Biosystems, Foster City, CA), with 2 forward and 2 reverse primers as described.21
Primers used for oligonucleotide-directed mutagenesis
| Variant plasmid . | Oligonucleotide sequence (5′ → 3′) . |
|---|---|
| γ379 | T A T T C C A T G T A G A A A A C C A C |
| γ384 | A C C A C T A T G T A G A T A A T C C C |
| γ385 | C C A C T A T G A A GT A A A T C C C A T T C A A C |
| γ386 | C A C T A T G A A G A T A T A G C C A T T C A A C A G A C |
| γ387 | C T A T G A A G A T A A T C T G A T T C A A C A G A C T C |
| γ390 | C C A T T C A A C T G A C T C A C A A T T G |
| γ395 | C A C A A T T G G A T A A G G A C A G C A |
| γ401 | C A A C A C C A C T A G G G G G G A G C |
| γ406 | G G A G C C A A A T A G G C T G G A G A |
| Variant plasmid . | Oligonucleotide sequence (5′ → 3′) . |
|---|---|
| γ379 | T A T T C C A T G T A G A A A A C C A C |
| γ384 | A C C A C T A T G T A G A T A A T C C C |
| γ385 | C C A C T A T G A A GT A A A T C C C A T T C A A C |
| γ386 | C A C T A T G A A G A T A T A G C C A T T C A A C A G A C |
| γ387 | C T A T G A A G A T A A T C T G A T T C A A C A G A C T C |
| γ390 | C C A T T C A A C T G A C T C A C A A T T G |
| γ395 | C A C A A T T G G A T A A G G A C A G C A |
| γ401 | C A A C A C C A C T A G G G G G G A G C |
| γ406 | G G A G C C A A A T A G G C T G G A G A |
The altered bases are indicated in bold characters.
Recombinant protein expression
The CHO cell lines that express normal human fibrinogen Aα and Bβ chains, AαBβ-CHO cells, were obtained by cotransfecting the plasmids pMLP-Aα, pMLP-Bβ, and pRSVneo into CHO cells; cells were cultured in Dulbecco modified Eagle medium Ham nutrient mixture F12 supplemented as described (DMEM-F12 medium).20 Each of the variant pMLP-γ vectors and original pMLP-γ vector20 was cotransfected with the histidinol selection plasmid (pMSVhis) into the AαBβ-CHO cell line, using the standard calcium-phosphate coprecipitation method.22 Colonies were selected on both G418 (Gibco BRL, Rockville, MD) and histidinol (Aldrich Chemical, Milwaukee, WI). Individual colonies were expanded in DMEM-F12 medium containing both G418 and histidinol and examined for fibrinogen synthesis as described.22
Immunoassays
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis was performed as described with minor modifications.22 Immunoblots were developed with a rabbit antihuman fibrinogen antibody (Dako, Carpinteria, CA) and cross-reacting species were visualized with horseradish peroxidase–conjugated goat antirabbit IgG antibody (Medical and Biological Laboratories, Nagoya, Japan) and enhanced chemiluminescence (ECL) detection reagents (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). Blots were exposed on Hyperfilm-ECL (Amersham Pharmacia Biotech). Alternatively blots were developed with a rabbit antihuman fibrinogen γ-chain antibody (Chemicon International, Temecula, CA), followed by alkaline phosphatase–conjugated goat antirabbit IgG antibody (EY Laboratories, San Mateo, CA), and developed as described previously.7,22 Fibrinogen concentrations in cell lysates or culture media were determined by enzyme-linked immunosorbent assay (ELISA) as described.7
Culture medium for immunologic analysis was prepared as follows. Cells were grown to confluence in 60-mm dishes (approximately 1.5-2.0 × 106 cells), and the conditioned medium was harvested 1 day after confluence (6-8 days after seeding) for immunoblot analysis or ELISA. Cell lysates were prepared from the same cultures in 60-mm dishes. The cells were harvested in trypsin-EDTA solution (Sigma, St Louis, MO), washed 3 times with phosphate-buffered saline (PBS), and lysed in either 50 μL Laemmli sample buffer for immunoblot analysis, or 250 μL 0.1% IGEPAL CA-630 (nonionic detergent; Sigma) and 10 mM phenylmethylsulfonyl fluoride (Sigma) for ELISA.
RNA isolation and quantitative reverse transcription–polymerase chain reaction
Cells were harvested and washed as described for the immunologic analyses and resuspended in 100 μL PBS. Total RNA was isolated using IsogenLS (Nippon Gene, Tokyo, Japan) according to the manufacturer's protocol. Isolated RNA (16-63 μg) was dissolved with 50 μL diethylpyrocarbonate solution containing RNase inhibitor and treated by RNase-free DNase I (Roche Diagnostics, Mannheim, Germany) at 37°C for 10 minutes, followed by 80°C for 10 minutes. Quantitative reverse transcription–polymerase chain reaction (RT-PCR) assay was carried out with gene-specific, double fluorescently labeled probes in an ABI PRISM 7700 Sequence detector (PE Applied Biosystems, Foster City, CA), using VIC or 6-carboxy fluorescein (FAM) as the 5′ fluorescent reporter, and tetramethylrhodamine (TAMRA) at the 3′ end as quencher. The primer and probe sequences were: human fibrinogen γ-chain forward primer, 5′-TTGAAGCACAGTGCCAGGAA-3′; human fibrinogen γ-chain reverse primer, 5′-CTCCCTTATTGGCAATGTCTTGAC-3′; human fibrinogen γ-chain probe, 5′-VIC-CTTGCAAAGACACGGTGCAAATCCATG-3′; Chinese hamster glyceraldehyde phosphate dehydrogenase (GAPDH) forward primer, 5′-GTATTGGACGCCTGGTTACCA-3′; Chinese hamster GAPDH reverse primer, 5′-GGTAGAGTCATACTGGAACATGTAGACC-3′; Chinese hamster GAPDH probe, 5′-FAM-TGGAAGTTGTTGCCATCAATGACCCC-3′. Transcribed messenger RNA (mRNA) was quantitated using 2 μL DNase I-treated RNA solution (corresponding to 1.5 μL original RNA solution) in a reaction mix (TaqMan One-step RT-PCR Master Mix reagent kit) with 10 mM Tris, pH 8.3; 50 mM KCl; 4 mM MgCl2 1 mM EDTA; 200 μM/L deoxynucleotide triphosphate; 0.25 U/μL reverse transcriptase from Moloney murine leukemia virus and 0.025 U/μL AmpliTaqGold DNA polymerase. Each primer and probe was used at a final concentration of 1000 nmol/L and 200 nmol/L, respectively. RT reactions were incubated at 50°C for 30 minutes and, after inactivation of RT at 95°C for 12 minutes, 50 cycles of amplification were carried out with denaturation at 95°C for 15 seconds, and annealing and extension at 60°C for 1 minute. Standard curves were constructed with the use of dilutions of an accurately determined pCR 2.1 plasmid vector (Invitrogen, San Diego, CA) containing the RT-PCR products of human fibrinogen γ-chain and Chinese hamster GAPDH. To compensate for differences in cell number and RNA recovery, the copy number of fibrinogen γ-chain mRNA was determined relative to Chinese hamster GAPDH mRNA assayed simultaneously; GAPDH mRNA in 1.5 μL original RNA solution was set at 5 × 106 copies.
Results
Synthesis and secretion of recombinant fibrinogen
To examine the role of the carboxyl-terminus of the γ chain in fibrinogen synthesis and secretion, we expressed 9 variant fibrinogens with γ chains ending at residues 379, 384, 385, 386, 387, 390, 395, 401 and 406. The truncations were introduced by oligonucleotide-directed mutagenesis of the γ-chain cDNA cloned in the previously described expression vector pMLP-γ.20Each altered vector was cotransfected with pMSVhis into a CHO cell line that expressed the normal Aα and Bβ chains of fibrinogen. Histidinol-resistant colonies were picked and expanded, and the culture media and cell lysates were assayed for fibrinogen as described in “Materials and methods.” Fibrinogen was detected in the media of 5 variants: γ387, γ390, γ395, γ401, and γ406. For each variant, except γ401, we selected at random 8 to 11 clones with rapidly dividing cells for further analysis. Because we developed clones expressing fibrinogen γ401 for a different project, we selected the 4 clones that had the highest fibrinogen levels in the culture medium. We also selected at random 22 clones expressing normal fibrinogen, γ411. From the remaining variants, γ379, γ384, γ385, and γ386, we analyzed 3 to 10 clones where fibrinogen was detected only in the cell lysate.
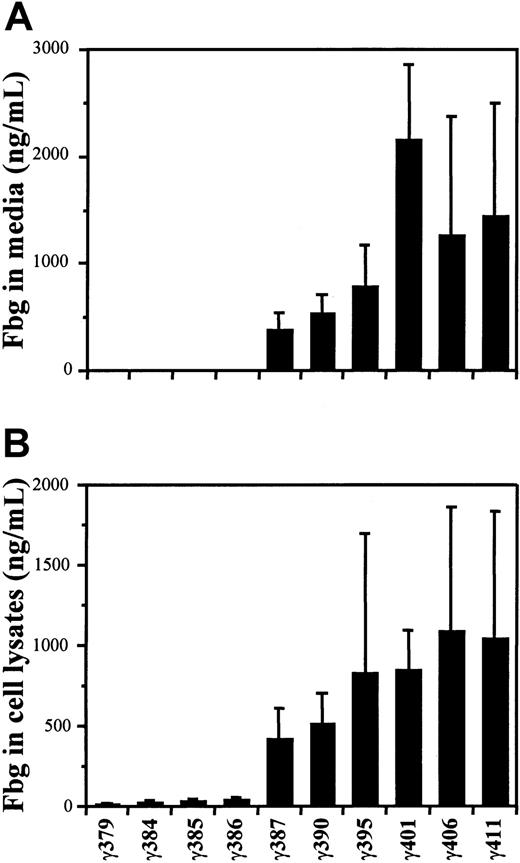
Fibrinogen concentrations were determined by ELISA, as described in “Materials and methods.” The concentrations found in the culture medium are presented in Figure 1A. For the 22 clones examined the concentration of normal fibrinogen (γ411) varied from 0.30 to 4.6 μg/mL, with a mean value of 1.4 μg/mL. The mean concentrations for the variants γ406, γ401, γ395, γ390, and γ387 were 1.3, 2.2, 0.79, 0.54, and 0.39 μg/mL, respectively. Excluding the data from fibrinogen γ401 because these clones were selected for high expression, the results demonstrated that the secretion of fibrinogen into the culture medium varied directly with the length of variant γ chain; clones with shorter chains contained less fibrinogen in the medium. Moreover, fibrinogen was not detected (< 10 ng/mL) in the medium from any of the 4 shorter variants with γ chains less than 387 residues.
Synthesis of variant fibrinogens in transfected CHO cells.
The concentrations of fibrinogen in the culture media (A) and cell lysates (B) were measured by ELISA as described in “Materials and methods.” The mean values are presented with SDs indicated by the error bars. Concentrations were determined for multiple isolates, indicated in parentheses, of the CHO lines γ379 (3), γ384 (9), γ385 (8), γ386 (10), γ387 (8), γ390 (11), γ395 (11), γ401 (4), γ406 (11), and γ411 (22).
Synthesis of variant fibrinogens in transfected CHO cells.
The concentrations of fibrinogen in the culture media (A) and cell lysates (B) were measured by ELISA as described in “Materials and methods.” The mean values are presented with SDs indicated by the error bars. Concentrations were determined for multiple isolates, indicated in parentheses, of the CHO lines γ379 (3), γ384 (9), γ385 (8), γ386 (10), γ387 (8), γ390 (11), γ395 (11), γ401 (4), γ406 (11), and γ411 (22).
The fibrinogen concentrations in cell lysates are shown in Figure 1B. For normal fibrinogen the levels varied from 0.19 to 3.2 μg/mL, with a mean of 1.0 μg/mL. The mean concentrations for the 5 longer variants, γ406, γ401, γ395, γ390, and γ387, were 1.1, 0.85, 0.83, 0.52, and 0.42 μg/mL, respectively. The mean concentrations for the shorter variants were markedly lower at 39, 30, 27, and 16 ng/mL for the variants γ386, γ385, γ384, and γ379, respectively. Thus, again, the shorter the γ chain, the less fibrinogen was synthesized. Furthermore, as was found in the medium, the amount of fibrinogen in cell lysates was gradually reduced with chain length up to 387 residues, but then markedly reduced when the chain was shortened from 387 to 386 residues. Finally, fibrinogen levels in these lysates were always greater than levels seen with the parent AαBβ-CHO cell lysates, where fibrinogen was below the 10 ng/mL detection limit of the assay.7 This finding suggests that the addition of γ chain stabilized the Aα or Bβ chains within the cells, although the synthesis of a third chain may simply have been sufficient to raise the levels above the detection limit.
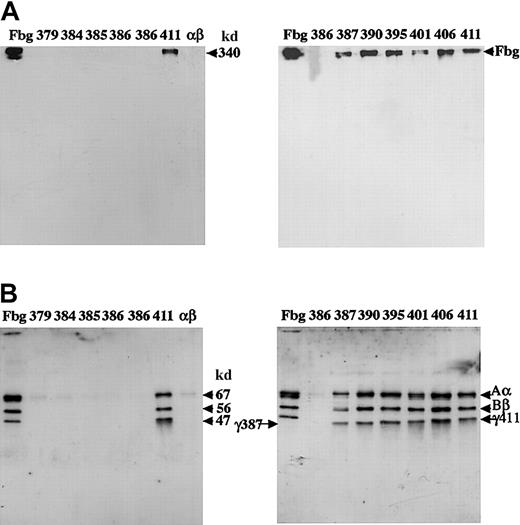
We examined the fibrinogen variants on immunoblots of SDS-acrylamide gels run under reducing and nonreducing conditions. Immunoblots with samples of the culture media from individual clones are shown in Figure2. As anticipated from the ELISA data, no bands were evident for the variants with less than 387 residues. We examined 3 independent cell lines of variant γ386, and in no case were bands evident. Under nonreducing conditions (Figure 2A), bands comparable to plasma fibrinogen and normal fibrinogen (γ411) were seen in the culture media of the variants γ387, γ390, γ395, γ401, and γ406. Under reducing conditions (Figure 2B), bands comparable to the normal Aα, Bβ, and γ chains were evident in all variants where fibrinogen was seen under nonreduced conditions, although the mobility of the γ chain increased as expected for the shorter variant chains.
Western blot analysis of the culture medium.
Samples of medium (5 μL) were subjected to 8% SDS-PAGE under nonreduced conditions (A) or 10% SDS-PAGE under reduced conditions (B). The blots were developed with a polyclonal antibody to fibrinogen and cross-reacting bands detected by chemiluminescence, as described in “Materials and methods.” Plasma fibrinogen (3 ng) was run in lanes labeled Fbg; medium from individual CHO lines were in lanes labeled 379: γ379-1; 384: γ384-33; 385: γ385-24; 386: from left to right, γ386-20, -37, and -39; 411: γ411-31; αβ: AαBβ-CHO cells; 387: γ387-5; 390: γ390-16; 395: γ395-2: 401: γ401-25; 406: γ406-7. Arrows at 340 kd, or 67 kd, 56 kd, and 47 kd indicate intact fibrinogen (A) or the normal Aα, Bβ, and γ chains (B). Arrows labeled γ387 and γ411 indicate this truncated and normal γ chain, respectively (B).
Western blot analysis of the culture medium.
Samples of medium (5 μL) were subjected to 8% SDS-PAGE under nonreduced conditions (A) or 10% SDS-PAGE under reduced conditions (B). The blots were developed with a polyclonal antibody to fibrinogen and cross-reacting bands detected by chemiluminescence, as described in “Materials and methods.” Plasma fibrinogen (3 ng) was run in lanes labeled Fbg; medium from individual CHO lines were in lanes labeled 379: γ379-1; 384: γ384-33; 385: γ385-24; 386: from left to right, γ386-20, -37, and -39; 411: γ411-31; αβ: AαBβ-CHO cells; 387: γ387-5; 390: γ390-16; 395: γ395-2: 401: γ401-25; 406: γ406-7. Arrows at 340 kd, or 67 kd, 56 kd, and 47 kd indicate intact fibrinogen (A) or the normal Aα, Bβ, and γ chains (B). Arrows labeled γ387 and γ411 indicate this truncated and normal γ chain, respectively (B).
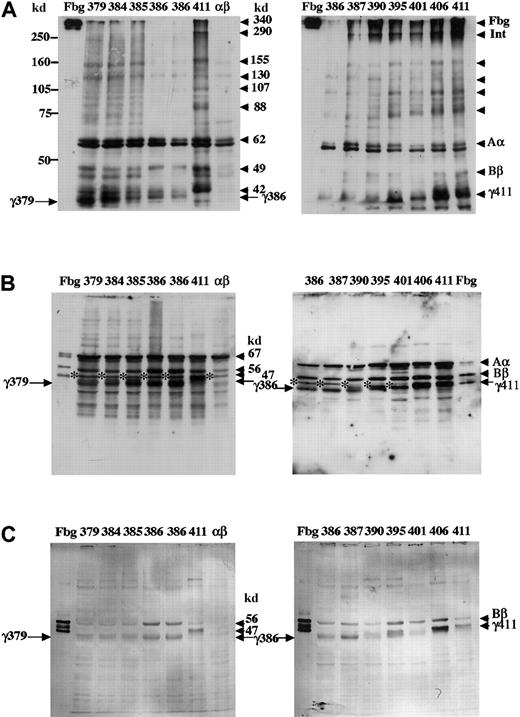
Immunoblots of cell lysates are shown in Figure3. When SDS-PAGE was run under nonreducing conditions and the blots were developed with an antifibrinogen antibody (Figure 3A), multiple bands were seen in all the CHO lysates. The parent CHO line that expressed only the Aα and Bβ chains (lane labeled αβ) showed a doublet of strong bands and weaker signals below and above these strong bands. The strong bands at about 62 kd and 59 kd were both Aα chain, because both reacted with an anti–Aα-chain antibody (data not shown). The smaller bands are likely Bβ chain, because bands equivalent to Bβ chain were also seen (Figure 3B). Because this antibody reacted most strongly with Aα chain, we presume that the bands larger than 62 kd were multimers of Aα chains; multimers of individual chains were seen by SDS-PAGE analysis of fibrinogen expressed in BHK cells.3,4 In addition to the bands seen in the AαBβ-CHO parent line, the cell line that synthesized normal fibrinogen (lanes labeled 411) showed normal γ chain (42 kd) and strong bands larger than 62 kd around 88 kd, 107 kd, 130 kd, 155 kd, 290 kd, and 340 kd. Based on the previous reports3 and the location of the fibrinogen standard (lanes labeled Fbg), we presume these bands were γγ, AαAα, Bβγ, Aαγ, AαBβγ (arrow labeled Int), and fibrinogen (arrow labeled Fbg), respectively. Synthesis of γ chain was evident in all clones that were selected following transfection with variant γ-chain expression plasmids (compare lanes γ379-γ406 to αβ). The mobility of the γ-chain band varied with the length of the encoded γ chain, as expected. Although bands larger than Aα chain were evident in all lysates, substantive bands at approximately 290 kd and 340 kd, analogous to those seen with normal fibrinogen (lanes labeled 411), were seen only in those clones that secreted fibrinogen (387-406). These results indicate that only the variants with γ chains of 387 or more residues were assembled into fibrinogen through intermediates analogous to those seen with normal fibrinogen.
Western blot analysis of CHO cell lysates.
Lysates were subjected to 8% SDS-PAGE under nonreduced conditions (A) or 10% SDS-PAGE under reduced conditions (B,C). Blots were developed with an antibody to fibrinogen (A,B) as described in the legend to Figure 2, or with a polyclonal antibody reacting with the fibrinogen Bβ and γ chains (C), as described in “Materials and methods.” Cross-reacting bands were detected by peroxidase-catalyzed chemiluminescence (A,B) or by alkaline phosphatase–catalyzed color development (C). The samples were as described in Figure 2 except the plasma fibrinogen (lanes Fbg) was 30 ng (C). Arrows labeled γ411, γ379, and γ386 indicate normal and truncated γ chains. The 47-kd band indicated with asterisk (B) was present in AαBβ-CHO cells.
Western blot analysis of CHO cell lysates.
Lysates were subjected to 8% SDS-PAGE under nonreduced conditions (A) or 10% SDS-PAGE under reduced conditions (B,C). Blots were developed with an antibody to fibrinogen (A,B) as described in the legend to Figure 2, or with a polyclonal antibody reacting with the fibrinogen Bβ and γ chains (C), as described in “Materials and methods.” Cross-reacting bands were detected by peroxidase-catalyzed chemiluminescence (A,B) or by alkaline phosphatase–catalyzed color development (C). The samples were as described in Figure 2 except the plasma fibrinogen (lanes Fbg) was 30 ng (C). Arrows labeled γ411, γ379, and γ386 indicate normal and truncated γ chains. The 47-kd band indicated with asterisk (B) was present in AαBβ-CHO cells.
When SDS-PAGE was run under reducing conditions and the blots were developed with an antifibrinogen antibody (Figure 3B), bands comparable to Aα, Bβ, and truncated or normal γ chains were seen in all transfected cell lysates (compare lanes γ379-γ406 to αβ). Similar blots developed with antibodies specific for the Aα chain showed that the 47-kd band, which is indicated by the asterisk in Figure 3B, was a degradation product of this chain (data not shown). Again, as expected, the mobility of the γ chain varied with the length of the encoded variant. Several smaller bands, which were not seen in plasma fibrinogen, were present in the cell lysates; these immunoreactive species may arise from premature termination of translation or proteolytic degradation. Similar blots of gels run under reduced conditions were developed with a polyclonal antibody raised against the fibrinogen γ chain; as reported previously7this antibody cross-reacted with both the Bβ and γ chains. As shown in Figure 3C, 2 bands were evident in every line except the parent AαBβ-CHO line where no bands were evident. Based on the mobility of these immunoreactive bands relative to the fibrinogen standard, we concluded that both Bβ and γ chains were present in these transfected cell lysates. Further, these blots clearly demonstrated that a normal or truncated γ chain was present in all the cell lysates, confirming that all clones synthesized γ chain irrespective of whether fibrinogen was assembled and secreted into the culture media.
Expression levels of variant mRNAs
Total RNA was prepared from each line and γ-chain mRNA levels were determined relative to GAPDH mRNA levels by quantitative RT-PCR as described in “Materials and methods.” The data are shown in Table2. The level of γ-chain mRNA was normalized to GAPDH mRNA, which was set at 5 × 106copies/1.5 μL total RNA solution. For comparison, the fibrinogen concentrations in the culture media and cell lysates are also shown in Table 2. Expression of γ-chain mRNA varied from 2.9 to 71 × 105 copies. This variation was not correlated with the different fibrinogen levels in either the cell lysates or the culture media. Comparing the 12 measurements and calculating the correlation coefficient, we confirmed the null hypothesis that there is no relationship between the relative mRNA concentration and the fibrinogen concentration in the cell lysates (P = .7823). Moreover, message levels did not correlate with secretion. In the 4 CHO lines, γ379, γ384, γ385, and γ386, that did not secrete fibrinogen, the level of γ-chain mRNA was 3.4 to 71 × 105 copies, whereas in the lines where fibrinogen was readily detected in the culture media the level of γ-chain mRNA was 2.9 to 42 × 105 copies. We also compared, using an unpaired t test, the fibrinogen concentrations in the lysates of cells expressing variants that were not secreted (γ379, γ384, γ385, and γ386) to those in the cells expressing variants that were secreted (γ387, γ390, γ395, γ401, γ406, and γ411) and found these levels are different (P = .0132). Thus, the differences in protein synthesis and secretion were not determined by differences in mRNA synthesis, although fibrinogen secretion levels were correlated with intracellular protein levels.
mRNA levels of fibrinogen γ-chain and fibrinogen antigen levels
| Cell line . | Concentration of RNA (μg/mL) . | GAPDH mRNA (× 105 copy) . | γ-Chain mRNA (× 105 copy) . | Relative γ-chain mRNA (× 105 copy)* . | Fibrinogen concentration in cell lysates (μg/mL) . | Fibrinogen concentration in media (μg/mL) . |
|---|---|---|---|---|---|---|
| γ379-1 | 0.55 | 57.0 | 6.1 | 5.4 | 0.015 | ND |
| γ384-33 | 1.1 | 12.0 | 11.0 | 48 | 0.045 | ND |
| γ385-24 | 1.2 | 12.0 | 17.0 | 71 | 0.04 | ND |
| γ386-20 | 0.58 | 48.0 | 7.5 | 7.8 | 0.036 | ND |
| γ386-37 | 0.32 | 64.0 | 4.4 | 3.4 | 0.058 | ND |
| γ386-39 | 0.41 | 54.0 | 8.2 | 7.7 | 0.032 | ND |
| γ387-5 | 1.3 | 76.0 | 11.0 | 7.3 | 0.81 | 0.62 |
| γ390-16 | 0.69 | 98.0 | 5.6 | 2.9 | 0.67 | 0.62 |
| γ395-2 | 0.97 | 82.0 | 23.0 | 14 | 3.2 | 1.7 |
| γ401-25 | 1.5 | 12.0 | 10.0 | 42 | 1 | 2.1 |
| γ406-7 | 1.2 | 71.0 | 58.0 | 41 | 3.2 | 3.7 |
| γ411-31 | 0.91 | 49.0 | 9.0 | 9.2 | 0.62 | 2.2 |
| Cell line . | Concentration of RNA (μg/mL) . | GAPDH mRNA (× 105 copy) . | γ-Chain mRNA (× 105 copy) . | Relative γ-chain mRNA (× 105 copy)* . | Fibrinogen concentration in cell lysates (μg/mL) . | Fibrinogen concentration in media (μg/mL) . |
|---|---|---|---|---|---|---|
| γ379-1 | 0.55 | 57.0 | 6.1 | 5.4 | 0.015 | ND |
| γ384-33 | 1.1 | 12.0 | 11.0 | 48 | 0.045 | ND |
| γ385-24 | 1.2 | 12.0 | 17.0 | 71 | 0.04 | ND |
| γ386-20 | 0.58 | 48.0 | 7.5 | 7.8 | 0.036 | ND |
| γ386-37 | 0.32 | 64.0 | 4.4 | 3.4 | 0.058 | ND |
| γ386-39 | 0.41 | 54.0 | 8.2 | 7.7 | 0.032 | ND |
| γ387-5 | 1.3 | 76.0 | 11.0 | 7.3 | 0.81 | 0.62 |
| γ390-16 | 0.69 | 98.0 | 5.6 | 2.9 | 0.67 | 0.62 |
| γ395-2 | 0.97 | 82.0 | 23.0 | 14 | 3.2 | 1.7 |
| γ401-25 | 1.5 | 12.0 | 10.0 | 42 | 1 | 2.1 |
| γ406-7 | 1.2 | 71.0 | 58.0 | 41 | 3.2 | 3.7 |
| γ411-31 | 0.91 | 49.0 | 9.0 | 9.2 | 0.62 | 2.2 |
γ-Chain mRNA relative to GAPDH mRNA level, as described in “Materials and methods.”
Discussion
Our studies demonstrated that residues near the C-terminus of the γ chain are essential for assembly, and thereby for secretion, of fibrinogen expressed in cultured CHO cells. Surprisingly, the loss of a single residue, γ387Ile, was found to markedly impair assembly and secretion. These results are consistent with previous studies demonstrating that the γ chain is critical for assembly and secretion not only in cultured cells but also in vivo. Recent studies of congenital afibrinogenemia indicate that this γ-chain C-terminal domain is critical for fibrinogen secretion from hepatocytes, because fibrinogen was not detected in plasma from patients who were homozygous for nonsense mutations with a stop codon in place of either residue γ19719 or γ231.12 We have also found that other shorter γ-chain variants are not secreted; fibrinogen was not detected in the culture media following transfection of pMLP-γ plasmids encoding γ142, γ190, γ355, or γ368 into AαBβ-CHO cells (N.O., unpublished observations, April 27, 2000). This attribute appears to be unique to the γ chain, because fibrinogens with truncated Aα or Bβ chains can be assembled and secreted. Fibrinogen was detected in the plasma of individuals who were homozygous for Aα chains truncated at Aα453,23,24Aα460,25 and Aα479.26 In addition, Zhang and Redman found that the C-terminal domain of the Bβ chain was not essential for assembly and secretion of recombinant fibrinogen following transient transfection of COS cells, because a variant expressing Bβ-chain truncated at residue 207 was secreted into the culture medium.27 This apparently unique function for γ chain may, nevertheless, arise simply from sampling, because other truncations of the Aα chain have been associated with defective secretion. That is, congenital afibrinogenemia has been found in individuals homozygous for several nonsense or frameshift mutations in the Aα-chain gene.19
A unique role for the γ chain is apparent in the model for fibrinogen chain assembly that was proposed by Huang et al3 in 1993. These investigators followed fibrinogen assembly and secretion in cultured human hepatocytes, HepG2 cells, and in a recombinant expression system with BHK cells. They monitored the formation of intermediates in assembly by 2-dimensional SDS-PAGE, detecting disulfide-linked intermediates in the first dimension under nonreducing conditions and the respective chain compositions in the second dimension under reducing conditions. These studies showed that γ chains were present in all assembly intermediates. Subsequent studies, which used directed mutagenesis to synthesize several variant fibrinogens, have shown that specific domains and some, but not all, disulfide linkages are important for assembly7,28-30 and subsequent secretion. These experiments suggest that impaired secretion emanated from impaired assembly, consistent with the data reported here. We22 and others3,29 have found that individual chains and some assembly intermediates, particularly Aα-γ chains, can be secreted from cultured cells, but at reduced levels relative to fully assembled fibrinogen. It is likely that the sensitivity of assays used here was insufficient to detect such low levels of secreted intermediates. Synthesis of some variant fibrinogens indicates that mechanisms other than assembly may impair secretion. Such other mechanisms were apparent from experiments designed to examine the basis of 2 afibrinogenemias associated with missense mutations in the Bβ chains, BβLeu353Arg and BβGly400Asp. In pulse-chase experiments using transiently transfected COS-1 cells, Duga et al demonstrated that both BβArg353 and BβAsp400 fibrinogens were assembled but not secreted.8 Together these studies support 2 conclusions: assembly intermediates are not efficiently secreted and apparently fully assembled fibrinogen is not always secreted. Our results suggest that γ-chain C-terminal residues have a role in assembly, and thereby in secretion. As γ-chain residues were lost from the C-terminus, assembly of fibrinogen decreased gradually until residue 386 was removed; thereafter, assembly into fibrinogen was undetectable. We postulate that a variant lacking γ387Ile was not able to form the necessary 2-chain intermediates and therefore fibrinogen was not assembled. In contrast, truncated Aα or Bβ chains may be incorporated into fibrinogen because a normal 2-chain intermediate can form between the normal γ chain and the normal Bβ or Aα chain, respectively. That is, the altered chain needs to participate only in the later steps of assembly to form the half-molecules and subsequently, fibrinogen.
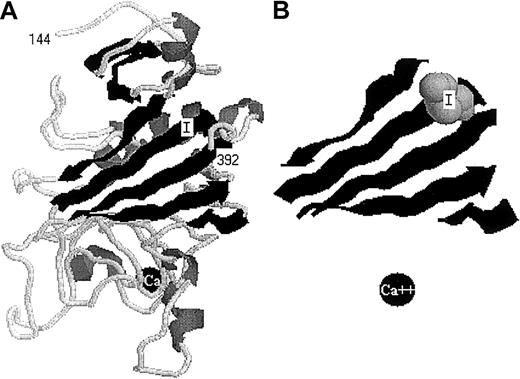
The remarkable finding that truncation at residue γ386 essentially abolished assembly (and thereby secretion) suggests either that γIle387 has a specific function in assembly or that the loss of this residue leads to a marked change in the domain structure. As shown in Table 3, residues in the C-terminal region of γ chain are highly conserved across species. Isoleucine is present at this position in chicken and mammals, but replaced by methionine and leucine in frog and lamprey fibrinogens. Thus, this domain and, perhaps this specific residue, likely have important functions. We believe that length is more critical than the specific residue and plan to test this by expressing variants with substitutions at this residue. Crystal structures of this γ chain domain show that γIle387 lies within a β strand composed of residues γ381-388, as depicted in Figure 4. This strand is unusual in that it inserts in an antiparallel fashion between strands formed by residues γ189-197 and γ246-252.31,32Recently, Medved and colleagues discovered that following plasmin cleavage of γ chain after Arg374, the plasmin-generated C-terminal peptide remained associated with the central γ-chain domain.33 Thermal-stability studies with the intact domain, the cleaved peptide-domain complex, and the cleaved domain without peptide showed that the loss of this region (γ374-411) markedly destabilized the domain structure. Subsequent studies with normal and truncated γ chains, which were synthesized inEscherichia coli, showed that truncation at γ373 resulted in destabilization, but not unfolding, of this central domain of the γ chain. In contrast, truncation at γ392 had properties indistinguishable form the intact chain. We propose that truncation at residue γ386 would have properties similar to truncation at γ373, such that this γ-chain domain is destabilized in CHO cells. Moreover, we propose that the destabilization of this structure in vivo either prevents assembly of αγ and βγ dimers or greatly enhances proteolytic degradation of these heterodimers. We prefer the former conclusion because the γ chains themselves appear stable in the cell lysates.
Alignment of carboxyl-terminal residues of fibrinogen γ chains from several species and the human β chain
| Fibrinogen chain . | 371381387391401411 . | |||||
|---|---|---|---|---|---|---|
| Human γ | TWKTRWYSMKKTTMKIIPFNRLTIGFGQQHHLGGAKQAGDV | |||||
| Bovine γ | TWKSRWYSMKKTTMKIIPLNRLAIGEGQQHQLGGAKQAGDV | |||||
| Rat γ | TWKTRWYSMKETTMKIIPFNRLSIGDGQQHHMGGSKQVGDM | |||||
| Chicken γ | TWRDRWYSMKKTTMKIIPFNRLSI DGQQHS GGLKQVGDS | |||||
| Frog γ | TWRRRWYSMKSVTMKIMPLNRYG AEGQQ TLGGSKKSDFENRGDF | |||||
| Lamprey γ | TWHDRWYSLKMTTMKLLPMGRDLSGHGGQQQSKGNSR GDN | |||||
| Human β | NWKGSWYSMRKMSMKIRPFFPQQ | |||||
| 439449459 461 | ||||||
| Fibrinogen chain . | 371381387391401411 . | |||||
|---|---|---|---|---|---|---|
| Human γ | TWKTRWYSMKKTTMKIIPFNRLTIGFGQQHHLGGAKQAGDV | |||||
| Bovine γ | TWKSRWYSMKKTTMKIIPLNRLAIGEGQQHQLGGAKQAGDV | |||||
| Rat γ | TWKTRWYSMKETTMKIIPFNRLSIGDGQQHHMGGSKQVGDM | |||||
| Chicken γ | TWRDRWYSMKKTTMKIIPFNRLSI DGQQHS GGLKQVGDS | |||||
| Frog γ | TWRRRWYSMKSVTMKIMPLNRYG AEGQQ TLGGSKKSDFENRGDF | |||||
| Lamprey γ | TWHDRWYSLKMTTMKLLPMGRDLSGHGGQQQSKGNSR GDN | |||||
| Human β | NWKGSWYSMRKMSMKIRPFFPQQ | |||||
| 439449459 461 | ||||||
The human γ and β chains are shown with the residue numbers above and below the alignment, respectively. Bold or italic characters indicate γ-chain residues common to all species or common to human, bovine, rat, and chicken, respectively.
The structure (PDB identification 3FIB) of the fibrinogen γ-chain C-terminal domain (γ144-392).
The image was constructed in display mode cartoon using the program CHIME at: www.umass.edu/microbio/chime. (A) The complete structure of this γ-chain domain, with residues 144 and 392 indicated, Ile387 labeled I and calcium indicated as a black ball labeled Ca. (B) The 5 strands that form a central part of this domain, depicting residues 190-197, 244-251, 257-263, 280-283, and 381-388. Ile387 is shown as a van der Waals space-filled model, and calcium as a black ball. Panel B is about 1.2 times the size of panel A.
The structure (PDB identification 3FIB) of the fibrinogen γ-chain C-terminal domain (γ144-392).
The image was constructed in display mode cartoon using the program CHIME at: www.umass.edu/microbio/chime. (A) The complete structure of this γ-chain domain, with residues 144 and 392 indicated, Ile387 labeled I and calcium indicated as a black ball labeled Ca. (B) The 5 strands that form a central part of this domain, depicting residues 190-197, 244-251, 257-263, 280-283, and 381-388. Ile387 is shown as a van der Waals space-filled model, and calcium as a black ball. Panel B is about 1.2 times the size of panel A.
Whether due to the stability of γ domain or specifically γIle387, the abrupt change seen with the loss of this residue suggests that this region is critical for normal fibrinogen assembly. Further studies are needed to determine whether this region affects interactions between the chains that are required for assembly, or interactions with chaperones, such as Bip, or other constituents that mediate assembly in the rough endoplasmic reticulum. In either case, our data reinforced the conclusion that the γ chain has a central function in assembly and identified the C-terminal region as critical.
We gratefully acknowledge Mrs K. Nakamura for excellent technical assistance. We also gratefully acknowledge Drs T. Katsuyama and M. Tozuka (Department of Laboratory Medicine, Shinshu University School of Medicine, Matsumoto) for helpful advice and encouragement and Dr Kelly Hogan (University of North Carolina, Chapel Hill) for advice on statistical analyses. We dedicate this publication to Hitoshi Tanaka, MD, who died in July 2000.
Supported in part by United States Public Health Services grant HL31048.
Hitoshi Tanaka died July 28, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Susan T. Lord, CB no. 7525, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7525; e-mail:stl@med.unc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal