The von Hippel-Lindau (VHL) tumor suppressor gene targets hypoxia-inducible transcription factors (HIFs) for proteasomal degradation. Erythrocytosis due to inappropriate production of erythropoietin (EPO), one of the HIF target genes, is a classic albeit rare finding in patients with renal cancer. We report the clinical to molecular analysis in a patient in whom a thrombotic myocardial infarction was the first manifestation of a clear cell renal carcinoma associated with an elevated serum EPO level (109 U/L) and erythrocytosis (hemoglobin 200 g/L [20 g/dL]). The tumor strongly expressed EPO messenger RNA and the 2 regulatory subunits HIF-1α and HIF-2α. Sequence analysis of tumor tissue identified a point mutation of the VHL gene (nucleotide 701 T>C) with a predicted amino acid exchange (Leu163Pro). This structural change, although located at distance to the HIF-binding region, was found to inhibit binding of HIF-1α to VHL, thus leading to accumulation of HIF, which drives EPO production.
Introduction
Erythropoietin (EPO) production in liver and kidneys is inversely related to oxygen availability, thus establishing a negative feedback control of erythropoiesis.1 Studies of EPO regulation led to the identification of the transcription factor hypoxia-inducible factor (HIF).2,3 HIF is composed of an HIFα and HIFβ subunit and binds to hypoxia response elements located in the vicinity of the EPO gene and several other genes induced by hypoxia. HIFα is the oxygen-regulated component, and 2 subunits with marked sequence homology have been described: HIF-1α and HIF-2α. In the presence of oxygen, HIFα is rapidly degraded by cellular proteasomes. The protein targeting HIFα for proteasomal degradation is the von Hippel-Lindau (VHL) protein, a tumor suppressor protein that is mutated in the germ line of patients affected by the VHL disease.4-6 Recent work has shown that oxygen-dependent hydroxylation of two proline residues of HIFα (Pro 402 and 564) essential for binding of HIF to pVHL and represent a critical component of the oxygen-sensing mechanism.7-9
Certain tumors that are associated with an inappropriate increase in EPO production can lead to erythrocytosis. Renal cancer is the most frequent cause of paraneoplastic polycythemia, and EPO expression has been demonstrated at the protein and messenger RNA (mRNA) level in renal tumors10,11 and tumor-derived primary cell lines.12,13 However, the mechanisms activating the EPO gene in association with malignant transformation have not been clarified. Given that somatic mutations of the VHL gene can be found in most clear cell renal carcinomas,5 the most frequent type of renal cancer, the discovery of the role of VHL in the cellular response to hypoxia raises the intriguing possibility of a link between VHL loss of function and overexpression of EPO. We have tested this hypothesis in a 50-year-old man, in whom a coronary thrombosis led to the diagnosis of a renal cell carcinoma. The patient was admitted with clinical, laboratory, and electrocardiogram evidence of acute myocardial infarction. He denied any diseases prior to admission, had no history of angina, and no cardiac risk factors. Laboratory findings revealed a marked erythrocytosis (Table1). Cardiac catheterization excluded any plaques, mild stenoses, or other signs of coronary artery disease. However, the distal left anterior descending artery was subtotally occluded by thrombotic material. A glycoprotein IIb/IIIa antagonist was given, and angioplasty was performed. An underlying stenosis could not be detected, no dissection was seen, and no stent was implanted. Fourteen days later, on control angiography, the left anterior descending artery appeared completely normal.
Laboratory values in a patient with an EPO-producing renal tumor and paraneoplastic myocardial infarction
| . | Normal range . | At admission . | 3 days before nephrectomy . | 1 day after nephrectomy . | 3 days after nephrectomy . | 4 weeks after nephrectomy . |
|---|---|---|---|---|---|---|
| Hemoglobin, g/L | 130 -160 | 204 | 184 | 178 | 168 | 131 |
| Hematocrit | 39 -49 | 66 | 59.8 | 58.2 | 55 | 42 |
| Erythrocyte count, × 1012/L | 4.3 -5.9 | 7.9 | 7.4 | 7.0 | 6.8 | 5.5 |
| Leukocyte count, × 109/L | 3.2 -9.8 | 8.7 | 5.0 | 7.9 | 7.6 | 8.0 |
| Platelet count, × 109/L | 130 -400 | 461 | 575 | 286 | 298 | 425 |
| EPO, U/L | 8.2 -21.4 | 109* | 53.6 | 25.7 | 6.0 | 6.8 |
| . | Normal range . | At admission . | 3 days before nephrectomy . | 1 day after nephrectomy . | 3 days after nephrectomy . | 4 weeks after nephrectomy . |
|---|---|---|---|---|---|---|
| Hemoglobin, g/L | 130 -160 | 204 | 184 | 178 | 168 | 131 |
| Hematocrit | 39 -49 | 66 | 59.8 | 58.2 | 55 | 42 |
| Erythrocyte count, × 1012/L | 4.3 -5.9 | 7.9 | 7.4 | 7.0 | 6.8 | 5.5 |
| Leukocyte count, × 109/L | 3.2 -9.8 | 8.7 | 5.0 | 7.9 | 7.6 | 8.0 |
| Platelet count, × 109/L | 130 -400 | 461 | 575 | 286 | 298 | 425 |
| EPO, U/L | 8.2 -21.4 | 109* | 53.6 | 25.7 | 6.0 | 6.8 |
First measurement of EPO was performed on the third day after admission.
Serum EPO was increased to 109 U/L (normal range, 8.2-21.4 U/L) (Table 1). Spirometry and blood gas analysis showed no evidence of a respiratory disorder. There was no sign of a primary hematologic disease. Ultrasonography revealed a mass (8 × 7 cm) at the top of the left kidney, which was verified on computed tomography scan (Figure1A). A renal cell carcinoma (RCC) with central necrosis was suspected. Metastatic lesions were not found. A small hypodense lesion in the spleen was interpreted as an infarction. Following nephrectomy, which confirmed the diagnosis of RCC (pT3a, pN0, R0, G3), EPO serum concentration decreased within 7 days and hemoglobin levels returned to normal (Table 1). The patient is well 9 months later. EPO serum concentration remains within normal range.
RCC with molecular characteristics that resulted in pronounced polycythemia.
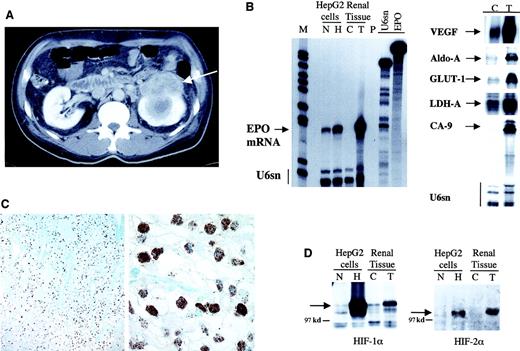
Computed tomography of the abdomen showing a left-sided renal mass (arrow) with central necrosis (A). Ribonuclease protection assay for EPO transcripts (B, left panel) and other hypoxia-inducible genes (B, right panel) demonstrated markedly elevated expression of these genes in the tumor (T) as compared with the control (C) adjacent nontumorous kidney tissue. Cell extracts from HepG2 cells were exposed to normoxia (N) or 16 hours of hypoxia (H) for comparison. U6sn served as internal control. Radiolabelled probes were protected from ribonuclease digestion by hybridization to 50 μg or 1 μg total RNA for HIF targets and U6sn, respectively. M indicates molecular weight marker; P, digested riboprobes; and U6sn and EPO, undigested riboprobes. Immunohistochemistry for HIF-1α of the tumor tissue shows homogenous nuclear staining of virtually every tumor cell (C). Sections were counterstained with Richardson's reagent. Magnifications are 100 × and 800 ×, respectively. Immunoblotting of protein extracts from tumor (T), control kidney tissue (C), and HepG2 cell extracts under normoxia (N) and 4 hours of hypoxia (H) demonstrates pronounced up-regulation of HIF-1α and HIF-2α proteins (arrows in panel D). The position of the 97 kd marker is indicated.
RCC with molecular characteristics that resulted in pronounced polycythemia.
Computed tomography of the abdomen showing a left-sided renal mass (arrow) with central necrosis (A). Ribonuclease protection assay for EPO transcripts (B, left panel) and other hypoxia-inducible genes (B, right panel) demonstrated markedly elevated expression of these genes in the tumor (T) as compared with the control (C) adjacent nontumorous kidney tissue. Cell extracts from HepG2 cells were exposed to normoxia (N) or 16 hours of hypoxia (H) for comparison. U6sn served as internal control. Radiolabelled probes were protected from ribonuclease digestion by hybridization to 50 μg or 1 μg total RNA for HIF targets and U6sn, respectively. M indicates molecular weight marker; P, digested riboprobes; and U6sn and EPO, undigested riboprobes. Immunohistochemistry for HIF-1α of the tumor tissue shows homogenous nuclear staining of virtually every tumor cell (C). Sections were counterstained with Richardson's reagent. Magnifications are 100 × and 800 ×, respectively. Immunoblotting of protein extracts from tumor (T), control kidney tissue (C), and HepG2 cell extracts under normoxia (N) and 4 hours of hypoxia (H) demonstrates pronounced up-regulation of HIF-1α and HIF-2α proteins (arrows in panel D). The position of the 97 kd marker is indicated.
Materials and methods
Informed consent was obtained for analysis of tissues and genotyping. Immediately after nephrectomy, specimens from healthy kidney and tumor were frozen in liquid nitrogen or fixed in 3% paraformaldehyde.
RNA analysis
Total RNA of tissues and HepG2 cells cultured under normoxia (21% oxygen) and hypoxia (1% oxygen; 16 hours) was extracted with RNAzol B (Biogenesis, Poole, United Kingdom). Ribonuclease protection assay was performed as described14for EPO, vascular endothelial growth factor (VEGF), glucose transporter-1 (GLUT-1), carbonic anhydrase-9, lactate dehydrogenase-A, aldolase A, and U6 small nuclear RNA (U6sn) as an internal control.
Immunohistochemistry
HIF-1α was detected on paraffin sections by mouse monoclonal antibody (α67, Novus Biologicals, Littleton, CO) using target retrieval solution and catalyzed signal enhancement system (DAKO, Hamburg, Germany).14
Immunoblotting
Protein extraction and immunoblotting were performed as described.14,15 For comparison, extracts of HepG2 cells cultured under normoxia (21% oxygen) and hypoxia (1% oxygen; 4 hours) were loaded alongside. HIF-1α and HIF-2α proteins were detected using mouse monoclonal antibodies (Transduction Laboratories, Lexington, KY, and 190b,15 respectively).
VHL mutation analysis
Genomic DNA was extracted from tumor, adjacent kidney tissue, and leukocytes with purification columns (Qiagen, Hilden, Germany). Single-strand conformation polymorphism analysis was performed to detect intragenic mutations. Exon 3, where an aberrant band in single-strand conformation polymorphism of tumor DNA was found, was sequenced on a semiautomated sequencer.
Site-directed mutagenesis and immunoprecipitation
The T>C mutation at position 701 of VHL (accession numberL15409) that was detected in the tumor (see below) was introduced into a wild-type cDNA expression plasmid of full-length VHL, tagged with hemagglutinin (HA) at the C-terminal end (wt-pVHL.HA) by standard site-directed mutagenesis, and was confirmed by sequencing.
Immunoprecipitation assays were performed essentially as described,8 using radiolabeled in vitro–transcribed and –translated HIF-1α, wt-VHL.HA, and mut-VHL.HA as binding proteins. For immunoprecipitation 2 μg monoclonal anti-HA antibody (12CA5, Roche, Mannheim, Germany) was used, and precipitates were denatured and resolved by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
Results
The resected tumor was a clear cell RCC. EPO mRNA was not detectable in normal kidney tissue but markedly up-regulated in the tumor (Figure 1B), the signal intensity being stronger to that derived from an equal amount of RNA from hepatoma cells exposed to severe hypoxia. Other hypoxia-inducible genes, including VEGF, GLUT-1, carbonic anhydrase-9, lactate dehydrogenase-A, and aldolase A,3 16 were also strongly induced in the tumor (Figure 1B).
Immunoblots revealed significant overexpression of the HIF-1α and HIF-2α subunits in the tumor, and immunohistochemistry performed for HIF-1α showed nuclear accumulation of the transcription factor in virtually every tumor cell (Figure 1C,D). As previously observed in clear cell RCC,14 17 the expression pattern is not different in the vicinity of blood vessels or at the tumor margin, suggesting that it is not affected by tissue oxygen gradients.
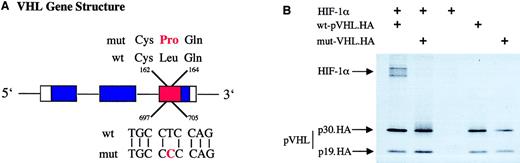
To explore the potential reason for HIF overexpression, we searched for mutational alteration of the VHL gene in tumor cells. A point mutation of nucleotide 701 (T>C, accession number L15409) was found in exon 3. This mutation has previously been identified in another RCC (http://www.umd.necker.fr18) and predicts an amino acid exchange at position 163 (leucine to proline, Figure2A). Leu163 lies within a surface α-helical region of the pVHL α-domain,19 which is part of the binding region to elongin C,20 bridging pVHL to the other known proteins of this E3 ubiquitin ligase. Although the substitution exchanges 2 hydrophobic amino acids, it might well have extensive effects on folding of the molecule because prolyl residues prevent α-helix formation.
Mutation analysis of tumor DNA reveals a point mutation in exon 3 of the VHL gene that loses the ability to bind HIF-1α.
Mutational analysis of genomic tumor DNA revealed a point mutation of the VHL gene in exon 3 at position 701. (A) The position of this mutation in relation to the intron/exon (boxes) boundaries and the functional domains of pVHL; α-domain, red; and β-domain, blue. Amino acid (top) and nucleotide (bottom) sequences are shown for wild-type (wt) and mutated (mut) VHL, illustrating the exchange of amino acid 163, leucine to proline. (B) An immunoprecipitation assay in which 10 μL of each radioactive in vitro–transcribed and –translated product of HIF-1α, wild-type pVHL (wt-pVHL.HA), and mutated pVHL (mut-pVHL.HA, Leu163Pro) were coincubated. HA tag–fused C-terminal to pVHL was used to immunoprecipitate pVHL with its potential binding partner using a specific anti-HA monoclonal antibody. Crosses indicate the input of respective proteins into the reaction. Immunoprecipitates were denatured and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Visualization was achieved by autoradiography. Arrows indicate HIF-1α and both positions of pVHL, full-length (p30.HA) and the truncated protein fragment, caused by an internal translation initiation site (p19.HA). Whereas wild-type pVHL.HA coimmunoprecipitates with HIF-1α, the mutated protein L163P shows no binding to HIF-1α.
Mutation analysis of tumor DNA reveals a point mutation in exon 3 of the VHL gene that loses the ability to bind HIF-1α.
Mutational analysis of genomic tumor DNA revealed a point mutation of the VHL gene in exon 3 at position 701. (A) The position of this mutation in relation to the intron/exon (boxes) boundaries and the functional domains of pVHL; α-domain, red; and β-domain, blue. Amino acid (top) and nucleotide (bottom) sequences are shown for wild-type (wt) and mutated (mut) VHL, illustrating the exchange of amino acid 163, leucine to proline. (B) An immunoprecipitation assay in which 10 μL of each radioactive in vitro–transcribed and –translated product of HIF-1α, wild-type pVHL (wt-pVHL.HA), and mutated pVHL (mut-pVHL.HA, Leu163Pro) were coincubated. HA tag–fused C-terminal to pVHL was used to immunoprecipitate pVHL with its potential binding partner using a specific anti-HA monoclonal antibody. Crosses indicate the input of respective proteins into the reaction. Immunoprecipitates were denatured and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Visualization was achieved by autoradiography. Arrows indicate HIF-1α and both positions of pVHL, full-length (p30.HA) and the truncated protein fragment, caused by an internal translation initiation site (p19.HA). Whereas wild-type pVHL.HA coimmunoprecipitates with HIF-1α, the mutated protein L163P shows no binding to HIF-1α.
To test for the functional significance of this mutation, we performed site-directed mutagenesis of a VHL expression plasmid and compared binding characteristics of the mutated protein (mut-pVHL) to the wild-type protein (wt-pVHL) in immunoprecipitation assays with HIF-1α. Whereas wt-pVHL coimmunoprecipitated with HIF-1α, mut-pVHL showed no binding (Figure 2B) indicating that mut-pVHL (Leu163Pro) loses its ability to target HIF-1α for destruction. Because HIFα binds to the β domain of pVHL,6 it is conceivable that the observed mutation in the α domain either leads to a confirmational change at a distance or that disturbed assembly of the E3 multiprotein complex indirectly effects binding of HIF-1α. Two nearby mutations (Leu158Pro and Arg167Gln) have recently been studied, and both ablate elongin C binding but, interestingly, only the former prevents HIFα chain association.21
Genotyping revealed that the mutant VHL was not present in other tissues of the patient (leukocytes and normal kidney), thus excluding a germ line mutation and VHL syndrome. Inactivation of both VHL alleles in sporadic clear cell RCC usually occurs through somatic mutations, promoter hypermethylation, or allele loss.5 Sequence analysis did not show evidence of loss of heterozygosity in tumor DNA from our case. This could indicate hypermethylation of the second allele but could also result from contamination with infiltrating leukocytes masking allele loss.
Erythrocytosis can occur in association with any of the tumors associated with VHL loss of function, which supports the significance of VHL-dependent suppression of EPO gene activity.22Although less than 5% of patients with renal cancer are polycythemic,23 studies suggest that an elevation of serum EPO levels is more frequent24-26 and cancer-related inhibition of erythropoiesis may blunt its biologic effect. Moreover, factors in addition to HIF accumulation, which are not ubiquitously operating in each tumor, may be required for increased EPO gene transcription. Of note, EPO expression in the kidney is normally restricted to peritubular fibroblasts,27,28 whereas RCCs are derived from tubular epithelial cells. Yet, renal tumor cells can produce EPO,10,11 and this production can be maintained in vitro after cell isolation12,13,29 and transplantation into nude mice.29-31 Thus, tumor-associated genetic events apparently relieve suppression of the EPO gene in some cases, so that transcription can be driven by stabilized HIF.
While erythrocytosis in the context of renal cancer has so far mainly been considered as a “tumor marker,” the present case shows that it may have prognostic implications due to thrombotic complications. In vitro data also suggest that renal carcinoma cells express EPO receptors and that their activation stimulates cell proliferation.32 Several other genes activated by HIF facilitate metabolic adaptation and neoangiogenesis. In a series of renal carcinomas, we have recently found that the mRNA expression of 2 of these target genes (VEGF, GLUT-1) is related to the abundance of HIF-1α, suggesting that this a dominant and relevant mechanism in vivo.14 Abnormal stabilization of HIF does therefore offer a unifying hypothesis for apparently diverse characteristics of renal tumors, such as erythrocytosis and pronounced vascularization.
We are indebted to M. Krüger and H. v. Randenborgh for help in the clinical workup and thank P. Ratcliffe (Oxford, United Kingdom) for riboprobes, pVHL.HA, and HIF-1α expression plasmids.
Supported by the German Research Foundation (DFG EC 87-3) and the German Ministry for Research and Technology (BMBF; Network RNA Technology, Berlin, Germany).
M.S.W. and M.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
K.-U. Eckardt, Dept of Nephrology and Medical Intensive Care, Charité, Campus Virchow-Klinikum, Augustenburger Platz 1, 13353 Berlin, Germany; e-mail: kai-uwe.eckardt@charite.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal