Glycoprotein (GP) IIb/IIIa antagonists are effective therapeutic agents, but elicit thrombocytopenia with a frequency that approaches 2%. Here, we provide evidence that thrombocytopenia in humans treated with the GP IIb/IIIa antagonist roxifiban is immune mediated. Two patients underwent conversion to a highly positive drug-dependent antibody (DDAB) status temporally associated with thrombocytopenia. Despite the continued presence of DDABs, the fall in platelet count was reversed by discontinuation of drug treatment, pointing to the exquisite drug dependency of the immune response. DDABs appear to bind to neoepitopes in GP IIb/IIIa elicited on antagonist binding. This information was used to develop an enzyme-linked immunosorbent assay (ELISA) for DDAB using solid-phase GP IIb/IIIa. A high level of specificity is indicated by the observation that DDAB binding is dependent on the chemical structure of the GP IIb/IIIa antagonist and that only 2% to 5% of human blood donors and 5% of chimpanzees present with pre-existing DDABs. Furthermore, none of 108 nonthrombocytopenic patients from the phase II roxifiban study showed an increase in antibody titer. Absorption of thrombocytopenia plasma with platelets reduced the DDAB ELISA signal, indicating that the test detects physiologically relevant antibodies. Screening patients for pre-existing or increasing DDAB titer during treatment with GP IIb/IIIa antagonists may reduce the incidence of drug-induced thrombocytopenia.
Introduction
Blockade of glycoprotein (GP) IIb/IIIa represents a major advancement in the management of patients with acute vascular disease (for reviews, see Cannon1 and Nurden et al2). However, thrombocytopenia complicates the treatment and some cases have been severe (nadir < 20 000 platelets/μL). Thrombocytopenic patients experienced more bleeding events and ischemic events.3,4 Thrombocytopenia has been observed with all intravenous agents currently in clinical use. For example, abciximab administration has been associated with the development of thrombocytopenia at rates between 2.5% and 5.6%, and with acute, potential life-threatening profound thrombocytopenia at rates between 0.3% to 0.8%.3,5-9 The rate of profound thrombocytopenia appears to be higher on abciximab readministration.10Similar rates of thrombocytopenia have been observed with eptifibatide.3,11 In general, patients with thrombocytopenia are older, weigh less, have a lower baseline platelet count, and are treated with bolus and infusion.3,4 To extend the benefits of the acute therapy to long-term care, a number of oral GP IIb/IIIa antagonists have undergone clinical trials. For example, RPR 109891 therapy was associated with a thrombocytopenia frequency of up to 13%.12 Lower frequencies have been observed for the other oral agents in clinical development. For sibrafiban, the thrombocytopenia frequency was not different compared to placebo,13 whereas in the case of orbofiban, increased frequencies were observed in the treatment arms.14 It is currently unclear whether thrombocytopenia frequencies are effected by pharmacokinetic and pharmacodynamic factors or inherent properties of the chemical entities.
The mechanism underlying severe thrombocytopenia after exposure to GP IIb/IIIa antagonists is not well defined. In vitro, exposure of platelets to arginine-glycine-aspartic acid (RGD) peptides or small-molecular GP IIb/IIIa antagonists induces alterations in the conformation of GP IIb/IIIa. These neoepitopes, also referred to as ligand-induced binding sites, have been monitored by using conformationally sensitive monoclonal antibodies15-19 or gel shift analysis.20 It is currently unknown whether similar conformationally sensitive antibodies can be elicited in response to GP IIb/IIIa antagonist therapy. Initial support for the concept of immune-mediated GP IIb/IIIa antagonist-induced thrombocytopenia has been provided.21 More specifically, chimpanzee A264 presented acute thrombocytopenia on dosing with the GP IIb/IIIa antagonists L-739758 and L-738167.21Fluorescence-activated cell sorting (FACS) analysis provided evidence for the presence of GP IIb/IIIa antagonist-dependent antiplatelet antibodies to GP IIb/IIIa antagonist that elicited thrombocytopenia, but not to antagonists that were well tolerated in the same chimpanzee. Moreover, this antibody was already present prior to first exposure to GP IIb/IIIa antagonists. Taken together, these data are consistent with the notion that the thrombocytopenia was immune mediated via pre-existing drug-dependent antibodies to platelet antigen(s).21 Limited information is currently available about the molecular mechanism of GP IIb/IIIa antagonist-induced thrombocytopenia in the clinic.
The mechanism for the development of drug-dependent antibodies (DDABs) to platelet antigens remains essentially unknown.22-28 No predisposing factors have been identified, and combined with the low incidence of thrombocytopenia with most drugs, has precluded populations at risk for this complication from being prospectively identified with clinical criteria.22 Thus, standard clinical practice continues to involve measuring the platelet counts of exposed individuals at various intervals and for varying duration. Numerous methods have been developed for the retrospective detection of platelet-associated DDABs in patients with drug-induced thrombocytopenia.24,25,29-37 However, the clinical utility appears to be limited due to requirements for specialized equipment, testing centers, and insufficient sensitivity and specificity of these methods. Importantly, the positive and negative predictive value of these assays is unknown in most settings.22
Here, we report data on 2 patients with drug-dependent antibodies elicited during therapy with the oral GP IIb/IIIa antagonist roxifiban. We provide evidence that the antibodies detect ligand-induced binding sites in GP IIb/IIIa. We describe the development of a rapid, sensitive, and specific DDAB ELISA that closely mimics the conditions on intact platelets without disturbing the expression of conformationally sensitive epitopes observed on the platelet surface on GP IIb/IIIa antagonist administration. This test may aid in the prospective identification of patients at risk for thrombocytopenia associated with GP IIb/IIIa antagonist therapy.
Materials and methods
Platelet-poor plasma sample preparations
Blood from normal subjects, chimpanzees (New Iberia Research Center, New Iberia, LA), and from a phase IIb study of roxifiban was collected into 1:10 volume of 3.2% sodium citrate. Platelet-poor plasma (PPP) was prepared by centrifugation at 1500g for 15 minutes and stored at less than −15°C or less than −60°C. In general, clinical samples were obtained at least 12 hours after the last dosing with roxifiban.
Synthesis of GP IIb/IIIa antagonists
XP280, a salt of the active, free acid form of the prodrug roxifiban38 has been described. Drs Gary Cain, Doug Batt, and Joanne Smallheer (Bristol-Myers Squibb, Wilmington, DE) are acknowledged for their preparation of orbofiban and sibrafiban. The GP IIb/IIIa antagonists L-739758, L-738167 and L-73421721 were kindly provided by Dr B. Bednar (Merck Research Laboratories, Rahway, NJ).
Purification of GP IIb/IIIa and gel shift analysis
Total human GP IIb/IIIa used in the initial enzyme-linked immunosorbent assay (ELISA) format was obtained from Enzyme Research Laboratories (South Bend, IN) or purified from platelets.20,39 For the improved ELISA format, RGD-retained GP IIb/IIIa was used and contaminating IgG was absorbed with protein G affinity (Pharmacia, Piscataway, NJ). The detergent concentration was determined by absorbance at 275 nm, and protein concentration was determined by bicinchoninic acid method (Pierce, Rockford, IL). Excess detergent was removed using EXTRACT-Gel (Pierce). Gel shift analysis was performed as described20 and for immunoblotting, polyvinylidene difluoride membranes were incubated with patient plasma, followed by horseradish peroxidase (HRP)–labeled antihuman IgG and DAB substrate (Biogenex, San Ramon, CA).
Differential DDAB ELISA
Improved ELISA format.
Costar ELISA plates (no. 3591) were coated (4°C, 16 hours) with 250 ng (2.5 μg/mL) RGD-retained GP IIb/IIIa in phosphate-buffered saline (PBS) containing 1 mM CaCl2 and 0.5 mM MgCl2(PBS/Ca/Mg) in the presence or absence of the indicated GP IIb/IIIa antagonist (100 nM; unless otherwise stated). After 3 washing steps with PBS/Ca/Mg containing 0.05% Tween 20 (vol/vol; PBST), the plates were blocked with 1% goat serum (VWR Scientific, Pittsburgh, PA) and 0.1% casein (Sigma, St Louis, MO) or Blotto (Bio-Rad, Hercules, CA) in PBST for 30 minutes at room temperature, followed by, the addition of PPP (diluted 1:10 in PBST, 1% vol/vol goat serum, 0.1% Blotto). The secondary antibody was diluted 1:5600 (depending on lot) in PBST, 0.1% Blotto, and 100 nM GP IIb/IIIa antagonist and added for 1 hour at room temperature. The bound peroxidase-labeled IgG was detected with 3,3′,5,5′-tetramethylbenzidine (Pierce). Changes in absorbance were determined kinetically for 5 minutes at 650 nM or until the change in absorbance reached 0.5 OD units. GP IIb/IIIa antagonist was present in the appropriate wells during all incubation and washing steps. For some experiments, thrombin inhibitors were added to the diluted PPP during the primary antibody incubation to prevent an infrequently observed clotting of the diluted recalcified PPP in the microtiter wells. Coating with 250 ng purified GP IIb/IIIa per well resulted in a maximal signal in the DDAB ELISA (not shown). The GP IIb/IIIa antagonists used have low nanomolar affinities for immobilized GP IIb/IIIa with the exception of orbofiban, and use of 100 nM antagonist is at least 20-fold above the concentration required to obtain a maximal signal (data not shown).
Initial ELISA format.
Early experiments were performed using an approximately 5-fold less sensitive ELISA format without plate blocking. Total GP IIb/IIIa was used along with a O-phenylamine detection system and a 15-minute kinetic read. Due to limited sample availability, some experiments could not be repeated with the improved ELISA format.
Immunoglobulin class.
The immunoglobulin class of DDABs was determined using peroxidase-labeled murine antibodies to human immunoglobulin (Zymed, San Francisco, CA) as the detection step and normal goat serum was replaced with normal mouse serum (Zymed).
Result reporting and quality assurance.
Results are expressed as delta ([Vmax plus compound]−[Vmax minus compound]). Plate acceptance criteria were established based on DDAB+ and DDAB− specimens and results were not normalized. Assays were performed with triplicate determinations with a coefficient of variation of less than 25%. When sufficient PPP samples were available, experiments were repeated at least once and representative results of a single experiment are presented. The DDAB titer was unchanged in samples stored for up to 2 years. Although 95% of plasmas had a background (minus compound) of less than 20 mOD/min, few specimens had backgrounds up to 100 mOD/min, possibly related to antibody to conformational changes in GP IIb/IIIa induced by the assay conditions. The matrix effect was studied using assay buffer and a high and low background DDAB− human PPP. The delta was found to be independent of the background and remained relatively unchanged in different matrices, whereas the signal to noise was reduced up to 5-fold using the high background PPP.
Platelet absorption studies and EDTA elution procedure
Solution-phase absorption was performed with platelets present in whole citrated anticoagulated blood, platelet-rich citrate plasma (PRP), or with gel-filtered platelets. In some experiments, the following inhibitors were added to prevent platelet aggregation and proteolysis: prostaglandin I2 (0.1 mM), apyrase (400 μg/mL), hirudin (1U/mL), and leupeptin (1 mM). Platelets were preincubated in the presence or absence of GP IIb/IIIa antagonists and PPP for 1 hour at room temperature or 37°C. Platelets were removed by centrifugation at 1500g for 10 minutes and the resulting PPP was analyzed for the presence of DDABs using ELISA.
For the recovery of platelet-bound DDAB, unfractionated citrate blood, citrated PRP, or gel-purified platelets were preincubated with DDAB containing PPP in the presence or absence of GP IIb/IIIa antagonist, and EDTA (10 mM final concentration) was added, followed by incubation at 37°C for up to 2 hours. The platelets were removed by centrifugation and the EDTA PPP recovered. The PPP was recalcified before addition to the GP IIb/IIIa-coated ELISA plates. FACS analysis was performed as described using a FACS scan flow cytometer (Becton Dickinson, Franklin Lakes, NJ).21 FITC fluorescence was read on FL1. Platelets were identified by their characteristic forward and side light scattering. Data from 10 000 platelets were obtained per sample and analyzed using Becton Dickinson CellQuest software. Dot plot data were analyzed as the sum of triplicates.
Results
Development of a solid-phase, differential ELISA for the detection of DDABs to GP IIb/IIIa antagonists
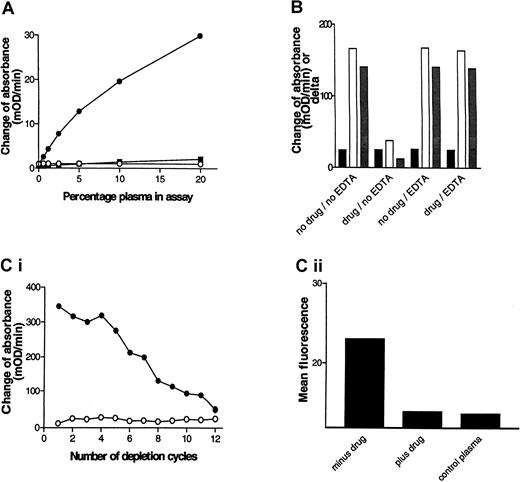
Chimpanzee A264 developed acute thrombocytopenia on dosing with L-739758, and FACS analysis provided evidence for GP IIb/IIIa antagonist-dependent DDABs.21 PPP from A264 was obtained several months after the thrombocytopenia episode and used to develop the initial version of a DDAB ELISA. Microtiter wells coated with purified GP IIb/IIIa in the presence or absence of the GP IIb/IIIa antagonist L-739758 were incubated with dilutions of chimpanzee A264 PPP. After washing, bound IgG was detected with HRP-labeled antihuman IgG antibodies, followed by substrate (Figure1A). A signal was detected in wells coated in the presence of GP IIb/IIIa antagonist, but not in the absence (note signal to noise > 10 at 5%). The assay was concentration dependent with respect to the plasma dilution and the lower detection limit was estimated to be a 1:160 dilution of PPP. In contrast, PPP derived from a nonthrombocytopenic control chimpanzee failed to elicit a positive response. Mixing experiments of DDAB+ and DDAB− PPP specimens excluded an inhibitory substance in DDAB− samples (not shown).
The DDAB ELISA detects pathophysiologically relevant antibodies.
(A) Detection of GP IIb/IIIa antagonist-dependent DDABs in chimpanzee. GP IIb/IIIa-coated wells were incubated with the indicated dilutions of chimpanzee A264 PPP in the presence or absence of L-739758 and analyzed using the initial ELISA version (see “Materials and methods”). The change of absorbance (mOD/min) of wells in the absence of drug (closed squares) and in the presence of GP IIb/IIIa antagonist (closed circles) is indicated. The change of absorbance in the presence of drug of a control chimpanzee PPP is indicated (open circles). (B) DDABs present in A264 PPP can be specifically depleted by platelets. A264 PPP was added to human DDAB− citrated whole blood in the absence (no drug) or presence (drug) of 50 nM L-739758 and incubated for 15 minutes at room temperature. EDTA (4.5 mM) was added to samples as indicated for an additional 15 minutes at room temperature. PPP was recovered and tested in the DDAB ELISA as in panel A (improved version). No drug wells were incubated with XP280 to prevent carry-over of L-739758. XP280 competes with L-739758 for GP IIb/IIIa binding but fails to elicit binding of chimpanzee A264 DDABs (Table 1). Black bars indicate mOD/min minus drug wells; open bars, mOD/min plus drug wells; gray bars, mOD/min L-739758 wells minus mOD/min XP280 wells. (C) Correlation between ELISA and FACS signal. (i) Wells were coated with GP IIb/IIIa in the absence (open circles) or presence (closed circles) of L-739758 and incubated with A264 PPP for 10 minutes, followed by PPP transfer to the next well. This depletion procedure was repeated 11 times. After 12 incubation steps, bound IgG on the depletion plate was detected (improved ELISA format). (ii) The ELISA-depleted PPPs were subsequently incubated with gel-filtered platelets in the presence of L-739758, followed by addition of FITC-labeled antihuman IgG and analyzed by FACS. The results are expressed as mean fluorescence of 10 000 events. Minus drug is A264 PPP incubated on ELISA plates in the absence of GP IIb/IIIa antagonist. Plus drug is A264 PPP incubated on ELISA plates in the presence of GP IIb/IIIa antagonist. Control PPP is ELISA DDAB− PPP from the platelet donor used for the FACS experiment.
The DDAB ELISA detects pathophysiologically relevant antibodies.
(A) Detection of GP IIb/IIIa antagonist-dependent DDABs in chimpanzee. GP IIb/IIIa-coated wells were incubated with the indicated dilutions of chimpanzee A264 PPP in the presence or absence of L-739758 and analyzed using the initial ELISA version (see “Materials and methods”). The change of absorbance (mOD/min) of wells in the absence of drug (closed squares) and in the presence of GP IIb/IIIa antagonist (closed circles) is indicated. The change of absorbance in the presence of drug of a control chimpanzee PPP is indicated (open circles). (B) DDABs present in A264 PPP can be specifically depleted by platelets. A264 PPP was added to human DDAB− citrated whole blood in the absence (no drug) or presence (drug) of 50 nM L-739758 and incubated for 15 minutes at room temperature. EDTA (4.5 mM) was added to samples as indicated for an additional 15 minutes at room temperature. PPP was recovered and tested in the DDAB ELISA as in panel A (improved version). No drug wells were incubated with XP280 to prevent carry-over of L-739758. XP280 competes with L-739758 for GP IIb/IIIa binding but fails to elicit binding of chimpanzee A264 DDABs (Table 1). Black bars indicate mOD/min minus drug wells; open bars, mOD/min plus drug wells; gray bars, mOD/min L-739758 wells minus mOD/min XP280 wells. (C) Correlation between ELISA and FACS signal. (i) Wells were coated with GP IIb/IIIa in the absence (open circles) or presence (closed circles) of L-739758 and incubated with A264 PPP for 10 minutes, followed by PPP transfer to the next well. This depletion procedure was repeated 11 times. After 12 incubation steps, bound IgG on the depletion plate was detected (improved ELISA format). (ii) The ELISA-depleted PPPs were subsequently incubated with gel-filtered platelets in the presence of L-739758, followed by addition of FITC-labeled antihuman IgG and analyzed by FACS. The results are expressed as mean fluorescence of 10 000 events. Minus drug is A264 PPP incubated on ELISA plates in the absence of GP IIb/IIIa antagonist. Plus drug is A264 PPP incubated on ELISA plates in the presence of GP IIb/IIIa antagonist. Control PPP is ELISA DDAB− PPP from the platelet donor used for the FACS experiment.
Platelet absorption experiments were performed to test whether the DDAB ELISA detects physiologically relevant, platelet-binding antibodies (Figure 1B). A264 PPP was incubated with whole blood from a DDAB− human volunteer in the absence or presence of L-739758. PPP was recovered by centrifugation and retested in the DDAB ELISA. Note that the ELISA foreground (open bars) signal was reduced by more than 75% in the preabsorbed PPP only in the presence, but not absence, of L-739758. In contrast, the background binding (closed bars) to the no-drug control wells were unchanged. As a consequence, the delta (mOD/min in plus drug minus mOD/min in minus drug; grayish bars) was correspondingly reduced. Similar results were obtained using gel-purified platelets (data not shown). These results indicate that the DDAB ELISA detects antibodies that bind to intact platelets.
The specificity of the GP IIb/IIIa antagonists used to demonstrate A264 antibody binding to purified GP IIb/IIIa and platelets suggests that GP IIb/IIIa is the molecular target for the DDABs. Two approaches were taken to further test this hypothesis. In the first, the ability of EDTA to dissociate platelet-bound DDABs was analyzed. EDTA treatment of platelets at 37°C has been reported to dissociate the GP IIb/IIIa complex, resulting in the dissociation of conformational sensitive antibodies.40 41-43 After the initial incubation with whole blood in the absence (Figure 1B, no drug/EDTA) or presence of L-739758 (drug/EDTA), EDTA was added for 15 minutes at room temperature, and PPP was prepared. DDABs were quantitatively recovered from the platelet surface by EDTA treatment, suggesting that conformational and calcium-sensitive GP IIb/IIIa epitopes are the molecular target. Similar results were obtained using gel-purified platelets (data not shown). In the second approach, the correlation between antibody binding to purified GP IIb/IIIa (ELISA) and platelets (FACS) was compared. Plasma was incubated on GP IIb/IIIa-coated plates in the presence of L-739758 in 12 consecutive incubation steps. The ELISA signal diminished with increasing number of absorption steps (Figure 1C). Analysis of the resulting ELISA-depleted PPP samples revealed a reduction of the FACS signal to control PPP levels only when depletion was performed in the presence of drug (Figure 1D). The mean fluorescence of the platelet donor PPP is shown for comparison (Figure1D). These results, taken together with the platelet absorption studies, indicate that the DDAB ELISA and FACS assay detect the same antibody population in the chimpanzee PPP. Moreover, all antibodies detected by the ELISA method bind to platelets and all platelet-bound antibodies bind to purified GP IIb/IIIa.
To further test the utility of the DDAB ELISA to detect pathophysiologically relevant DDABs, the reactivity of chimpanzee A264 PPP with different GP IIb/IIIa antagonists was analyzed (Table1). Although the ELISA assay detected DDABs using 2 compounds that elicited a thrombocytopenic response (L-739758 and L-738167), no antibodies were detectable using L-734217, a chemically distinct GP IIb/IIIa antagonist that did not cause thrombocytopenia in A264.21 In addition, no reactivity was observed with XP280, the active form of the prodrug roxifiban or a number of GP IIb/IIIa antagonists previously in development (Table 1). It should be noted that experiments were performed with saturating concentrations of GP IIb/IIIa antagonists, and in the case of XP280 where a tritiated analog was available, a maximum occupancy of available binding sites could be demonstrated on completion of the ELISA (not shown).
Reactivity of thrombocytopenia PPPs with chemically distinct IIb/IIIa antagonists
| . | A264 . | 307 . | 330 . |
|---|---|---|---|
| 739758 | 100 | 8 | 36 |
| 734217 | 0 | 5 | 0 |
| 738167 | 60 | 0 | 0 |
| Roxifiban* | 0 | 100 | 100 |
| Sibrafiban* | 0 | 0 | 0 |
| Orbofiban* | 0 | 70 | 6 |
| . | A264 . | 307 . | 330 . |
|---|---|---|---|
| 739758 | 100 | 8 | 36 |
| 734217 | 0 | 5 | 0 |
| 738167 | 60 | 0 | 0 |
| Roxifiban* | 0 | 100 | 100 |
| Sibrafiban* | 0 | 0 | 0 |
| Orbofiban* | 0 | 70 | 6 |
The reactivity of thrombocytopenia PPPs with chemically distinct GP IIb/IIIa antagonists was tested in the improved DDAB ELISA version. The reactivity (delta) toward the antagonist that was dosed during the thrombocytopenia episode is set as 100%. The reactivity with the other antagonists is expressed as percentage of the maximal response (delta) observed with the GP IIb/IIIa antagonist dosed during thrombocytopenia (indicated by boldface type).
Name designates prodrugs; in vitro experiments were performed with the active forms.
Frequency of pre-existing DDABs in the general population
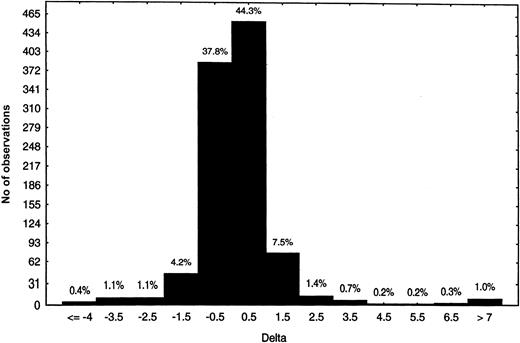
Chimpanzee A264 developed immune-mediated thrombocytopenia within hours of dosing and predose PPP was already positive for DDABs.21 We hypothesized that pre-existing DDAB could be prospectively used to identify patients at risk for thrombocytopenia. To begin to test this hypothesis, the frequency of DDABs present in citrated PPP from nondosed human volunteers (Figure2) and chimpanzees was evaluated. Plasma samples from 1000 blood donors were analyzed in the DDAB ELISA using roxifiban. Overall, the majority of the PPPs were negative for pre-existing DDABs. The deltas were centered on 0, and only 1% of the population presented deltas of more than 7 (Figure 2). In addition, 41 different chimpanzee PPPs were analyzed with the initial version of the DDAB ELISA in the presence of L-739758. Two of 41 chimpanzees had a delta or ratio above the 95th percentile. Taken together, these observations point to the specificity of the DDAB ELISA to identify patients with pre-existing DDAB prior to dosing with GP IIb/IIIa antagonists.
Prevalence of pre-existing DDABs to XP280 in the general population.
Citrate PPP (n = 1000) was analyzed with the initial ELISA format using XP280, the active form of roxifiban. The number of observations is indicated on the y-axis, whereas the delta range is indicated on the x-axis. The percentage of total is indicated above the bars.
Prevalence of pre-existing DDABs to XP280 in the general population.
Citrate PPP (n = 1000) was analyzed with the initial ELISA format using XP280, the active form of roxifiban. The number of observations is indicated on the y-axis, whereas the delta range is indicated on the x-axis. The percentage of total is indicated above the bars.
Utility of the DDAB ELISA to identify the mechanism of human GP IIb/IIIa antagonist-induced thrombocytopenia
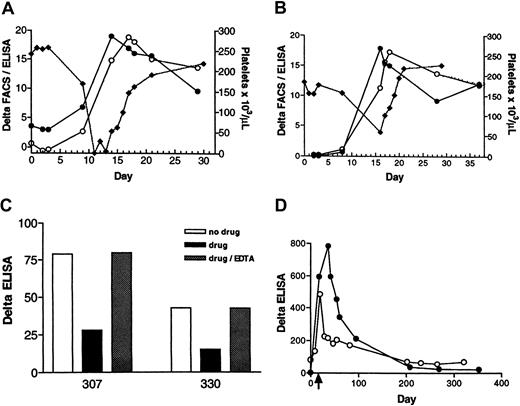
Two patients, designated 307 and 330, developed thrombocytopenia in a phase IIB study of roxifiban. The details of the clinical manifestation of the thrombocytopenia are summarized here. In patient 307 (Figure 3A; closed diamonds), the platelet count was unchanged from baseline, at day 3 (257 000/μL) and fell to 168 000 on day 9. Platelets dropped precipitously on day 11 to undetectable levels. Drug treatment was stopped. The patient received platelet transfusion on days 11 and 13. The platelet count normalized by day 21. In patient 330 (Figure 3B; closed diamonds), the platelet number was constant through day 8 (157 000/μL), but was 59 000 on day 16. Drug treatment was terminated. The platelet count increased to 101 000/μL on day 17. Normal levels (194 000/μL) were observed on day 20.
DDAB development in patients 307 and 330.
Patient 307 (A) and patient 330 (B). The time course of DDAB development was monitored by the initial version of the DDAB ELISA (closed circles) and by FACS (open circles) versus decrease in platelet count (closed diamonds) during treatment with roxifiban. Antibody binding, denoted as delta, is measured by the difference between IgG bound to purified IIb/IIIa (ELISA) or platelets (FACS) in the presence of XP280 to that in the absence. Note that the increase in DDABs coincides with the decrease in platelet number. (C) Gel-purified platelets were incubated with 307 (left bars) or 330 (right bars) PPPs in the absence (no drug; open bars) or presence of XP280 (drug; filled bars). Dotted bars indicate that samples were incubated with XP280, but EDTA (9 mM) was added after the initial platelet-binding step. Platelets were removed by centrifugation and the resulting PPP tested in the DDAB ELISA. To prevent drug-dependent antibody binding of IgG to the no-drug wells, these wells were incubated with a GP IIb/IIIa antagonist that competes with XP280 for GP IIb/IIIa binding but fails to elicit binding of 307 or 330 DDABs. (D) The time courses of the DDAB titer rise and decline were analyzed in patients 307 (open circles) and 330 (closed circles) using the improved ELISA version. The PPP samples were diluted to be in the linear, dynamic range of the assay. The time of thrombocytopenia is indicated by an arrow.
DDAB development in patients 307 and 330.
Patient 307 (A) and patient 330 (B). The time course of DDAB development was monitored by the initial version of the DDAB ELISA (closed circles) and by FACS (open circles) versus decrease in platelet count (closed diamonds) during treatment with roxifiban. Antibody binding, denoted as delta, is measured by the difference between IgG bound to purified IIb/IIIa (ELISA) or platelets (FACS) in the presence of XP280 to that in the absence. Note that the increase in DDABs coincides with the decrease in platelet number. (C) Gel-purified platelets were incubated with 307 (left bars) or 330 (right bars) PPPs in the absence (no drug; open bars) or presence of XP280 (drug; filled bars). Dotted bars indicate that samples were incubated with XP280, but EDTA (9 mM) was added after the initial platelet-binding step. Platelets were removed by centrifugation and the resulting PPP tested in the DDAB ELISA. To prevent drug-dependent antibody binding of IgG to the no-drug wells, these wells were incubated with a GP IIb/IIIa antagonist that competes with XP280 for GP IIb/IIIa binding but fails to elicit binding of 307 or 330 DDABs. (D) The time courses of the DDAB titer rise and decline were analyzed in patients 307 (open circles) and 330 (closed circles) using the improved ELISA version. The PPP samples were diluted to be in the linear, dynamic range of the assay. The time of thrombocytopenia is indicated by an arrow.
Plasma samples obtained from both patients were analyzed retrospectively for the presence of GP IIb/IIIa antagonist-dependent DDABs. For patient 307, the ELISA signal was positive prior to dosing (Figure 3A; closed circles). This result was confirmed with the more sensitive, improved version of the DDAB ELISA (Figure 3D; open circles). The DDAB titer increased during dosing, and this rise was temporally correlated with a fall in platelet count. Analysis of the PPP by FACS revealed a similar profile of DDAB development (Figure 3A; open circles). Moreover, platelet absorption studies revealed that the ELISA+ DDABs bound to gel-purified platelets in the presence, but not absence, of XP280 (Figure 3C; left panels). Moreover, antibodies could be dissociated under conditions known to disrupt the GP IIb/IIIa complex (EDTA treatment at 37°C for 1 hour; Figure 3C).
For patient 330, the DDAB titer was below quantifiable levels prior to dosing (Figure 3B; closed circles), and the absence of detectable predose titer was confirmed using the more sensitive version of the DDAB ELISA (Figure 3D; closed circles). Similar to patient 307, an increase in titer was detected by ELISA and confirmed by FACS (Figure3B; open circles). This increase correlated with a decrease in platelet count (Figure 3B; closed diamonds). Again, platelet absorption studies revealed that the ELISA+ DDABs bound to gel-purified platelets (Figure 3C; right panels). The extent of depletion was concentration dependent with respect to the platelet number (not shown). Moreover, platelet-bound DDABs could be dissociated by EDTA treatment (Figure 3C). In addition, identity of the ELISA+antibodies with the FACS signal was established using methods described for the chimpanzee antibody by absorption of 330 DDABs to ELISA plates, followed by FACS analysis (not shown). Taken together, these observations strongly suggest that the DDAB ELISA is detecting physiologically relevant antibodies.
If the delayed-onset thrombocytopenia is immune mediated, then from a mechanistic perspective, a rise in DDABs could precede a fall in platelet count (see “Discussion”). For patient 330, a small but reproducible increase in DDABs was observed on day 8, 8 days before thrombocytopenia occurred (day 16; Figure 3B). For patient 307, even though a rise in DDAB was evident coinciding with the fall in platelet counts, samples at the critical time (4-9 days) were not available (Figure 3A). Taken together, these results provide strong circumstantial evidence that the thrombocytopenia observed with these patients was immune mediated.
Both patients have participated in follow-up studies by providing blood samples on a regular basis. The time course of the DDAB decline is indicated in Figure 3D. A time-dependent decrease in DDAB titer was evident over a period of 12 months. Patient 330 (closed circles) had a higher titer at the time of thrombocytopenia than patient 307 (open circles). An initial fast decline was observed. The titer stabilized around zero about a year after thrombocytopenia and thus was not different from predose values. The titer of patient 307 decreased with similar kinetics. It should be noted that a positive titer, similar to the predose signal, was still evident 12 months after thrombocytopenia.
To further analyze the specificity of the immune response in both patient plasmas, the reactivity with a panel of GP IIb/IIIa antagonists was tested (Table 1). Experiments were performed under saturating concentrations of GP IIb/IIIa antagonists. The DDAB ELISA detected strong reactivity with roxifiban, whereas a graded response was observed with antagonists of different chemical classes. This observation supports the exquisite drug specificity of the immune response.
The improvement in the sensitivity of the DDAB ELISA enabled the detection of the immunoglobulin classes of the DDABs. The sensitivity of the IgG class-specific antibodies was lower than the pan IgG antiserum (data not shown). The antibodies used to determine Ig classes had comparable sensitivity when tested using known amounts of immobilized Ig standards (not shown). Samples were tested with antibodies to human IgG 1 to 4, IgM, IgA, and IgE. In addition, the IgG light chain class was determined. Peak titer PPP from patient 307 was reactive only with antibodies directed to IgG 1, κ light chain, whereas patient 330 was IgG 1, λ light chain. Chimpanzee A264 reacted only with IgG1 and κ light chain antibodies. Thus, within the detection limit of the Ig class-specific antibodies, only a single antibody class was detectable.
For the DDAB ELISA to have utility to identify the risk of thrombocytopenia based on an increase in DDAB titer during dosing, the specificity of this increase should be high. The change of DDAB titer during dosing was monitored in 108 nonthrombocytopenic patients from the same phase IIB study in which patients 307 and 330 were participating (Figure 4). None of the nonthrombocytopenic patients presented with an increase in antibody titer during dosing with roxifiban, indicating a high specificity of the DDAB ELISA. However, one patient had a high (> 150 mOD/min) initial titer that did not increase during dosing. In contrast to the thrombocytopenic patients, no increase in DDAB titer was observed during dosing. A number of reasons may explain why this patient did not develop thromboctyopenia, including lower levels of antibodies compared to the thrombocytopenic patients, affinity of the ELISA detectable antibodies for platelets, and capacity of the bone marrow to compensate for potential increased platelet turnover. Insufficient PPP was available to further characterize this antibody. Taken together, the results suggest that an increase in DDAB titer may serve as a prognostic marker to identify patients at risk for thrombocytopenia during treatment with GP IIb/IIIa antagonists.
Predictive value of an increase in DDAB titer for thrombocytopenia.
The 108 PPPs from patients of a phase IIB study of roxifiban who did not develop thrombocytopenia were analyzed by the DDAB ELISA at multiple time points prior to and during dosing. The first day of dosing is indicated (day 0). Note that the DDAB titers are relatively constant during the 30 days of dosing.
Predictive value of an increase in DDAB titer for thrombocytopenia.
The 108 PPPs from patients of a phase IIB study of roxifiban who did not develop thrombocytopenia were analyzed by the DDAB ELISA at multiple time points prior to and during dosing. The first day of dosing is indicated (day 0). Note that the DDAB titers are relatively constant during the 30 days of dosing.
Evidence that DDABs detect neoepitopes in GP IIb/IIIa
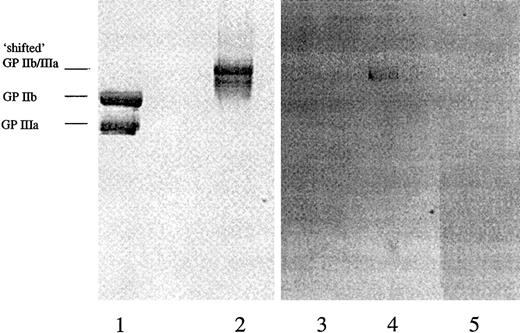
To investigate the hypothesis that the DDABs in thrombocytopenia PPPs detect XP280-induced neoepitope in GP IIb/IIIa rather than an epitope that includes drug per se bound to GP IIb/IIIa, the following experiments were conducted. The drug concentration used in the DDAB ELISA was varied over a large concentration range from 5 nM to 10 μM. The prediction is that if the drug is part of the epitope, the signal may be reduced at higher XP280 concentrations. This was not observed experimentally (data not shown). In addition, purified GP IIb/IIIa was preincubated with XP280 and fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), at room temperature without boiling the samples, and followed by protein staining (Figure 5). Preincubation of GP IIb/IIIa with XP280 changed the electrophoretic mobility of the receptor (Figure 5). More specifically, while under these SDS-PAGE conditions in the absence of drug, the 2 subunits of the receptor were separated (Figure 5, lane 1), XP280 preincubation resulted in a mobility shift of the protein, indicating that the 2 subunits remained associated (Figure 5, lane 2). Immunoblotting analysis using patient 307 high-titer PPP showed that the DDABs bound to the conformational altered form of the receptor (Figure 5, lane 4), but not to the unshifted GP IIb/IIIa (Figure 5, lane 3). Similar results were obtained with patient 330 PPP (not shown). Very-low-titer predrug PPP samples showed no detectable staining (Figure 5, lane 5). Parallel experiments revealed that radiolabeled XP280 failed to remain associated with the conformationally altered form of GP IIb/IIIa after SDS-PAGE (not shown), suggesting that the drug-induced neoepitope and not the drug itself was the recognition site for DDAB binding. In addition, incubation of the membrane with XP280 failed to restore DDAB binding to the nonshifted GP IIb/IIIa species (not shown).
DDABs detect neoepitopes in conformational altered GP IIb/IIIa.
Purified GP IIb/IIIa was fractionated by SDS-PAGE without (lanes 1 and 3) or after preincubation with XP280 (lanes 2, 4, and 5). Lanes 1 and 2 were analyzed by protein stain, lanes 3 and 4 by immunoblotting using patient 307 peak titer PPP, and lane 5 by immunoblotting using patient 307 predose PPP. The mobilities of the “shifted” GP IIb/IIIa and the GP IIb and IIIa subunits are indicated.
DDABs detect neoepitopes in conformational altered GP IIb/IIIa.
Purified GP IIb/IIIa was fractionated by SDS-PAGE without (lanes 1 and 3) or after preincubation with XP280 (lanes 2, 4, and 5). Lanes 1 and 2 were analyzed by protein stain, lanes 3 and 4 by immunoblotting using patient 307 peak titer PPP, and lane 5 by immunoblotting using patient 307 predose PPP. The mobilities of the “shifted” GP IIb/IIIa and the GP IIb and IIIa subunits are indicated.
Discussion
The results presented here are consistent with the notion that thrombocytopenia in humans dosed with the small molecule GP IIb/IIIa antagonist roxifiban is due to immune-mediated platelet destruction or clearance or both. The antigen recognized by the roxifiban-dependent DDABs appears to be a conformationally altered form of GP IIb/IIIa rather than a drug-GP IIb/IIIa complex. Fibrinogen binding to GP IIb/IIIa induces conformational changes, collectively termed ligand-induced binding sites.15-19 Besides fibrinogen, small molecule GP IIb/IIIa antagonists, including RGD peptides, induce similar conformational changes. The observation that the DDABs analyzed here detect GP IIb/IIIa-antagonist complexes over a wide agonist concentration range suggests that the drug may not be part of the epitope. Furthermore, GP IIb/IIIa antagonists induce SDS-stable complexes of IIb and IIIa. Although the drug-dependent antibodies present in patient 330 failed to detect individual subunits of GP IIb/IIIa by immunoblotting after SDS-PAGE, strong reactivity with the SDS-stable complexes was observed. The data are consistent with the idea that roxifiban-induced conformational changes in GP IIb/IIIa comprise the epitope for DDABs.
Based on the PPPs obtained from the 2 human thrombocytopenia donors and the chimpanzee thrombocytopenia PPP, we developed an ELISA for the rapid detection of small molecule GP IIb/IIIa antagonist-dependent DDABs. The ELISA signal correlates extremely well with the FACS assay during the temporal conversion to a highly positive DDAB status. The antibodies detected by ELISA bind to platelets and can be specifically eluted with calcium chelators known to disrupt the structure of GP IIb/IIIa. Moreover, reciprocal absorption experiments revealed that the FACS signal is depleted by immobilized GP IIb/IIIa and the ELISA signal is depleted by incubation with platelets. In addition, platelet-depleted thrombocytopenia PPP is determined to be negative by repeated FACS analysis using fresh platelets. Thus, ELISA and FACS are detecting the same antibody population. The improved ELISA is clearly more sensitive than FACS and may allow the detection of low titer antibodies before development of thrombocytopenia.
These considerations point to the potential utility of the ELISA test in the management of patients dosed with small-molecule GP IIb/IIIa antagonists. Based on the limited database provided here, we propose to provisionally categorize GP IIb/IIIa antagonist-induced thrombocytopenia into 3 classes. The first class appears to be represented by chimpanzee A264. The chimpanzee had a high titer prior to first dosing with GP IIb/IIIa antagonist.21 Consistent with the expected antibody dose dependency of immune-mediated platelet destruction, a decrease in platelet counts was observed within hours after dosing.21 Translated to patient management, a prescreen test could identify the increased risk for acute immune-mediated thrombocytopenia. The second class appears to be represented by patient 307. This patient presented with low but detectable titer. Antibodies increased on dosing, coinciding with the thrombocytopenia. A “307”-like patient could also be identified and excluded by a prescreen. The third class appears to be presented by patient 330. This patient had no pre-existing titer prior to dosing, even with the most improved version of the ELISA. However, an increase in titer was detectable several days prior to the thrombocytopenia. A second test performed during the first 10 days of treatment with GP IIb/IIIa antagonist could have detected “330”-like patients. The drug therapy could have been terminated, possibly preventing thrombocytopenia. Further experience with GP IIb/IIIa antagonist-mediated thrombocytopenia and antibody testing will tell whether a DDAB ELISA holds promise of reducing the incidence of thrombocytopenia in roxifiban-treated patients. Clinical studies to test the utility of the DDAB ELISA are under way.
Submitted July 30, 2001; accepted January 15, 2002.
From the Brystol-Myers Squibb Company, Wilmington, DE.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dietmar Seiffert, Department of Chemical Enzymology, Bristol- Myers Squibb Pharma Company, Experimental Station, E400/3255, PO Box 80400, Wilmington, DE 19880-0400; e-mail:dietmar.seiffert@BMS.com.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal