Blast crisis is the most advanced stage of chronic myelogenous leukemia (CML) and is highly refractory to therapy. CML is caused by expression of the chimeric BCR-ABL tyrosine kinase oncogene, the product of the t(9;22) Philadelphia translocation. Imatinib (Glivec, formerly STI571) is a rationally developed, orally administered inhibitor of the Bcr-Abl tyrosine kinase. A total of 260 patients with CML were enrolled in a phase II trial, of whom 229 had a confirmed diagnosis of CML in blast crisis. Patients were treated with imatinib in daily oral doses of 400 mg or 600 mg. Imatinib induced hematologic responses in 52% of patients and sustained hematologic responses lasting at least 4 weeks in 31% of patients, including complete hematologic responses in 8%. For patients with a sustained response, the estimated median response duration was 10 months. Imatinib induced major cytogenetic responses in 16% of patients, with 7% of the responses being complete. Median survival time was 6.9 months. Nonhematologic adverse reactions were frequent but generally mild or moderate. Episodes of severe cytopenia were also frequent and were attributable to the underlying condition and treatment with imatinib. Drug-related adverse events led to discontinuation of therapy in 5% of patients, most often because of cytopenia, skin disorders, or gastrointestinal reactions. These results demonstrate that imatinib has substantial activity and a favorable safety profile when used as a single agent in patients with CML in blast crisis. Additional clinical studies are warranted to explore the efficacy and feasibility of imatinib used in combination with other antileukemic drugs.
Introduction
Blast crisis is the terminal phase of chronic myelogenous leukemia (CML), a clonal neoplastic disorder of hematopoietic stem cells.1,2 Blast crisis is usually defined as the presence of 30% or more blasts in peripheral blood or bone marrow.3-5 Clinically, blast crisis is characterized by such signs and symptoms as fever, sweats, pain, weight loss, cytopenia, hepatosplenomegaly, enlarged lymph nodes, and extramedullary disease (chloromas). Blast crisis is also marked by karyotypic evolution, or the accumulation of multiple characteristic genetic abnormalities.1 2
In nearly all patients, blast crisis is preceded by an initial period of chronic-phase CML, typically 3 to 7 years in duration, in which malignant progenitor cells proliferate rapidly but retain much of their ability to differentiate.1,2 The appearance of numerous blasts during CML progression is due to the gradual loss of the differentiation potential of malignant cells coincident with their karyotypic evolution. The transition between the chronic and blastic phases of CML is frequently gradual and apparent as an accelerated-disease phase that precedes blast crisis by 2 to 15 months.6-8 Blast crisis is usually fatal within 3 to 6 months of onset in patients with the myeloid phenotype.9 10
The causative event in the initiation of CML is a genetic translocation resulting in the fusion of genetic sequences to form theBCR-ABL oncogene, which codes for a constitutively active Bcr-Abl tyrosine kinase that mediates cellular transformation.1,11 In more than 90% of patients, theBCR-ABL fusion gene is associated with a t(9;22)(q34;q11) reciprocal translocation (Philadelphia [Ph] translocation), which is the most characteristic feature of CML.12 Expression of the BCR-ABL gene is sufficient to cause chronic-phase CML, whereas progression of disease to blast crisis is thought to depend on the development of additional genetic changes leading to loss of differentiation and an increasingly aggressive clinical presentation.1 13-16
There is no single standard therapy for patients with CML at this advanced stage. Treatment usually comprises combination chemotherapy regimens developed for acute leukemias, with the most common therapy using an anthracycline with cytarabine. There is no consensus on how a hematologic response should be defined in these patients. Whereas the criteria for a complete hematologic response (CHR) are similar among studies, the criteria for incomplete response vary greatly. With the specific nature of the blast crisis, which arises as a terminal event of a years-long myeloproliferative disorder, taken into account, these incomplete responses have been described as either “partial response,” “hematologic improvement,” “minor or minimal response,” or “return to chronic phase.” Against this background of limitations regarding criteria, reported hematologic response rates in patients with myeloid blast crisis range from 9% to 65%, but complete responses occur in only 10% to 20% of patients, and median survival time is 3 to 6 months.9,10,17-24Allogeneic stem cell transplantation induces durable remission in fewer than 10% of patients. However, pretransplantation therapy leading to a return from blast crisis to chronic phase is associated with a greatly improved transplantation outcome.25-29
Imatinib (imatinib mesylate; formerly STI571; Glivec in Europe; Gleevec in the United States; Novartis Pharmaceuticals, Basel, Switzerland) is a rationally designed, potent competitive inhibitor of the Bcr-Abl protein tyrosine kinase. In preclinical studies, imatinib showed specific antileukemic activity both in vitro and in vivo againstBCR-ABL–positive cells, including eradication of leukemias induced by injection of cell lines derived from patients with blast-crisis CML.30-34 In an ascending-dose clinical phase I study, imatinib induced substantial and durable responses, with minimal toxicity, when used in daily doses of 300 mg or higher in nearly all patients with chronic-phase CML.35 Imatinib used in daily doses of 300 to 1000 mg also induced hematologic responses, including 4 CHRs (11% of patients), in 21 of 38 patients (55%) with CML in myeloid blast crisis.36 No dose-limiting toxicity was observed in these studies. These phase I results indicated that selective inhibition of Bcr-Abl tyrosine kinase can arrest the progression of CML, even in patients with blast crisis, with minimal toxicity. Accordingly, we conducted a phase II trial to confirm the activity and safety of imatinib in a larger population of patients with blast crisis and to characterize prognostic factors associated with a favorable outcome.
Patients and methods
Patients
Male or female patients were eligible for inclusion in this study if they were at least 18 years of age and had Ph chromosome–positive (Ph-positive) CML in myeloid blast crisis. The study was designed mainly to evaluate treatment in patients with newly diagnosed blast crisis, defined as patients who had not received specific therapy for advanced CML (either in blast crisis or in accelerated phase) except for interferon α (IFN-α) and palliative therapy with hydroxyurea or low-dose cytosine arabinoside (ara-C; < 30 mg/m2 of body-surface area every 12 to 24 hours administered daily). However, to allow a preliminary investigation of imatinib in a heavily pretreated population, enrollment was also open to patients who had previously received therapy for advanced CML.
CML in blast crisis was defined as at least 30% blasts in peripheral blood or marrow or the presence of extramedullary disease (other than liver or spleen enlargement). Presence of the myeloid phenotype was to be confirmed by flow cytometry and required myeloperoxidase positivity, presence of standard myeloid markers, and not more than one lymphoid marker. This definition of CML blast crisis is more strict than the recently proposed World Health Organization criterion of at least 20% blasts in peripheral blood or marrow.37
Patients were required to be free of marked liver or kidney disease as indicated by levels of serum transaminases (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) not higher than 3 times the upper-normal limit if liver involvement with leukemia was not suspected or not higher than 5 times the upper-normal limit if liver involvement was suspected, serum total bilirubin levels not higher than 3 times the upper-normal limit, and serum creatinine levels not higher than twice the upper-normal limit. Women of childbearing potential were required to have a negative pregnancy test before starting treatment, and both male and female patients were required to use barrier contraceptive measures throughout therapy with imatinib. Patients were excluded from the trial if they had an Eastern Cooperative Oncology Group performance status of 3 or higher, grade 3 or 4 cardiac disease, or any serious concomitant medical condition. Patients were to have ceased any prior treatment for CML within a minimum period established according to the nature of the treatment. For hydroxyurea treatment, this period was 24 hours; for IFN-α, 48 hours; for ara-C, 7 days if low dose (< 30 mg/m2 every 12 to 24 hours administered daily), 14 days if medium dose (100-200 mg/m2 for 5 to 7 days), and 28 days if high dose (1-3 g/m2 every 12 to 24 hours administered for 6 to 12 doses); for homoharringtonine, 14 days; for anthracyclines, mitoxantrone, or etoposide, 21 days; for any other investigational agent, 28 days; and for busulfan or any hematopoietic stem cell transplantation, 6 weeks. Patients were also excluded from enrollment if they had a history of noncompliance with therapy or if they were considered by the investigator to be potentially unreliable with respect to compliance.
All patients gave written informed consent to participate in the study before entry, and the study was reviewed and approved by a recognized ethics review committee at each trial center. The study was performed in accordance with the Declaration of Helsinki (as amended in Tokyo, Venice, and Hong Kong).
Study design and treatment
This study was an open-label, nonrandomized, multicenter, phase II trial designed to evaluate the clinical efficacy of imatinib, as determined by the rate of hematologic response lasting at least 4 weeks, and the safety of treatment.
Initially, enrolled patients received treatment with orally administered imatinib in daily doses of 400 mg. When phase I dose-escalation data demonstrating the safety of prolonged treatment with higher doses became available, the initial daily dose was increased by protocol amendment to 600 mg. For patients who relapsed, dose escalation (initially to 600 mg daily and increased by protocol amendment to 400 mg twice daily) was permitted at the discretion of the investigator. Dose escalation was also permitted for patients who did not achieve a hematologic response after at least 1 month of therapy, on a case-by-case basis following discussion between the investigator and sponsor. Patients received treatment for 24 weeks, with subsequent indefinite prolongation in cases in which the investigator judged that further treatment was of clinical benefit.
Treatment was interrupted or reduced in response to nonhematologic, hepatic, or hematologic toxicity, graded according to National Cancer Institute (NCI)–National Institutes of Health common toxicity criteria (CTC). For patients requiring dose reduction, daily doses were reduced from 800 mg (400 mg twice daily) to 600 mg, from 600 to 400 mg, or from 400 to 300 mg. Further dose reductions were permitted by the protocol, but in practice, therapy was interrupted rather than reduced to doses below 300 mg daily. If CTC grade 2 nonhematologic toxicity occurred, therapy was interrupted until recovery to grade 1 or lower and then resumed at the original dose. If grade 2 toxicity recurred following treatment resumption, treatment was again interrupted until recovery and then resumed at a reduced dose. If grade 3 or 4 nonhematologic toxicity occurred, therapy was interrupted until recovery to grade 1 or lower and then resumed at a reduced dose.
Specific dose-reduction rules for hepatic toxicity were applied to patients who enrolled with elevated baseline transaminase levels (3 fold to 5 fold above upper-normal limits). If such patients had increases of more than 3 fold in one or more transaminase levels, therapy was interrupted until levels returned to baseline and then resumed at a reduced dose. For such patients who had clinically relevant but less than 3-fold increases in transaminase levels, treatment was interrupted until recovery and then resumed at the same dose. If patients had a subsequent clinically relevant increase in transaminase levels, treatment was interrupted until recovery and then resumed at a reduced dose.
Dose reductions for hematologic toxicity were considered only for patients with grade 4 neutropenia (neutrophil counts < 0.5 × 109/L) lasting at least 2 weeks and were based on marrow hypocellularity and disease status as determined by bone marrow biopsies done after a minimum of 28 days of therapy. Biopsy specimens were to be obtained at 2-week intervals until recovery from grade 4 neutropenia, but in practice, they were obtained less frequently. For patients with persistent marrow cellularity values below 10% and blast values below 10%, the daily dose was reduced successively at 2-week intervals or therapy was interrupted until recovery of neutropenia to grade 2 or higher (neutrophil counts > 1.0 × 109/L). On recovery, treatment was resumed at the full initial dose. Treatment was not interrupted or reduced for patients with marrow cellularity or blast values above 10%.
Concomitant administration of anticancer drugs or use of procedures was not permitted, except for hydroxyurea, anagrelide, or leukopheresis within the first 28 days of treatment if required to control elevated blast levels or platelet counts. Within the first 28 days of treatment, hydroxyurea could be given at a maximum dose of 5 g daily for up to a total of 7 days. For leukopheresis, a maximum of 2 procedures per week or 4 procedures during the first 28 days was allowed. Treatment with allopurinol (300 mg daily) was suggested until stabilization of white blood cell (WBC) counts. Investigators could prescribe colony-stimulating factors for neutropenic fever.
Evaluation of patients
Patients were evaluated for hematologic and cytogenetic responses and relapse at specified intervals. Peripheral blood samples were obtained and analyzed at baseline, 3 times weekly for the first 4 weeks, weekly between weeks 5 and 13, every 2 weeks after week 13, and on the last day of treatment. Bone marrow aspirations, and in some institutions, bone marrow biopsies were also to be performed at screening; at weeks 5, 9, and 13; and every 3 months thereafter. Bone marrow biopsies or aspirations were also to be done as indicated and to evaluate hematologic toxicity. Extramedullary leukemic involvement was assessed primarily by physical examination at screening, every 4 weeks during therapy, and on the last day of treatment. Patients discontinuing treatment were followed up for survival monthly for the first 3 months after treatment and every 3 months thereafter. Treatment toxicity was evaluated by patient interview at each office visit. Toxicity was graded according to the NCI CTC scale.
The primary efficacy end point in this study was sustained hematologic response lasting at least 4 weeks, assessed by the investigator as (1) CHR, (2) marrow response, or (3) return to chronic phase (RTC). CHR was defined according to conventional criteria as a blast value below 5% in bone marrow, with no circulating peripheral blood blasts; a neutrophil count of at least 1.5 × 109/L and a platelet count of at least 100 × 109/L; and no evidence of extramedullary involvement. In patients not achieving a CHR, marrow response was defined as a blast value below 5% in bone marrow, with no circulating peripheral blood blasts; a neutrophil count of at least 1.0 × 109/L and a platelet count of at least 20 × 109/L (without platelet transfusion and without evidence of bleeding); and no evidence of extramedullary involvement. By exclusion of patients with features of accelerated-phase CML as defined in a parallel phase II study,38 an RTC was defined as below 15% blasts in peripheral blood and bone marrow, with below 30% blasts plus promyelocytes in the peripheral blood and bone marrow; below 20% basophils in peripheral blood; and no extramedullary disease except liver or spleen enlargement. In other studies of CML blast crisis, these incomplete responses were termed partial. Sustained responses were required to be observed at 2 consecutive evaluations done at least 4 weeks apart. According to this definition, “sustained” response is identical to “confirmed” response, a nomenclature that is also used in clinical trials of treatment for leukemia and solid tumors.
Secondary efficacy end points were the induction of cytogenetic response, duration of hematologic response, and overall survival (OS). Cytogenetic response was based on the prevalence of Ph-positive metaphases among at least 20 metaphases investigated in each bone marrow sample and was defined as complete (0% Ph-positive cells), partial (1%-35%), minor (36%-65%), minimal (66%-95%), or none (> 95%). Duration of response was calculated from the first reported date of response to the earliest date of reported relapse or death. Duration of response was censored at the last examination date for patients with an ongoing response or patients who discontinued treatment for reasons other than adverse events, progression, or death. A single determination not fulfilling the criteria for RTC was considered a loss of hematologic response. OS was calculated from the time of the start of treatment with imatinib to the date of death. Survival was censored at the time treatment was discontinued to allow bone marrow transplantation or at the last recorded contact or evaluation for patients alive at time of analysis.
Statistical analysis
This study was designed to demonstrate whether the overall hematologic response rate among patients with no prior treatment for advanced CML was at least 15%. A required sample size of 79 evaluable patients was based on the Fleming single-stage procedure and tested the following: H0: P ≤ 15% and H1:P ≥ 30%, with α = 2.5% (one-sided) and a power of 90%. To allow for premature withdrawals from the study, the planned sample size was 100 patients with CML in blast crisis. The protocol provided for the additional inclusion of 50 patients previously treated for advanced CML (either in blast crisis or accelerated phase); this sample size was based on practical considerations rather than a formal sample-size calculation. Response rates are reported as an intent-to-treat analysis. Patients who withdrew from treatment before a confirmed response was reported were counted as nonresponders. A landmark analysis of survival was performed, including only patients who had an assessment of hematologic response at 2 and 3 months, at which time most of the responders had achieved a sustained response; survival results were then presented according to response status (no response, RTC, CHR, or marrow response) at 2 months. Response duration and survival were computed by using standard Kaplan-Meier methods. Safety results are reported for all enrolled patients who received at least 1 dose of imatinib.
Univariate and multivariate analyses were conducted to test for effects of possible prognostic factors on OS. Prognostic factors and criteria were consistent with those described in earlier clinical studies of other antileukemic agents8-10 to facilitate comparison with the results of those trials. The log rank test was used to identify prognostic factors at a significance level of P less than .2. Factors meeting this criterion were included as terms in a multivariate Cox regression model. Terms with no significant effect at a level of P less than .1 in multivariate analysis were removed, whereas factors remaining in the multivariate model were interpreted as independently predictive of survival outcome.
Results
Patients and treatment
A total of 260 patients were enrolled at 27 centers in France, Germany, Italy, Switzerland, the United Kingdom, and the United States from August 1999 to June 2000, and efficacy and safety data for analysis were collected through the end of July 2001. Patient enrollment was allowed to exceed the original planned accrual when follow-up data from an earlier phase I study became available and provided increasing evidence of the activity and safety of imatinib in patients with CML blast crisis.36 Patients were given a diagnosis of CML in blast crisis during the screening period for patient selection. A central review of data from screening and baseline tests showed that 229 patients (88%) had an ongoing diagnosis of CML in blast crisis at the time imatinib therapy was started, whereas this stage of disease could not be confirmed at the start of treatment in 31 patients (12%). For these 31 patients, disease status at the start of therapy was consistent with accelerated phase (16 patients) or chronic phase (4 patients) or could not be determined from reported data (11 patients).
Of the 260 patients enrolled, 37 (14%) started therapy with imatinib at a daily dose of 400 mg, which was the highest dose adequately tested for safety at the time of their enrollment. The remaining 223 patients (86%) started treatment at a daily dose of 600 mg because phase I data available after the start of this study demonstrated that treatment with this higher dose was feasible and possibly associated with greater activity.36
Table 1 shows a summary of patient characteristics and disease history at baseline for all 260 enrolled patients and for the 229 patients with a confirmed diagnosis overall and according to prior treatment for advanced CML. Patient demographic and disease characteristics were typical for patients with CML in blast crisis. Clonal evolution with consistent chromosomal aberrations in addition to the Ph translocation in at least 2 metaphases was reported in 111 patients with a confirmed diagnosis of blast crisis. Aneuploidy was found in 70 patients, with 28 patients having trisomy 8; 26 patients, a second Ph chromosome; 16, trisomy 19; 10, trisomy 21; and 3, loss of a sex chromosome. A complex Ph translocation with involvement of chromosomes other than 9 or 22 was discovered in 15 cases. In 33 cases, aberrations involving chromosome 17, including iso,17 occurred. Additional translocations were detected in 43 cases. Thirty-two patients had a complex karyotype with at least 3 additional chromosomal aberrations.
Patient and disease characteristics at baseline
| Characteristic . | Patients with a confirmed diagnosis . | All enrolled patients (n = 260) . | ||
|---|---|---|---|---|
| Total (n = 229) . | Previously untreated (n = 148) . | Previously treated (n = 81) . | ||
| Median age, y (range) | 56 (19-81) | 57 (19-81) | 52 (20-73) | 56 (19-81) |
| Sex: M/F | 128/101 | 81/67 | 47/34 | 136/124 |
| ECOG score (no. [%] of patients) | ||||
| 0-1 | 135 (59) | 90 (61) | 45 (56) | 150 (58) |
| 2 | 85 (37) | 52 (35) | 33 (41) | 97 (37) |
| 3 | 2 (1) | 1 (1) | 1 (1) | 4 (2) |
| Median y since initial diagnosis of CML (25th-75th percentile) | 3.4 (1.4-5.8) | 3.1 (1.2-6.0) | 3.5 (1.7-5.7) | 3.4 (1.5-5.8) |
| Median mo since diagnosis of blast crisis (25th-75th percentile) | 0.6 (0.2-2.3) | 0.4 (0.2-0.9) | 2.8 (0.8-5.6) | 0.6 (0.3-2.5) |
| Extramedullary involvement (no. [%] of patients) | 160 (70) | 108 (73) | 52 (64) | 179 (69) |
| Splenomegaly ≥ 10 cm below costal margin | 61 (27) | 42 (28) | 19 (23) | 65 (25) |
| Lymph node | 24 (10) | 20 (14) | 4 (5) | 24 (9) |
| Other (chloroma) | 7 (3) | 4 (3) | 3 (4) | 7 (3) |
| Median hemoglobin, g/L (25th-75th percentile) | 93 (85-104) | 94 (86-107) | 92 (85-99) | 92 (85-103) |
| Median WBC count, × 109/L (25th-75th percentile) | 31 (13-67) | 27 (11-56) | 42 (13-80) | 29 (10-66) |
| Median platelet count, × 109/L (25th-75th percentile) | 66 (29-194) | 80 (30-204) | 63 (28-168) | 75 (29-208) |
| Median blasts in bone marrow, % (25th-75th percentile) | 50 (35-70) | 48 (33-70) | 57 (39-71) | 46 (32-70) |
| Median blasts in peripheral blood, % (25th-75th percentile) | 35 (13-61) | 34 (11-57) | 37 (14-68) | 30 (10-56) |
| Initial dose of imatinib (no. [%] of patients) | ||||
| 400 mg | 32 (14) | 10 (7) | 22 (27) | 37 (14) |
| 600 mg | 197 (86) | 138 (93) | 59 (73) | 223 (86) |
| Characteristic . | Patients with a confirmed diagnosis . | All enrolled patients (n = 260) . | ||
|---|---|---|---|---|
| Total (n = 229) . | Previously untreated (n = 148) . | Previously treated (n = 81) . | ||
| Median age, y (range) | 56 (19-81) | 57 (19-81) | 52 (20-73) | 56 (19-81) |
| Sex: M/F | 128/101 | 81/67 | 47/34 | 136/124 |
| ECOG score (no. [%] of patients) | ||||
| 0-1 | 135 (59) | 90 (61) | 45 (56) | 150 (58) |
| 2 | 85 (37) | 52 (35) | 33 (41) | 97 (37) |
| 3 | 2 (1) | 1 (1) | 1 (1) | 4 (2) |
| Median y since initial diagnosis of CML (25th-75th percentile) | 3.4 (1.4-5.8) | 3.1 (1.2-6.0) | 3.5 (1.7-5.7) | 3.4 (1.5-5.8) |
| Median mo since diagnosis of blast crisis (25th-75th percentile) | 0.6 (0.2-2.3) | 0.4 (0.2-0.9) | 2.8 (0.8-5.6) | 0.6 (0.3-2.5) |
| Extramedullary involvement (no. [%] of patients) | 160 (70) | 108 (73) | 52 (64) | 179 (69) |
| Splenomegaly ≥ 10 cm below costal margin | 61 (27) | 42 (28) | 19 (23) | 65 (25) |
| Lymph node | 24 (10) | 20 (14) | 4 (5) | 24 (9) |
| Other (chloroma) | 7 (3) | 4 (3) | 3 (4) | 7 (3) |
| Median hemoglobin, g/L (25th-75th percentile) | 93 (85-104) | 94 (86-107) | 92 (85-99) | 92 (85-103) |
| Median WBC count, × 109/L (25th-75th percentile) | 31 (13-67) | 27 (11-56) | 42 (13-80) | 29 (10-66) |
| Median platelet count, × 109/L (25th-75th percentile) | 66 (29-194) | 80 (30-204) | 63 (28-168) | 75 (29-208) |
| Median blasts in bone marrow, % (25th-75th percentile) | 50 (35-70) | 48 (33-70) | 57 (39-71) | 46 (32-70) |
| Median blasts in peripheral blood, % (25th-75th percentile) | 35 (13-61) | 34 (11-57) | 37 (14-68) | 30 (10-56) |
| Initial dose of imatinib (no. [%] of patients) | ||||
| 400 mg | 32 (14) | 10 (7) | 22 (27) | 37 (14) |
| 600 mg | 197 (86) | 138 (93) | 59 (73) | 223 (86) |
ECOG indicates Eastern Cooperative Oncology Group; CML, chronic myelogenous leukemia; and WBC, white blood cell.
At the time of data analysis, the median duration of treatment for all enrolled patients in the 400-mg–dose group was 3.7 months (25%-75% quartiles, 1.5-7.6 months), whereas that in the 600-mg–dose group was 4.0 months (25%-75% quartiles, 1.9-9.3 months); 21% of the patients were treated for more than a year. The median actual dose intensities were 400 mg and 600 mg daily, as planned. In about 50% of the patients in each dose group, treatment was reduced or interrupted at least once, but 58% of the patients in the 400-mg–dose group and 40% of the patients who started with the 600-mg dose had their dose escalated to 600 mg and 800 mg, respectively, at least once during the study. Of the 260 patients enrolled, 220 (85%) have withdrawn from treatment. Primary reasons for withdrawal were disease progression or unsatisfactory therapeutic effect (151 patients [58%]), adverse events or laboratory test results (23 [9%]), death during therapy (24 [9%]), bone marrow transplantation (14 [5%]), protocol violation (3 [1%]), and withdrawal of consent (5 [2%]).
Efficacy
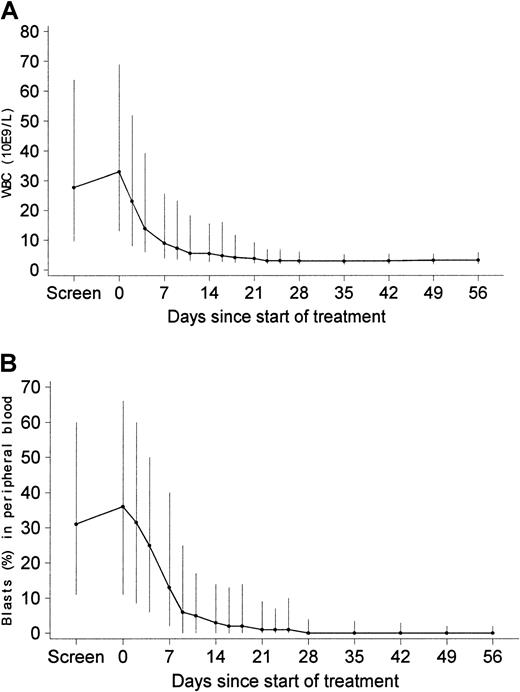
Efficacy analyses included the 229 patients with a confirmed diagnosis of myeloid blast crisis. Among these 229 patients, blast crisis was newly diagnosed in 148 patients (65%), whereas 81 patients (35%) had received previous therapy for advanced CML (other than IFN-α, hydroxyurea, or palliative ara-C). Data shown in Figure1 indicate that treatment with imatinib led to a rapid decrease in leukocyte counts (panel A) and blast levels in peripheral blood (panel B) and that this pharmacodynamic effect was maintained with prolonged treatment in patients remaining in the study. After 1 month, more than 80% of patients with available values had a peripheral blood blast level below 15%.
Leukocyte counts (109/L) and peripheral blood blast levels (%) during the first 8 weeks of treatment with imatinib in patients with a confirmed diagnosis and available values for WBC (blasts).
Values were 213 (199) on day 7, 207 (194) on day 14, 196 (181) on day 28, 173 (156) on day 42, and 160 (147) on day 56. Values are median values, with vertical lines extending to 25th and 75th percentiles.
Leukocyte counts (109/L) and peripheral blood blast levels (%) during the first 8 weeks of treatment with imatinib in patients with a confirmed diagnosis and available values for WBC (blasts).
Values were 213 (199) on day 7, 207 (194) on day 14, 196 (181) on day 28, 173 (156) on day 42, and 160 (147) on day 56. Values are median values, with vertical lines extending to 25th and 75th percentiles.
Table 2 shows a summary of hematologic response rates for all 229 patients with a confirmed diagnosis and for patients according to their prior treatment. Values represent the best response observed at any time during therapy. Of the 229 patients, 119 (52%) had reductions in blast values in peripheral blood and bone marrow features corresponding to a hematologic response on at least one occasion. Thirty-five patients (15%) had a CHR, 55 (24%) had a CHR or marrow response, and 64 (28%) met the criteria for an RTC. Sustained hematologic responses lasting at least 4 weeks were reported for 31% of patients, including 8% of patients with a CHR or 12% with either a CHR or a marrow response and 18% with an RTC. Responses usually occurred soon after the start of treatment: of the 70 patients with a sustained hematologic response, 45 (64%) achieved their first response within 1 month after starting imatinib therapy, corresponding to the first scheduled evaluation of response, and an additional 15 (21%) had a response within 2 months. In 3 patients, a hematologic response was achieved only after dose escalation (from 400 to 600 mg in 1 patient and from 600 to 800 mg in 2). In a multivariate analysis, 4 factors were independently predictive of a higher likelihood of sustained hematologic response: initial dose of imatinib (34% with a dose of 600 mg and 9% with 400 mg), hemoglobin value of at least 100 g/L, platelet count of at least 100 × 109/L, and blood blast level below 50%.
Hematologic response according to duration and previous treatment
| Hematologic response . | All responses (total n = 229) . | Patients with a sustained response (≥ 4 wk) . | ||
|---|---|---|---|---|
| Total (n = 229) . | Previously untreated (n = 148) . | Previously treated (n = 81) . | ||
| Overall* | 119 (52.0) | 70 (30.6) | 53 (35.8) | 17 (21.0) |
| 95% CI (%) | 45.3-58.6 | 24.7-37.0 | 28.1-44.1 | 12.7-31.5 |
| Complete | 35 (15.3) | 18 (7.9) | 13 (8.8) | 5 (6.2) |
| Marrow response | 20 (8.7) | 10 (4.4) | 8 (5.4) | 2 (2.5) |
| Return to chronic phase | 64 (27.9) | 42 (18.3) | 32 (21.6) | 10 (12.3) |
| No response | 108 (47.2) | 152 (66.4) | 92 (62.2) | 60 (74.0) |
| Not evaluable | 2 (0.9) | 7 (3.0) | 3 (2.0) | 4 (4.9) |
| Hematologic response . | All responses (total n = 229) . | Patients with a sustained response (≥ 4 wk) . | ||
|---|---|---|---|---|
| Total (n = 229) . | Previously untreated (n = 148) . | Previously treated (n = 81) . | ||
| Overall* | 119 (52.0) | 70 (30.6) | 53 (35.8) | 17 (21.0) |
| 95% CI (%) | 45.3-58.6 | 24.7-37.0 | 28.1-44.1 | 12.7-31.5 |
| Complete | 35 (15.3) | 18 (7.9) | 13 (8.8) | 5 (6.2) |
| Marrow response | 20 (8.7) | 10 (4.4) | 8 (5.4) | 2 (2.5) |
| Return to chronic phase | 64 (27.9) | 42 (18.3) | 32 (21.6) | 10 (12.3) |
| No response | 108 (47.2) | 152 (66.4) | 92 (62.2) | 60 (74.0) |
| Not evaluable | 2 (0.9) | 7 (3.0) | 3 (2.0) | 4 (4.9) |
Values are numbers (%) of patients unless otherwise indicated. CI indicates confidence interval.
Overall hematologic response was a complete hematologic response, a marrow response, or a return to chronic phase.
Major cytogenetic responses were reported for 37 patients (16%) and 7% of those responses were complete (Table3). A major, minor, or minimal cytogenetic response was reported in 71 patients (31%). The median time to major cytogenetic response was approximately 3 months, corresponding to the first assessment of response in most patients. The initial dose of imatinib had a strong effect on response: major cytogenetic responses were reported in 18% of patients treated with 600 mg daily and in 6% given 400 mg daily.
Cytogenetic response according to previous treatment
| Cytogenetic response3-150 . | Total (n = 229) . | Patients previously untreated (n = 148) . | Patients previously treated (n = 81) . |
|---|---|---|---|
| Major3-151 | 37 (16.2) | 23 (15.5) | 14 (17.3) |
| 95% CI (%) | 11.6-21.6 | 10.1-22.4 | 9.8-27.3 |
| Complete | 17 (7.4) | 12 (8.1) | 5 (6.2) |
| Partial | 20 (8.7) | 11 (7.4) | 9 (11.1) |
| Minor | 4 (1.7) | 3 (2.0) | 1 (1.2) |
| Minimal | 30 (13.1) | 22 (14.9) | 8 (9.9) |
| No response | 151 (65.9) | 97 (65.5) | 54 (66.7) |
| Not evaluable | 7 (3.1) | 3 (2.0) | 4 (5.0) |
| Cytogenetic response3-150 . | Total (n = 229) . | Patients previously untreated (n = 148) . | Patients previously treated (n = 81) . |
|---|---|---|---|
| Major3-151 | 37 (16.2) | 23 (15.5) | 14 (17.3) |
| 95% CI (%) | 11.6-21.6 | 10.1-22.4 | 9.8-27.3 |
| Complete | 17 (7.4) | 12 (8.1) | 5 (6.2) |
| Partial | 20 (8.7) | 11 (7.4) | 9 (11.1) |
| Minor | 4 (1.7) | 3 (2.0) | 1 (1.2) |
| Minimal | 30 (13.1) | 22 (14.9) | 8 (9.9) |
| No response | 151 (65.9) | 97 (65.5) | 54 (66.7) |
| Not evaluable | 7 (3.1) | 3 (2.0) | 4 (5.0) |
Values are numbers (%) of patients unless otherwise indicated. CI indicates confidence interval.
A cytogenetic response was defined by the prevalence of Philadelphia-chromosome-positive metaphases, as follows: 0%, complete response; 1%-35%, partial response; 36%-65%, minor response; 66%-95%, minimal response; and more than 95%, no response.
A major cytogenetic response was a complete or partial response.
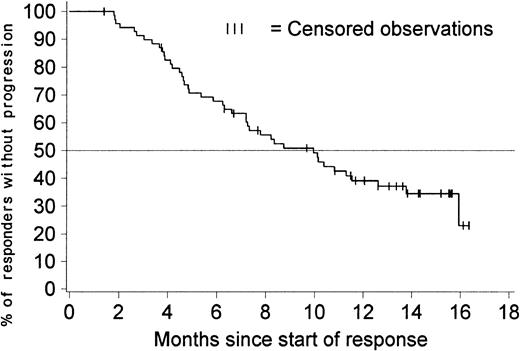
Figure 2 shows the duration of hematologic response for patients with a confirmed diagnosis of blast crisis. Only patients with responses lasting at least 4 weeks were included in this analysis. The estimated median duration of response was 10 months (95% confidence interval [CI], 7.2-12.6 months), with comparable response durations in previously treated and untreated patients. The duration of hematologic response exceeded 6 months in 68% of the 70 patients with a response (95% CI, 57%-79%).
Duration of hematologic response (sustained responses).
The duration of response is censored for 27 of the 70 patients who had a sustained hematologic response; 3 of them discontinued imatinib therapy to undergo stem cell transplantation, 1 withdrew consent to participate after about 1 year, and the remaining 23 patients are still in response between 7 and 16 months after response was first recorded. The estimated percentage of patients without disease progression at 6 months is 68% (95% CI, 57%-79%). The estimated median duration of response is 10 months (95% CI, 7.2-12.6 months).
Duration of hematologic response (sustained responses).
The duration of response is censored for 27 of the 70 patients who had a sustained hematologic response; 3 of them discontinued imatinib therapy to undergo stem cell transplantation, 1 withdrew consent to participate after about 1 year, and the remaining 23 patients are still in response between 7 and 16 months after response was first recorded. The estimated percentage of patients without disease progression at 6 months is 68% (95% CI, 57%-79%). The estimated median duration of response is 10 months (95% CI, 7.2-12.6 months).
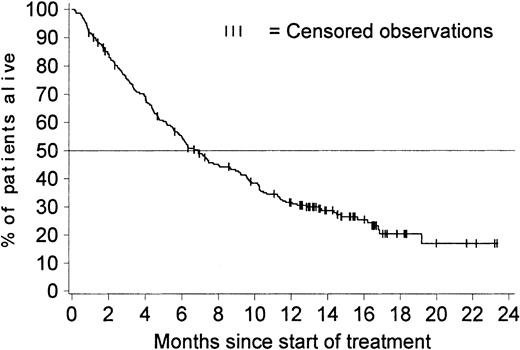
Figure 3 shows OS for all 229 patients with a confirmed diagnosis. The Kaplan-Meier estimated median survival time was 6.9 months (95% CI, 5.7-8.7 months), and the estimated survival rates were 43% at 9 months (95% CI, 36%-49%), 32% at 12 months (95% CI, 25%-38%), and 20% at 18 months (95% CI, 15% to 27%). These estimates remained the same when survival data for the 10 patients who discontinued therapy to undergo bone marrow transplantation were included (4 of these 10 patients were alive at the time of analysis). The estimated median survival time for previously untreated patients was 7.5 months, whereas that for patients who had previously received treatment for advanced CML was 5.6 months.
OS in all 229 patients with a confirmed diagnosis.
Survival was censored for 65 of the 229 patients with a confirmed diagnosis; 10 stopped treatment to undergo stem cell transplantation; 21 patients are still alive after they stopped treatment, and 34 are still receiving treatment at between 12.6 and 23.2 months. The estimated percentage of patients alive at 12 months is 32% (95% CI, 25%-38%). The median survival time is 6.9 months (95% CI, 5.7-8.7 months).
OS in all 229 patients with a confirmed diagnosis.
Survival was censored for 65 of the 229 patients with a confirmed diagnosis; 10 stopped treatment to undergo stem cell transplantation; 21 patients are still alive after they stopped treatment, and 34 are still receiving treatment at between 12.6 and 23.2 months. The estimated percentage of patients alive at 12 months is 32% (95% CI, 25%-38%). The median survival time is 6.9 months (95% CI, 5.7-8.7 months).
Univariate (log rank) analyses and Cox proportional hazards regression analyses were used to test for the effects of several baseline variables on survival. Table 4 shows the prognostic variables included in these analyses, the cut-off values used to define patient subgroups, and the results. Because data for 2 variables (blasts in bone marrow and other cytogenetic abnormalities) were unavailable in a substantial number of patients, separate analyses were performed for these variables. The main analysis excluded these factors but included all patients with a confirmed diagnosis to obtain a final predictive model. To this model, each of the other 2 factors was individually added to explore their additional predictive benefit. There were 200 patients with an assessment of blasts in bone marrow and 178 in the analysis of other chromosomal abnormalities.
Prognostic factors tested for association with survival
| Factor and category . | No. of patients . | No (%) of deaths . | Median survival time (mo) . | P (log rank)4-150 . | P (Wald χ)4-150 . | Relative risk . |
|---|---|---|---|---|---|---|
| Disease group | ||||||
| Untreated | 147 | 102 (69) | 7.5 | .32 | .80 | 0.96 |
| Pretreated | 76 | 57 (75) | 5.6 | — | — | |
| Initial dose of imatinib | ||||||
| 400 mg | 31 | 24 (77) | 4.5 | .40 | — | — |
| 600 mg | 192 | 135 (70) | 7.3 | — | — | |
| Age | ||||||
| < 60 y | 137 | 98 (72) | 6.3 | .72 | — | — |
| ≥ 60 y | 86 | 61 (71) | 7.2 | — | — | |
| Sex | ||||||
| Male | 124 | 94 (76) | 6.2 | .09 | — | — |
| Female | 99 | 65 (66) | 9.2 | — | — | |
| Weight | ||||||
| < 70 kg | 122 | 86 (70) | 6.3 | .72 | — | — |
| ≥ 70 kg | 101 | 73 (72) | 7.2 | — | — | |
| Hepatomegaly | ||||||
| No | 150 | 108 (72) | 6.7 | .80 | — | — |
| Yes | 73 | 51 (70) | 6.9 | — | — | |
| Splenomegaly | ||||||
| No | 89 | 67 (75) | 6.2 | .35 | — | — |
| Yes | 134 | 92 (69) | 7.2 | — | — | |
| Time since blast-crisis diagnosis | ||||||
| < 1 mo | 135 | 91 (67) | 7.5 | .07 | — | — |
| ≥ 1 mo | 88 | 68 (77) | 5.4 | — | — | |
| Hemoglobin | ||||||
| < 100 g/L | 151 | 116 (77) | 5.6 | .004 | — | — |
| ≥ 100 g/L | 72 | 43 (60) | 10.3 | — | — | |
| WBC count | ||||||
| < 50 × 109/L | 148 | 104 (70) | 7.5 | .15 | — | — |
| ≥ 50 × 109/L | 75 | 55 (73) | 6.0 | — | — | |
| Platelet count | ||||||
| < 100 × 109/L | 130 | 110 (85) | 4.5 | < .001 | < .001 | 2.60 |
| ≥ 100 × 109/L | 93 | 49 (53) | 14.5 | — | — | |
| Basophils in PB | ||||||
| < 10% | 195 | 142 (73) | 6.3 | .20 | — | — |
| ≥ 10% | 28 | 17 (61) | 8.7 | — | — | |
| Blasts in PB blood | ||||||
| < 50% | 149 | 93 (62) | 9.6 | < .001 | < .001 | 0.51 |
| ≥ 50% | 74 | 66 (89) | 4.0 | — | — | |
| Blasts in BM4-151 | ||||||
| < 50% | 94 | 54 (57) | 9.6 | < .001 | — | — |
| ≥ 50% | 106 | 89 (84) | 5.2 | — | — | |
| Other chromosomal abnormalities4-151 | ||||||
| No | 67 | 42 (63) | 10.3 | .003 | — | — |
| Yes | 111 | 86 (77) | 5.5 | — | — |
| Factor and category . | No. of patients . | No (%) of deaths . | Median survival time (mo) . | P (log rank)4-150 . | P (Wald χ)4-150 . | Relative risk . |
|---|---|---|---|---|---|---|
| Disease group | ||||||
| Untreated | 147 | 102 (69) | 7.5 | .32 | .80 | 0.96 |
| Pretreated | 76 | 57 (75) | 5.6 | — | — | |
| Initial dose of imatinib | ||||||
| 400 mg | 31 | 24 (77) | 4.5 | .40 | — | — |
| 600 mg | 192 | 135 (70) | 7.3 | — | — | |
| Age | ||||||
| < 60 y | 137 | 98 (72) | 6.3 | .72 | — | — |
| ≥ 60 y | 86 | 61 (71) | 7.2 | — | — | |
| Sex | ||||||
| Male | 124 | 94 (76) | 6.2 | .09 | — | — |
| Female | 99 | 65 (66) | 9.2 | — | — | |
| Weight | ||||||
| < 70 kg | 122 | 86 (70) | 6.3 | .72 | — | — |
| ≥ 70 kg | 101 | 73 (72) | 7.2 | — | — | |
| Hepatomegaly | ||||||
| No | 150 | 108 (72) | 6.7 | .80 | — | — |
| Yes | 73 | 51 (70) | 6.9 | — | — | |
| Splenomegaly | ||||||
| No | 89 | 67 (75) | 6.2 | .35 | — | — |
| Yes | 134 | 92 (69) | 7.2 | — | — | |
| Time since blast-crisis diagnosis | ||||||
| < 1 mo | 135 | 91 (67) | 7.5 | .07 | — | — |
| ≥ 1 mo | 88 | 68 (77) | 5.4 | — | — | |
| Hemoglobin | ||||||
| < 100 g/L | 151 | 116 (77) | 5.6 | .004 | — | — |
| ≥ 100 g/L | 72 | 43 (60) | 10.3 | — | — | |
| WBC count | ||||||
| < 50 × 109/L | 148 | 104 (70) | 7.5 | .15 | — | — |
| ≥ 50 × 109/L | 75 | 55 (73) | 6.0 | — | — | |
| Platelet count | ||||||
| < 100 × 109/L | 130 | 110 (85) | 4.5 | < .001 | < .001 | 2.60 |
| ≥ 100 × 109/L | 93 | 49 (53) | 14.5 | — | — | |
| Basophils in PB | ||||||
| < 10% | 195 | 142 (73) | 6.3 | .20 | — | — |
| ≥ 10% | 28 | 17 (61) | 8.7 | — | — | |
| Blasts in PB blood | ||||||
| < 50% | 149 | 93 (62) | 9.6 | < .001 | < .001 | 0.51 |
| ≥ 50% | 74 | 66 (89) | 4.0 | — | — | |
| Blasts in BM4-151 | ||||||
| < 50% | 94 | 54 (57) | 9.6 | < .001 | — | — |
| ≥ 50% | 106 | 89 (84) | 5.2 | — | — | |
| Other chromosomal abnormalities4-151 | ||||||
| No | 67 | 42 (63) | 10.3 | .003 | — | — |
| Yes | 111 | 86 (77) | 5.5 | — | — |
WBC indicates white blood cell; PB, peripheral blood; and BM, bone marrow.
Results for 223 patients with available data are shown for univariate (log rank) analysis and multivariate (Wald χ) analysis. Factors significant at P < .2 in univariate analysis were included in the multivariate model. Factors in the multivariate model were sequentially removed in order of least significance until the final model included only factors showing an effect withP < .1.
Multivariate analysis was limited to patients with available data for all prognostic factors.
Results of log rank analyses (Table 4) indicated that 5 baseline variables were predictive of longer survival (P < .05). These were a hemoglobin value of at least 100 g/L, a platelet count of at least 100 × 109/L, a blast level below 50% in either peripheral blood or bone marrow, and absence of chromosomal abnormalities suggesting clonal evolution. Additional factors withP less than .2 results in the log rank analysis were included in the initial proportional hazards analysis. In the final regression model, the only 2 factors independently predictive of longer survival were a platelet count of at least 100 × 109/L and a peripheral blood blast level below 50% (Table 4). In exploratory analyses, the use of a different cut-off value for hemoglobin (< 110 g/L) led to inclusion of a high hemoglobin value in the final multivariate model as an additional factor predictive of favorable survival. Previous treatment for advanced CML (blast crisis or accelerated phase) was retained in all models because it was a study-design feature, but it was not significantly predictive of survival. When the indicator of level of blasts in bone marrow was added to this model, it was not an independently significant factor, presumably because of the high correlation with blasts in peripheral blood. Similarly, other cytogenetic abnormalities did not add to the predictive value of the final regression model obtained for all patients. Whereas the median survival time was only 4 months in the 51 patients with all 3 unfavorable prognostic factors (hemoglobin < 100 g/L, platelets < 100 × 109/L, and ≥ 50% peripheral blasts at baseline), it was not reached (estimated 12-month survival rate, 77%) in the 32 patients with none of these criteria at baseline. The remaining 140 patients (with only 1 or 2 of these unfavorable prognostic values) had a median survival time of 7.2 months.
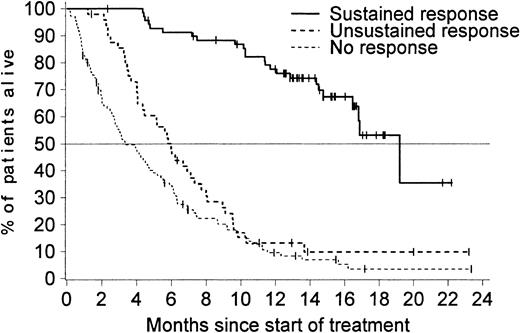
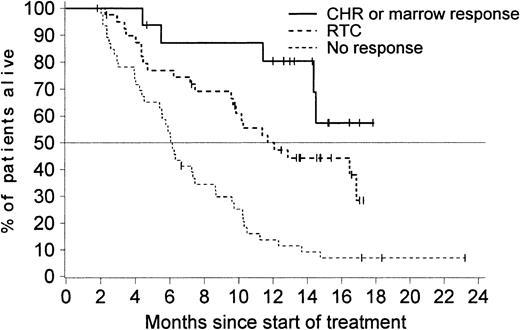
As expected, patients who had a sustained hematologic response benefited most from imatinib therapy (Figure4). Notably, patients with an unsustained response had a survival time similar to that of patients who did not have any response. Patients who showed a hematologic response during the second month of treatment (either a CHR, marrow response, or an RTC) had a markedly improved overall survival compared with patients with available assessments at 2 months indicating no hematologic response (Figure 5). Similar results were observed in a separate analysis considering a landmark at 3 months (data not shown). The achievement of a major cytogenetic response was also associated with an improved survival. The median survival time was 12 months among the 37 patients who achieved a major cytogenetic response and only 6 months in patients without a response.
Median survival time according to response.
Median survival time was 19 months (95% CI, 16.8 months–not reached) for the 70 patients with a sustained response, 6 months (95% CI, 4.3-7.3 months) for the 49 patients with an unsustained response, and only 3 months (95% CI, 2.7-4.7 months) in the 110 patients with no documentation of response during treatment with imatinib.
Median survival time according to response.
Median survival time was 19 months (95% CI, 16.8 months–not reached) for the 70 patients with a sustained response, 6 months (95% CI, 4.3-7.3 months) for the 49 patients with an unsustained response, and only 3 months (95% CI, 2.7-4.7 months) in the 110 patients with no documentation of response during treatment with imatinib.
Survival results for 103 patients who had an available assessment of hematologic response (including bone marrow response) at 2 months.
Of the 16 patients with a CHR or marrow response, 11 (69%) are still alive, compared with 16 (40%) of the 40 patients with an RTC (median survival, 11.7 months) and only 5 (11%) of the 47 patients with no documented response at 2 months (median survival, 6.1 months;P < .001 on log rank test).
Survival results for 103 patients who had an available assessment of hematologic response (including bone marrow response) at 2 months.
Of the 16 patients with a CHR or marrow response, 11 (69%) are still alive, compared with 16 (40%) of the 40 patients with an RTC (median survival, 11.7 months) and only 5 (11%) of the 47 patients with no documented response at 2 months (median survival, 6.1 months;P < .001 on log rank test).
Efficacy results for all 260 enrolled patients were similar to those for the 229 patients with a confirmed diagnosis of blast crisis. For the 260 enrolled patients, the overall rate of hematologic response lasting at least 4 weeks was 31%, including a CHR or marrow response in 12% of patients. The rate of major cytogenetic response was 15%, with 7% complete cytogenetic responses. The estimated duration of hematologic response was also 10 months. The estimated median overall survival time was 6.9 months, and the estimated survival rate at 12 months was 32%.
Safety
Analyses of safety were based on data from all 260 enrolled patients. The safety profile of imatinib in this trial was generally similar to that observed in a previous phase I study with comparable doses. Table 5 shows treatment-related adverse events (adverse reactions) reported in at least 5% of patients. The most frequently reported adverse reactions were gastrointestinal disorders (nausea and vomiting), edema, muscle cramps, diarrhea, and dermatologic events. Grade 1 or 2 edema was more frequent in the 600-mg–dose group (61% compared with 24% in the 400-mg–dose group), but the incidences of other grade 1 or 2 reactions and of all grade 3 or 4 reactions were comparable in the 2 dose groups.
Nonhematologic adverse events related to treatment with imatinib in all patients (n = 260)
| Adverse event . | All grades of adverse events . | Grade 3 or 4 adverse events . |
|---|---|---|
| Nausea | 164 (63) | 5 (1.9) |
| Fluid-retention events | 149 (57) | 15 (5.8) |
| Superficial edema | 144 (55) | 9 (3.5) |
| Other fluid-retention events | 24 (9) | 8 (3.1) |
| Vomiting | 114 (44) | 3 (1.2) |
| Muscle cramps | 65 (25) | 2 (0.8) |
| Diarrhea | 62 (24) | 2 (0.8) |
| Dermatitis/rash | 59 (23) | 11 (4.2) |
| Musculoskeletal pain | 30 (12) | 2 (0.8) |
| Abdominal pain | 27 (10) | 2 (0.8) |
| Headache | 26 (10) | 2 (0.8) |
| Hemorrhage | 27 (10) | 6 (2.3) |
| Arthralgia | 21 (8) | 3 (1.2) |
| Fatigue | 20 (8) | 4 (1.5) |
| Dyspepsia | 18 (7) | 0 |
| Myalgia | 15 (6) | 0 |
| Adverse event . | All grades of adverse events . | Grade 3 or 4 adverse events . |
|---|---|---|
| Nausea | 164 (63) | 5 (1.9) |
| Fluid-retention events | 149 (57) | 15 (5.8) |
| Superficial edema | 144 (55) | 9 (3.5) |
| Other fluid-retention events | 24 (9) | 8 (3.1) |
| Vomiting | 114 (44) | 3 (1.2) |
| Muscle cramps | 65 (25) | 2 (0.8) |
| Diarrhea | 62 (24) | 2 (0.8) |
| Dermatitis/rash | 59 (23) | 11 (4.2) |
| Musculoskeletal pain | 30 (12) | 2 (0.8) |
| Abdominal pain | 27 (10) | 2 (0.8) |
| Headache | 26 (10) | 2 (0.8) |
| Hemorrhage | 27 (10) | 6 (2.3) |
| Arthralgia | 21 (8) | 3 (1.2) |
| Fatigue | 20 (8) | 4 (1.5) |
| Dyspepsia | 18 (7) | 0 |
| Myalgia | 15 (6) | 0 |
Values are numbers (%) of patients.
Table 6 shows a summary of the incidence of grade 3 or 4 hematologic toxicity. Values for each variable represent the numbers of patients who had normal or not worse than grade 2 findings before therapy and in whom grade 3 or 4 abnormalities developed during treatment with imatinib. Incidences were comparable for patients treated with 400 mg and 600 mg of imatinib, and the most common grade 4 abnormalities were neutropenia and thrombocytopenia. Table 6 also shows a summary of the time to nadir values of neutrophil and platelet counts (for all patients) and the duration of grade 3 or 4 abnormalities (based on all episodes).
Hematologic abnormalities during treatment with imatinib
| Abnormality6-150 . | All patients (n = 260) . | 400-mg-dose group (n = 37) . | 600-mg-dose group (n = 223) . |
|---|---|---|---|
| Anemia | |||
| Grade 36-151 | 106 (41) | 16 (43) | 90 (40) |
| Grade 46-151 | 29 (11) | 6 (16) | 23 (10) |
| Neutropenia | |||
| Grade 36-151 | 41 (16) | 5 (14) | 36 (16) |
| Grade 46-151 | 124 (48) | 20 (54) | 104 (47) |
| Median duration, d (range)6-152 | 19 (1-268) | 19 (1-205) | 19 (1-268) |
| Median time to nadir, d (25th-75th percentile) | 36 (17-77) | 26 (14-107) | 38 (18-77) |
| Thrombocytopenia | |||
| Grade 36-151 | 75 (29) | 12 (32) | 63 (28) |
| Grade 46-151 | 85 (33) | 12 (32) | 73 (32) |
| Median duration, d (range)6-152 | 31 (1-445) | 28 (1-202) | 31 (1-445) |
| Median time to nadir, d (25th-75th percentile) | 37 (15-81) | 29 (10-71) | 39 (15-81) |
| Abnormality6-150 . | All patients (n = 260) . | 400-mg-dose group (n = 37) . | 600-mg-dose group (n = 223) . |
|---|---|---|---|
| Anemia | |||
| Grade 36-151 | 106 (41) | 16 (43) | 90 (40) |
| Grade 46-151 | 29 (11) | 6 (16) | 23 (10) |
| Neutropenia | |||
| Grade 36-151 | 41 (16) | 5 (14) | 36 (16) |
| Grade 46-151 | 124 (48) | 20 (54) | 104 (47) |
| Median duration, d (range)6-152 | 19 (1-268) | 19 (1-205) | 19 (1-268) |
| Median time to nadir, d (25th-75th percentile) | 36 (17-77) | 26 (14-107) | 38 (18-77) |
| Thrombocytopenia | |||
| Grade 36-151 | 75 (29) | 12 (32) | 63 (28) |
| Grade 46-151 | 85 (33) | 12 (32) | 73 (32) |
| Median duration, d (range)6-152 | 31 (1-445) | 28 (1-202) | 31 (1-445) |
| Median time to nadir, d (25th-75th percentile) | 37 (15-81) | 29 (10-71) | 39 (15-81) |
Values are numbers (%) of patients unless otherwise indicated.
Abnormalities occurring during treatment or worsening from baseline level to the indicated grade.
National Cancer Institute-National Institutes of Health common toxicity criteria (CTC). For CTC grade 3, the neutrophil count was 0.5 to < 1.0 × 109/L; the platelet count, 10.0 to < 50.0 × 109/L; and the hemoglobin level, < 65 to 80 g/L. For CTC grade 4; the neutrophil count was < 0.5 × 109/L; the platelet count, < 10.0 × 109/L; and the hemoglobin level, < 65 g/L.
The duration of cytopenias refers to all episodes of grade 3 or 4 abnormalities.
In one patient, a grade 4 abnormality in ALT developed during treatment. Routine laboratory tests revealed newly occurring grade 3 abnormalities in AST in 2% of patients, ALT in another 2%, and bilirubin in 4% of patients during treatment.
Adverse events led to a temporary or permanent reduction in the initial dose of imatinib on one or more occasions in 17 patients (46%) who started treatment with imatinib given at 400 mg daily and 105 patients (47%) who started treatment with 600 mg daily. Drug-related adverse events led to termination of imatinib therapy in 13 patients (5%)—all in the 600-mg–dose group—and consisted of neutropenia or pancytopenia (4 patients), dermatitis or rash (3 patients), gastrointestinal disorders (hematemesis, melena, or vomiting; 2 patients), cardiac failure (2 patients), headache, edema, abnormal hepatic function, hemothorax, and acute renal failure (1 patient each). Some patients discontinued treatment because of multiple adverse events.
Serious adverse events related to treatment were reported for 47 patients (18%) and were most frequently hematologic events, including neutropenia, thrombocytopenia, and febrile neutropenia or neutropenic sepsis (16 patients); gastrointestinal events, including nausea, vomiting, gastric or esophageal irritation, and hemorrhage (15 patients); general disorders, including fever, fatigue or hemorrhage, bone pain, and dehydration (11 patients); cardiac disorders (3 patients, including one with concomitant renal failure); skin disorders, including dermatitis or rash (6 patients); and fluid retention (7 cases of ascites, pleural effusion, or edema). Some patients had more than one serious adverse event. One death, which was caused by renal and cardiac failure due to pleural effusion and ascites, was suspected to be related to therapy.
Discussion
We conducted this phase II study to determine whether imatinib, a potent inhibitor of the oncogenic Bcr-Abl tyrosine kinase, could induce sustained hematologic responses lasting at least 4 weeks in at least 15% of patients with CML in previously untreated myeloid blast crisis, when administered at well-tolerated doses defined in an earlier phase I study.35,36 We found that orally administered imatinib induced a sustained hematologic response in 36% of previously untreated patients, including a CHR in 9% of patients. Remarkably, treatment with imatinib also induced major cytogenetic responses in 16% of patients, including a complete cytogenetic response in 7%. Rates of sustained hematologic response and major cytogenetic response were markedly higher in patients treated with an initial imatinib dose of 600 mg daily than in those given 400 mg daily. The results of this study are consistent with those of an earlier phase I trial in which 38 patients with CML in myeloid blast crisis were treated with imatinib in daily doses of 300 to 1000 mg.36The demographic features, disease history, baseline characteristics, and major prognostic factors of the patients enrolled in this trial appear to be consistent with those described in other studies of patients with blast crisis.9,10,37 39 Therefore, these encouraging results observed with imatinib should not be attributable to a bias induced through selection of patients with an unusually favorable prognosis.
The induction of major cytogenetic responses in 16% of patients treated with imatinib is remarkable, since transient cytogenetic responses are only rarely reported with other treatments.10,18,23 Whether these results translate into a clear survival advantage remains to be proved through further follow-up. However, the estimated median survival time of 7.5 months for previously untreated patients observed in this trial compares favorably with the median overall survival time of 3 to 5 months observed with other therapies in patients with newly diagnosed myeloid blast crisis.9 10 Several patients who achieved a sustained hematologic response are still alive after up to 23 months (Figure 5), but longer-term follow-up is required to determine whether treatment with imatinib leads to long-term disease stabilization and survival in a fraction of patients.
Imatinib therapy was associated with numerous adverse events, but this was expected because advanced CML is associated with considerable morbidity. Most of the nonhematologic adverse events that appeared to be drug related (edema, gastrointestinal disorders, and muscle and joint pain) were seldom severe and rarely required discontinuation of treatment. A fluid-retention syndrome involving disorders of pleural effusion, pulmonary edema, acute respiratory distress syndrome, ascites, congestive heart failure, or edema was identified as a possible adverse drug reaction. Although uncommon, this syndrome is potentially serious, as was shown by its implication in the death of one patient, and it should be considered when a patient presents with a sudden weight gain or respiratory distress.
Episodes of severe cytopenias were frequent. Most cases of cytopenia are probably due to the direct pharmacologic effect of imatinib on leukemic cells and the lack of bone marrow reserve in severely ill patients. Accordingly, cytopenia may in many cases reflect treatment efficacy, especially during the first weeks of therapy, and does not necessarily require withdrawal of therapy or dose reduction. Continuation of therapy despite cytopenia may be desirable in some patients and may be associated with less risk in view of the nonspecific cytotoxic effects of alternative therapies, which entail severe myelosuppression leading to febrile neutropenia in more than 80% of patients.9 In this study, imatinib therapy was withdrawn because of cytopenia in only 9 patients, and the primary reason for discontinuation in 8 of these cases was disease progression.
An analysis of prognostic factors revealed that platelet counts of at least 100 × 109/L and peripheral blood blast values below 50% at baseline were independently predictive of favorable survival outcome in this trial. These prognostic factors are similar to those identified in a retrospective study of 121 patients with blast crisis who were treated with either decitabine or combination chemotherapy.10 Furthermore, the achievement of any hematologic response sustained for 4 weeks, including a reduction in blast levels to below 15% (termed RTC in this analysis), was significantly associated with improved survival.
Mechanisms of resistance to imatinib remain to be fully elucidated but do not appear to involve drug absorption or metabolism.36Instead, plausible resistance mechanisms are postulated to involve drug efflux, amplification of the BCR-ABL fusion gene or increased expression of Bcr-Abl protein, or decreased cellular bioavailability of imatinib.40-43 Amplification and mutations of the BCR-ABL gene have been observed in samples from patients.44-47 Further studies are warranted to clarify the clinical relevance of the different specific molecular mechanisms of resistance to imatinib.
In conclusion, imatinib provides hematologic control in blast-crisis CML with an acceptable level of toxicity. In addition, imatinib specifically suppresses leukemia precursor cells, thereby inducing cytogenetic responses even at this late stage of CML. The results of this trial suggest that imatinib is a valuable treatment alternative in patients with this disorder.
Because imatinib is well tolerated and less myelosuppressive than current conventional chemotherapy agents, it may be feasible to combine imatinib with existing agents used to treat CML in blast crisis or to use it as an adjunct to bone marrow transplantation. Patients with blast crisis often respond well to fludarabine, high-dose cytarabine, or decitabine. In vitro studies have revealed significant cytotoxic synergic or additive effects between imatinib and commonly used antileukemic agents in cells positive for BCR-ABLexpression.48 49 Accordingly, further studies are warranted to test the optimal doses of imatinib used in combination with chemotherapeutic and other antileukemic agents.
We thank the numerous coinvestigators, nursing staff, and clinical trial monitors who participated in this study; the data managers and programmers at Novartis Pharmaceuticals for their contributions; David Parkinson and Greg Burke for invaluable support; Nick Shand, John Ford, and Elisabeth Wehrle for collaboration in implementing the protocol and reporting the study results; and Thomas Brown for assistance in preparing the manuscript.
Sponsored by Novartis Pharmaceuticals AG, Basel, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Charles L. Sawyers, 11-934 Factor Building, UCLA, 10833 Le Conte Ave, Los Angeles, CA 90095; e-mail:csawyers@mednet.ucla.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal