The glycoprotein CD47 (integrin-associated protein, IAP) is present on the surface of virtually all cells, including red blood cells (RBCs). CD47 acts like a marker of self by ligating the macrophage inhibitory receptor signal regulatory protein α (SIRPα). In this manner mild reactivity of wild-type RBCs with macrophage phagocytic receptors is tolerated, whereas otherwise identical CD47-deficient RBCs are rapidly eliminated. We show here that virtually all CD47-deficient nonobese diabetic (NOD) mice spontaneously develop severe lethal autoimmune hemolytic anemia (AIHA) at 180 to 280 days of age, whereas none of the control CD47+ NOD mice develop lethal AIHA at least during the first year of life. This phenotype is at least partially due to a markedly increased rate of elimination of opsonized CD47−/− compared to CD47+ RBCs. Similarly, CD47−/−C57BL/6 mice were much more sensitive than their wild-type counterparts to experimental passive AIHA induced by anti-RBC monoclonal antibodies. Thus, CD47-SIRPα signaling can have a profound influence on the severity of AIHA, making manipulation of this signaling pathway a theoretically appealing avenue in the treatment of the disease.
Introduction
Autoimmune hemolytic anemia (AIHA) and other immune cytopenias are common in autoimmune diseases and lymphoid malignancies. AIHA is defined as an increased destruction of red blood cells (RBCs) due to the presence of anti-RBC autoantibodies.1 RBC autoantibodies are classified as warm or cold because they react optimally at temperatures between 35°C and 40°C or below 30°C, respectively.1 Warm autoantibodies are mostly IgG but may sometimes also include IgA or IgM or both.1 Binding of warm IgG autoantibodies to RBCs does not itself damage the RBCs and may or may not activate complement.1 IgG-opsonized RBCs are captured by cells of the monocyte-macrophage phagocytic system, mainly in the spleen and liver, following Fc-receptor engagement on the phagocytic cells.2-4 Treatment is immunosuppressive (cytotoxic drugs, splenectomy) or Fc receptor–competitive (IgG).5 A more complete understanding of the regulatory pathways involved in target cell recognition and elimination may provide additional and better treatment modalities.
CD47 (integrin-associated protein) is a surface glycoprotein exposed on virtually all cells including RBCs.6,7 Although CD47 has been described to interact with the integrins αvβ3, αIIbβ3, and α2β1,8-11 no association with integrins has been described for CD47 on RBCs. Expression of CD47 on RBCs seems to be associated with the Rh protein complex,12 and individuals with the rare Rhnull phenotype also show profound (≥ 75%) reduction in the expression of CD47 on RBCs, whereas CD47 expression is normal on other cells.6 Humans with completely CD47-deficient RBCs have not been described.
We have recently shown that normal RBCs are recognized by splenic macrophages and would be phagocytosed, were it not for the presence of CD47 on the RBC surface.13 This mechanism is based on the binding of CD47 on RBCs to signal regulatory protein α (SIRPα) on the macrophage, which inhibits macrophage activation and phagocytosis.13 Thus, these data indicate that splenic red pulp macrophages have a phagocytic receptor(s) capable of recognizing normal circulating RBCs, and the CD47-SIRPα signal is sufficient to counteract phagocytosis.13 Because the nature of this phagocytic receptor(s) is still under investigation, we refer to this functional activity as the “RBC receptor.”
After ligation and cross-linking, SIRPα is tyrosine phosphorylated by Src family kinases at its intracellular immunoreceptor tyrosine-based inhibitory motifs (ITIMs).14 Next, these phosphorylated motifs bind the src-homology 2 domain-containing tyrosine phosphatases SHP-1 and SHP-2. Although both SHP-1 and SHP-2 seem to be present in macrophages, SHP-1 predominates.14 The importance of SHP-1 for the CD47-SIRPα system is further shown by the fact that CD47-SIRPα signaling is strongly reduced in motheaten viable mice,15 having a mutation resulting in reduced (about 20% of normal) SHP-1 levels but normal SHP-2 levels.16 17
Our previous data showed that CD47-SIRPα signaling can also attenuate prophagocytic signals from macrophage Fcγ and complement receptors challenged with opsonized RBCs.15 This observation suggests that this mechanism might also be of importance in AIHA. Because it has been shown that nonobese diabetic (NOD) mice spontaneously develop mild AIHA at the age of 300 to 550 days,18 we explored the possibility that the impairment of CD47-SIRPα signaling could accelerate the development and progression of AIHA in NOD mice. We show here that CD47−/− NOD mice spontaneously develop severe lethal AIHA at 180 to 280 days of age, in contrast to the later development of mild anemia in CD47+ NOD mice.18 In addition, we probe the role of CD47 in the disease using transfusion experiments and a model of passive experimental AIHA.
Materials and methods
Reagents
The 5/6-carboxyfluorescein diacetate succimidyl ester (CFSE) was from Molecular Probes (Eugene, OR). The monoclonal antibody (mAb) TER-119 for detection of murine erythropoietic cells was from Pharmingen (San Diego, CA). Mouse antimouse RBC mAbs 34-3C (IgG2a) and 105-2H (IgG1), established from autoimmune-prone NZB mice,19 were purified from hybridoma supernatants by ammonium sulfate precipitation and protein A chromatography (Pharmacia, Piscataway, NJ). PKH26, secondary antibodies, and other reagents were from Sigma-Aldrich (St Louis, MO).
Mice
Male and female CD47−/− NOD mice, back-crossed to NOD (Jackson Laboratory, Bar Harbor, ME ) for 15 generations, CD47−/− C57BL/6J mice, back-crossed to C57BL/6J (Jackson Laboratory) for 16 or more generations, and their heterozygous and homozygous littermates were from our own breeding colony. Animals were kept in accordance with National Institutes of Health and local guidelines and maintained in a specific pathogen-free barrier facility.
Flow cytometric detection of anti-RBC antibodies
Blood (5 μL) collected during hematocrit measurements was washed twice in 1 mL phosphate-buffered saline (PBS). Surface-bound RBC autoantibodies were then detected using fluorescein isothiocyanate–conjugated F(ab′)2 specific goat antimouse IgG or goat antimouse IgM antibodies. After washing off unbound secondary antibodies, the RBCs were analyzed using flow cytometry (Epics XL; Coulter, Hialeah, FL).
Measurement of hematocrit and reticulocyte count
Blood (70 μL) was collected into heparinized capillary tubes (Fisher Scientific, Pittsburgh, PA), from the retro-orbital vein plexa of anesthetized mice. The hematocrit tubes were spun in a hematocrit centrifuge (ICN, Needham Heights, MA), and the hematocrit was determined as the relative height of the RBC column expressed in percent.18 For reticulocyte counts, blood smears were air dried, fixed in methanol, and stained for 20 minutes in 10% modified Wright-Giemsa stain. The fraction of reticulocytes was then determined by light microscopy analysis of at least 100 RBCs/slide and expressed as percent of total RBCs.18
Experimental AIHA
Studies on experimental AIHA were performed essentially as previously described.2 Weight and hematocrit were determined for recipient mice immediately before an intraperitoneal injection of 0.25, 0.5, 1, or 4 μg mAb 34-3C or mAb 105-2H/g body weight. Weight and hematocrit were determined daily. Mice showing a weight loss of more than 20% were considered moribund and killed.
In vivo RBC clearance assay
Clearance of CD47−/− or wild-type (CD47+/+) RBCs was determined as previously described.13 RBCs were stained with the green fluorescent dye CFSE or with the red fluorescent dye PKH26 following the manufacturer's protocol and resuspended in sterile pyrogen-free 0.9% NaCl to a hematocrit of 30%. Recipients were given 200 μL of the CFSE-stained or PKH26-stained RBCs and the clearance of fluorescent RBCs was followed using flow cytometry (Epics XL, Coulter) using 5-μL blood samples collected from a tail vein at the time points indicated. The fraction of fluorescent RBCs at 30 minutes after the RBC injection was used as a reference. Control experiments showed that the choice of dye (CFSE or PKH26) did not affect clearance kinetics13,15(not shown). Use of a 5-minute reference point gave similar results, although the experimental variation was higher this early after injection15 (not shown).
Results
Incidence and clinical signs of AIHA in CD47−/− NOD mice
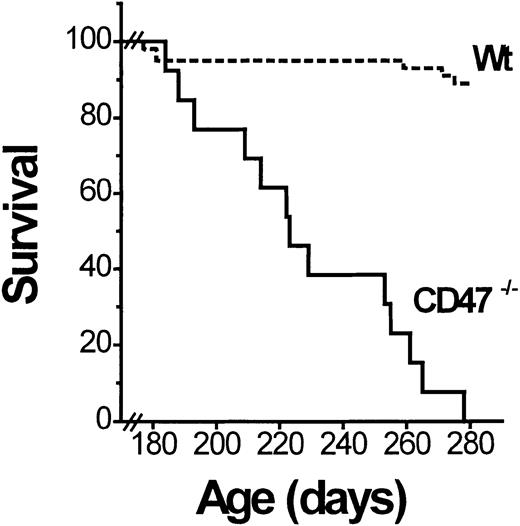
The NOD mice spontaneously develop mild nonlethal AIHA at the age of 300 to 550 days (up to 75% incidence in nondiabetic NOD mice at the age of 550 days).18 As part of a longitudinal diabetes study (manuscript in preparation), we followed a cohort of female CD47−/− and sibling control (CD47+/+ and CD47+/−) NOD mice to determine the possible effect of CD47 deficiency on development of AIHA. Mice that became diabetic well before 180 days of age were excluded from this study. The remaining mice were observed until they were 1 year old. In contrast to the mild hemolytic anemia described in old (300-550 days) wild-type NOD mice,18 the anemia in CD47−/− NOD mice was severe and rapidly fatal. Virtually all CD47−/− mice developed lethal anemia (hematocrit below 15%) with icterus between the ages of 180 and 280 days (Figure 1). This was neither seen in any CD47+ NOD mice (at least not until 365 days) nor in any CD47−/− C57BL/6J mice. Necropsy of deceased CD47−/− NOD mice showed a massively enlarged spleen (1.8 ± 0.4 g; about 10 times that normally seen in similarly aged NOD mice).20 Flow cytometric analysis revealed that a vast majority of the spleen cells of anemic CD47−/− NOD mice were TER-119+ erythropoietic cells (not shown). There was also evidence of extreme erythroid expansion in vertebrae, sternum, rib tips, and long bone marrow cavities. No evidence of bleeding was seen. Thus, the severe anemia appeared to be secondary and due to a rapid destruction of RBCs.
Nondiabetic CD47−/− but not wild-type (wt) NOD mice succumb to AIHA.
A cohort of CD47−/− (n = 18) and wild-type (CD47+/+ or CD47+/−; Wt; n = 44) NOD mice was followed for development of lethal AIHA. Survival data are shown as a Kaplan-Meier plot. All CD47−/− mice in this figure developed AIHA, whereas the small loss of wild-type mice was unrelated and due to the development of diabetes.
Nondiabetic CD47−/− but not wild-type (wt) NOD mice succumb to AIHA.
A cohort of CD47−/− (n = 18) and wild-type (CD47+/+ or CD47+/−; Wt; n = 44) NOD mice was followed for development of lethal AIHA. Survival data are shown as a Kaplan-Meier plot. All CD47−/− mice in this figure developed AIHA, whereas the small loss of wild-type mice was unrelated and due to the development of diabetes.
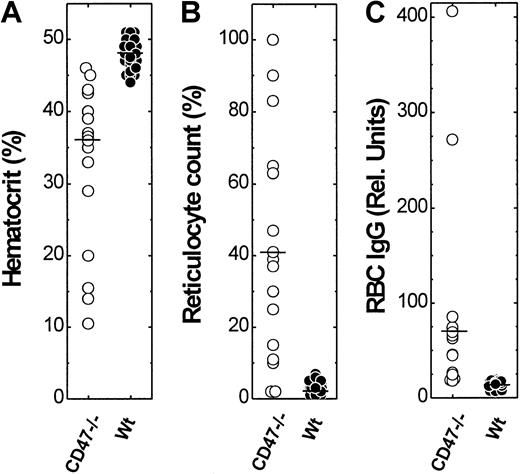
We next determined hematocrit and reticulocyte count of all the mice in the cohort at 194.4 ± 38.4 (CD47−/−) and 206.1 ± 43.6 (control CD47+/+ and CD47+/−) days of age. Control NOD mice had normal hematocrit (47.9% ± 1.8%), whereas virtually all CD47−/− mice had a reduced hematocrit (33.0% ± 10.9%; Figure2). Peripheral blood smears from control NOD mice showed normal RBC morphology, whereas blood smears from CD47−/− NOD mice were abnormal showing polychromasia and macrocytosis (not shown). As expected, there was an increased reticulocyte count (44.3% ± 33.6%) in CD47−/− NOD mice, as compared with control NOD mice having a normal reticulocyte count (2.5% ± 1.5%; Figure 2). Hematocrit and reticulocyte count in CD47−/− NOD mice showed a very strong inverse correlation (r = −0.99). Flow cytometric analysis showed high levels of both IgG and IgM on the RBCs of anemic CD47−/− NOD mice (Figure 2). Such antibodies were not detected on RBCs from mice with normal hematocrit and reticulocyte counts. Notably, anti-RBC antibodies were not seen in any CD47+/− or CD47+/+ NOD mice at an age of 180 to 280 days (Figure 2). Thus, CD47−/− NOD mice show both accelerated development of autoimmune anti-RBC antibodies and accelerated destruction of RBCs.
Hematocrit, reticulocyte count, and relative amount of anti-RBC IgG in CD47−/− and wild-type (CD47+) NOD mice.
A cohort of CD47−/− (n = 18) and wild-type (CD47+/+ or CD47+/−; n = 44) NOD mice, at 194.4 ± 38.4 (CD47−/−) and 206.1 ± 43.6 (CD47+/+ and CD47+/−) days of age was studied. The median hematocrit, reticulocyte counts, and anti-RBC IgG levels are indicated in the figure by a horizontal bar.
Hematocrit, reticulocyte count, and relative amount of anti-RBC IgG in CD47−/− and wild-type (CD47+) NOD mice.
A cohort of CD47−/− (n = 18) and wild-type (CD47+/+ or CD47+/−; n = 44) NOD mice, at 194.4 ± 38.4 (CD47−/−) and 206.1 ± 43.6 (CD47+/+ and CD47+/−) days of age was studied. The median hematocrit, reticulocyte counts, and anti-RBC IgG levels are indicated in the figure by a horizontal bar.
The lack of CD47 on RBCs is responsible for the severity of AIHA in CD47−/− NOD mice
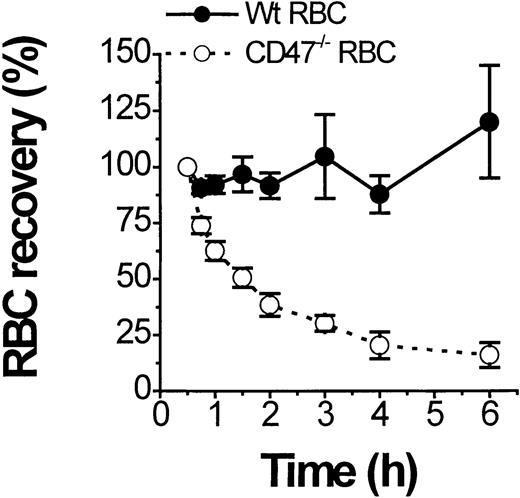
CD47/SIRPα modulates Fcγ and complement receptor–dependent macrophage activation.15 It is therefore conceivable that the markedly accelerated development of AIHA in CD47−/−NOD mice is at least in part due to the lack of CD47 on RBCs and the resulting absence of CD47/SIRPα-dependent inhibitory signaling in macrophages. To test this possibility, we followed clearance of fluorescently labeled CD47−/− and wild-type (CD47+/+) RBCs from healthy mice in CD47−/−NOD mice with a hematocrit below 25% (Figure3). Labeled CD47−/− RBCs showed a very short half-life (1.5 hours) in the circulation of anemic CD47−/− mice, whereas wild-type RBCs were not significantly cleared during the 6-hour experiment (Figure 3). Incubation of wild-type and CD47−/− RBCs with serum from anemic CD47−/− NOD mice resulted in identical levels of IgG and IgM deposition as assayed by flow cytometry (not shown). Thus, under otherwise identical conditions, opsonized CD47−/−RBCs are much more rapidly eliminated in CD47−/− NOD mice than equally opsonized wild-type cells.
Rapid clearance of transfused CD47−/− but not of transfused wild-type (CD47+/+) RBCs in CD47−/− NOD mice with overt AIHA.
Clearance of fluorescent-labeled RBCs from normal nonanemic CD47−/− (open circles) or wild-type (CD47+/+) mice (closed circles) was followed in anemic CD47−/− NOD mice with a hematocrit below 25%. Data are mean ± SD from 3 separate recipients and results were independent of the dye used to label the RBCs.
Rapid clearance of transfused CD47−/− but not of transfused wild-type (CD47+/+) RBCs in CD47−/− NOD mice with overt AIHA.
Clearance of fluorescent-labeled RBCs from normal nonanemic CD47−/− (open circles) or wild-type (CD47+/+) mice (closed circles) was followed in anemic CD47−/− NOD mice with a hematocrit below 25%. Data are mean ± SD from 3 separate recipients and results were independent of the dye used to label the RBCs.
Increased sensitivity to experimentally induced AIHA in CD47−/−C57BL/6J mice
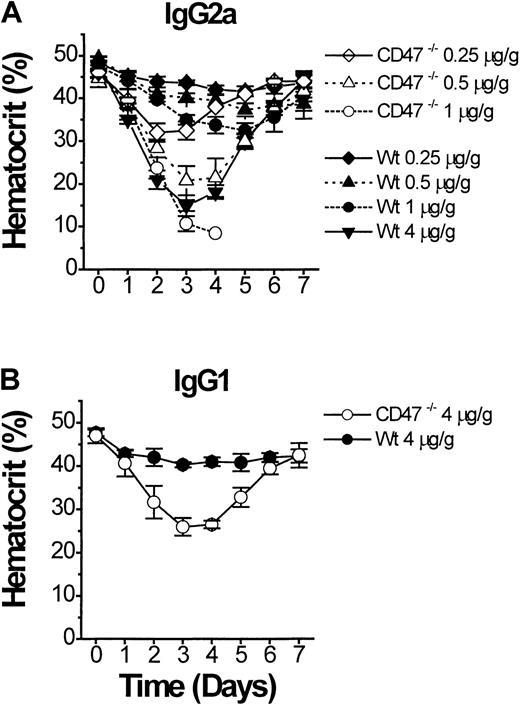
If CD47−/− mice are more sensitive to the pathogenic effect of anti-RBC antibody, this should also be apparent in models of secondary AIHA.2 Eight-week-old CD47−/− or wild-type C57BL/6J mice were monitored after a single intraperitoneal injection of purified antimurine RBC mAb 34-3C (IgG2a).3,19 Compared to wild-type mice, CD47−/− mice showed an extreme dose-dependent sensitivity to experimental AIHA, with a lethal outcome at doses of 1 μg mAb 34-3C/g body weight or above (Figure 4A). A dose of 4 μg/g body weight in wild-type mice was required to induce an anemia of similar severity as seen with 0.5 μg/g body weight in CD47−/− mice (Figure 4A). Experiments with another antimurine RBC mAb 105-2H (IgG1), which is less pathogenic than the 34-3C mAb, but induces anemia through FcγR-dependent erythrophagocytosis,3 19 confirmed the accelerated development of AIHA in CD47−/− mice (Figure 4B).
Experimental AIHA in CD47−/− and wild-type (CD47+/+) C57BL/6J mice.
Experimental AIHA was induced in CD47−/− (open symbols) or wild-type (closed symbols) C57BL/6J mice by using (A) the antimouse RBC mAb 34-3C (mouse IgG2a), or (B) the antimouse RBC mAb 105-2H (mouse IgG1). The indicated amount of mAb 34-3C or 105-2H per gram body weight was given as an intraperitoneal injection on day 0. The mice were then followed daily for weight and hematocrit for 7 days after injection. CD47−/− mice that received 1 μg mAb 34-3C/g body weight (panel A, open circles) succumbed at day 3 to 4 of the experiment. Data are mean ± SD for 3 to 4 mice in each group.
Experimental AIHA in CD47−/− and wild-type (CD47+/+) C57BL/6J mice.
Experimental AIHA was induced in CD47−/− (open symbols) or wild-type (closed symbols) C57BL/6J mice by using (A) the antimouse RBC mAb 34-3C (mouse IgG2a), or (B) the antimouse RBC mAb 105-2H (mouse IgG1). The indicated amount of mAb 34-3C or 105-2H per gram body weight was given as an intraperitoneal injection on day 0. The mice were then followed daily for weight and hematocrit for 7 days after injection. CD47−/− mice that received 1 μg mAb 34-3C/g body weight (panel A, open circles) succumbed at day 3 to 4 of the experiment. Data are mean ± SD for 3 to 4 mice in each group.
Discussion
Our data show that the lack of the expression of CD47 promotes a markedly accelerated development of spontaneous AIHA in NOD mice and of experimental AIHA induced by 2 different anti-RBC monoclonal autoantibodies in nonautoimmune C57BL/6 mice. This indicates that the interaction of RBC CD47 with macrophage SIRPα plays an important role in regulating the autoimmune elimination of opsonized RBCs in AIHA. Because CD47 is expressed on virtually all host cells, but not on foreign microorganisms, CD47-SIRPα signaling is critically involved not only in prevention against autoantibody-mediated autoimmune manifestations but also in host defense against foreign antigens, by modulating the phagocytic signal mediated through the activation of Fcγ and complement receptors.15
Accordingly, a small degree of opsonization might suffice to trigger phagocytic activation when present on a foreign (CD47−) particle, whereas a self particle, such as a CD47+ RBC, would escape phagocytosis. The latter notion is particularly significant, because this would avoid unnecessary autoimmune cellular damages by autoantibodies induced nonspecifically and transiently during microbial infections. However, sufficiently high opsonization and Fcγ receptor signal occurring in autoimmune diseases such as AIHA can override the CD47-SIRPα signal, thereby mediating clinically overt AIHA.
NOD mice, due to a specific major histocompatibility complex class II variant (I-Ag7) and a number of other interacting loci, frequently develop autoimmune destruction of pancreatic β cells and insulin-dependent diabetes.21 I-Ag7 is also associated with other autoimmune manifestations including rheumatoid arthritis and AIHA.18,22 We find that female CD47−/− NOD mice develop AIHA earlier and more frequently than reported for wild-type NOD mice. The disease in CD47−/− mice is almost 100% penetrant and leads to death before 1 year of age in virtually all animals. In wild-type NOD mice, AIHA is reportedly more common in diabetic than in nondiabetic mice.18 However, because a group of mice that became diabetic earlier in life was excluded from the analysis of the present study, a direct comparison of incidence is not possible. It should be stressed that anemia and anti-RBC antibodies were not found in CD47−/− C57BL/6 mice, which are not predisposed to autoimmune diseases, further emphasizing a critical role of the NOD genetic background. Thus, our data indicate that the absence of CD47 by itself on RBCs is not sufficient to trigger anti-RBC autoimmune responses, but is rather implicated in the accelerated development and progression of AIHA in NOD mice.
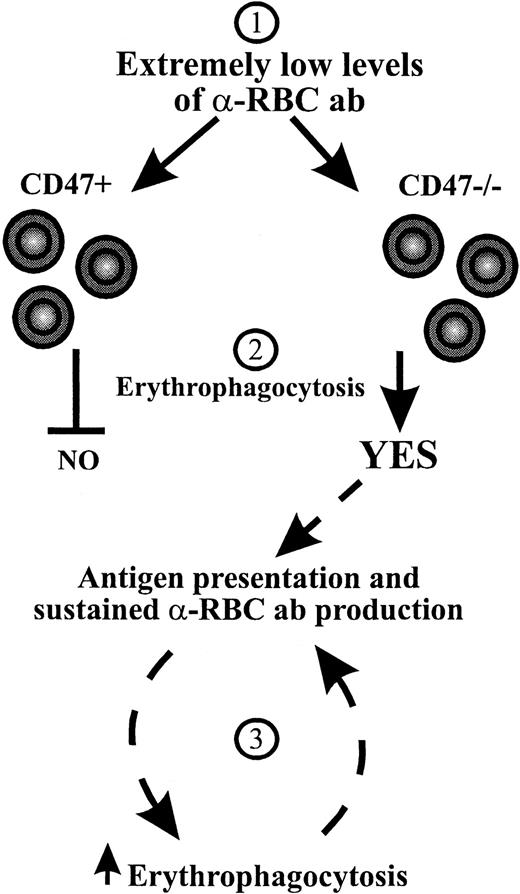
At present we do not know why CD47−/− NOD mice are more prone to develop a more severe form of AIHA. Our present results, however, suggest that the effect of CD47 deficiency on AIHA in NOD mice may be 2-fold. First, CD47−/− RBCs are more susceptible to autoantibody-mediated immune destruction in NOD mice, because the absent interaction between CD47 and SIRPα enhances prophagocytic signals induced by Fc and complement receptors.15 Results obtained with 2 different anti-RBC monoclonal autoantibodies inducing anemia through FcγR-dependent erythrophagocytosis demonstrate that CD47−/− RBCs are cleared rapidly even at very low levels of opsonization in nonautoimmune C57BL/6 mice (Figure 4). This is also consistent with our previous observations that the critical factor for the accelerated clearance of opsonized RBCs is the presence or absence of CD47 on the RBCs rather than on other cells.15 Second, the absence of CD47 on RBCs may promote the spontaneous production of anti-RBC autoantibodies in mice predisposed to AIHA such as NOD mice. In fact, the analysis of in vivo opsonized RBC has demonstrated higher levels of antibody-bound RBCs in CD47−/− NOD mice than in wild-type NOD mice at an age of 180 to 280 days (Figure 2), suggesting that the onset of anti-RBC autoantibody production is accelerated in CD47−/− NOD mice. We do not know the mechanism underlying the accelerated production of anti-RBC autoantibodies in CD47−/− versus CD47+ NOD mice. Possibly, low initial levels of anti-RBC autoantibodies, probably below the detection limit of the assay used here, are produced both in CD47−/− and CD47+NOD mice. In CD47+ NOD mice, RBCs will not be phagocytosed due to the inhibitory CD47-SIRPα signaling system (Figure5). However, in CD47−/− NOD mice this mechanism will not come into play and the RBCs will be phagocytosed. This increased rate of RBC destruction may then theoretically promote antigen presentation of pathogenic self peptides, thereby creating a closed circuit that leads to accelerated generation of autoimmune anti-RBC antibodies in CD47−/− NOD mice (Figure 5). Such a scenario may also explain why CD47+ NOD mice develop a less severe AIHA at a significantly older age.
Hypothesis for a mechanism behind the accelerated development of AIHA in CD47−/− NOD mice.
At low levels of anti-RBC autoantibodies (1) inhibitory CD47-SIRPα signaling prevents erythrophagocytosis in CD47+ NOD mice, whereas the absence of CD47-SIRPα signaling in CD47−/−NOD mice results in erythrophagocytosis (2).15 This may result in increased antigen presentation of self RBC peptides and sustained autoantibody production, which through increased RBC opsonization will lead to accelerated erythrophagocytosis and progressive exacerbation of AIHA in CD47−/− NOD mice (3).
Hypothesis for a mechanism behind the accelerated development of AIHA in CD47−/− NOD mice.
At low levels of anti-RBC autoantibodies (1) inhibitory CD47-SIRPα signaling prevents erythrophagocytosis in CD47+ NOD mice, whereas the absence of CD47-SIRPα signaling in CD47−/−NOD mice results in erythrophagocytosis (2).15 This may result in increased antigen presentation of self RBC peptides and sustained autoantibody production, which through increased RBC opsonization will lead to accelerated erythrophagocytosis and progressive exacerbation of AIHA in CD47−/− NOD mice (3).
In humans, it has long been suggested that a majority of anti-RBC autoantibodies are related to Rh.23 Therefore, based on the known association between CD47 and the Rh protein complex, it cannot be excluded that some of these autoantibodies do also interfere with CD47-SIRPα interaction. Although there are today no data to support such a hypothesis, it is tempting to speculate that this can be one reason why some human anti-RBC autoantibodies are more hemolytic than others. However, this hypothesis needs further investigation.
Regardless of the mechanism by which CD47 deficiency contributes to the accelerated development of AIHA, the markedly reduced clearance of CD47+ compared to CD47−/− RBCs in the hemolytic anemia models suggests that enhanced SIRPα signaling might be useful to decrease the severity of AIHA and possibly also of other autoimmune cytopenias. Thus, CD47 mimetics might be an alternative or complement to treatments aimed at reducing the amount of antibody, reducing the Fcγ receptor signal, or eliminating the clearance system.
Acknowledgments
We thank Drs John P. Atkinson and Thomas H. Steinberg for critical evaluation of the manuscript and Eric Ford and Anna Oldenborg for excellent technical assistance.
Supported by grants from the Swedish Medical Research Council (06P-14098), the Swedish Society of Medicine, the Umeå University-Washington University exchange program, National Institutes of Health (GM57573-01), the American Diabetes Association, the Washington University-Monsanto Research Agreement, a pilot grant from the Howard Hughes Medical Institute, the Medical Research Service of the Department of Veterans Affairs, and a grant from the Swiss National Foundation for Scientific Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Per-Arne Oldenborg, Department of Integrative Medical Biology, Section for Histology and Cell Biology, Umeå University, SE-901 87 Umeå, Sweden; e-mail:per-arne.oldenborg@histocel.umu.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal