It was previously reported that treatment with the sulfated polysaccharide fucoidan or the structurally similar dextran sulfate increased circulating mature white blood cells and hematopoietic progenitor/stem cells (HPCs) in mice and nonhuman primates; however, the mechanism mediating these effects was unclear. It is reported here that plasma concentrations of the highly potent chemoattractant stromal-derived factor 1 (SDF-1) increase rapidly and dramatically after treatment with fucoidan in monkeys and in mice, coinciding with decreased levels in bone marrow. In vitro and in vivo data suggest that the SDF-1 increase is due to its competitive displacement from heparan sulfate proteoglycans that sequester the chemokine on endothelial cell surfaces or extracellular matrix in bone marrow and other tissues. Although moderately increased levels of interleukin-8, MCP1, or MMP9 were also present after fucoidan treatment, studies in gene-ablated mice (GCSFR−/−, MCP1−/−, or MMP9−/−) and the use of metalloprotease inhibitors do not support their involvement in the concurrent mobilization. Instead, SDF-1 increases, uniquely associated with sulfated glycan–mobilizing treatments and not with several other mobilizing agents tested, are likely responsible. To the authors' knowledge, this is the first published report of disrupting the SDF-1 gradient between bone marrow and peripheral blood through a physiologically relevant mechanism, resulting in mobilization with kinetics similar to other mobilizing CXC chemokines. The study further underscores the importance of the biological roles of carbohydrates.
Introduction
Stromal-derived factor 1 (SDF-1) is a highly potent chemoattractant both in vitro and in vivo for mature leukocytes and hematopoietic progenitor/stem cells (HPCs), which carry its receptor CXCR4.1-7 This highly conserved chemokine is constitutively expressed by virtually all tissues,8including bone marrow (BM).3 It is expressed as 2 alternatively spliced isoforms, the predominant α form and the β form containing 4 additional amino acids at the C terminus, each possessing a heparin-binding domain.9,10 The SDF-1–CXCR4 interaction plays a dominant role in hematopoiesis, and mice deficient in either gene die in utero exhibiting defects in B-cell lymphopoiesis and BM myelopoiesis.4,5 Additionally, a critical role for CXCR4 on human cells in engraftment to the BM of nonobese diabetic/severe combined immunodeficiency mice6,7 has been shown. Although involvement of SDF-1 in mobilization—the egress of HPCs from the BM to the peripheral blood (PB)—has also been speculated, direct evidence has only recently been obtained in mice using a synthetic SDF-1 analog11 or following injection with an adenovirus expressing human SDF-1.12
Previously, we reported that the sulfated polysaccharide fucoidan (FucS) and the structurally similar dextran sulfate (DexS) can elevate circulating white blood cells (WBCs) and mobilize HPCs within hours in a selectin-independent manner in mice and nonhuman primates.13 A subsequent report has confirmed FucS-induced mobilization in mice.14 We now report that these sulfated glycans also dramatically increase the plasma concentrations of SDF-1 in both monkeys and mice, in the latter coinciding with decreased levels of BM SDF-1. Using in vitro studies and gene-deficient mouse models, we investigated the mechanism of SDF-1 release and provide evidence for its etiologic involvement in sulfated glycan–induced mobilization.
Materials and methods
Reagents
Commercially available glycans were purchased from Sigma Chemical (St Louis, MO). DexS was cell-culture grade, and FucS tested negative for endotoxins (Aldevron, Fargo, ND). Granculocyte colony-stimulating factor (G-CSF) (filgrastim) was from Amgen (Thousand Oaks, CA). A fucosylated chondroitin sulfate15 was a kind gift from Dr P. A. S. Mourão, Institute de Ciências Biomédicas, Brazil. Matrix metalloproteinase inhibitors (MMP) BB94 16 and AG3340 17 were kindly provided by Dr A. Janowska-Wieczorek, University of Alberta, BC, Canada. The monoclonal antibodies specific for SDF-1β (BAF351) or SDF-1α (BAF310) used in in vitro assays (with capture antibody MAB350) or in vivo studies were from R&D Systems (Minneapolis, MN).
Animals and treatment schedules
BDF1 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and 129S7SvEv and B6/129 mice from Taconic Labs (Ventura, CA). G-CSF receptor (GCSFR)−/−mice18 were kindly provided by Dr D. Link and MMP9−/− mice19 by Dr R. Senior, both of Washington University School of Medicine (St Louis, MO), and monocyte chemoattractant protein (MCP)1−/− mice20 by Dr B. Rollins, Harvard Medical School, Boston, MA. Mice were housed at the specific pathogen-free facility of the University of Washington, approved by the American Association for the Accreditation of Laboratory Animal Care. For mobilization studies, mice were injected intravenously with saccharides diluted in phosphate-buffered saline (PBS++) (with Ca++ and Mg++). Metalloproteinase inhibitors were diluted in PBS++ and injected intraperitoneally immediately before injection with FucS. Inhibiting antibodies were diluted in PBS++ and coinjected with FucS. Treatment and mobilization in mice treated with G-CSF or G-CSF plus Flt3 ligand have been published previously.21 PB was collected into preservative-free heparin from the retro-orbital plexus of anesthetized animals.
Primates were housed in the accredited Regional Primate Research Center at the University of Washington, and protocols were approved by the Institutional Review Board and by the Animal Care and Use Committee. Treatment and dosing schedules for mobilization studies performed in primates have been published13 21-23: 100 mg/kg FucS or DexS, 100 μg/kg/d G-CSF for 5 days, 100 μg/kg/d G-CSF plus 200 μg/kg/d Flt3 ligand for 5 days, 1 injection of 1 μg/kg interleukin-1β (IL-1β) or antibodies against CD18 (60.3, 2 mg/kg/d for 2 days) or CD49d (humanized HP1/2, 1 mg/kg/d for 3 days).
Clonogenic assays
A total WBC count from an aliquot of anticoagulated mouse blood was measured using a Coulter counter (Hialeah, FL). The remainder was centrifuged, the plasma removed and frozen, and the cells cultured in methylcellulose-containing media with growth factors as previously described.21 Colonies were counted based on morphologic criteria under a dissecting microscope. All colonies (erythroid burst-forming units; granulocyte, erythroid, macrophage, and megakaryocyte colony-forming units; and granulocyte macrophage colony-forming units) were totaled and reported as colony-forming cells (CFCs). Granulocytic cells in PB were determined by direct staining with fluorescent-labeled GR-1 antibody from BD-Pharmingen (La Jolla, CA) and evaluated using a flow cytometer (Becton Dickinson, San Jose, CA).
Complete blood cell counts with chemistries were performed on ethylenediaminetetraacetic acid blood samples from primates. A second blood sample, drawn into preservative-free heparin, was centrifuged, and the plasma was removed and stored at −80°C. Clonogenic assays were performed on the remaining pellet and the results published elsewhere.13,21 22
Plasma cytokine and chemokine assays
SDF-1 levels in murine or primate plasma from PB or BM were analyzed by enzyme-linked immunosorbent assay at the Cytokine Analysis Laboratory, Fred Hutchinson Cancer Center, Seattle, WA, using antibodies and protocols from R&D Systems. Samples in BM were prepared for analysis by flushing the contents of 2 femurs directly into 0.4 mL sample buffer (0.1% BSA, 0.05% Tween 20 in 20 mM Trizma base, 150 mM NaCl, pH 7.3) and centrifuged. Supernatants were then analyzed for SDF-1 concentrations, diluting further as needed.
In vitro SDF-1 binding studies
Heparin binding studies were performed as previously described in detail.9 10 Briefly, varying concentrations of synthetic SDF-1, kindly provided by Dr Francoise Baleux, Pasteur Institute, were incubated with or without sulfated glycans and then reacted with heparin immobilized on a Biacore sensorchip (Uppsala, Sweden). Affinities were evaluated using BIA evaluation software (Biacore).
Results
Treatment with sulfated glycans increases plasma levels of SDF-1 in mice
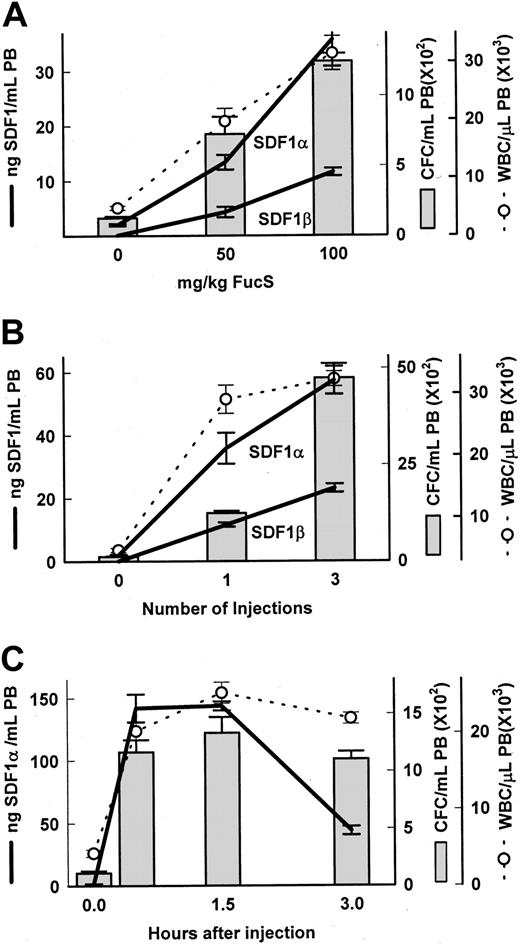
To explore the mechanism involved in selectin-independent sulfated glycan–induced mobilization, and taking into account the fast kinetics of mobilization, we considered the involvement of chemokines. Because SDF-1 is a potent chemoattractant for hematopoietic cells, we examined the plasma levels of SDF-1 in animals treated with FucS. The high degree of homology between the mouse and human proteins allowed us to use commercially available antibodies in an enzyme-linked assay system to directly measure SDF-1 concentrations. Dose-dependent increases of the chemokine were apparent in PB of BDF1 mice 3 hours after one injection of FucS, with peaks in animals treated with 100 mg/kg averaging 35.8 ng/mL for SDF-1α and 11.6 ng/mL for the SDF-1β isotype, rising from baselines of 2 ng/mL or negligible, respectively (Figure 1A). These increases corresponded to dose-dependent increases in both CFCs and WBCs (Figure 1A). When treatment was increased to 100 mg/kg once a day for 3 days, the levels of both SDF-1α and SDF-1β were comparably higher at 57.4 and 23.1 ng/mL, respectively, 3 hours after the last injection and were associated with even higher increases in circulating CFCs and WBCs (Figure 1B). In an additional experiment with 4 mice (data not shown), a single injection of 100 mg/kg resulted in increases at 3 hours in SDF-1α averaging 29.0 ± 0.5 ng/mL, with increases of CFCs and WBCs of 549 ± 111/mL and 14.5 × 109 ± 2.2 × 109/L (14.5 × 103 ± 2.2 × 103/μL), respectively. All of these values also increased significantly (compared with one injection) after treating these animals a total of 3 times with 100 mg/kg/d and bleeding at 3 hours after the last injection (SDF-1α 46.5 ± 6.5 ng/mL, P < .05; CFCs 3855 ± 802/mL, P < .001; and WBCs 26.4 × 109 ± 2.2 × 109/L,P < .05). Thus, a total of 21 mice in 6 experiments were injected once with 100 mg/kg, and all exhibited an average increase in SDF-1α from baseline less than 2 ng/mL to 41.2 ± 2.8 ng/mL with accompanying increases in circulating CFCs (1049 ± 99/mL) and WBCs (24.1 × 109 ± 1.9 × 109/L). A total of 19 mice in 3 experiments treated with 3 injections of 100 mg/kg over 3 days and bled 3 hours after the last injection also had increased levels of SDF-1α (68.7 ± 7.1 ng/mL), CFCs (3630 ± 332/mL), and WBCs (32.7 × 109 ± 2.2 × 109/μL) in PB. DexS at a dose of 50 mg/kg was also able to elicit increases in plasma levels of SDF-1α (9.4 ± 1.3 ng/mL) and SDF-1β (4.2 ± 0.8 ng/mL) after 1 injection and even higher (25.5 ± 2.5 and 8.7 ± 1.6 ng/mL, respectively) after 3 injections, indicating that the ability to increase SDF-1 levels, as well as mobilize HPCs,13 is shared by this related glycan.
Increases in SDF-1, CFCs, and WBCs in blood after FucS treatment.
Plasma levels of SDF-1α and SDF-1β (heavy lines), total WBCs (open circle and dashed line), and CFCs (bars) were measured in untreated BDF1 mice or after treatment with FucS. (A) Mice were intravenously injected with 50 or 100 mg/kg FucS and bled at 3 hours. Differences were statistically significant by the Student t test between groups treated with 50 or 100 mg/kg (n = 5 per group, CFCs and WBCs,P < .01; SDF-1α, P < .005; SDF-1β,P < .001). (B) Mice were bled 3 hours after the last of 1 or 3 intravenous injections of 100 mg/kg FucS at 1 injection per day. Differences were statistically significant between groups treated once or thrice (n = 5 per group, SDF-1α, P < .02; CFCs,P < .000 01; WBCs and SDF-1β,P < .0001). All treated to untreated (n = 6) values were significant (P < .000 01) in panels A and B. (C) Untreated mice (0 hours) and mice intravenously injected once with 100 mg/kg FucS were bled at the indicated times. All treated to untreated values were significant statistically (n = 5 per group;P < .0001). Error bars represent the mean ± SEM.
Increases in SDF-1, CFCs, and WBCs in blood after FucS treatment.
Plasma levels of SDF-1α and SDF-1β (heavy lines), total WBCs (open circle and dashed line), and CFCs (bars) were measured in untreated BDF1 mice or after treatment with FucS. (A) Mice were intravenously injected with 50 or 100 mg/kg FucS and bled at 3 hours. Differences were statistically significant by the Student t test between groups treated with 50 or 100 mg/kg (n = 5 per group, CFCs and WBCs,P < .01; SDF-1α, P < .005; SDF-1β,P < .001). (B) Mice were bled 3 hours after the last of 1 or 3 intravenous injections of 100 mg/kg FucS at 1 injection per day. Differences were statistically significant between groups treated once or thrice (n = 5 per group, SDF-1α, P < .02; CFCs,P < .000 01; WBCs and SDF-1β,P < .0001). All treated to untreated (n = 6) values were significant (P < .000 01) in panels A and B. (C) Untreated mice (0 hours) and mice intravenously injected once with 100 mg/kg FucS were bled at the indicated times. All treated to untreated values were significant statistically (n = 5 per group;P < .0001). Error bars represent the mean ± SEM.
To further assess the kinetics of the SDF-1 increases and the associated mobilization, we studied mice over a 3-hour period after a single injection of FucS. We found increases in SDF-1α levels, rising as early as 0.5 hours to an average of 142 ng/mL, remaining high over the next hour, and eventually beginning to fall by 3 hours to 44 ng/mL (Figure 1C). Furthermore, early increases were also accompanied by high numbers of WBCs and CFCs circulating in the periphery. WBC counts rose from a baseline of 4.3 × 109/L to 20.2 × 109/L (4.3 × 103/μL to 20.2 × 103/μL) at 0.5 hours, increased significantly (P < .05) at 1.5 hours to 25.2 × 109/L, and remained elevated at 3 hours. CFCs in PB also increased as early as 0.5 hours, peaking at 1.5 hours to 1333/mL (12-fold over untreated controls) and were still high at 3 hours. Similar time-dependent results were apparent in another mouse strain (129S7SvEv) at 2 hours after a single injection with the SDF-1α levels, increasing from a baseline of 2 ng/mL to an average 133 ± 11 ng/mL, remaining high at 3 hours at 117 ± 1 ng/mL, with CFCs of 1361 ± 206/mL and 1298 ± 313/mL at 2 and 3 hours, respectively.
These data illustrate the ability of FucS to induce and sustain high plasma levels of SDF-1 in a dose- and time-dependent fashion with corresponding increases in circulating CFCs and WBCs. The immediate increase at 0.5 hours implies a mechanism of quick release of the chemokine rather than de novo synthesis.
FucS competitively binds to SDF-1 at its heparin-binding domain, a likely mechanism of release
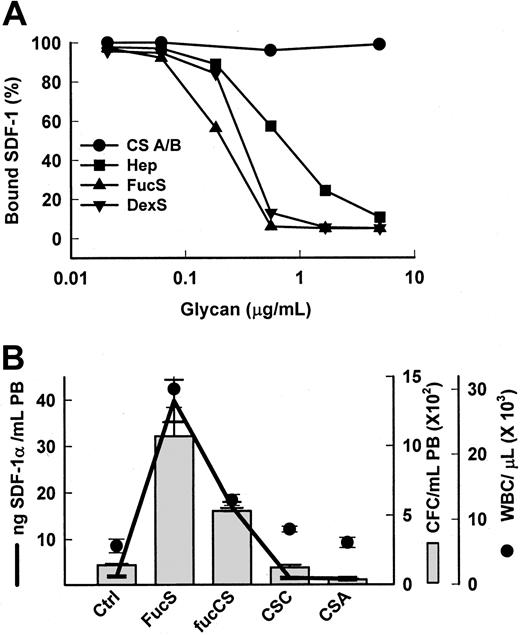
Chemokines or cytokines,24-30 including SDF-1,9,10 are anchored to proteoglycans (PG) on the membrane of stromal cells, endothelial cells, or the extracellular matrix. This occurs via binding between a specific sequence of positively charged amino acids termed the heparin-binding domain (HBD) on the protein and specific, negatively charged chains of heparan sulfate (HS) on the PG25 for SDF-1 containing essential 2-O and N-sulfate groups.10 Heparin competitively inhibits cytokine/chemokine binding to HBD in vitro,9,25,27,28releases them from cells,27 and alters their activities in vivo.26,29,30 FucS, which carries mainly 2-O sulfate groups,31 has been shown in vitro to bind to HBD on some chemokines/cytokines similarly, or even more strongly, than heparin,28 providing a potential mechanism of release for these proteins. Using a Biacore sensorchip, we determined that both FucS and DexS bound to SDF-1 in vitro in a dose-dependent manner and inhibited its binding to immobilized heparin better than soluble heparin (Figure 2A). In contrast, chondroitin sulfates A and B, glycans that generally do not bind to HBD, did not bind SDF-1 or inhibit SDF-1/heparin binding (Figure 2A). Correspondingly, chondroitin sulfates also had no effect on SDF-1α levels in vivo (Figure 2B) and, moreover, were unable to mobilize CFCs or increase WBCs in the PB (Figure 2B).13 However, the presence of a sulfated fucose chain, as found in a fucosylated chondroitin sulfate (fucCS) isolated from echinoderm,15confers both SDF-1 releasing activity and mobilizing capability to chondroitin sulfate. Mice treated with this fucCS exhibited significantly increased plasma levels of SDF-1α averaging 17 ng/mL at 3 hours accompanied by a 4-fold rise in circulating CFCs to 532 per mL and a 2.4-fold increase in WBCs to 13.0 × 109/L (13.0 × 103/μL) (Figure 2B). The results of these 2 experiments bolster the hypothesis that, in vivo, certain sulfated glycans specifically displace sequestered chemokines/cytokines, especially SDF-1, from HSPG anchors, increasing circulating levels.
Specific sulfated glycans bind to SDF-1 in vitro and release SDF-1 in vivo.
(A) Inhibition of SDF-1α/heparin binding by sulfated glycans. SDF-1α (100 nM) was coincubated with sulfated glycans in increasing concentrations and then injected over a heparin-activated sensorchip. CS A/B indicates chondroitin sulfates A or B; Hep, heparin. (B) Increases in SDF-1α levels (line) in mice 3 hours after one intravenous injection of 100 mg/kg FucS, (n = 8), fucosylated chondroitin sulfate (fucCS) (n = 3), or chondroitin sulfate C or A (CS C/A, n = 3 per group) were associated with increases in CFCs (bars) and WBCs (circles) as compared with untreated controls (n = 11). The error bars represent the mean ± SEM. All values compared with untreated controls were statistically significant by the Student t test for FucS (P < .000 001 for all) and fucCS (CFCs, P < .02; WBCs,P < .01; SDF-1α, P < .000 001). Ctrl indicates control mice.
Specific sulfated glycans bind to SDF-1 in vitro and release SDF-1 in vivo.
(A) Inhibition of SDF-1α/heparin binding by sulfated glycans. SDF-1α (100 nM) was coincubated with sulfated glycans in increasing concentrations and then injected over a heparin-activated sensorchip. CS A/B indicates chondroitin sulfates A or B; Hep, heparin. (B) Increases in SDF-1α levels (line) in mice 3 hours after one intravenous injection of 100 mg/kg FucS, (n = 8), fucosylated chondroitin sulfate (fucCS) (n = 3), or chondroitin sulfate C or A (CS C/A, n = 3 per group) were associated with increases in CFCs (bars) and WBCs (circles) as compared with untreated controls (n = 11). The error bars represent the mean ± SEM. All values compared with untreated controls were statistically significant by the Student t test for FucS (P < .000 001 for all) and fucCS (CFCs, P < .02; WBCs,P < .01; SDF-1α, P < .000 001). Ctrl indicates control mice.
SDF-1α levels are reduced in BM of FucS-treated animals
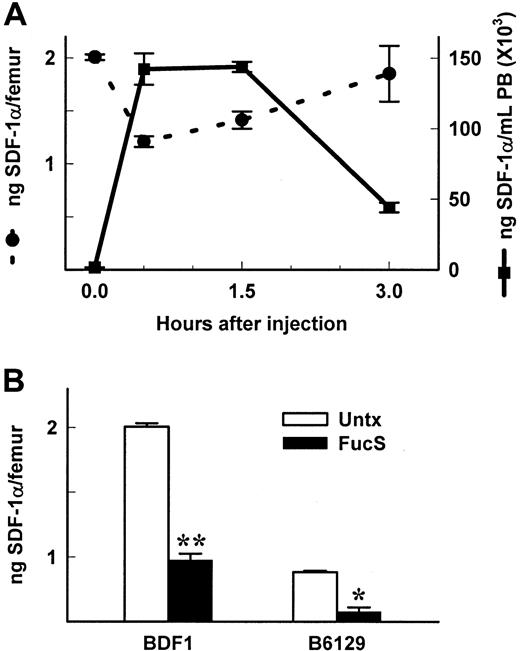
SDF-1α is known to be constitutively expressed in the BM3 and elsewhere.8 We postulated that release of SDF-1 would occur in the BM (as well as other tissues), thereby setting up a gradient favorable to the PB. We measured levels of SDF-1α in femurs of mice treated with FucS. At various time points after one injection of 100 mg/kg FucS, PB from mice was assayed for progenitor levels, WBC counts, and SDF-1α levels (Figure 1C). Femurs and tibiae were removed at the same time, and total cellularity, progenitor content, and SDF-1α levels were measured. Correlating with the time-dependent increases in SDF-1α in PB plasma, SDF-1α levels in BM rapidly decreased (Figure 3A) to 60% compared with levels in untreated mice (P < 0.000 01) at 0.5 hours. After this initial drop, levels began to rise again at 1.5 hours to 70% of controls, approaching baseline levels by 3 hours. This scenario likely reflects a quick release followed by up-regulated expression of the chemokine. The decline in BM SDF-1 levels was accompanied by a decrease in total cellularity to 66% compared with controls (P < .05) at 0.5 hours and 64% at 1.5 hours (P < 0.05) with a nonsignificant loss of progenitors to 82% at 0.5 hours. After 3 injections over 3 days, the 3-hour levels of SDF-1 in the PB were further increased (Figure 1B). Correspondingly, SDF-1 levels in BM of BDF1 or B6/129 mice treated 3 times (Figure 3B) were also reduced to 48.7% and 60%, respectively, as compared with untreated controls. These data provide evidence that SDF-1α is rapidly released from the BM into the periphery, generating a disturbance in the SDF-1α gradient. The evidence gives further credence to the theory that both a decrease in extracellular matrix or membrane presentation of SDF-1 within BM and an increase of soluble SDF-1 in PB are causally related to the movement of progenitors and mature cells from the BM into the PB.
Levels of SDF-1 are reduced in BM of FucS-treated animals.
(A) SDF-1α levels in BM (circles and dashed lines) and PB (squares and solid lines) taken from the same BDF1 mice shown in Figure1C. Values at 0.5 and 1.5 hours compared with untreated controls (n = 5 per group) were statistically significant by the Studentt test for BM (P < .0001) and PB (P < .000 001) and at 3 hours for PB (n = 5;P < .000 001) but not for BM (n = 3). (B) SDF-1α levels in BM of BDF1 or B6/129 mice untreated (open bars) or 3 hours after the last of 3 intravenous injections of 100 mg/kg/d (closed bars). Values of treated compared with untreated controls were statistically significant by the Student t test (*P < .001, **P < .000 001, all groups n = 5).
Levels of SDF-1 are reduced in BM of FucS-treated animals.
(A) SDF-1α levels in BM (circles and dashed lines) and PB (squares and solid lines) taken from the same BDF1 mice shown in Figure1C. Values at 0.5 and 1.5 hours compared with untreated controls (n = 5 per group) were statistically significant by the Studentt test for BM (P < .0001) and PB (P < .000 001) and at 3 hours for PB (n = 5;P < .000 001) but not for BM (n = 3). (B) SDF-1α levels in BM of BDF1 or B6/129 mice untreated (open bars) or 3 hours after the last of 3 intravenous injections of 100 mg/kg/d (closed bars). Values of treated compared with untreated controls were statistically significant by the Student t test (*P < .001, **P < .000 001, all groups n = 5).
Increases in plasma levels of SDF-1 are unique to treatment with sulfated glycans
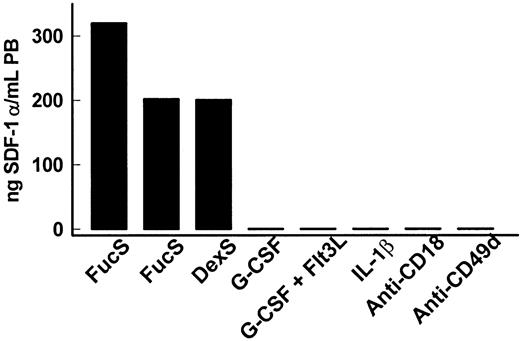
We have previously reported that in primates a single injection of FucS and DexS mobilized HPCs and increased circulating WBCs (with augmentation in granulocytes, monocytes, and lymphocytes), with associated increases in plasma levels of certain chemokines/cytokines.13 Plasma from these same animals were also tested for SDF-1. We found that SDF-1 levels increased dramatically from undetectable baseline values. In one FucS-treated (100 mg/kg, 1 injection) Macaca nemestrina, SDF-1α levels rose as early as 1 hour after treatment to 54 ng/mL, increasing steadily over time and peaking at 6 hours at 202 ng/mL (Figure4), with SDF-1β increasing to 45 ng/mL. A second M nemestrina similarly treated with FucS exhibited even higher increases of SDF-1α to 320 ng/mL at 6 hours (Figure 4). Plasma from an M nemestrina treated with 100 mg/kg DexS was also tested and found to have SDF-1α levels of 200 ng/mL (Figure 4) and SDF-1β to 44 ng/mL 6 hours after treatment. In plasmas taken from animals 24 hours after treatment and no longer exhibiting increased CFCs, SDF-1 levels had dropped to undetectable baseline values.
SDF-1 levels increase in primates treated with sulfated glycans but not other mobilizing agents.
SDF-1α levels in primates intravenously injected with 100 mg/kg FucS or DexS or injected with G-CSF, G-CSF plus Flt3 ligand, IL-1β, or antibodies against CD18 or CD49d. Plasma levels of SDF-1α (black bars) peaked at 6 hours with maximum HPC mobilization in 3 M nemestrina treated with sulfated glycans. Other treated primates were tested for SDF-1α when increased numbers of CFCs were highest:M nemestrina treated with G-CSF on day 6, G-CSF/Flt3 ligand on day 5, anti-CD18 on day 3, and baboons treated with humanized anti-CD49d on day 8 or with IL-1β at 2 and 6 hours. The primates were also tested for SDF-1β, but all values remained at baseline levels at all time points tested (G-CSF at 0, 3, 4, 5, 6 days; G-CSF/Flt3 ligand at 0, 4, 5, 6 days; IL-1β at 0, 2, 6, 12 hours, 5 or 7 days; anti-CD18 at 0, 2, 3, 4, 8 days; anti-CD49d at 0, 5, 7 days).
SDF-1 levels increase in primates treated with sulfated glycans but not other mobilizing agents.
SDF-1α levels in primates intravenously injected with 100 mg/kg FucS or DexS or injected with G-CSF, G-CSF plus Flt3 ligand, IL-1β, or antibodies against CD18 or CD49d. Plasma levels of SDF-1α (black bars) peaked at 6 hours with maximum HPC mobilization in 3 M nemestrina treated with sulfated glycans. Other treated primates were tested for SDF-1α when increased numbers of CFCs were highest:M nemestrina treated with G-CSF on day 6, G-CSF/Flt3 ligand on day 5, anti-CD18 on day 3, and baboons treated with humanized anti-CD49d on day 8 or with IL-1β at 2 and 6 hours. The primates were also tested for SDF-1β, but all values remained at baseline levels at all time points tested (G-CSF at 0, 3, 4, 5, 6 days; G-CSF/Flt3 ligand at 0, 4, 5, 6 days; IL-1β at 0, 2, 6, 12 hours, 5 or 7 days; anti-CD18 at 0, 2, 3, 4, 8 days; anti-CD49d at 0, 5, 7 days).
To test whether SDF-1 increases are unique to sulfated glycan treatment or can be observed in other mobilization schemes, plasmas from animals treated with known mobilizing agents21-23 were tested. In contrast to the remarkable increases seen in sulfated glycan–treated animals, enhanced SDF-1 levels were not found in primates after mobilization treatment with G-CSF, combined G-CSF/Flt3 ligand, IL-1β, or anti-integrin (CD18 or CD49d) antibodies. Neither SDF-1α nor SDF-1β rose above 1 ng/mL in these animals, although multiple time points in 2 primates were measured for each treatment (Figure 4). Four mice treated with G-CSF and 2 mice treated with G-CSF/Flt3 ligand to induce mobilization also exhibited no increases in plasma SDF-1 levels from baseline levels of less than 2 ng/mL. These data strongly suggest that SDF-1 release is, in fact, uniquely associated with specific sulfated glycan treatment and unveil a novel means of SDF-1 release from its physiologic anchors in BM or other tissues.
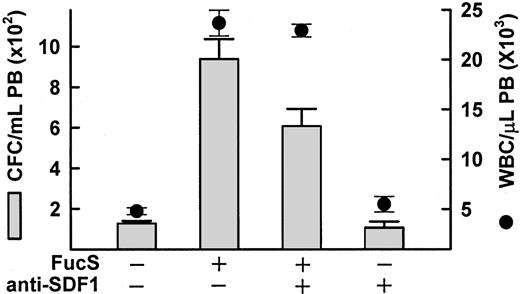
Anti–SDF-1 antibody inhibits FucS-induced mobilization
Because mice lacking either SDF-1 or CXCR4 do not survive beyond birth, these mice are not an option for studying the effects of FucS-induced SDF-1 increases on mobilization. To test whether the inhibition of SDF-1 inhibits sulfated glycan–induced mobilization, we used an anti–SDF-1 antibody with and without FucS. Because plasma SDF-1 concentrations reach enormous levels after treatment with FucS, we used the lower dose of 50 mg/kg. In the presence of 2 mg/kg anti–SDF-1, FucS-induced mobilization decreased by 35% from an average CFCs of 938/mL to 607/mL (P < .001; Figure5). There was no significant decrease, however, in the WBC counts. It is unlikely that all the SDF-1α available was bound by the antibody. SDF-1α is thought to dimerize in solution and may not be as readily recognized by the antibody in vivo, or the FucS may interfere with the antibody recognition (see “Discussion”). Nevertheless, mobilization was partially inhibited by the antibody, indicating that the plasma level increases of SDF-1 resulting from FucS treatment are at least in part responsible for mobilization.
Inhibition of FucS-induced mobilization by anti–SDF-1 antibodies.
Mobilization of CFCs (bars) or WBCs (circles) in PB of BDF1 mice 3 hours after one intravenous injection of 50 mg/kg FucS in the absence or presence of 2 mg/kg anti–SDF-1 antibody as compared with untreated animals (n = 6) or animals treated with antibody alone (n = 3). The inhibition of FucS-induced mobilization of CFCs in the presence of antibody was statistically significant (n = 5 for each group,P < .05) by the Student t test.
Inhibition of FucS-induced mobilization by anti–SDF-1 antibodies.
Mobilization of CFCs (bars) or WBCs (circles) in PB of BDF1 mice 3 hours after one intravenous injection of 50 mg/kg FucS in the absence or presence of 2 mg/kg anti–SDF-1 antibody as compared with untreated animals (n = 6) or animals treated with antibody alone (n = 3). The inhibition of FucS-induced mobilization of CFCs in the presence of antibody was statistically significant (n = 5 for each group,P < .05) by the Student t test.
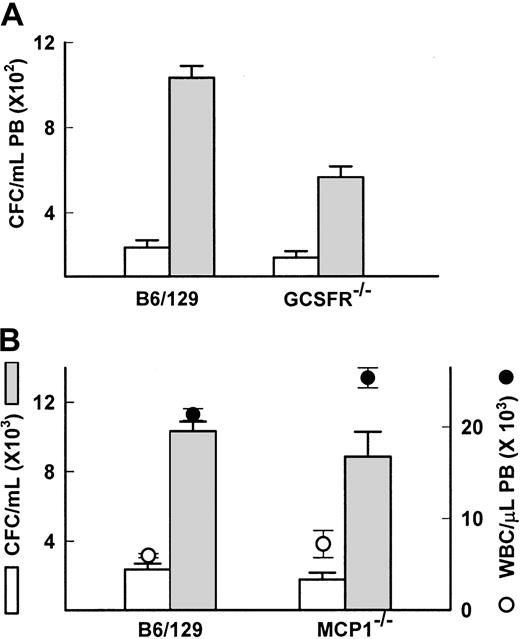
Studies in gene-deficient models indicate that other chemokines or cytokines released by FucS are not necessary for mobilization
In the primates treated with the sulfated glycans, we observed and reported increases in plasma levels of the chemokines IL-8, IL-6, and MCP1, and the cytokines kit ligand (KL), G-CSF, and macrophage (M)-CSF.13 The increases were transient, mostly peaking at 3 hours after injection, except KL, which remained elevated for 24 hours in the FucS-treated animals but did not increase in the DexS-treated animals. Although IL-6, KL, and G-CSF are known to induce mobilization, their kinetics of HPC mobilization are slow, acting over days.32-34 The rapid kinetics of the sulfated glycans in inducing mobilization resemble more those of chemokines such as IL-8.33-36 IL-8 itself increased as high as 4.1 ng/mL in glycan-treated primates.13 IL-8 is known to require functionally competent neutrophils expressing the GCSFR for chemotactic response37 and will not mobilize neutrophils or HPCs in GCSFR-deficient mice.18 To determine if IL-8 increases were responsible for FucS-induced mobilization, we examined GCSFR−/− mice for response to FucS. Both mature leukocytes and stem/progenitor cell numbers increased in PB of GCSFR−/− mice 3 hours after a single injection of FucS. The average values for the treated GCSFR−/− mice appeared to be less than its wild-type control (565 vs 1033 CFCs/mL), but the average-fold increase in individual mice for GCSFR−/−(4.0 ± 1.0-fold) and wild-type (4.6 ± 0.5-fold) mice did not differ significantly (Figure 6A). The WBCs, on the other hand, had similar counts after treatment, but the average increase per mouse in the wild-type (3.6 ± 0.1-fold) was significantly higher (P < .000 01) than the deficient mice (1.9 ± 0.1-fold). SDF-1 levels in treated GCSFR−/− mice were significantly higher (97 ± 8 ng/mL) than in the wild-type controls (38 ± 3 ng/mL,P < .001), possibly due to a decrease in SDF-1 inactivation by granulocytic enzymes.38 In GCSFR−/− mice treated 3 times, the CFC increases did not differ significantly either in values (1208 ± 133 CFCs/mL for GCSFR−/− and 1428 ± 221 CFCs/mL for wild type) or in-fold difference (10.0 ± 2.1-fold and 6.5 ± 1.4-fold, respectively). In contrast, the WBC increase remained significantly lower (P < .002) in the deficient mice (2.9 ± 0.05-fold) than in the wild type (7.5 ± 1.3-fold). Despite the differences mentioned here, the data with the GCSFR−/− mice make it unlikely that IL-8 is responsible for the mobilization induced by FucS.
IL-8, G-CSF, and MCP1 are not responsible for FucS-induced mobilization.
(A) CFCs in PB of wild-type B6/129 controls (n = 4) or GCSFR−/− mice (n = 9) before (open bars) or after one intravenous injection of 100 mg/kg FucS (closed bars). Differences were statistically significant by the Student t test between untreated and treated (P < .0001) in both groups. (B) Total numbers of WBCs (circles) and CFCs (bars) in PB of wild-type B6/129 controls (n = 4) or MCP1−/− mice (n = 5) before (open circles and bars) or after one intravenous injection of 100 mg/kg FucS (closed circles and bars). Error bars represent the mean ± SEM. Differences were statistically significant by the Student t test between untreated and treated for both CFCs and WBCs (P < .0001) in both groups but not between wild-type and MCP1−/−.
IL-8, G-CSF, and MCP1 are not responsible for FucS-induced mobilization.
(A) CFCs in PB of wild-type B6/129 controls (n = 4) or GCSFR−/− mice (n = 9) before (open bars) or after one intravenous injection of 100 mg/kg FucS (closed bars). Differences were statistically significant by the Student t test between untreated and treated (P < .0001) in both groups. (B) Total numbers of WBCs (circles) and CFCs (bars) in PB of wild-type B6/129 controls (n = 4) or MCP1−/− mice (n = 5) before (open circles and bars) or after one intravenous injection of 100 mg/kg FucS (closed circles and bars). Error bars represent the mean ± SEM. Differences were statistically significant by the Student t test between untreated and treated for both CFCs and WBCs (P < .0001) in both groups but not between wild-type and MCP1−/−.
The chemokine MCP1 was the only other chemokine to increase above 10 ng/mL upon treatment with FucS, rising as high as 86 ng/mL, although DexS elicited only modest increases. This chemokine is known to have chemotactic properties. To explore the possibility that it may have a role in FucS-induced mobilization, we treated mice deficient in the gene for MCP1.20 We found that 3 hours after a single treatment with 100 mg/kg FucS, both WBCs and CFCs were elevated in these animals to nearly identical levels as in their wild-type controls (Figure 6B). Average WBC counts for MCP1−/− mice increased 3.5-fold and for wild type 3.6-fold. Baseline CFCs in the MCP1−/− mice were lower than their wild-type controls but increased 5-fold comparable to wild-type mice, which increased 4.4-fold. As in other strains, SDF-1α levels in both strains rose from baseline values below 3 ng/mL to 40 ± 1 ng/mL for wild-type and a slightly higher 58 ± 6.8 ng/mL for the MCP1−/− mice 3 hours after treatment, although this difference was not significant. After 3 injections, increases in both CFCs and WBCs remained equivalent as well (data not shown). This experiment provides conclusive evidence that MCP1 is not involved in the mobilization of CFC or in WBC increases induced by the FucS.
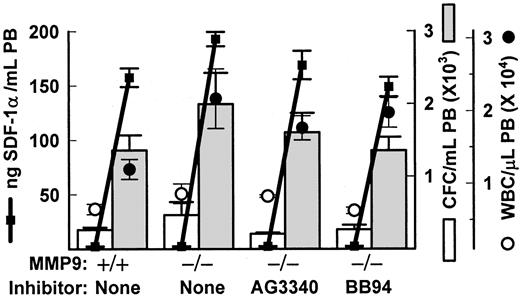
FucS-induced mobilization and SDF-1 release does not require metalloproteinases
Previous in vivo studies with inhibiting anti-MMP9 antibodies have concluded that mobilization of HPCs induced by the CXC chemokines IL-834 or GROβ35 can be attributed to increases in MMP9. We also observed increases in MMP9 activity after sulfated glycan treatment, especially in monkeys.13 To examine the importance of this protease in FucS-induced mobilization, we treated mice lacking the gene for MMP919 with FucS. These mice responded equally well to FucS as did their wild-type controls 2 hours after treatment of 100 mg/kg, with CFCs per milliliter averaging 5.4-fold and 5.3-fold higher from their untreated values, respectively (Figure 7). The total WBCs increased more in the deficient animals (2.8-fold) than controls (1.9-fold; Figure 7), but this was not statistically significant for either total numbers or ratios, and such a differential increase was not apparent in animals at 3 hours after a single injection. In 2 additional experiments a total of 6 MMP9−/− mice bled 3 hours after a single injection of 100 mg/kg FucS did not differ significantly from 6 wild-type controls in CFCs (3.0 ± 1.1-fold vs 4.1 ± 0.8-fold increase) or WBCs (3.4 ± 0.5-fold vs 3.5 ± 0.6-fold increase). MMP9−/− mice also responded to 3 injections of FucS and did not differ significantly from their wild-type controls in numbers of CFCs mobilized or WBC elevations (data not shown).
FucS-induced mobilization occurs in the absence of metalloproteases.
(A) Total numbers of WBCs (circles), CFCs (bars), and SDF-1α levels (solid squares and heavy lines) in PB of wild-type or MMP9−/− mice before (open circles and bars) or 2 hours after one intravenous injection of 100 mg/kg FucS (closed circles and bars) in the absence or presence of metalloprotease inhibitors; n = 3 animals per group. MMP9−/− and wild-type data are representative of 3 separate experiments. All values after treatment were statistically significant to pretreatment values (WBCs and CFCs,P < .05 to P < .005; SDF-1,P < .000 001).
FucS-induced mobilization occurs in the absence of metalloproteases.
(A) Total numbers of WBCs (circles), CFCs (bars), and SDF-1α levels (solid squares and heavy lines) in PB of wild-type or MMP9−/− mice before (open circles and bars) or 2 hours after one intravenous injection of 100 mg/kg FucS (closed circles and bars) in the absence or presence of metalloprotease inhibitors; n = 3 animals per group. MMP9−/− and wild-type data are representative of 3 separate experiments. All values after treatment were statistically significant to pretreatment values (WBCs and CFCs,P < .05 to P < .005; SDF-1,P < .000 001).
It is possible that other metalloproteases, such as MMP2, may be up-regulated in the absence of MMP9 to compensate for its loss. To address this possibility as well as exclude other MMPs acting independently, we coinjected the MMP9−/− mice with FucS and a broad-spectrum metalloproteinase inhibitor, either 100 mg/kg AG3340 or 30 mg/kg BB94, known to inhibit in vivo.17 18 As illustrated in Figure 7, presence of the inhibitors did not impede the increases in circulating CFCs or WBCs induced by FucS in these mice. Average CFC numbers in PB did not differ significantly between groups, increasing by 7.5-fold (AG3340) and 5.4-fold (BB94) over untreated values, similar to values from MMP9−/− and wild-type mice treated in the absence of inhibitors. Elevated WBC numbers were also without remarkable differences in the presence of either inhibitor, as compared with the control groups with AG3340 rising 2.1-fold and BB94 3.2-fold from untreated baselines.
In vitro studies in CD34+ cells have proposed a role for the release of MMP9 in SDF-1 chemotaxis and in cell migration induced by IL-8 and MIP-1α.39 40 We also measured SDF-1 plasma levels in FucS-treated MMP9−/− and wild-type mice. SDF-1 levels were slightly higher in deficient mice (193 ng/mL) than in controls (157 ng/mL) or in mice treated with inhibitors BB94 (149 ng/mL) or AG3340 (163 ng/mL), although the last was not significant (Figure 7). The higher values in MMP9−/− mice are likely due to the absence of this protease, which may cleave SDF-1 or another proenzyme upstream. The inhibitors may also be suppressing some upstream protease inhibitor and/or activators. Although MMP9 and related metalloproteinases do not appear to be necessary for FucS-induced mobilization, a role for other proteases cannot be ruled out and remains a possible mechanistic step in this mobilization.
Discussion
SDF-1, like other chemokines, has a very low molecular weight (8-12 kd) and is easily degraded, making direct studies with the native protein difficult. In this report we document rapid and dramatic increases in circulating SDF-1 following treatment with certain sulfated glycans, ie, FucS, DexS, and fucCS. Equally important, no such SDF-1 increases were seen in other mobilization schemes, thus uncovering a unique mechanistic step elicited by sulfated glycans in the release of SDF-1 into circulation.
The rapid buildup of SDF-1 argues for a mechanism of immediate release of the chemokine from pre-existing sources rather than de novo synthesis. SDF-1, like other chemokines or cytokines, is anchored to the membrane of stromal cells, endothelial cells, or extracellular matrix by specifically binding to HSPG.9,10,24-30Tethering protects chemokines/cytokines from degradation,24,29 increases their local concentrations,9,24,25 and allows them to oligomerize,9,27 facilitating binding to receptors and enhancing receptor-signaling capacity,9,24-27 thereby influencing downstream events, presumably for SDF-1 integrin modulation and cell adhesion or migration.3,4 41 Our in vivo and in vitro data support our thesis that certain sulfated glycans can specifically displace sequestered chemokines/cytokines, especially SDF-1, from their HSPG anchors, leading to their release into circulation. Release is likely followed by increased production and additional displacement, producing a rapid and sustained increase in plasma levels.
SDF-1 plasma levels above 100 ng/mL, as documented here after treatment with sulfated glycans, would be comparable to microgram-per-kilogram doses of other CXC chemokines used for mobilization.35,36Is SDF-1, then, responsible for mobilization induced by sulfated glycans? Several observations presented herein strengthen this view. (1) After treatment with FucS, SDF-1 levels in BM dropped rapidly to 60% accompanied by a drop in total cellularity and total progenitor count. Concurrently, SDF-1 levels in PB plasma rose and mobilization occurred. According to this scenario, both the decrease of bound SDF-1 within BM and the disruption of the physiologic chemokine gradient after release of soluble SDF-1 into the periphery (from BM or other tissues) could trigger mobilization of HPCs and mature cells to the periphery. Nucleated cell numbers in the PB remain high while the circulating SDF-1 levels are sustained, possibly protected from degradation by its association with FucS,24,25,29 or bolstered with additional SDF-1 release. (2) Chondroitin sulfates, which generally do not bind to HBD, did not bind to SDF-1 in vitro, increase SDF-1 levels, or mobilize HPCs. However, a naturally occurring chondroitin sulfate that bears a sulfated fucose chain did elicit both SDF-1 increase and mobilization. (3) Partial inhibition of FucS-induced mobilization was obtained with anti–SDF-1 antibody in mice treated with 50 mg/kg FucS. There are several possible reasons that only partial effects are obtained with anti–SDF-1. It would be difficult to bind and inhibit all the circulating SDF-1, especially if SDF-1 is dimerized,42 possibly aided in this configuration by FucS.9,10,27 Furthermore, the HBD in SDF-1α, to which FucS likely binds, has been determined to involve amino acids 24 to 27 with a role for the N-terminal amino acid.10 The receptor binding site is outside this domain, and HS does not interfere with receptor-ligand interaction.9 However, the antibody used in these studies was raised against the full-length recombinant protein expressed in Escherichia coli and may interact with the HBD, although its exact epitope has not been determined. When a similar antibody was used in capture assays for quantification of SDF-1 in plasmas of FucS-treated animals, binding was also inhibited at low dilutions (< 1:50) and the SDF-1 levels were underestimated. Additionally, higher amounts of anti–SDF-1 antibody administered in the presence of 100 mg/kg FucS still inhibited by 25%, but SDF-1 levels increased slightly, supporting the likelihood that the antibodies were also binding to the HBD. Finally, WBC elevations were not inhibited in the presence of the anti–SDF-1 antibody. This may be related to higher affinities of mature cells than HPCs for SDF-1 when competing with the antibody or related to their source of release (marginal tissue pools vs BM) or their egress under distinct mechanistic steps.
Recently, 2 reports of engineered increases in SDF-1 have been associated with mobilization.11,12 First, increases in circulating progenitors were noted 24 to 48 hours after injection of high amounts of an N-terminal–modified analog of SDF-1, MetSDF-1β, which has enhanced functional activity.11 Similar increases were not observed with the use of native SDF-1β, although early kinetics were not examined for either treatment. MetSDF-1β is protected from inactivating N-terminal degradation38and/or may induce mobilization by a different mechanism. The authors attribute the cause of mobilization solely to the functional down-modulation of CXCR4 that follows binding of the MetSDF-1. In support of their claim, they cite decreased responsiveness to SDF-1 of G-CSF–mobilized CD34+ cells.2,11 However, numerous, disparate reports of CXCR4 expression on G-CSF–mobilized HPCs in humans have been published,2,3,11,43-45 and reports of relatively lower SDF-1 levels (0.4-0.2 pg/mL) in PB of good G-CSF “mobilizers”44 and those describing inhibition of SDF-1 production in BM 24 hours after G-CSF treatment45create confusion regarding the involvement of SDF-1 in G-CSF mobilization.
In the second study, increases in circulating WBCs and HPCs were observed in nonobese diabetic/severe combined immunodeficiency mice injected with an adenoviral vector expressing recombinant human SDF-1.12 CFU-S12 (spleen colony-forming unit, day 12) peaked at day 5, concurrent with peak SDF-1 plasma levels. However, the progressive increases over several days in the virally expressed SDF-1 and progressive increases in mobilization make it difficult to compare with mobilization kinetics following a single injection of CXC chemokines. Additionally, peak SDF-1 levels achieved are rather low (2.5 ng/mL), equivalent to baseline plasma levels in some strains of mice (Figures 1, 2, 6, and 7), raising the possibility that in this model additional parameters may be at play.
The interpretation of our data with sulfated glycans is complicated by the fact that, in addition to SDF-1, other chemokines (IL-6, MCP1, IL-8) or cytokines (G-CSF, KL, M-CSF) and MMP9 are also increased after FucS treatment.13 MCP1 increased significantly, but studies here in genetically deficient mice make its contribution unlikely. IL-8 and another CXC chemokine, GROβ, are known to mobilize HPCs in mice and primates, and their action is inhibited by anti-MMP9 antibodies.35,36 However, FucS induced mobilization in MMP9-deficient mice. The increase in MMP9 activity observed in primates was not as apparent in mice and may be secondary to increases in IL-8. IL-8 may itself be released from HSPG anchors or from SDF-1–stimulated mast cells46 or from activated neutrophils or endothelial cells. Neutrophils from GCSFR−/− mice do not respond to IL-8 stimulation in vitro,37 and in vivo GCSFR−/− mice do not mobilize with IL-8.18These mice do, however, respond to FucS, although with lower increases of WBCs and HPCs. The reason for this decreased response will require further study but may be inherent to the model's decreased progenitor pool because a similar decrease was apparent in mobilization with Flt-3 ligand.18
In vitro, FucS did not directly activate neutrophils to release either cytokines or MMP9 (data not shown). However, the possibility that a protease other than a metalloprotease is involved in FucS-induced mobilization cannot be excluded. In fact, several promising candidates are now being considered. For example, neutrophil elastase, released upon degranulation, has been implicated in vascular cell adhesion molecule-1 cleavage with subsequent disruption of very late activation antigen-4–vascular cell adhesion molecule-1 interactions followed by mobilization.47 Studies in mice with upstream protease deficiencies, such as those lacking the gene for dipeptidyl peptidase I,48 may shed some light on this issue.
Other cytokines increased by FucS treatment,13 possibly also released from HSPG, are considered unlikely to be causative agents in mobilization, yet they may have supportive roles. For instance, IL-6 and KL both increase CXCR4 expression on progenitors, improving their engraftment in vivo.6 KL can augment the chemotactic properties of SDF-1 in culture by downstream signaling events,49 including integrin function.50Interestingly, though, in vivo studies of combined treatments with FucS and anti–very late activation antigen-4 showed only additive and not synergistic effects, and those with FucS and anti-Mac1 showed no influence of the antibody on FucS-induced mobilization (data not shown).
In summary, we report here that certain sulfated glycans (FucS, DexS, fucCS) dramatically increase and sustain SDF-1 levels in plasma of treated animals. This increase is likely due to competitive displacement of SDF-1 from HSPG, its physiologic anchor within the BM environment, underscoring an important biological role for carbohydrates. A decrease in bound SDF-1 within BM and/or a SDF-1 chemotactic gradient favoring the plasma may induce mobilization of both WBC and stem/progenitor cells. Whether this process requires the cooperation of additional molecules, apart from those already examined, will require further studies.
The authors are grateful to Dr Paulo Mourão, Institute de Ciências Biomédicas, Brazil, for his gift of fucosylated chondroitin sulfate; Dr Anna Janowska-Wieczorek, University of Alberta, for protease inhibitors; and Dr Francoise Baleux, Pasteur Institute, for synthetic SDF-1. Drs Daniel C. Link and Robert M. Senior, both of Washington University School of Medicine, are thankfully acknowledged for providing GCSFR−/− mice and MMP9−/−mice, respectively, and Dr Barrett Rollins, Harvard Medical School for MCP1−/− mice. The authors are also grateful to Dr Robert Winn, University of Washington, and Dr R. R. Lobb, Biogen, for providing anti-CD18 (60.3) and anti-CD49d (humanized HP1/2) antibodies, respectively. The technical assistance of Vivian Zafiropoulos is appreciated.
Fom the Department of Medicine, Division of Hematology, University of Washington, Seattle, WA; and the Institut de Biologie Structurale, Grenoble, France.
Supported by National Institutes of Health grant HL46557.
Submitted June 15, 2001; accepted August 21, 2001.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thalia Papayannopoulou, Div of Hematology, Dept of Medicine, University of Washington, Box 357710, Seattle, WA 98195-7710; e-mail: thalp@u.washington.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal