Germ-line events, such as paternal mutation or genomic imprinting, contribute to the early onset of childhood cancers such as retinoblastoma, Wilms tumors, and neuroblastoma. Given the high frequency of deletion involving chromosome 9p in childhood acute lymphoblastic leukemia (ALL), this study investigated whether 9p deletion might reflect preexisting germ-line gene inactivation. To do this the parental origin of deletion was determined in 10 cases of ALL with 9p21 loss of heterozygosity. Of these cases, 9 showed loss of the maternally derived allele, suggesting that a germ-line event involving a 9p gene may play a role in the onset of childhood ALL.
Introduction
The young age of onset of childhood cancers can often be attributed to a constitutional genetic predisposition. In some cases the predisposition results from the inheritance of a familial mutation, whereas in other cases it reflects the occurrence of de novo germ-line mutation, as observed in some cases of retinoblastoma,1 or the involvement of an imprinted gene, as seen in Wilms tumors and some neuroblastomas.2 Although childhood acute lymphoblastic leukemia (ALL) is seldom familial, we hypothesized that a constitutional predisposition might explain its early age of onset. The chromosome 9p21 region, which containsCDKN2A and CDKN2B, is a candidate region for a predisposing genetic event because deletion of this area is the most common known molecular abnormality in childhood ALL, occurring in 35% to 60% of cases.3 4 To determine whether constitutional modification of a chromosome 9p21 gene might be involved in childhood ALL, we determined the parental origin of the deleted 9p21 allele in a series of 48 cases of childhood ALL.
Study design
A total of 48 children with ALL were recruited at pediatric oncology clinics throughout New Zealand. Archived slides of bone marrow aspirate previously obtained at presentation and at remission were collected after obtaining informed consent in accord with the instructions of regional ethics committees. The age, diagnosis, proportion of blasts, and the reported karyotype are shown in Table1. Given that children were recruited at follow-up clinics up to 3 years after diagnosis, there may have been a bias toward cases with longer survival.
The age, diagnosis, percentage of bone marrow blasts, reported karyotype, and allele ratios (lost-retained allele) at tetranucleotide markers D9S1679, D9S746, and D9S761 for the 10 cases of ALL showing loss of heterozygosity
| Case no. . | Age at diagnosis (y, mo) . | ALL type . | % BM blasts . | D9S1679 . | D9S746 . | D9S761 . | Karyotype . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Ratio . | POL . | Ratio . | POL . | Ratio . | POL . | |||||
| 22 | 2, 4 | Pre-B | >99 | 0.17 | M | 0.06 | M | 0.02 | NI | 56-57,XXY,[cp3]/46,XY[9] |
| 20 | 3, 7 | Pre-B | 95 | 0.27 | M | 0.05 | M | 0.02 | NI | 46,XX[2] |
| 29 | 3, 1 | Common | >90 | 0.13 | M | 0.84 | 0.81 | 46,XY[20] | ||
| 12 | 4, 7 | Common | 95 | 0.02 | ni | 0.08 | M | 0 | M | 53-59,XXXX,+6,+21,inc[cp2]/46,XX[8] |
| 16 | 4, 7 | Common | 95 | NI | 0.1 | M | 0.13 | M | 45,XX,der(9)t(9;21)(p11;q11.1)t(9;22)(q34;q11),-21[12]/ 46,XX[3] | |
| 21 | 1, 7 | Common | 81-96 | 0.1 | M | 0.2 | M | NI | 46,XX[3] | |
| 15 | 4, 9 | Common | 98 | 0.03 | M | NI | NI | 20-40,X,add(11)(p11),inc[cp2]/46,XX[11] | ||
| 17 | 6, 1 | T | 96 | 0.03 | M | 0.02 | M | 0.95 | 46,X,t(X;1)(p22;p32),t(4;17)(p16;q?21),del(6)(q?23), del(9)(p?22),del(17)(p12)[cp8]/46,XX[2] | |
| 53 | 6, 4 | Common | 93 | 0.44 | M | 0.02 | M | 0.04 | M | 50-56[5], poor quality |
| 24 | 2, 10 | Common | >98 | 0 | P | 0.09 | P | 0.1 | P | 46,XX[9] |
| Case no. . | Age at diagnosis (y, mo) . | ALL type . | % BM blasts . | D9S1679 . | D9S746 . | D9S761 . | Karyotype . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Ratio . | POL . | Ratio . | POL . | Ratio . | POL . | |||||
| 22 | 2, 4 | Pre-B | >99 | 0.17 | M | 0.06 | M | 0.02 | NI | 56-57,XXY,[cp3]/46,XY[9] |
| 20 | 3, 7 | Pre-B | 95 | 0.27 | M | 0.05 | M | 0.02 | NI | 46,XX[2] |
| 29 | 3, 1 | Common | >90 | 0.13 | M | 0.84 | 0.81 | 46,XY[20] | ||
| 12 | 4, 7 | Common | 95 | 0.02 | ni | 0.08 | M | 0 | M | 53-59,XXXX,+6,+21,inc[cp2]/46,XX[8] |
| 16 | 4, 7 | Common | 95 | NI | 0.1 | M | 0.13 | M | 45,XX,der(9)t(9;21)(p11;q11.1)t(9;22)(q34;q11),-21[12]/ 46,XX[3] | |
| 21 | 1, 7 | Common | 81-96 | 0.1 | M | 0.2 | M | NI | 46,XX[3] | |
| 15 | 4, 9 | Common | 98 | 0.03 | M | NI | NI | 20-40,X,add(11)(p11),inc[cp2]/46,XX[11] | ||
| 17 | 6, 1 | T | 96 | 0.03 | M | 0.02 | M | 0.95 | 46,X,t(X;1)(p22;p32),t(4;17)(p16;q?21),del(6)(q?23), del(9)(p?22),del(17)(p12)[cp8]/46,XX[2] | |
| 53 | 6, 4 | Common | 93 | 0.44 | M | 0.02 | M | 0.04 | M | 50-56[5], poor quality |
| 24 | 2, 10 | Common | >98 | 0 | P | 0.09 | P | 0.1 | P | 46,XX[9] |
The allele ratios were calculated by using RFLPscan PLUS 3.0 and were normalized against paired remission samples, after electrophoresis on a LI-COR DNA sequencer. BM, bone marrow; POL, parental origin of loss; M, maternal; P, paternal; NI, not informative; T, T-cell lineage; Pre-B, cIg+μB lineage.
DNA was extracted from unfixed archived slides of bone marrow aspirate with the use of a previously published method5 except that 20 mM EDTA was substituted for the 2.5 mM MgCl2 in the extraction buffer, and the proteinase K digestion was performed at 56°C overnight, followed by an additional 2 hours at 56°C with fresh proteinase K. Parental DNA was extracted from peripheral blood leukocytes.
Loss of heterozygosity
Loss of heterozygosity (LOH) was assessed by using 3 polymorphic tetranucleotide repeat markers D9S1679, D9S746, and D9S761 located on 9p, which are currently mapped to approximately 3, 6, and 14 Mb centromeric of CDKN2A,respectively, within the region showing a high frequency of LOH.3 Polymerase chain reaction (PCR) primers used were D9S746, ACAACTGCTGTCACTCACTGA, AACACAGCGAGACTCCATCTC; D9S761, GCCAAGACCACCGCAGTAC, GGAAGGGAACCCCCATACG; and D9S1679, CACCTCTGCCTGCCAA, TGCTGTGGACCTAACAAAAA. Forward primers were labeled with IRD 800. The relative intensities of the microsatellite alleles amplified from presentation bone marrow slides were quantified by using a LI-COR (Lincoln, NE) DNA sequencer model 4000L and normalized against paired remission bone marrow samples. Samples were scored as having LOH when the relative allele intensity was less than 0.3, an arbitrary cutoff level based on the occurrence of LOH in at least 80% of leukemic cells in a sample containing 10% normal cells. By examining the parental microsatellite bands, the origin of the lost allele was determined. Note that in samples with homozygous loss at a marker, there would be no evidence of any deletion because amplified bands will be derived from residual normal bone marrow cells.
Sequencing
Exon 1-β of CDKN2A was amplified by PCR, using primers as previously described,6 with the addition of a T3 sequence 5′ of the forward primer. PCR products were sequenced by using Amersham Thermosequenase cycle sequencing kit with IRD-labeled T3 primer. Sequencing products were visualized on a LI-COR DNA sequencer model 4000L.
Results and discussion
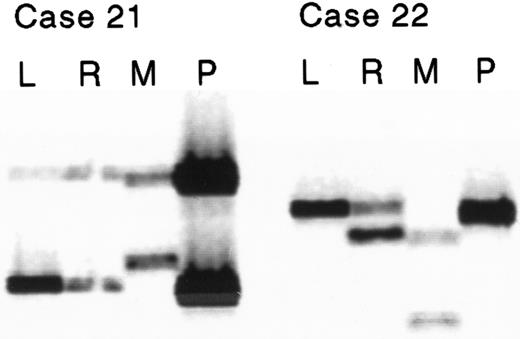
Of the 48 cases of ALL, 10 showed LOH at one or more of the 3 polymorphic markers on chromosome 9p21 (Table 1, Figure1). In 9 of these 10 cases the percentage of leukemic blasts in the bone marrow had been reported as more than 90% (for case 21, 81% blasts were reported). Apart from the results for case 53 at D9S1679, the amount of the deleted allele present was consistent with the proportion of normal cells in the bone marrow aspirate biopsy. The parental origin of the lost allele was determined, and in 9 of the 10 cases with LOH the lost allele was maternally derived (P = .02). In 8 of the 10 cases the parental origin of the lost allele was confirmed at more than one locus.
Two examples showing loss of the maternally inherited allele at marker D9S746.
The lanes show leukemic (L), remission (R), maternal (M), and paternal (P) microsatellite PCR products as imaged on a LI-COR DNA sequencer.
Two examples showing loss of the maternally inherited allele at marker D9S746.
The lanes show leukemic (L), remission (R), maternal (M), and paternal (P) microsatellite PCR products as imaged on a LI-COR DNA sequencer.
The preferential loss of the maternally derived allele in leukemic cells suggests that a parent-specific process modifies chromosome 9p and that germ-line events may play a role in the leukemogenic pathway. This strong bias toward loss of the maternally derived 9p allele is supported by a previous small study by Heyman et al7 in which 4 of 5 cases of childhood ALL showed loss of the maternal allele (combined probability = 0.007).
The identity of the leukemogenic gene or genes in the 9p21 region is not yet certain, although good candidates exist. The region contains genes for at least 3 cell cycle regulatory proteins p15, p16, and p14ARF and there is good evidence for the existence of another tumor suppressor gene (TSG) nearby.8 An additional complexity with respect to this region is that some cases of ALL show hemizygous deletion, whereas others show homozygous deletion. Acquisition of homozygous loss during tumor evolution suggests that nullisomy for 9p21 may not be necessary for leukemia initiation, but that progressive loss of genes may enhance malignant growth.9-11 Because analysis of leukemic cells at clinical presentation will reflect the summation of all previous genomic events, the interpretation of observations from 9p21 requires caution. However, we propose 3 models to explain the parental bias of LOH, each of which is consistent with the involvement of a TSG on chromosome 9p and in each the maternal deletion provides the “second hit,” unmasking prior changes on the paternal allele.
In the first model, maternal LOH unmasks genetic mutation of a 9p TSG on the paternal allele. A precedent for this model is provided by bilateral retinoblastoma in which paternal de novo RBmutations are followed by somatic loss of the maternally derived allele.1 For childhood ALL, mutations of the candidate genes CDKN2A and CDKN2B are rare, although exon 1-β of CDKN2A that encodes the p14ARF protein has not yet been extensively investigated.12-14 Because of this we sequenced exon 1-β in the 10 cases of ALL showing LOH by using primers as previously described,6 but no mutations were detected. However, a model involving genetic mutation would appear to conflict with observations on the lack of heritability of ALL. That is, very few familial cases of ALL have been reported, and no increase in the rate of malignancy in the offspring of children with ALL has been observed.15
A second model that could account for the apparent germ-line origin but the lack of heritability assumes that the paternal mutation is epigenetic. A pathologic epigenetic event, in which a gene is inactivated by inappropriate methylation during spermatogenesis, provides a plausible explanation because epigenetic inactivationCDKN2B, through promoter methylation, has been observed in some cases of ALL. In these cases, about half of the leukemic DNA samples from leukemias with LOH of 9p genes showed methylation of the remaining allele.16
The third model proposes that the paternally derived gene is not inactivated by mutation but by the normal physiologic process of genomic imprinting. A precedent for this model is the preferential deletion of the maternal copy of p73, an imprinted TSG involved in childhood neuroblastoma.2 Although studies in mice provide no evidence to support genomic imprinting ofCdkn2a or Cdkn2b, it is interesting that imbalances between the relative expression levels of CDKN2Aalleles, which might reflect genomic imprinting, have been reported.17
Our data together with previous data from Heyman et al7 provide strong evidence for the involvement of a paternal germ-line factor that predisposes to a proportion of cases of childhood ALL. If this factor involves a pathologic process, such as genetic or epigenetic mutation, then targeted epidemiologic studies of the paternal environment in the subgroup of children with 9p LOH may provide a useful way forward to understanding the environmental factors involved in childhood ALL.
Supported by the Cancer Society of New Zealand, the New Zealand Lottery Grants Board, and the Child Cancer Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ian M. Morison, Cancer Genetics Laboratory, Department of Biochemistry, University of Otago, PO Box 56, Dunedin, New Zealand; e-mail: ian.morison@otago.ac.nz.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal