Low-grade marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) type can transform into high-grade diffuse large B-cell lymphoma (DLBCL). Up to 60% of the MALT lymphomas contain the recently described t(11;18). However, this translocation has not been detected in any DLBCL so far. To elucidate the pathogenesis of these tumors, microsatellite screening of 24 gastric MALT lymphomas was performed and the results were compared with aberrations detected in a previous study on gastric DLBCL. The most frequent aberration, found in 21% of the MALT lymphomas that were exclusively t(11;18)-negative cases, was amplification of the 3q26.2-27 region (harboring the locus of the BCL6 gene). Allelic imbalances in regions 3q26.2-27, 6q23.3-25, 7q31, 11q23-24, and 18q21 were shared by both MALT lymphoma and DLBCL. Loss of heterozygosity in regions 5q21 (APC gene locus), 9p21 (INK4A/ARF), 13q14 (RB), and 17p13(p53) and allelic imbalances in 2p16, 6p23, and 12p12-13 occurred exclusively in DLBCL. Only one of 10 t(11;18)-positive MALT lymphomas showed an additional clonal abnormality. These tumors thus display features of a clonal proliferation characterized by the presence of the t(11;18). However, they only rarely display secondary aberrations and do not seem to transform into DLBCL. In contrast, t(11;18)-negative MALT lymphomas show numerous allelic imbalances—some of them identical with aberrations seen in DLBCL—suggesting that this group is the source of tumors eventually transforming into high-grade DLBCL.
Introduction
Primary extranodal gastric marginal zone B-cell lymphoma (MZBCL) of mucosa-associated lymphoid tissue (MALT) type attracted much attention recently. The disease originates on inflammatory background brought about by a chronic Helicobacter pylori infection that initiates buildup of MALT in originally lymphoid follicle-free stomach. Further development of lymphoma out of the MALT is the result of continuous antigen-dependent growth of B lymphocytes in the early phase that then progresses into a stage of autonomous proliferation of a true low-grade lymphoma. That lymphoma can and in some cases does develop into a high-grade lymphoma.1-3 This sequence of events makes this disease an attractive model on which the development of neoplasia out of a chronic inflammatory disease can be studied.
As in other neoplasms, MALT lymphoma development is marked by a series of genomic aberrations that contribute, step by step, to increasing genomic instability (GI) and establishment of a population of autonomously growing neoplastic cells at the end. Recently, the translocation t(11;18)(q21;q21) was identified in a fraction of low-grade MALT lymphoma cases, ranging from 21% to 60% of examined tumors.4,5,API2 and MALT1, genes affected by this translocation, have been cloned and the breakpoints characterized.6-8 How the t(11;18) contributes to the MALT lymphoma development and which regulatory pathways are affected by altered expression of these 2 genes is still a subject of investigation. Cytogenetic and fluorescence in situ hybridization (FISH) studies documented the presence of other abnormalities in MZBCL of MALT type as well.9 Trisomy 3 was reported with frequency ranging from 20%10 to 85% of tumors.11 Translocation t(1;14) was described as another characteristic abnormality, albeit much less frequently (in only 6 MALT-type lymphomas so far).12-14 A previous study suggested microsatellite instability (MSI) to play an important role in lymphomagenesis,15 but the high prevalence of MSI-positive MALT lymphomas could not be confirmed by us and others.16-19
Recently, we analyzed a group of 31 gastric diffuse large B-cell lymphomas (DLBCLs) for loss of heterozygosity (LOH) and amplification of genomic DNA with a panel of 73 microsatellite markers.20 Presuming that the same aberrations we saw in the DLBCLs could occur in the low-grade MZBCL of MALT type, we established a smaller panel containing 39 frequently affected microsatellites with which to analyze the low-grade lymphomas. Here, we report results of screening 24 such MALT lymphomas with this marker panel and compare the aberrations detected with the results of the high-grade DLBCL study.
Patients, materials, and methods
Patients and samples
Twenty-four extranodal gastric low-grade MZBCLs of MALT type from the lymph node registry at the Institute of Pathology in Würzburg on whom fresh frozen tissue (16 tumors) or formalin-fixed/paraffin-embedded tissue (8 tumors) was available were selected for the study. The diagnosis was established according to the criteria of the Revised European-American Lymphoma Classification21,22 by morphologic and immunophenotypic analyses of paraffin-embedded and fresh frozen tissue sections using standard staining methods as described recently.23 The tumors were localized at presentation, with no clinical evidence of generalized disease. The low-grade MALT lymphoma patients were in various disease stages; 5 subjects were in stage EI1, 5 in stage EI2, 8 in stage EII1, 2 in stage EII2, 1 in stage EIII, and no staging data were available on the remaining 3 cases.24 The patient population showed an age distribution from 32 to 75 years, with a mean of 54 years. The male to female ratio was 2:1; there were 16 males and 8 females. DLBCL cases used for comparison were described previously.20
Microscopic dissection and DNA extraction
In each case, 16 serial 10-μm–thick tissue sections were cut. The first and last cuts were stained with hematoxylin and eosin to assure high tumor content and as a guidance for the following microdissection. The fresh frozen tissue sections were visualized under polarized light, and an area showing low-grade lymphoma was scraped to be used for microsatellite analysis. In a similar way, control genomic DNA was derived from separate tissue blocks not involved by the tumor. Paraffin-embedded/formalin-fixed tissue sections were additionally stained by Nuclear–Fast Red to precisely delineate tumor-containing areas and the collected tissue deparaffinized using xylene before digestion. DNA extraction was performed using proteinase K and phenol-chloroform according to routine molecular biology protocols.25 A polymerase chain reaction (PCR)–based clonality analysis assay for immunoglobulin heavy chain rearrangement was performed on all control DNA samples to assure they did not contain the lymphoma clone.26
Microsatellite analysis
Primer sequences for the amplification of microsatellite repeats listed in Table 1 were retrieved from Genome Database (http://gdbwww.gdb.org). PCR primers were synthesized at MWG Biotech (Munich, Germany) and one oligonucleotide of each primer pair labeled with fluorescent dye phosphoramidites FAM, TAMRA, NED, ROX, or HEX. Paired normal and tumor DNA samples from each patient were amplified with the AmpliTaq Gold DNA polymerase (ABI, Foster City, CA) in multiplex PCR reactions using 50 ng genomic DNA as template under conditions specified by the Genome Database. Thirty cycles were carried out in a PE-2400 thermal cycler (ABI) in a total volume of 20 μL. Aliquots of the PCR reactions were then mixed with size standard and formamide, denatured, and subjected to electrophoresis on an ABI 377 DNA Sequencer (ABI). The automatically collected data were analyzed using GeneScan and Genotyper software as described in the manufacturer's manual. Only patients heterozygous for a given locus were regarded to be informative; homozygosity and MSI rendered the particular locus unevaluable for LOH or amplification. In heterozygous genotypes, ratios of both alleles in normal and tumor tissues were calculated. If these ratios showed a difference of more than 20%, the locus was further evaluated for possible allelic imbalance. For determination of LOH or amplification in a locus, first the unchanged allele was identified (by comparison with other microsatellites showing no change in the same multiplex PCR), and then the ratios of the allele showing decreased or increased signal to the unchanged allele were calculated, first for control DNA and then for the tumor. Increase of the ratio by 40% in the tumor (as compared with the control) was called amplification; decrease by 40%, LOH. All aberrations were confirmed 2 times.
Thirty-nine microsatellite markers used in the analysis of gastric MZBCL of MALT type and their chromosomal locations
| Marker . | Location . |
|---|---|
| D1S237,*D1S2827* | 1q32.1 |
| D2S391* | 2p16-22 |
| D3S4103,*D3S1300* | 3p14.2 |
| D3S1261* | 3p14.1 |
| D3S1229,* D3S3715 | 3q26.2-27 |
| D3S1530,*D3S1262, D3S1580,* D3S1314,*D3S1311* | 3q27-qter |
| D5S82,*D5S346* | 5q21 |
| D6S1721* | 6p23 |
| D6S447* | 6q21-q22.1 |
| D6S310* | 6q23.3-q25 |
| D6S441* | 6q24-q25.3 |
| D6S297* | 6q27 |
| D7S501* | 7q31 |
| D7S486* | 7q31.1 |
| D9S2136* | 9p21 |
| D11S1356,*D11S1345* | 11q23-24 |
| D12S89,*D12S98 | 12p12-13 |
| D13S153* | 13q14 |
| D17S250* | 17p12 |
| TP53CA,*P53p | 17p13.1 |
| D18S474,* D18S1099, D18S484, D18S1156, D18S35,* D18S1127, D18S1144, D18S1129 | 18q21 |
| Marker . | Location . |
|---|---|
| D1S237,*D1S2827* | 1q32.1 |
| D2S391* | 2p16-22 |
| D3S4103,*D3S1300* | 3p14.2 |
| D3S1261* | 3p14.1 |
| D3S1229,* D3S3715 | 3q26.2-27 |
| D3S1530,*D3S1262, D3S1580,* D3S1314,*D3S1311* | 3q27-qter |
| D5S82,*D5S346* | 5q21 |
| D6S1721* | 6p23 |
| D6S447* | 6q21-q22.1 |
| D6S310* | 6q23.3-q25 |
| D6S441* | 6q24-q25.3 |
| D6S297* | 6q27 |
| D7S501* | 7q31 |
| D7S486* | 7q31.1 |
| D9S2136* | 9p21 |
| D11S1356,*D11S1345* | 11q23-24 |
| D12S89,*D12S98 | 12p12-13 |
| D13S153* | 13q14 |
| D17S250* | 17p12 |
| TP53CA,*P53p | 17p13.1 |
| D18S474,* D18S1099, D18S484, D18S1156, D18S35,* D18S1127, D18S1144, D18S1129 | 18q21 |
The 29 repeats employed in the comparison of MZBCL and DLBCL.
Fluorescence in situ hybridization for t(11;18)
The interphase t(11;18) FISH assay was established by selecting yeast artificial chromosome (YAC) clones flanking the breakpoint region on 11q21.27 YAC clone 805c4 was chosen on the telomeric side; for the region centromeric to the breakpoint, YAC clones 963c8 and 966e4 were pooled to enhance signal intensity. All YAC clones were obtained from CEPH (Paris, France). After amplification of human sequences by Alu-PCR,28 probes were generated by nick translation with biotin-16-dUTP or digoxigenin-11-dUTP (Roche Diagnostics, Mannheim, Germany). FISH was performed on cytogenetic preparations or tumor cells isolated from fresh frozen tumor tissue according to standard protocols.29 In normal interphase cells, hybridization resulted in a close spatial relation of the differentially labeled YAC clones leading to 2 red/green signal pairs per cell. In tumors carrying the t(11;18), a derivative signal constellation with one signal pair and one separate red and green signal per nucleus was observed. To determine the cutoff level for normal interphase nuclei, cytogenetic preparations of 5 reactive lymph node specimens served as a negative control. At least 100 (in most cases 200) intact nuclei per slide were evaluated under a Zeiss Axiophot fluorescence microscope (Zeiss, Jena, Germany). Illustrations were generated using the ISIS imaging system (MetaSystems, Altlussheim, Germany).
RT-PCR for t(11;18)
All tumors were further evaluated for the presence of theAPI2-MALT1 fusion RNA product. Tumors from which fresh frozen tissue was available were analyzed using a test developed by Kalla et al.8 Paraffin-embedded/formalin-fixed tumors were analyzed by a reverse transcriptase (RT)–PCR approach described by Inagaki et al.30 However, to maximize the sensitivity of the latter reaction and precisely size the products, the second-round 5′ primers were labeled with fluorescent dyes FAM or HEX and the products separated using the ABI 377 DNA Sequencer (ABI).
Results
Gastric MZBCL of MALT type shows low frequency of genomic aberrations
We collected 24 MALT-type MZBCLs of the stomach to be examined for signs of GI using microsatellite analysis. Thirty-nine microsatellite markers were chosen to be used in this study (Table 1); 29 of them were those markers having shown frequent allelic imbalance or MSI in a previous allelotype analysis of extranodal gastric high-grade DLBCL.20 To distinguish LOH from amplification of genomic DNA, multiplex PCRs with the analyzed marker and at least one other marker used as an internal control were performed. The overall level of GI in the low-grade MALT lymphomas was fairly low. Only 10 (42%) patients or 37 (5.9%) genotypes out of 623 informative analyses showed an allelic imbalance.
Aberrations in regions 3q26.2-27, 11q23-24, and 18q21 are the most common allelic imbalances detected in MALT lymphoma
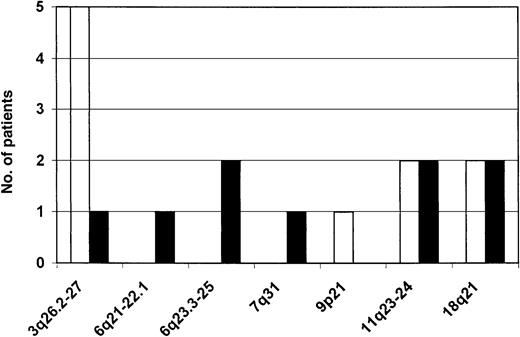
Several loci on various chromosomes showed allelic imbalances (Figure 1). The most frequent amplification of genomic material occurred in the 3q26.2-27 region (Figure 2). It was detected in 5 patients (21% of informative cases). The smallest segment showing amplification was flanked by markers D3S3715 and D3S1262 and contains the locus of the BCL6 gene. Region 11q23-24, including the locus of theMLL gene, was screened for the amplification we found in DLBCL previously with just 2 markers; 2 amplifications and 2 LOHs were detected. Four patients revealed an allelic imbalance in the 18q21 region, twice an amplification and twice an LOH. The smallest amplified segment was flanked by markers D18S474 and D18S484; it does not contain the loci of the MALT1 and BCL2 genes. Another 2 cases had an LOH on the long arm of chromosome 6, region 6q23.3-25, assayed for by markers D6S310 and D6S441, a hot spot of deletions in gastric DLBCL. One of the deletions was extremely large and encompassed also the 6q21-22.1 region. There were sporadic imbalances on 7q31 and 9p21—one case of each.
Chromosomal regions showing allelic imbalance in gastric MZBCL of MALT type.
Amplified regions are depicted as empty bars; regions showing LOH, black bars. The left-hand side shows the absolute frequency of aberrations expressed as the number of patients showing an aberration in a particular region.
Chromosomal regions showing allelic imbalance in gastric MZBCL of MALT type.
Amplified regions are depicted as empty bars; regions showing LOH, black bars. The left-hand side shows the absolute frequency of aberrations expressed as the number of patients showing an aberration in a particular region.
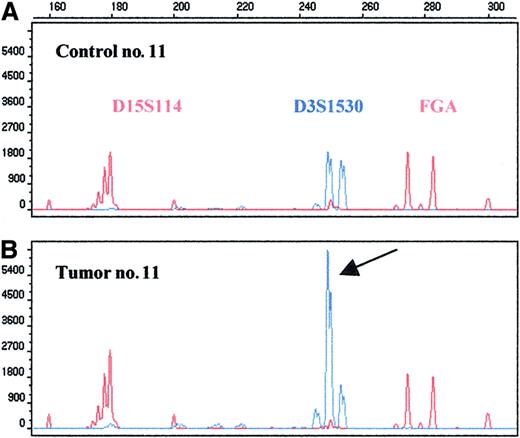
High-level amplification in the 3q26.2-27 region as detected by microsatellite D3S1530.
Repeats D3S1530, D15S114, and FGA were amplified in a multiplex PCR; the last 2 markers were used as an internal control. The 250–base pair allele of the D3S1530 shows 3.5-fold amplification (arrow).
High-level amplification in the 3q26.2-27 region as detected by microsatellite D3S1530.
Repeats D3S1530, D15S114, and FGA were amplified in a multiplex PCR; the last 2 markers were used as an internal control. The 250–base pair allele of the D3S1530 shows 3.5-fold amplification (arrow).
MZBCL reveals a very low level of MSI
Included in the microsatellite screening panel were markers that had shown a considerable degree of MSI in our previous study on high-grade DLBCL of the stomach.20 However, the level of MSI in the low-grade MALT lymphomas proved to be extremely low. We detected only 5 (0.6%) genotypes revealing MSI: 2 in case no. 11 and the remaining 3 in 3 other patients. All MSI events were type II mutations (only one novel allele occurred per marker), they did not cluster with any particular marker, all 5 affected a different microsatellite.
Additional clonal aberrations associate with t(11;18)-negative status
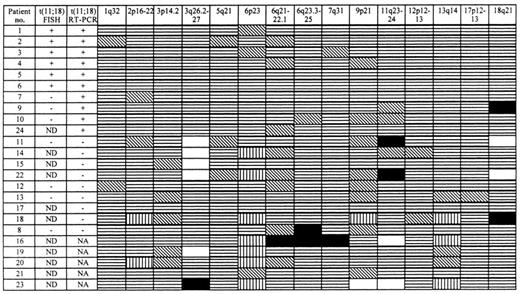
FISH and RT-PCR analyses for determination of the t(11;18) were performed on most fresh frozen tumors or all studied tumors, respectively. By RT-PCR, 10 lymphomas were positive for the t(11;18) and 9 were negative. When the patients were grouped according to their t(11;18) status, it became obvious that the cases positive for the translocation only rarely manifested any additional clonal aberration (Figure 3). In contrast, 6 (67%) of the t(11;18)-negative tumors revealed an allelic imbalance. The association of the t(11;18)-negative status with the manifestation of additional clonal aberrations proved to be statistically significant (P = .01, χ2 test).
Pattern of allelic imbalances in gastric MZBCL of MALT type and their relationship to the t(11;18).
Chromosomal regions analyzed are shown at the top of each column. Patients are listed in the first column with their t(11;18) status as detected by FISH (ND = not done) or RT-PCR (NA = no amplificate) given in the second and third columns, respectively. Status of each locus is indicated: (▤), retention of heterozygosity; (▧), not informative; (▥), no amplificate; (□), genomic DNA amplification; and (■), LOH.
Pattern of allelic imbalances in gastric MZBCL of MALT type and their relationship to the t(11;18).
Chromosomal regions analyzed are shown at the top of each column. Patients are listed in the first column with their t(11;18) status as detected by FISH (ND = not done) or RT-PCR (NA = no amplificate) given in the second and third columns, respectively. Status of each locus is indicated: (▤), retention of heterozygosity; (▧), not informative; (▥), no amplificate; (□), genomic DNA amplification; and (■), LOH.
MGII in low-grade MALT lymphoma is significantly lower than in high-grade DLBCL
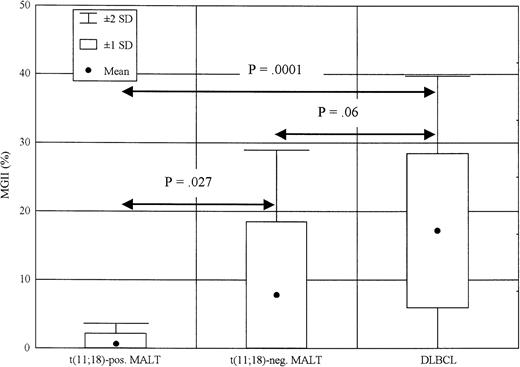
We used the results of a previous DLBCL study20 to compare the degree of GI in extranodal gastric MZBCL of MALT type and DLBCL. These tumors were analyzed using 38 and 73 microsatellites, respectively; however, both microsatellite panels contained an identical core set of 29 repeats used for the comparison (Table 1). These 29 markers would detect all consistent aberrations in both lymphoma types. We designed a so-called microsatellite genomic instability index (MGII) to quantify the level of GI in these cases. The MGII is the percentage of microsatellites showing any clonal aberration—either allelic imbalance or MSI—out of the total number of repeats analyzed. Using such a GI measure, we compared GI levels of the MALT lymphomas positive or negative for the t(11;18) and the DLBCL (Figure 4). The t(11;18)-positive MZBCL of MALT type (10 cases) showed very low MGII, with mean of 0.7% and SD of 1.48%. The MALT lymphomas negative for the translocation (9 cases) displayed only a low-level GI, with an MGII mean of 9.3% and SD of 10.22%. The group showing the highest MGII (17.15% ± 11.13%) were the high-grade DLBCL patients (31 cases). The MGII difference between the t(11;18)-positive MALT lymphomas and the DLBCLs proved to be statistically significant (P = .0001, Mann-WhitneyU test). There was a significant trend to increased MGII values in the t(11;18)-negative MALT lymphomas when compared with the t(11;18)-positive cases (P = .027, Mann-WhitneyU test).
MGII in low-grade MALT lymphomas positive or negative for the t(11;18) as compared with DLBCL.
MGII was calculated as a percentage of loci showing an allelic imbalance or MSI out of the total number of repeats analyzed. The differences in MGII between depicted groups of patients were statistically evaluated with the Mann-Whitney Utest.
MGII in low-grade MALT lymphomas positive or negative for the t(11;18) as compared with DLBCL.
MGII was calculated as a percentage of loci showing an allelic imbalance or MSI out of the total number of repeats analyzed. The differences in MGII between depicted groups of patients were statistically evaluated with the Mann-Whitney Utest.
Amplification in region 3q26.2-27 seems to be a crucial step in the development of t(11;18)-negative MZBCL
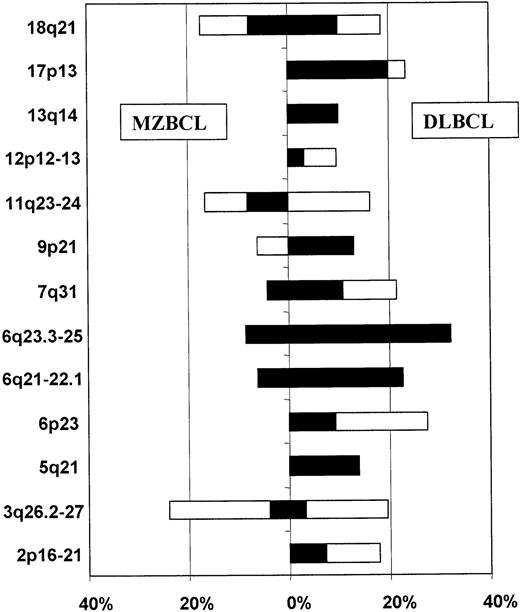
To identify chromosomal regions whose losses or amplifications are key steps in the extranodal gastric lymphoma development, we compared the allelotypes of the low-grade gastric MZBCL of MALT type and the high-grade gastric DLBCL. Only the aforementioned core set of 29 repeats was used for the comparison (Table 1). Microsatellite analysis results for 13 chromosomal regions showing consistent aberrations were plotted in a bar diagram and the frequencies of allelic imbalance compared (Figure 5). The regions could be divided into 3 groups. The first group consisted of regions 3q26.2-27, 11q23-24, and 18q21, showing allelic imbalances at about the same frequency in both low- and high-grade lymphomas. The second group consisted of regions 6q21-22.1, 6q23.3-25, and 7q31, showing rare aberrations in the low-grade tumors and much higher frequency of abnormalities in the high-grade lymphomas. However, only LOH in the 6q23.3-25 region occurred in more than one of the low-grade tumors; the 6q21-22.1 and 7q31 aberrations were displayed by just one patient each. The third group contained aberrations occurring exclusively in the high-grade lymphomas: LOH in the 5q21 (APC gene locus), 9p21 (INK4A/ARF), 13q14 (RB), and 17p13 (p53) regions and allelic imbalances in the 2p16-21, 6p23, and 12p12-13 regions.
Comparison of the allelotypes of low-grade MZBCL of MALT type and high-grade DLBCL.
Frequency of allelic imbalance (percentage of informative analyses) in individual regions is expressed as a bar diagram: open bar, amplification; black bar, LOH. The left-hand side shows results for MZBCL; the right-hand side, results for DLBCL.
Comparison of the allelotypes of low-grade MZBCL of MALT type and high-grade DLBCL.
Frequency of allelic imbalance (percentage of informative analyses) in individual regions is expressed as a bar diagram: open bar, amplification; black bar, LOH. The left-hand side shows results for MZBCL; the right-hand side, results for DLBCL.
Discussion
Several consistent cytogenetic abnormalities have been associated with particular subtypes of non-Hodgkin lymphoma, the most notorious example being t(14;18) found in follicular lymphoma.31Recently, t(11:18)(q21;q21) was identified in 21% to 60% of extranodal low-grade MZBCLs of MALT type4,5,32; it seems to be the most frequent translocation found in this non-Hodgkin lymphoma subtype. The identification of genes involved in this translocation (the apoptosis inhibitor gene API2 and a novel gene of unknown function called MALT1) suggests that the t(11;18) may result in a survival advantage for MALT lymphoma B-cell clones.6-8,33 However, at least 40% of the same low-grade MALT lymphomas do not feature this translocation. Moreover, none of the extranodal high-grade DLBCLs was found to harbor the t(11;18)—even high-grade DLBCLs with a concomitant low-grade MALT component.27 In a previous study,20 we identified by microsatellite analysis several genetic aberrations frequently appearing in gastric high-grade DLBCL. Assuming that the low-grade counterpart of the high-grade lymphoma—the gastric MZBCL of MALT type—could feature analogous aberrations, we performed a similar study on 24 gastric low-grade MALT lymphomas.
Altogether, the frequency of aberrations in the MZBCL of MALT type was much lower than in the DLBCL. Only 5.9% of the analyses or 42% of patients showed an allelic imbalance. Amplification of the 3q26.2-3q27 region, the most frequent consistent abnormality, was detected in 21% of patients. The smallest amplified segment was flanked by markers D3S3715 and D3S1262 (lying about 10 cM apart). Previously, trisomy 3 had been reported to be the most frequent abnormality in this lymphoma, with prevalence ranging from 20%10,34 up to approximately 60% of cases.35 However, it is also one of the most common numerical abnormalities described in other subtypes of non-Hodgkin lymphoma as well. The genetic mechanism by which trisomy 3 or amplification of the 3q26.2-27 region may contribute to lymphomagenesis is not known. An increased gene dosage effect resulting from higher copy numbers of gene(s) relevant to B-cell development or proliferation in general has been favored to explain the biological consequences underlying chromosomal trisomies. Several candidate genes are located in the D3S3715-D3S1262 amplicon of the 3q26.2-27 region, the most promising being the gene coding for phosphatidylinositol-3 kinase p110 catalytic subunit (PIK3CA), implicated as an oncogene in ovarian cancer,36 and the BCL6 gene that functions as a transcriptional switch controlling germinal center formation. Because a number of nodal DLBCLs derive from germinal-center B cells,37 deregulated BCL6 expression might contribute to lymphomagenesis by preventing postgerminal center differentiation.38 Indeed, some of the nodal DLBCLs and all normal germinal center B cells analyzed by complementary DNA microarrays39 showed overexpression of BCL6and, interestingly, overexpression of PIK3CA messenger RNAs as well.
Other chromosomes suffered less frequent aberrations in the low-grade MALT lymphomas. The 11q23-24 region revealed allelic imbalance in 4 (17%) patients, 2 with amplifications and 2 with deletions. Four cases revealed an allelic imbalance in the 18q21 region—2 an amplification and 2 an LOH. The amplifications were flanked by markers D18S474 and D18S484 (2 cM apart). The amplified region does not contain any well-known oncogenes, only several expressed sequence tags of unknown function. The BCL2 and MALT1 genes are not a part of the amplicon; they are located about 10 cM farther toward the telomere. Trisomy 18 has been described less frequently in MALT lymphomas,40-42 and no particular candidate genes have been identified besides BCL2. The frequency of alterations on the long arm of chromosome 6 was much lower in the MALT lymphomas than in the DLBCLs. Only 2 cases had an LOH in the region 6q23.3-25, which we identified recently as a hot spot of deletions in gastric DLBCL. In one of them, the deletion was so large that it also encompassed the region 6q21-22.1. Just one case revealed an aberration in the region 7q31. The frequency of MSI was very low in MZBCL of MALT type, verifying our results on high-grade DLBCL showing the tumor suppressor pathway to play a decisive role in lymphomagenesis. However, there was still about a 7-fold increase in MSI frequency from the low- to high-grade lymphomas (from 0.6% to 4% of MSI-positive markers).16
Of 19 cases in whom the results of t(11;18) RT-PCR analyses were available, 10 revealed this translocation. When the studied patients were grouped according to their t(11;18) status (Figure 3), the t(11;18)-negative cases interestingly showed distinctively more clonal aberrations as detected by microsatellite analysis. According to the level of allelic imbalance displayed, gastric MZBCL of MALT type can be thus divided into 2 groups: the first characterized by the t(11;18) and rare additional clonal aberrations, the second missing the t(11;18) but revealing significantly more other genetic aberrations (P = .01, χ2 test). These results are supported by cytogenetic analyses published on these lymphomas so far,5 albeit the methodology used in this study is much more sensitive in detecting aberrations than cytogenetics. Compared with DLBCLs, both the t(11;18)-positive and -negative MALT lymphomas displayed lower levels of GI. We quantified the level of GI in these lymphomas using an MGII, a percentage of microsatellites showing any aberration (either allelic imbalance or MSI) out of the total of all repeats analyzed. Indeed, the low-grade lymphomas positive for the t(11;18) showed very low MGII values (mean 0.7%, SD = 1.48%). The t(11;18)-negative MALT lymphomas revealed higher MGII values (9.3% ± 10.22%), and the high-grade DLBCLs showed the highest values (17.15% ± 11.3%) (Figure 4). The MGII values in these 3 lymphoma groups thus follow the model of increased GI in a high-grade disease when compared with its low-grade counterpart. Accumulation of additional genetic aberrations and increasing GI are typical features in the transformation of any low- into a high-grade disease, the best example being follicular lymphoma. In follicular lymphoma, the product of the t(14;18) alone is insufficient for lymphomagenesis. Over time, additional genetic events occur, contributing to more aggressive behavior and occasionally to frank histologic transformation into a diffuse large cell lymphoma.43 DLBCLs of the stomach may develop in certain cases by clonal evolution from a pre-existing low-grade MALT lymphoma.44-46 However, the t(11;18) detected in the low-grade MALT tumors was identified in neither primary (de novo) nor secondary (derived from low-grade MALT lesions) high-grade lymphomas.27 It cannot be ruled out that the t(11;18) just disappeared during tumor progression from low- to high-grade lymphoma; however, such a loss of primary lesion would be a rather uncommon finding. A more plausible explanation for the absence of the t(11;18) in the DLBCLs could be that the exclusive origin of such secondary high-grade lymphomas are the low-grade tumors negative for the t(11;18). All aberrations seen in the low-grade t(11;18)-negative MALT lymphomas were also found in the high-grade lymphomas, suggesting a clonal progression from low- to high-grade lymphoma.
To identify genetic aberrations playing a role in the progression of low-grade lymphoma and early stages of high-grade lymphoma, we compared the allelotype of low-grade gastric MZBCL of MALT type that we established in this work with the allelotype of high-grade gastric DLBCL we published recently (Figure 5). Allelic imbalances in the 3q26.2-27, 11q23-24, and 18q21 regions occurred with comparable frequencies in both low- and high-grade tumors. In contrast, aberrations in regions 6q23.3-25 and 7q31 were shared by both tumor types but occurred with much higher frequency in the DLBCLs. Only LOH in the 6q23.3-25 region occurred in more than one of the low-grade tumors and thus does not appear to occur purely by chance. The last group of aberrations occurred exclusively in the DLBCLs. Among them were LOHs in the 5q21 (APC tumor suppressor gene), 9p21(INK4A/ARF), 13q14 (RB), and 17p13(p53) regions and allelic imbalances in the 2p16-21, 6p23, and 12p12-13 regions.
Several of the just-mentioned regions harboring known tumor suppressor genes were already shown to play a role in the low- to high-grade lymphoma transition. Du et al47 concluded that partial inactivation of the p53 gene by mutation or deletion might play an important role in the development of low-grade MALT lymphoma, whereas complete inactivation seen in 29% of DLBCLs might be associated with high-grade transformation in at least some cases. Inactivation of the INK4A gene, a cyclin-dependent kinase inhibitor and negative regulator of the cell cycle, has also been described as a possibly important event in the progression from low- to high-grade lymphoma.48,49 Two of 6 high-grade DLBCL cases analyzed by Calvert et al revealed LOH in the APC gene locus.50 However, none of these abnormalities occurred at such a high frequency as the 6q aberrations reported in our DLBCL study. The results of our present analysis suggest that the 6q23.3-25 deletion occurs even before the loss of the p53,INK4A/ARF, or APC gene functions as evidenced by the LOH in regions harboring these genes.
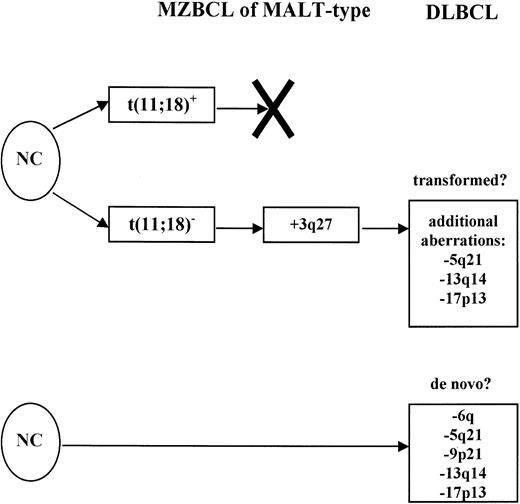
On the basis of these data, we propose the following model of MZBCL of MALT-type development (Figure 6). There seem to be 2 pathways of MALT lymphoma development and progression. One group of tumors develops along the pathway determined by the dysregulation of the API2 and MALT1 genes brought about by the t(11;18). These tumors do not accumulate enough secondary genetic aberrations to transform into DLBCL and remain in the stage of MZBCL. Other MALT lymphomas characterized by the absence of the t(11;18) and increased accumulation of various clonal genetic aberrations, most frequently the 3q26.2-27 amplification, could be the source of tumors that eventually do transform into high-grade DLBCL. Curiously, 2 groups of tumors could be identified among the DLBCLs we analyzed previously; they were characterized by 3q26.2-27 and 6q aberrations and represent 16% and 42% of tumors, respectively (with one overlapping case only). Similarly, among the MALT lymphomas, there were 5 tumors displaying the 3q26.2-27 aberration and 2 tumors with the 6q aberration. These data invite the hypothesis that possibly the 3q26.2-27 DLBCL group could encompass secondary DLBCL arising by transformation from a pre-existing MZBCL showing the same aberration. The minority of DLBCL is secondary disease transformed from its low-grade counterpart. However, we also detected 2 low-grade lymphomas showing the 6q aberration. These 2 cases were morphologically low-grade tumors but still could be evolving primary DLBCL. How far this hypothesis is correct in describing the transformation of MZBCL into DLBCL will need to be investigated next. It is quite possible that the DLBCL development is not as simple and there is even more heterogeneity in the group of lymphomas currently lumped together under the label of MZBCL of MALT type.
Two pathways of gastric MZBCL of MALT-type development from normal cells (NC).
The first one is characterized by the t(11;18); peculiarly, these lymphomas only rarely accumulate additional genetic aberrations and do not seem to transform into DLBCL. On the other side, the t(11;18)-negative tumors acquire various genetic aberrations exemplified by the 3q26.2-27 amplification, and some of them may eventually transform into high-grade DLBCL (listed are some of the most common additional aberrations). The 6q aberration displaying cases might be primary DLBCL.
Two pathways of gastric MZBCL of MALT-type development from normal cells (NC).
The first one is characterized by the t(11;18); peculiarly, these lymphomas only rarely accumulate additional genetic aberrations and do not seem to transform into DLBCL. On the other side, the t(11;18)-negative tumors acquire various genetic aberrations exemplified by the 3q26.2-27 amplification, and some of them may eventually transform into high-grade DLBCL (listed are some of the most common additional aberrations). The 6q aberration displaying cases might be primary DLBCL.
The heterogeneity of genetic aberrations we found in the MZBCL has immediate clinical implications. Namely, the extranodal lymphomas of MALT type characterized by the t(11;18) are unlikely to transform into high-grade lymphomas, although they may clinically present with early dissemination or advanced tumor stages. It has been shown in 2 recent studies51,52 that these are the cases resistant toH pylori antibiotic eradication therapy. This resistance, however, does not mean that these lymphomas automatically have an aggressive course. With the spreading popularity of stomach-conserving approaches, prospective studies evaluating the prognostic significance of the t(11;18) will be of fundamental significance for the further treatment of such patients. Increased attention should be devoted to cases that are t(11;18) negative. Some of them will respond to antibiotics; a recent study has shown that even some high-grade DLBCL cases could undergo remission after eradication of H pylori.53 Patients who are negative for the t(11;18) and do not respond to Helicobacter eradication therapy should be put on a regimen of intensive surveillance. Specifically, these lymphomas could be the primary candidates for transformation into DLBCL.
Supported by grants from the Interdisziplinäres Zentrum für Klinische Forschung (B3) and Sander Stiftung (no. 94.025.3).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Petr Starostik, Institute of Pathology, Würzburg University, Luitpoldkrankenhaus, Josef-Schneider-Strasse 2, D-97080 Würzburg, Germany; e-mail:petr.starostik@mail.uni-wuerzburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal