Unlike other leukemia types in which the bone marrow findings are diagnostic, the bone marrow pathology of T-cell granular lymphocytic leukemia (GLL) is subtle and ill-defined. In this study, bone marrow biopsy specimens from 36 patients with T-cell GLL and from 25 control patients with cytopenias and relative or absolute increases in blood large granular lymphocytes were studied by immunohistochemistry using antibodies to the cytolytic lymphocyte antigens CD8, CD56, CD57, TIA-1, and granzyme B. The goals were to clarify the bone marrow pathology of T-cell GLL and to refine the diagnostic criteria for T-cell GLL. Most bone marrow specimens from the T-cell GLL patients contained interstitially distributed clusters of at least 8 CD8+(83%) or TIA-1+ (75%) lymphocytes or clusters of at least 6 granzyme B+ (50%) lymphocytes. Interstitial clusters of CD8+, TIA-1+, or granzyme B+ cells were present in 36%, 12%, and 0%, respectively, of the control bone marrows (all values significantly different, P < .001). An additional T-cell GLL disease-specific finding was the presence of linear arrays of intravascular CD8+, TIA-1+, or granzyme B+ lymphocytes, found in 67% of cases of T-cell GLL and in none of the 25 control samples (P < .001). Staining for CD56 and CD57 was noncontributory. These findings clarify the bone marrow histopathology of T-cell GLL and provide an additional tool by which the discrete, abnormal lymphocyte population required for a diagnosis of T-cell GLL can be identified.
Introduction
Granular lymphocytic leukemia (GLL), also termed large granular lymphocyte leukemia, is a chronic, indolent lymphoproliferative disorder with distinctive clinical and laboratory manifestations.1-5 The hallmark feature of GLL is expansion of a discrete, or clonal, population of cytolytic lymphocytes (CTLs) in the peripheral blood. These lymphocytes have characteristic morphologic features, including the presence of abundant cytoplasm containing a variable number of azurophilic granules and relatively small nuclei with inconspicuous nucleoli. In GLL the expansion of large granular lymphocytes (LGLs) often occurs in individuals with autoimmune disorders and is associated with variably severe neutropenia, anemia, and thrombocytopenia, which are responsible for most of the disease-associated morbidity and mortality. Most GLL cases can be subclassified into cytolytic T-cell type for the cases in which the neoplastic cells express CD3 and exhibit clonal T-cell antigen receptor gene rearrangements.1,6-9 This disorder is now referred to as T-cell granular lymphocytic leukemia in the current World Health Organization classification of diseases of the hematopoietic and lymphoid tissues.10 11
The individual neoplastic cells in GLL have few, if any, cytologic features that distinguish them from benign granular lymphocytes.12-14 This attribute makes a morphologic distinction between GLL and conditions associated with a reactive, polyclonal increase in granular lymphocytes almost impossible in most cases. Because of the lack of morphologic specificity, the diagnostic criteria for T-cell GLL require an increased blood LGL population, demonstration of T-cell clonality and, probably, demonstration of an immunophenotypically distinct peripheral blood T-cell population by flow cytometry.15-17 These diagnostic criteria fail to account for those T-cell GLL cases in which the patients present with cytopenias associated with clonal T-cell antigen receptor gene rearrangements but have only marginally elevated LGL counts and do not have a phenotypically aberrant peripheral blood cell population by flow cytometry.
As opposed to virtually all other lymphoproliferative disorders, there is a paucity of information regarding the bone marrow findings in T-cell GLL, at least in part because the distinctive cytologic features of granular lymphocytes are impossible to recognize in hematoxylin and eosin–stained bone marrow biopsy specimens.18,19 A number of antibodies to cytotoxic lymphocyte-associated antigens that react in paraffin-embedded tissues have recently become available; these include antibodies to CD8,20 CD56,21CD57,22 and the cytotoxic granule proteins TIA-1 (also known as gmp-17)23,24 and granzyme B.25 These reagents could potentially facilitate recognition of normal and neoplastic cytolytic T cells in bone marrow biopsy specimens.
In this study we examined the pathologic features of bone marrow involvement by T-cell GLL in established cases, employing immunohistochemistry with antibodies to CTLs as an ancillary tool for the identification of the neoplastic lymphocytes in fixed, paraffin-embedded bone marrow biopsy specimens. These findings were compared with similarly evaluated bone marrow specimens from patients with cytopenias and relatively increased peripheral blood granular T lymphocytes that failed to meet the minimal criteria for a diagnosis of T-cell GLL due to the absence of clonal T-cell antigen receptor gene rearrangements. Our goal was to identify specific morphologic and immunophenotypic features that characterize T-cell GLL and that could potentially contribute to refining current diagnostic criteria for this disorder.
Materials and methods
The Mayo Clinic files were reviewed over a time interval from 1983 to 1999 for cases of established T-cell GLL. From the initially identified cases, 36 were selected for further study based on availability of a complete clinical record with patient follow-up, availability of peripheral blood smears, bone marrow smears and biopsy specimens for morphologic review, and availability of paraffin-embedded tissue from the bone marrow biopsy specimens on which to perform immunoperoxidase stains (see below). In addition, in each case molecular genetic studies analyzing the T-cell antigen receptor genes and routine cytogenetic studies had been performed on a peripheral blood or bone marrow aspirate specimen. The molecular genetics studies were performed by previously published methods.26 A seminested polymerase chain reaction technique was used to evaluate the T-cell receptor (TCR)γ chain gene, or a Southern blot technique was employed to evaluate the TCRβ and TCRγ chain genes. In all cases clonal TCR gene rearrangements were identified by polymerase chain reaction or Southern blot. Cytogenetics studies were done by both a direct and a short-term unstimulated culture technique. For each specimen an attempt was made to analyze 20 metaphases with trypsin-Giemsa banding and/or banding with quinacrine mustard. No cytogenetic abnormalities were identified in any of these cases.
The published diagnostic criteria for T-cell GLL and the phenotypic evaluation of the blood and bone marrow specimens varied over the long time interval during which these cases were initially diagnosed. Therefore, for the purposes of this study, the minimal criteria for the diagnosis of T-cell GLL included the following for each case: clinical features that in the opinion of a hematologist were compatible T-cell GLL; a persistent absolute or relative increase in peripheral blood LGLs; presence of anemia, neutropenia, and/or thrombocytopenia; clonal TCR rearrangements demonstrated from a blood or bone marrow specimen; and absence of myelodysplasia and absence of myelodysplasia-associated cytogenetic abnormalities in the bone marrow specimen. The study included 36 cases of T-cell GLL that met these criteria. In addition, in 20 of the 36 cases blood or bone marrow specimens were analyzed by flow cytometry by previously described methods.27 A distinct, abnormal CD3+ and CD8+peripheral blood T-cell population was found in 15 of the 20 cases. All 5 blood or bone marrow specimens tested for CD16 contained an abnormal CD16+ T-cell population. Four specimens had normal T-cell phenotypes. Phenotypic analysis of one additional spleen specimen demonstrated an abnormal CD8+ T-cell population distributed within the red pulp.
As control cases, 25 additional peripheral blood and bone marrow specimens were also selected for analysis. In each of the control cases, there was a relative increase in peripheral blood LGLs associated with peripheral blood cytopenias. Myelodysplasia was not identified by morphology in any of the studied bone marrow specimens from these patients. Cytogenetic analysis revealed no clonal karyotypic abnormality, and molecular genetics studies performed as above on blood or bone marrow from all cases failed to identify clonal TCR gene rearrangements. These cases were classified as “nonclonal reactive T-cell LGL disorders.” In 10 of the 25 patients, flow cytometric analysis was performed on the blood or bone marrow as above, and in none was a phenotypically aberrant, distinct T-cell population identified. In 10 of the 25 patients with reactive conditions, follow-up that included evaluation of subsequent bone marrow specimens suggested the following etiologies for the observed cytopenias: myelodysplasia (n = 2), pure red blood cell aplasia (n = 2), aplastic anemia (n = 1), temporal arteritis (n = 1), primary biliary cirrhosis (n = 1), drug hypersensitivity (n = 1), cutaneous T-cell lymphoma after therapy (n = 1), and familial immunodeficiency of unspecified type (n = 1). In the remaining 15 patients, the etiology of the cytopenias was never determined or the patients were lost to follow-up.
Immunohistochemistry was performed on B5-fixed, decalcified, paraffin-embedded bone marrow biopsy specimens after deparaffinization and rehydration. The basic method has been previously published.28 In brief, the slides were pretreated by steaming for 30 minutes while immersed in either citrate buffer (1 mM/L, pH 6.0) or ethylenediaminetetraacetic acid (EDTA) buffer (1 mM/L, pH 8.0) as indicated in Table 1. After antigen retrieval the slides were rinsed with water and then treated with methanolic peroxide to block endogenous peroxidase activity. The slides were then rinsed, and the primary antibody was applied. The source of each primary antibody used and the staining method are also shown in Table 1. On the Ventana instrument (Ventana Medical Systems, Tucson, AZ) antibody incubations were performed at 42°C, and the labeled streptavidin-biotin-peroxidase method was used. The BioTek instrument (BioTek Solutions, Santa Barbara, CA) performed antibody incubations at room temperature and used an avidin-biotin complex method. For the manual method, incubations were performed at room temperature, and the labeled streptavidin-biotin-peroxidase method was used. For all of the primary antibodies the chromogen used was 3-amino-9-diethylcarbazole with hematoxylin counterstain. The immunoperoxidase-stained bone marrow biopsy specimens were reviewed, and the number and distribution of CD3+, CD8+, TIA-1+, granzyme B+, and CD20+cells were determined for each case.
Primary antibodies and immunoperoxidase stain methods
| Antibody . | Clone . | Source . | Pretreatment . | Method . |
|---|---|---|---|---|
| CD3 | Polyclonal | Dako* | EDTA | Ventana |
| CD8 | C8/144B | Dako | EDTA | Ventana |
| CD20 | L26 | Dako | Citrate | Ventana |
| CD56 | 123C3 | Caltag† | EDTA | Ventana |
| CD57 | HNK-1 | Becton Dickinson‡ | EDTA | Ventana |
| TIA-1 | 266A10FS | Coulter1-153 | EDTA | Manual |
| Granzyme B | GRB-7 | Caltag | EDTA | BioTek |
| Antibody . | Clone . | Source . | Pretreatment . | Method . |
|---|---|---|---|---|
| CD3 | Polyclonal | Dako* | EDTA | Ventana |
| CD8 | C8/144B | Dako | EDTA | Ventana |
| CD20 | L26 | Dako | Citrate | Ventana |
| CD56 | 123C3 | Caltag† | EDTA | Ventana |
| CD57 | HNK-1 | Becton Dickinson‡ | EDTA | Ventana |
| TIA-1 | 266A10FS | Coulter1-153 | EDTA | Manual |
| Granzyme B | GRB-7 | Caltag | EDTA | BioTek |
Carpinteria, CA.
San Francisco, CA.
San Jose, CA.
Westbrook, ME.
This study was approved by the Mayo Clinic Institutional Review Board, and all patients consented for use of their medical records for research.
Results
Clinical and hematologic data
The clinical and laboratory features of the patients with T-cell GLL and with nonclonal reactive T-cell LGL disorders are summarized in Table 2. Marked neutropenia (absolute neutrophil count < 0.5 × 109/L) was seen in 11 (31%) GLL patients and in none of the nonclonal T-cell LGL disorder patients; the mean hemoglobin values and platelet counts were similar between the 2 groups (Table 2). The GLL patients demonstrated a higher average absolute lymphocyte count and a higher absolute granular lymphocyte count than that seen in the nonclonal T-cell LGL disorder group. Despite this trend, however, the absolute granular lymphocyte count was less than 2 × 109/L in 21 (58%) of the GLL patients. The frequency or degree of cytopenias did not differ significantly between GLL patients with less than or more than 2 × 109/L granular lymphocytes (data not shown). None of the nonclonal LGL disorder patients had an absolute granular lymphocyte count more than 2 × 109/L, with a highest observed granular lymphocyte count being 1.65 × 109/L in this group.
Clinical and hematologic data
| . | T-GLL . | Reactive . |
|---|---|---|
| No. of patients | 36 | 25 |
| Age, mean (range), y | 65 (28-87) | 56 (29-76) |
| Sex: male, female | 20, 16 | 9, 16 |
| Rheumatoid arthritis, no. (%) | 7 (19) | 0 (0) |
| Hemoglobin, mean (range), g/L | 103 (34-145) | 112 (71-158) |
| Hemoglobin, no. (%), less than 120 g/L | 25 (69) | 16 (67) |
| Hemoglobin, no. (%), less than 80 g/L | 7 (19) | 2 (8) |
| ANC, mean (range), × 109/L | 1.92 (0.02-12.08) | 2.62 (0.83-7.72) |
| ANC, no. (%), less than 2 × 109/L | 23 (64) | 13 (54) |
| ANC, no. (%), less than 0.5 × 109/L | 11 (31) | 0 (0) |
| Platelet count, mean (range), × 109/L | 231 (30-634) | 180 (39-508) |
| Platelet count, no. (%), less than 100 × 109/L | 7 (19) | 4 (17) |
| Lymphocyte count, mean (range), × 109/L | 3.26 (0.62-10.77) | 1.44 (0.43-3.74) |
| LGL count, mean (range), × 109/L | 1.99 (0.29-7.12) | 0.58 (0.22-1.65) |
| LGL count, no. (%), more than 2 × 109/L | 15 (42) | 0 (0) |
| . | T-GLL . | Reactive . |
|---|---|---|
| No. of patients | 36 | 25 |
| Age, mean (range), y | 65 (28-87) | 56 (29-76) |
| Sex: male, female | 20, 16 | 9, 16 |
| Rheumatoid arthritis, no. (%) | 7 (19) | 0 (0) |
| Hemoglobin, mean (range), g/L | 103 (34-145) | 112 (71-158) |
| Hemoglobin, no. (%), less than 120 g/L | 25 (69) | 16 (67) |
| Hemoglobin, no. (%), less than 80 g/L | 7 (19) | 2 (8) |
| ANC, mean (range), × 109/L | 1.92 (0.02-12.08) | 2.62 (0.83-7.72) |
| ANC, no. (%), less than 2 × 109/L | 23 (64) | 13 (54) |
| ANC, no. (%), less than 0.5 × 109/L | 11 (31) | 0 (0) |
| Platelet count, mean (range), × 109/L | 231 (30-634) | 180 (39-508) |
| Platelet count, no. (%), less than 100 × 109/L | 7 (19) | 4 (17) |
| Lymphocyte count, mean (range), × 109/L | 3.26 (0.62-10.77) | 1.44 (0.43-3.74) |
| LGL count, mean (range), × 109/L | 1.99 (0.29-7.12) | 0.58 (0.22-1.65) |
| LGL count, no. (%), more than 2 × 109/L | 15 (42) | 0 (0) |
ANC indicates absolute neutrophil count.
Bone marrow morphology
Bone marrow morphology is described in (Table3). The bone marrow aspirate and biopsy specimens in the T-cell GLL patients were usually hypercellular, although both normocellular and hypocellular specimens were encountered. In aspirate specimens, the cytoplasm of granular lymphocytes appeared less voluminous than that of LGLs in blood, and the azurophilic granules were easily overlooked. Interstitial lymphocytic infiltrates were commonly seen in the bone marrow biopsy specimens from the T-cell GLL patients (25 samples, 69%). The infiltrates were typically composed of small groups of lymphocytes with small, minimally irregular nuclei and sparse cytoplasm. They were subtle and easy to overlook in hematoxylin and eosin–stained sections. Large, discrete, lymphoid aggregates were present in only 5 T-cell GLL bone marrow specimens. Evaluation of the individual hematopoietic cell lineages in both aspirate and biopsy specimens demonstrated a frequent increase in erythroid precursors with normal maturation, but in occasional cases an increase in the proportion of pronormoblasts and basophilic normoblasts was observed. In contrast, when abnormal, the granulocyte precursors were most often numerically decreased and demonstrated left-shifted maturation. In most cases, the megakaryocytes were numerically and morphologically unremarkable.
Bone marrow morphologic findings
| . | Cellularity . | Red cell precursors . | Granulocyte precursors . | Megakaryocytes . |
|---|---|---|---|---|
| T-cell GLL (n = 36) | ||||
| Increased | 20 (56%) | 16 (44%) | 9 (25%) | 3 (8%) |
| Decreased | 5 (14%) | 10 (28%) | 14 (39%) | 3 (8%) |
| Normal | 11 (31%) | 10 (28%) | 13 (36%) | 30 (83%) |
| Immature precursors3-150 | — | 6 (17%) | 13 (36%) | — |
| Reactive (n = 25) | ||||
| Increased | 5 (20%) | 6 (24%) | 3 (12%) | 2 (8%) |
| Decreased | 9 (36%) | 10 (40%) | 10 (40%) | 4 (16%) |
| Normal | 11 (44%) | 9 (36%) | 12 (48%) | 19 (76%) |
| Immature precursors3-150 | — | 2 (8%) | 2 (8%) | — |
| . | Cellularity . | Red cell precursors . | Granulocyte precursors . | Megakaryocytes . |
|---|---|---|---|---|
| T-cell GLL (n = 36) | ||||
| Increased | 20 (56%) | 16 (44%) | 9 (25%) | 3 (8%) |
| Decreased | 5 (14%) | 10 (28%) | 14 (39%) | 3 (8%) |
| Normal | 11 (31%) | 10 (28%) | 13 (36%) | 30 (83%) |
| Immature precursors3-150 | — | 6 (17%) | 13 (36%) | — |
| Reactive (n = 25) | ||||
| Increased | 5 (20%) | 6 (24%) | 3 (12%) | 2 (8%) |
| Decreased | 9 (36%) | 10 (40%) | 10 (40%) | 4 (16%) |
| Normal | 11 (44%) | 9 (36%) | 12 (48%) | 19 (76%) |
| Immature precursors3-150 | — | 2 (8%) | 2 (8%) | — |
For red cell precursors, immature precursors refers to identification of increased pronormoblasts and basophilic normoblasts. For granulocyte precursors, immature precursors refers to left-shifted maturation.
In contrast to the T-cell GLL bone marrow specimens, those in the nonclonal LGL disorder group were often hypocellular or normocellular. In this group both the erythroid and granulocyte precursors were often decreased in number and demonstrated normal maturation. In the 2 patients with pure red blood cell aplasia, erythroid precursors were virtually absent. An interstitial lymphocytic infiltrate was seen much less commonly in the nonclonal reactive LGL disorder bone marrows (2 specimens, 8%) than in those from the T-cell GLL group. Discrete lymphoid aggregates were observed in bone marrow biopsy specimens from 4 of the nonclonal LGL disorder patients.
Bone marrow immunohistochemistry
Sections of the decalcified, paraffin-embedded bone marrow biopsy specimens were stained for CD3 and CD20 and for the CTL-associated membrane antigens CD8, CD56, CD57, and cytotoxic granule proteins TIA-1 (gmp-17) and granzyme B (Table 4). This antibody panel revealed 2 distinct but not mutually exclusive patterns of marrow involvement by T-cell GLL. The first pattern was characterized by the presence of interstitial clusters of CTLs. This pattern was easy to recognize and could be found in multiple foci in all of the studied biopsy specimens. The interstitial CTL clusters contained confluent groups of 8 or more cells positive for either CD8 (Figure 1A) or TIA-1 or confluent groups of 6 or more granzyme B+ cells (Figure2A). The numbers 8 CD8+ or TIA-1+ cells and 6 granzyme B+ cells were chosen because they provided optimal separation of the T-cell GLL cases from the nonclonal reactive LGL disorder cases (Table 4). The CTL clusters in T-cell GLL were CD8+, TIA-1+, and granzyme B+ in 17 (47%) cases; CD8+, TIA-1+, and granzyme B− in 9 cases (25%); TIA-1+, granzyme B+, and CD8− in 1 case (3%); and only positive for CD8 in 4 cases (11%). In occasional cases intense staining of granulocytes for TIA-1 was observed. However, in most cases TIA-1 staining of the granulocyte precursors did not interfere with interpretation because the T cells had coarse granular TIA-1 positivity in contrast to the fine, dusty, cytoplasmic positivity of the granulocyte precursors.
Frequency of interstitial CTL clusters (at least 8 cells per cluster) and of intravascular lymphocytes positive for CTL antigens
| . | CD8 . | TIA-1 . | Granzyme B . | Intravascular4-150 . | CD57 . |
|---|---|---|---|---|---|
| T-cell GLL (n = 36) | 30 (83%) | 27 (75%) | 18 (50%) | 24 (67%) | 6 (17%) |
| Reactive (n = 25) | 9 (36%) | 3 (12%) | 0 (0%) | 1 (4%) | 0 (0%) |
| P4-151 | < .0001 | < .0001 | < .0001 | < .0001 | .072 |
| . | CD8 . | TIA-1 . | Granzyme B . | Intravascular4-150 . | CD57 . |
|---|---|---|---|---|---|
| T-cell GLL (n = 36) | 30 (83%) | 27 (75%) | 18 (50%) | 24 (67%) | 6 (17%) |
| Reactive (n = 25) | 9 (36%) | 3 (12%) | 0 (0%) | 1 (4%) | 0 (0%) |
| P4-151 | < .0001 | < .0001 | < .0001 | < .0001 | .072 |
Positive for CD8, TIA-1, and/or granzyme B.
T-cell GLL versus reactive using the Fisher exact test.
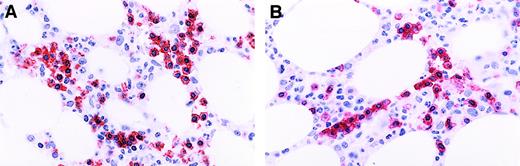
Patterns of staining for CD8 in T-cell GLL.
(A) Immunoperoxidase stain for CD8 demonstrating distinct membrane staining in confluent aggregates of more than 8 CD8+ lymphocytes. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 150. (B) Immunoperoxidase stain CD8 demonstrating a linear array of CD8+ intravascular T cells. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 240.
Patterns of staining for CD8 in T-cell GLL.
(A) Immunoperoxidase stain for CD8 demonstrating distinct membrane staining in confluent aggregates of more than 8 CD8+ lymphocytes. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 150. (B) Immunoperoxidase stain CD8 demonstrating a linear array of CD8+ intravascular T cells. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 240.
Patterns of staining for granzyme B in T-cell GLL.
(A) Immunoperoxidase stain for granzyme B demonstrating an interstitial cluster of more than 6 granzyme B+ T cells. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 240. (B) Immunoperoxidase stain for granzyme B demonstrating the intravascular location of the granzyme B+ lymphocytes. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 240.
Patterns of staining for granzyme B in T-cell GLL.
(A) Immunoperoxidase stain for granzyme B demonstrating an interstitial cluster of more than 6 granzyme B+ T cells. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 240. (B) Immunoperoxidase stain for granzyme B demonstrating the intravascular location of the granzyme B+ lymphocytes. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 240.
The second observed staining pattern was more subtle and required careful examination of the immunoperoxidase-stained slides to recognize. It was characterized by linear arrangements of CTLs with an immunophenotype identical to the interstitial CTL clusters (Figures 1B and 2B). Detailed morphologic evaluation revealed these linear configurations to be due to accumulation of CTLs within marrow microvascular structures. Only in isolated instances were the interstitial CTL clusters or intravascular CTLs positive for CD57 (Table 4). CD56+ CTLs were not present in any of the bone marrow specimens. The phenotypes of the lymphocytes within discrete lymphoid aggregates were the same in both the LGL leukemia patient group and the reactive LGL disorder patient group (Figure3). Cells in the centers of the aggregates were CD20+ small lymphocytes. CD8−, TIA-1−, granzyme B−, and CD3+ T cells surrounded them. Thus, the lymphoid aggregates were considered to represent nonspecific lymphoid hyperplasia. Finally, specimens from both T-cell GLL and nonclonal reactive LGL disorder cases contained overlapping numbers of CD3+ lymphocytes such that numbers and distribution of CD3+ cells did not provide a discriminant between the T-cell GLL and the nonclonal reactive LGL disorder groups (Figure 4A).
Aggregate of nonneoplastic lymphocytes in a bone marrow specimen involved by T-cell GLL.
(A) Immunoperoxidase stain for CD3. Most of the lymphocytes in the aggregate are CD3+ T cells. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 80. (B) Immunoperoxidase stain for CD20. The lymphoid aggregate contains few CD20+ B cells. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 80. (C) Immunoperoxidase stain for granzyme B. Virtually none of the neoplastic granzyme B+ granular lymphocytes are present in the lymphoid aggregate, but they can be seen in the bone marrow interstitium and the blood vessels at the periphery of the aggregate. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 80.
Aggregate of nonneoplastic lymphocytes in a bone marrow specimen involved by T-cell GLL.
(A) Immunoperoxidase stain for CD3. Most of the lymphocytes in the aggregate are CD3+ T cells. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 80. (B) Immunoperoxidase stain for CD20. The lymphoid aggregate contains few CD20+ B cells. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 80. (C) Immunoperoxidase stain for granzyme B. Virtually none of the neoplastic granzyme B+ granular lymphocytes are present in the lymphoid aggregate, but they can be seen in the bone marrow interstitium and the blood vessels at the periphery of the aggregate. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 80.
CTL immunostaining in nonclonal T-cell LGL disorder.
Immunoperoxidase stains for CD3 (A) and CD8 (B) demonstrating increased numbers of singly distributed cells in the marrow interstitium. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 240. (C) Immunoperoxidase stain for granzyme B demonstrating isolated positive cells. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 240.
CTL immunostaining in nonclonal T-cell LGL disorder.
Immunoperoxidase stains for CD3 (A) and CD8 (B) demonstrating increased numbers of singly distributed cells in the marrow interstitium. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 240. (C) Immunoperoxidase stain for granzyme B demonstrating isolated positive cells. Aminoethyl carbazol chromogen, hematoxylin counterstain; original magnification: × 240.
To determine if the immunohistochemical findings were disease specific, the bone marrow biopsy specimens from the nonclonal reactive T-cell LGL disorder group were stained with the same antibody panel (Figure 4). While an increase in interstitially distributed CTLs was present, distinct clusters or intravascular staining of cells positive for CD8, TIA-1, and granzyme B was rarely seen in these cases. Comparison of the T-cell GLL group and the nonclonal reactive T-cell LGL disorder group revealed that both interstitial CTL clusters and intravascular CTL staining were preferentially seen in T-cell GLL cases (Table 4). The most dramatic differences were seen in the presence or absence of granzyme B+ interstitial cell clusters and the presence of cytolytic T cells in the intravascular spaces.
The data indicated that interstitial CTL clusters and intravascular CTL distribution were findings associated with T-cell GLL. However, given the lower average granular lymphocyte count in our group of reactive cases, it remained possible that these immunohistochemical findings were secondary to the number of circulating LGLs. For this reason the occurrence of these staining patterns was stratified by the peripheral blood LGL counts (Table 5). This analysis revealed that while intravascular and interstitial clusters of CTLs were more common in T-cell GLL cases with more than 2 × 109/L circulating granular lymphocytes, both were present in most T-cell GLL cases regardless of the granular lymphocyte count. The most prominent difference between the high and low granular lymphocyte groups was seen when the biopsies were stained with antibodies to granzyme B. In cases with a high LGL count, the CTL clusters were most often granzyme B+, whereas in LGL cases with less than 2 × 109/L granular lymphocytes in the peripheral blood the CTL clusters often did not stain with antibodies to granzyme B. The severity of the peripheral blood anemia, granulocytopenia, and thrombocytopenia did not correlate with the cytolytic T-cell phenotype (granzyme B+ vs granzyme B−) or with the pattern of bone marrow involvement (interstitial clusters alone vs intravascular CTLs) in either T-cell GLL or nonclonal reactive LGL patient groups (data not illustrated).
Frequency of CTL clusters in T-cell GLL with various absolute granular lymphocyte counts
| Granular lymphocytes, × 109/L . | CD8 . | TIA-1 . | Granzyme B . | Intravascular5-150 . | CD57 . |
|---|---|---|---|---|---|
| LGL at least 2 (n = 15) | 13 (87%) | 13 (87%) | 12 (80%) | 13 (87%) | 3 (15%) |
| LGL at least 0.5 and less than 2 (n = 12) | 10 (83%) | 8 (67%) | 4 (33%) | 7 (58%) | 2 (17%) |
| LGL less than 0.5 (n = 9) | 7 (78%) | 6 (67%) | 2 (22%) | 4 (44%) | 1 (11%) |
| Granular lymphocytes, × 109/L . | CD8 . | TIA-1 . | Granzyme B . | Intravascular5-150 . | CD57 . |
|---|---|---|---|---|---|
| LGL at least 2 (n = 15) | 13 (87%) | 13 (87%) | 12 (80%) | 13 (87%) | 3 (15%) |
| LGL at least 0.5 and less than 2 (n = 12) | 10 (83%) | 8 (67%) | 4 (33%) | 7 (58%) | 2 (17%) |
| LGL less than 0.5 (n = 9) | 7 (78%) | 6 (67%) | 2 (22%) | 4 (44%) | 1 (11%) |
Positive for CD8, TIA-1, and/or granzyme B.
Discussion
Unlike most types of leukemia, in which bone marrow findings are dramatic, relatively specific for the leukemia type, and well described, there are few detailed accounts of the bone marrow pathology of GLL.18,19 Most studies report relatively nonspecific findings, including LGL lymphocytosis on blood and bone marrow aspirate smears and subtle interstitial lymphocytic infiltrates or lymphoid aggregates in bone marrow biopsy specimens.1 4 A detailed morphologic review of the bone marrow specimens in this study disclosed similar findings. LGLs were subtle and difficult to detect in both the bone marrow aspirate and biopsy specimens irrespective of the degree of peripheral blood granular lymphocytosis. The bone marrow biopsy specimens from T-cell GLL patients contained subtle interstitial lymphoid infiltrates rather than the dense interstitial and paratrabecular lymphoid aggregates that characterize bone marrow involvement by B-cell lymphoproliferative disorders. Indeed, discrete lymphoid aggregates, previously hypothesized to be a bone marrow manifestation of GLL, were shown in this study to be present in only a subset of T-cell GLL cases and to be composed of nonneoplastic B cells and T cells that did not exhibit a cytolytic phenotype. These bone marrow morphologic findings overlap substantially with various reactive processes, including infectious and autoimmune diseases, and with the bone marrow findings of the patients with nonneoplastic LGL disorders in this study. Thus, by itself bone marrow morphology is insufficiently distinctive to support a suspected diagnosis of T-cell GLL. Given the lack of unique morphologic features, the immunohistochemical approach employed here clearly illuminated the characteristics of bone marrow involvement by LGL leukemia.
Of the antibodies used in our study, the most useful in identifying CTLs in B5-fixed, decalcified bone marrow biopsy specimens were those specific for CD8 and for the cytotoxic granule proteins TIA-1 and granzyme B. These stains highlighted interstitial clusters of CD8+, TIA-1+, and/or granzyme B+lymphocytes in a high percentage of the T-cell GLL bone marrow biopsy specimens. Interestingly, in 2 of the 3 reactive cases with interstitial CTL clusters a final diagnosis of pure red cell aplasia was made. GLL is found in a significant subset of patients with pure red cell aplasia,29 raising the possibility that these “false positive” reactive cases may actually represent T-cell GLL with “false negative” T-cell antigen receptor gene rearrangement studies or natural killer (NK) cell GLL in which clonal T-cell antigen receptor gene rearrangements are expected to be absent. Finally, intravascular localization of TIA-1+, granzyme B+, and/or CD8+ CTLs in the bone marrow biopsy specimens provided a very strong indicator that a case represented T-cell GLL.
Few previous studies have commented upon the frequency and distribution of cytolytic T cells in bone marrow specimens from patients with T-cell GLL. Picker and colleagues demonstrated increased CD8+lymphocytes in bone marrows from patients with neutropenia and identified a subgroup that they suggested was similar to patients with GLL who had increased HNK-1+ lymphocytes in bone marrow biopsy specimens.30 In addition, Felgar and colleagues showed TIA-1 positivity in lymphocytes in bone marrow biopsy specimens from patients with GLL,24 and Evans and colleagues report an increase in CD3+ and CD57+ lymphocytes in the bone marrows of patients with GLL compared with neutropenic control patients.19 However, this is the first comprehensive analysis of the morphologic and phenotypic features of T-cell GLL compared with a control group of patients who have presented with clinical and hematologic findings that overlap with GLL.
Although GLL has been extensively studied, uncertainty remains about the minimal features that are required to establish this diagnosis.2-4,13-15 The initially proposed diagnostic criteria included the presence of more than 2 × 109/L granular lymphocytes in the peripheral blood for more than 6 months.4 However, shortly after these criteria were proffered it became evident that a number of bona fide GLL cases did not have this degree of peripheral blood granular lymphocytosis.15 At our institution a potential diagnosis of T-cell GLL was entertained in all cases with unexplained cytopenias and a relative increase in granular lymphocytes even if the absolute LGL count was only slightly elevated. A diagnosis of T-cell GLL was considered to be confirmed if a case exhibited clonal T-cell antigen receptor gene rearrangements.1 For this reason, a significant number of the T-cell GLL cases in this study had peripheral LGL counts of less than 2 × 109/L. A high diagnostic premium has also been placed on identifying a phenotypically distinctive T-cell population in addition to demonstrating clonal T-cell antigen receptor gene rearrangements prior to accepting a T-cell GLL diagnosis. In particular, normal peripheral blood does not contain large populations of CD16+ T cells.16,17,31,32When it does, this phenotype correlates tightly with the ability to demonstrate clonal T-cell antigen receptor gene rearrangements and seems to characterize T-cell GLL.16 Another phenotypic attribute that has been proposed as a relatively specific finding in T-cell GLL is coexpression of CD57 by T cells.2 19 It is not clear why cells from most of the T-cell GLL cases in this study were CD57− in the immunoperoxidase stains performed on the bone marrow biopsy specimens. All specimens contained at least a few strongly CD57+ lymphocytes, providing internal positive controls for the immunohistochemistry methods. In our experience (unpublished observations, October 2000), flow cytometry frequently reveals a broad range of CD57 staining intensity of T-GLL cells. CD57 immunohistochemistry may only detect those cells with high levels of CD57 staining. Our results indicate that CD57 staining in bone marrow biopsy specimens cannot be used reliably as a sensitive diagnostic marker of T-cell GLL.
In cases with a pronounced increase in phenotypically abnormal T LGLs and molecular genetic evidence of T-cell clonality, the aforementioned diagnostic parameters are readily used. However, when the blood granular lymphocyte count is only mildly elevated and a phenotypically distinct lymphocyte population cannot be recognized by flow cytometric analysis, achieving a diagnosis of T-cell GLL is more difficult. Even evidence of T-cell clonality may not be synonymous with T-cell malignancy because minor T-cell clones can be found in oligoclonal immune reactions.33-39 Such clones can be demonstrated in the contracted T-cell repertory of elderly40,41 or immunosuppressed individuals,40 and they can be found in blood or bone marrow specimens from patients with autoimmune disorders42-46 and in other conditions such as myelodysplasia (C.A.H., manuscript in preparation). Thus, it seems that finding a phenotypically distinct lymphocyte population becomes a critical criterion for a T-cell GLL diagnosis. The presence of disease-specific immunohistochemical features in the bone marrow biopsies of T-cell GLL cases with a broad range of peripheral blood granular lymphocyte counts supports the notion that these all represent a uniform disease process regardless of the degree of peripheral blood involvement. Furthermore, these findings suggest that immunohistochemical studies on bone marrow biopsy specimens may provide a useful tool that can be employed in conjunction with other clinical, morphologic, flow cytometric, and molecular genetic studies to help determine if T-cell GLL is present.
Although the bone marrow immunohistochemical studies described here are diagnostically useful, even they appear to lack sufficient diagnostic sensitivity to identify every case of T-cell LGL leukemia. Hopefully, evaluation of other genes and gene products expressed by CTLs will prove useful in determining if a distinct granular lymphocyte population is present. The killing inhibitory receptors (KIRs) are a family of cell-surface receptor molecules that are clonotypically expressed by NK cells and a subset of cytolytic T cells, making them an excellent candidate for such studies.47-52 The KIRs bind to specific class I MHC molecules. A distinct gene encodes for each KIR member, and individual NK cells and cytolytic T cells appear to express a distinct array of these molecules. Evaluation of KIR expression may potentially provide another tool in the armamentarium of tests used in evaluating T-cell GLL patients.53 54 Indeed, one of the patients in this study with confirmed T-cell GLL had restricted KIR expression demonstrated by flow cytometry in the blood LGL population.
The authors thank Barbara Crawford and the immunoperoxidase stain laboratory personnel for technical assistance and Jessica Walters for preparation of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William G. Morice, Div of Hematopathology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail:morice.william@mayo.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal