Tissue inhibitor of metalloproteinases (TIMP)-1 and TIMP-2 are proteins with proteinase-inhibiting and cytokine properties. TIMP-1 is active primarily in B cells and B-cell lymphomas, whereas TIMP-2 expression is restricted to T cells. The expression of TIMP-1 and TIMP-2 in lymph nodes from patients with Hodgkin disease (HD) and in Hodgkin-derived cell lines was investigated. In situ hybridization showed TIMP-1 RNA expression in 3% to 80% of Hodgkin/Reed-Sternberg (H/R-S) cells from 14 of 15 patients, with results in one patient being at the lowest detection limit; no expression of TIMP-2 in H/R-S cells; and only weak expression of TIMP-2 in reactive lymphoid tissue. Production of TIMP-1 protein by H/R-S cells was accordingly found on immunohistochemical analysis of lymph nodes from patients with HD. There was only low expression of matrix metalloproteinase (MMP)-2, which is mainly inhibited by TIMP-2; no expression of MMP-1 and MMP-3 in reactive lymphoid tissue; and no expression of these MMPs in H/R-S cells. Thus, TIMP-1 expression in lymph nodes was not correlated with metalloproteinase expression. Five of 7 Hodgkin-derived cell lines expressed TIMP-1 at the protein level. Only one of these cell lines expressed TIMP-2, at the lowest detection limit. TIMP-1 levels in plasma from patients with HD were within the same range as those in plasma from healthy controls. Recombinant human TIMP-1 inhibited induced cell death in Hodgkin-derived cell lines in vitro. TIMP-1 and TIMP-2 inhibited T-cell cytotoxicity against autologous cells presenting tumor-associated antigens and in allogeneic mixed lymphocyte cultures. Thus, TIMP-1, aside from its role in proteinase equilibrium, is an autocrine and paracrine survival factor for H/R-S cells and an immunosuppressive protein expressed in Hodgkin lymphomas.
Introduction
Hodgkin disease (HD) comprises a lymphoma entity1 and has different histological subtypes. Cytokines play a major role in HD.2-5 Some cytokines are thought to produce symptoms such as fever, night sweats, and weight loss, and some have been described as autocrine or paracrine growth factors. The histopathological presentation of the HD subtypes of nodular sclerosis (NS) and mixed cellularity (MC) is characterized by a few neoplastic Hodgkin/Reed-Sternberg (H/R-S) cells and an abundant infiltration with immune cells, eosinophils, histiocytes, stroma cells, and extracellular matrix (ECM). These features are thought to be related partly to cytokines such as interleukin-1 (IL-1), IL-5, IL-7, IL-8, lymphotoxin-α, tumor necrosis factor (TNF)–α, transforming growth factor-β (TGF-β), hematopoietic growth factors, and eotaxin.2,6-9 Because of the extensive infiltration of lymph nodes in patients with HD by cells of the immunosurveillance system, mechanisms of escape from the immune response are important in explaining disease progression. Most lymphocytes in HD lesions are CD4+ and have a T-helper 2 (Th2) cytokine-expression pattern.10 H/R-S cells express several members of the TNF receptor family, such as the FAS ligand (CD95L), that may induce apoptosis of activated FAS+/CD8+ T cells and natural killer (NK) cells.10 Furthermore, H/R-S cells produce TGF-β and IL-10, which have been found to down-modulate the Th1 response.10
Remodeling of ECM is important during normal development and under pathologic conditions. The matrix metalloproteinases (MMPs) are a family of zinc-dependent peptidases, including collagenases, gelatinases, and stromelysins, that degrade components of ECM and thus play a role in tissue remodeling as well as morphogenesis, angiogenesis, tissue repair, and particularly, tumor invasion.11-18 Most MMPs are secreted as zymogen and activated by other proteases. The activities of MMPs are regulated by a family of specific inhibitors called tissue inhibitors of metalloproteinases (TIMPs). Four TIMPs have been cloned: TIMP-1,19 TIMP-2,20 TIMP-3,21-24and TIMP-4.25 The structural properties of some TIMPs have been studied in detail,26,27 as has their mechanism of MMP inhibition by complex formation.28 A balance between MMPs and TIMPs is physiologically important, and TIMP levels are regulated by steroids, growth factors, and cytokines such as IL-1, IL-6, IL-10, leukemia inhibitory factor, ciliary neurotrophic factor, oncostatin M, TNF-α, and epidermal growth factor.29-36
Only a few studies of the expression and function of TIMPs in lymphoid cells or lymphoma have been described and none investigated HD. Trafficking of lymphocytes is controlled by the balance between MMPs and TIMPs, both of which are produced by these cells.37,38TIMP-1 expression is elevated in patients with malignant non-Hodgkin lymphoma (NHL) and correlates with clinical aggressiveness of the disease.39 TIMP-1 expression in NHL is regulated by IL-6.40 Interestingly, TIMP-1 expression was reported to be restricted to the B-cell lineage and TIMP-2 expression to the T-cell lineage.41 In accordance with results of studies reporting on cytokinelike activities of TIMPs in a wide variety of cell types,32,42-48 Guedez et al observed that TIMP-1 was a survival factor for B cells and induced differentiation in such cells.49,50
To understand further the role of TIMPs in the NS compared with the MC subtype of HD, we studied expression of some members of the MMP and TIMP families in HD. Furthermore, we tested TIMP function in the proliferation and survival of H/R-S cells in vitro and in allogeneic mixed lymphocyte cultures (MLC) and autologous antitumor T-cell cytotoxicity in vitro. Our results show that the proteinase inhibitor TIMP-1 is an autocrine and paracrine survival factor that inhibits induced cell death in H/R-S cells. Surprisingly, we also found TIMP-1 to be an immunosuppressive molecule expressed by HD lymphoma cells in situ and in vitro.
Patients, materials, and methods
Patients
Lymph nodes from 8 patients with the NS subtype and 7 patients with the MC subtype of HD (no further selection) were obtained at diagnosis (before treatment) and subsequently analyzed by using in situ hybridization (ISH). The lymph node specimens were fixed in formalin and embedded in paraffin with use of standard procedures. Additionally, snap-frozen lymph node samples from 13 other patients with HD (6 with NS, 5 with MC, and 2 with lymphocyte-depleted disease), stored at −80°C for up to 4 years, were obtained from the Gerhard Domagk Institute, Muenster, Germany, for supplementary immunohistological studies.
Heparin-treated plasma from 46 patients with HD treated in studies of the German Hodgkin Lymphoma Study Group was obtained either while active disease was present (DP) or during complete remission (CR; NS or MC subtype; no further selection), stored frozen, and used in enzyme-linked immunosorbent assay (ELISA) analysis. Control plasma was obtained from healthy volunteers.
Cells
Cell lines derived from H/R-S cells were either established by one of the authors (V.D.) or provided by Dr D. B. Jones (Southampton, United Kingdom) and Dr H. Kamesaki (Kyoto, Japan). Morphologic characteristics and immunophenotypes of these cell lines were described previously.51 Cell lines were cultured in AIM V medium (Gibco BRL, Gaithersburg, MD) that contained neither TIMPs nor fetal-calf serum (FCS). The K562 (human chronic myelogenous leukemia, blast crisis) and Jurkat (T-cell lymphoma) cell lines served as controls (American Type Culture Collection [ATCC], Manassas, VA). Cell lines were tested repeatedly to ensure absence ofMycoplasma infection during the experiments. For cytotoxicity studies, we generated autologous and allogeneic lymphocytes as effector and target cells.
In situ hybridization
TIMP-1 and TIMP-2 gene probes were generated by oligo(dt)-primed reverse transcription of total-cell RNA prepared from human placentas and HT144 melanoma cells (HTB 63; ATCC), respectively, with subsequent amplification using specific oligodeoxyribonucleotide primers. Primers for TIMP-1 corresponded to nucleotides 69 to 116 and 645 to 665 and those for TIMP-2 to nucleotides 490 to 514 and 981 to 1001 of the published sequences.19,20,52 Sequence analysis was done to verify authenticity of the amplification products. After linearization of plasmids (pAMP1; Gibco BRL) containing specific sequences of the genes for TIMP-1 and TIMP-2, sulfur 35–labeled run-off antisense and sense (control) transcripts were generated by using Sp6 and T7 RNA polymerases (Gibco BRL). MMP-1, MMP-2, and MMP-3 gene probes were obtained and used as described previously.53,54 ISH for detection of RNA transcripts was done as described previously.55 Briefly, dewaxed and rehydrated paraffin sections were exposed to 0.2 N hydrochloric acid (HCl) and 0.125 mg/mL pronase (Boehringer Mannheim, Germany). This was followed by acetylation with 0.1 M triethanolamine (pH 8.0) and 0.25% (vol/vol) acetic anhydride and dehydration through graded ethanols. Slides were hybridized to a level of 2 to 4 × 105 cpm of labeled probes overnight at 54°C. Washing and autoradiography was done as described previously.56
All sections were processed in parallel by using the same batches of reagents and probes. Incubation of sections with Micrococcusnuclease (Boehringer Mannheim) before ISH resulted in extinction of the specific autoradiographic signal, thereby establishing that RNA sequences were the targets of the hybridization procedure.57 ISH results were quantitated by directly visualizing and assessing the percentage of positive H/R-S cells, with results verified by a second person.
Immunohistochemical analyses
TIMP-1–specific immunoreactivity was assessed by using a human TIMP-1–specific monoclonal antibody (mAb; clone 147-6D11; Oncogene, Boston, MA) on acetone-fixed frozen sections, and CD30 staining was done in adjacent serial sections with the mAb Ber-H2 (Dako, Hamburg, Germany). The TIMP-1–specific antibody was also used in a 1:30 dilution for immunostaining of HD-derived cell lines after cytocentrifugation and fixation in acetone. Immunostaining was developed by the alkaline phosphatase antialkaline phosphatase (APAAP) method using new fuchsin as the chromogen.
ELISA analysis
TIMP levels in plasma and cell-culture supernatants were measured by using ELISA kits (R&D Systems, Wiesbaden, Germany) as described by the manufacturer. Plasma (stored at −80°C) was measured either directly or after additional dilution. Cells from cell lines were washed and cultured at a concentration of 106 cells/20 mL of AIM V medium for 48 hours (pH 7.2, 37°C, 5% carbon dioxide [CO2], and high humidity). Subsequently, culture supernatants were harvested, stored at −80°C, and either used directly for ELISA or assayed after additional dilution.
Cell-proliferation assays
Cell lines derived from H/R-S cells were assayed for proliferation in the presence of recombinant human (rh) TIMP-1, rhTIMP-2 (Oncogene Products, Calbiochem-Novabiochem, Bad Soden, Germany), and TIMP-1 mAb (clone 7-6C1, Oncogene) by using tritium-thymidine uptake as a read-out. For the tritium-thymidine–uptake assay, 6.6-μL aliquots of rhTIMP-1 or of anti–TIMP-1 antibody and bovine serum albumin (BSA) in AIM V medium were seeded into 6 wells/test group of 96-well flat-bottomed microtiter plates (NUNC, Kamstrup, Denmark) at various concentrations. The wells already contained 200 μL of the cell suspension (1 × 104 cells/well). Controls contained 6.6 μL phosphate-buffered saline with 0.1% BSA vehicle without TIMP or antibody. The values reported are the final TIMP or antibody concentrations. Plates were incubated at 37°C (pH 7.2) in an atmosphere of 5% CO2 and high humidity for up to 48 hours. The cultures were pulsed for the last 6 hours with 0.25 μCi (0.00925 MBq) tritium-thymidine per well (specific activity, 21.0 mCi/mg [777MBq/mg]; Amersham Pharmacia Biotech Europe, Freiburg, Germany). The samples were then processed and counted (Trilux liquid scintillation and luminescence counter [1450 Microbeta]; Wallac Distribution, Freiburg, Germany). The values reported are means ± SD.
MLC and chromium 51–release assay
MLC were established essentially as described previously.58 59 Briefly, blood was drawn from histoincompatible healthy donors (stimulator and responder) and subjected to Ficoll treatment, and the interphase cells were washed twice. Stimulator mononuclear cells were irradiated (30 Gy). In one experiment, stimulation was done with CD14-enriched cells (by using a magnetically activated cell sorter and CD14 microbeads; Miltenyi Biotech, Bergisch-Gladbach, Germany) and irradiated cells. Irradiated stimulator cells (8 × 106) and viable responder cells (2 × 107) were coincubated (pH 7.2, 37°C, 5% CO2, and high humidity) for 5 days in aliquots of 15 mL RPMI 1640 medium supplemented with 2 mM glutamine and 10% FCS in 50-mL Falcon tubes with repeated, gentle agitation (once a day). On the fifth day, fresh, nonirradiated stimulator cells (obtained as described above; 2 × 106 cells/100 μL) were labeled for 90 to 120 minutes (pH 7.2, 37°C, 5% CO2, and high humidity) with 100 μCi (3.6 MBq) sodium chromium 51 (51Cr)–chromate (100-μL volume; specific activity; 471.2 mCi/mg [17435.1 MBq/mg]; Amersham). Labeled cells were washed 3 times in 1640 RPMI medium with 10% FCS. Meanwhile, effector cells were preincubated with rhTIMP-1 or rhTIMP-2 in various concentrations or with control vehicle for 3 hours. Subsequently, each V-shaped bottom well of the 96-well microtiter plates received a total volume of 150 μL labeled stimulator/target cells and responder/effector cells in the selected effector-to-target ratios, with either rhTIMP-1 or rhTIMP-2 in the concentrations indicated or control vehicle. Microplates were then incubated for 4 hours (pH 7.2, 37°C, 5% CO2, and high humidity) and centrifuged for 5 minutes at 200g. Aliquots of the supernatants were assayed for radioactivity. Maximum Cr release was assayed after Triton X-100 treatment of the wells. Spontaneous chromium (Cr) release from target cells was assayed in medium only. Mean results from triplicates were expressed as the percentage of specific Cr release (experimental Cr release [in cpm] minus spontaneous Cr release [in cpm] times 100 divided by maximum Cr release [in cpm] minus spontaneous release).
Autologous antitumor T-cell cytotoxicity
In one experiment, we used autologous (HLA-A2–positive) phytohemagglutinin-stimulated lymphocytes pulsed with a melanoma-associated nonapeptide (Ile-Met-Asp-Gln-Val-Pro-Phe-Ser-Val, a gp100 peptide modified in position 2 to yield a higher affinity for HLA-A*0201–binding anchor sites than the native peptide60) as a stimulator/target cell and lymphocytes (after 3 periods of stimulation by nonapeptide-presenting cells [autologous to the effector lymphocytes]) as responder/effector cells; other variables were as described above for the MLC.
Apoptosis assays
Annexin V and propidium iodide staining and DNA fragmentation studies with fluorescence-activated cell-sorter scanning analysis.
To assay the influence of rhTIMP-1 on radiation-induced apoptosis of cell lines derived from H/R-S cells, 1 × 106 cells/mL were incubated (37°C, pH 7.2, 5% CO2, and high humidity) with either 500 ng/mL rhTIMP-1 or control medium without TIMP-1, subsequently irradiated (30-90 Gy), and subjected to FACS analysis for annexin V and propidium iodide (PI) staining after additional incubation periods of 4, 12, and 24 hours. Apoptosis was assayed by annexin V, and PI staining (Becton Dickinson, Mountain View, CA) was done to identify apoptotic and dead cells. The cell suspension was kept at 4°C in the dark for 1 hour. The cell samples were then measured by using fluorescence-activated cell-sorter scanning (FACS) analyzer (FACS Calibur; Becton Dickinson) and analyzed with CellQuest and Paint-A-Gate 3.0 software on a Macintosh PC. To assay DNA fragmentation under the same conditions, degraded chromatin was detected by using terminal deoxynucleotidyltransferase deoxyuridine triphosphate nick-end labeling (TUNEL) with the APO-BRDU kit (Pharmingen, San Diego, CA), according to the manufacturer's instructions.
Tritium-thymidine DNA fragmentation assay.
The method used in these experiments was adapted from a protocol described by Brown and Phipps.61 Briefly, 2 × 107 Hodgkin cells (cell line L591) were labeled with 10 μCi tritium-thymidine in a total volume of 20 mL AIM V, 10% FCS, and 2% glutamine for 3.5 hours at 37°C. Cells were then washed twice and resuspended in a concentration of 150 × 103cells/200 μL in AIM V, 10% FCS, and 2% glutamine. Subsequently, cells were incubated with or without 500 ng rhTIMP-1/mL for 1 hour before addition of 500 μM hydrogen peroxide (H2O2) or irradiation (90 Gy) for induction of apoptosis and cell death. Forty-eight hours after this treatment, cells were centrifuged and the supernatant was retained. The cell pellet was resuspended in 0.5 mL lysis buffer containing 5 mM Tris-HCl (pH 8.0), 20 mM EDTA [pH 8.0] and 0.5% Triton X-100. The lysate was centrifuged at 12 000g, and the supernatant was retained. The cell pellet was harvested on a filter mat (Wallac Distribution). The samples were then processed and counted (Trilux liquid scintillation and luminescence counter [1450 Microbeta]). The percentage of DNA fragmentation was calculated as the cpm for fragmentation for the reagent minus the cpm for fragmentation for the control, divided by the total cpm minus the cpm for fragmentation of the control, where the cpm for fragmentation was the cpm in the culture supernatant plus the cpm in the 12 000g supernatant, and the total cpm was the cpm for fragmentation plus the cpm in the 12 000g pellet.
Statistical analysis
Results were evaluated by using the Mann-Whitney test.P values lower than .05 were considered to represent a significant difference.
Results
TIMP-1 RNA expression in sections of lymph node tissue from patients with the NS and MC subtypes of HD
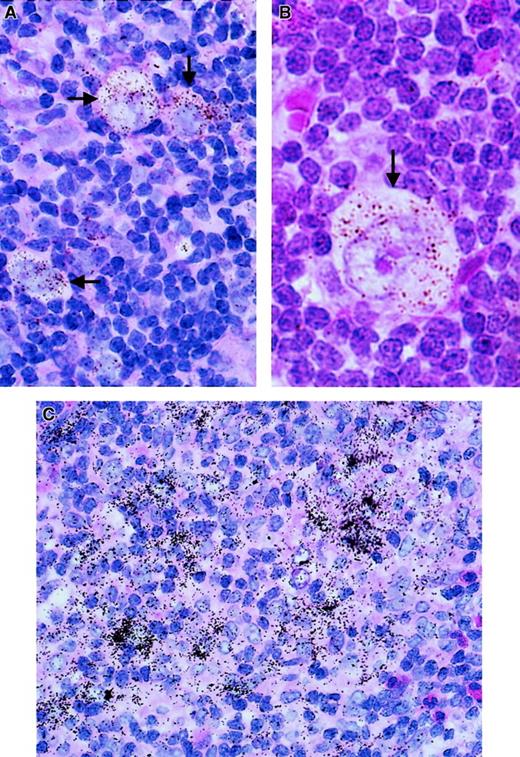
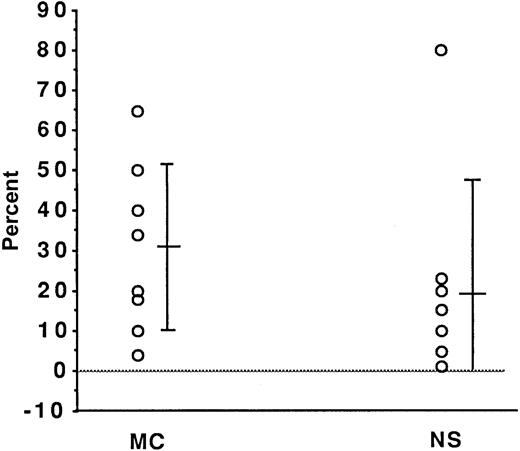
In a set of experiments using paraffin-embedded lymph node tissue sections from 8 patients with the NS subtype of HD and 7 patients with the MC subtype, we conducted assays for RNA expression of TIMP-1. TIMP-1 expression was detected in the samples from 14 of the 15 patients; in one additional sample, expression at the lowest detection limit was observed (Figure 1A and B and Figure 2). In every sample in which expression was observed, the H/R-S cells showed expression of TIMP-1. However, among these cases, various percentages—between 3% and 80%—of H/R-S cells were positive (Figure 2). Although there was a trend toward a higher percentage of H/R-S cells expressing TIMP-1 in samples from patients with the MC subtype, there was no significant difference in TIMP-1 expression between the NS and MS histological subtypes (Figure 2). Distinct, though light, positive signals were also detected in reactive lymphoid tissue distributed throughout the lymph nodes from all patients (both HD subtypes; Figure 1). These signals were most pronounced in areas of active tissue remodeling (Figure1C).
TIMP-1 expression (ISH analysis) in sections of lymph nodes from patients with HD.
Positive cells are characterized by an accumulation of black or brown stains (arrows). (A,B) TIMP-1 expression in H/R-S cells, and TIMP-1 expression in reactive lymphoid tissue. (C) TIMP-1 expression in areas of tissue remodeling.
TIMP-1 expression (ISH analysis) in sections of lymph nodes from patients with HD.
Positive cells are characterized by an accumulation of black or brown stains (arrows). (A,B) TIMP-1 expression in H/R-S cells, and TIMP-1 expression in reactive lymphoid tissue. (C) TIMP-1 expression in areas of tissue remodeling.
TIMP-1 expression (ISH analysis) in H/R-S cells from patients with HD of the NS subtype (n = 7) compared with the MC (n = 8) subtype.
Each circle represents one patient. The y-axis shows the mean ± SD percentage of positive cells. There was no significant difference between the histological subtypes (P = .35 on Mann-Whitney testing).
TIMP-1 expression (ISH analysis) in H/R-S cells from patients with HD of the NS subtype (n = 7) compared with the MC (n = 8) subtype.
Each circle represents one patient. The y-axis shows the mean ± SD percentage of positive cells. There was no significant difference between the histological subtypes (P = .35 on Mann-Whitney testing).
TIMP-2 RNA expression in sections of lymph node tissue from patients with the NS and MC subtypes of HD
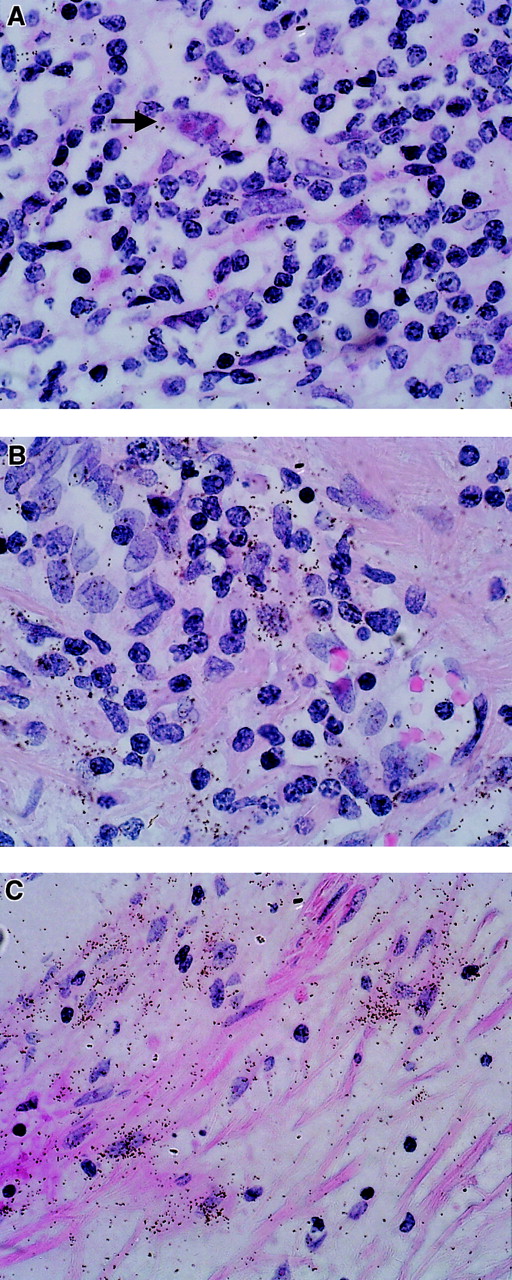
Weak expression of TIMP-2 RNA was observed in all samples examined. In contrast to the results for TIMP-1, H/R-S cells were negative for TIMP-2 RNA (Figure 3A). On the other hand, reactive lymphoid tissue showed faint expression of TIMP-2 in some areas of tissue remodeling (Figure 3B). There was clear expression in fibrotic areas and lymph node capsules (Figure3C).
TIMP-2 expression (ISH analysis) in sections of lymph nodes from patients with HD.
Positive cells are characterized by an accumulation of black stains. (A) TIMP-2–negative H/R-S cells (arrow). (B) TIMP-2 expression in reactive lymphoid tissue. (C) TIMP-2 expression in fibrotic areas.
TIMP-2 expression (ISH analysis) in sections of lymph nodes from patients with HD.
Positive cells are characterized by an accumulation of black stains. (A) TIMP-2–negative H/R-S cells (arrow). (B) TIMP-2 expression in reactive lymphoid tissue. (C) TIMP-2 expression in fibrotic areas.
MMP-1, MMP-2, and MMP-3 RNA expression in sections of lymph node tissue from patients with the NS and MC subtypes of HD
There was only low expression of MMP-2, which is mainly inhibited by TIMP-2, in reactive lymphoid tissue, and no expression of MMP-2 in H/R-S cells. Furthermore, we observed no expression of MMP-1 or MMP-3 in H/R-S cells or reactive lymphoid tissue (data not shown). Thus, TIMP-1 expression in the lymph nodes was not correlated with expression of these MMPs.
Presence of TIMP-1 protein in sections of lymph node tissue from patients with HD
To assay lymph nodes of patients with HD for the presence of TIMP-1 protein, we used the APAAP technique to conduct immunohistochemical studies using specific antibodies against TIMP-1. Because we could not find TIMP-1 antibodies that were useful in studies in paraffin-embedded tissues, we tested 13 snap-frozen HD tissue samples. The antibody produced a diffuse background staining in 11 of 13 samples, with enhanced reactivity on a proportion of plasma cells and cell clusters comprising mononuclear cells, spindle-shaped cells, and a few H/R-S cells, as verified by colocalized CD30 reactivity in serial sections. In 2 samples, one each of the NS and the MC subtype, stored for shorter periods at −80°C, background staining was reduced, and a distinct specific, primarily cytoplasmic staining was observed in cell clusters containing H/R-S cells (Figure4). To check the validity of the immunohistochemical analysis, we also tested our cell lines with this technique (see below).
Immunostaining of TIMP-1 protein.
Specific anti–TIMP-1 antibody (A,B) and CD30 antibody (C) were used on acetone-fixed, frozen lymph node sections from patients with HD.
Immunostaining of TIMP-1 protein.
Specific anti–TIMP-1 antibody (A,B) and CD30 antibody (C) were used on acetone-fixed, frozen lymph node sections from patients with HD.
TIMP-1 and TIMP-2 levels in cell lines derived from H/R-S cells
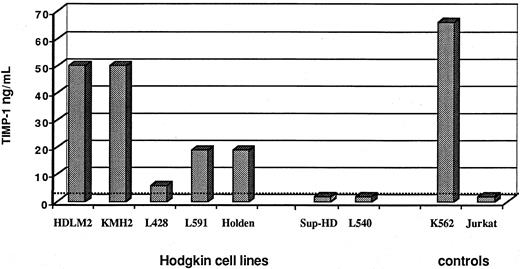
Next, we used ELISA to analyze TIMP-1 and TIMP-2 levels in supernatants of 7 cell lines derived from H/R-S cells. Supernatants of 5 of the 7 cell lines examined showed TIMP-1 protein in various concentrations (Figure 5). For 2 of those 5 cell lines, TIMP-1 levels were about 50 ng/mL. These experiments were reproduced twice, with similar results: the SD was less than 10%. In these experiments, we used as controls the K562 myeloid cell line, which is known to produce TIMP-1, and the Jurkat T-cell line, which is known to be negative for TIMP-1.41 Interestingly, when the serum in the growth conditions for the H/R-S–derived cell lines was reduced, cell supernatants of some cell lines showed considerably more TIMP-1 production on ELISA than those with growth conditions that included serum (the percentage increment in the absence of serum was 20% for the KMH2 cell line, 55% for the Holden line, and 77% for the HDLM2 line).
TIMP-1 concentration (ELISA; nanograms per milliliter) in supernatants of H/R-S–derived cell lines.
TIMP-1 concentration (ELISA; nanograms per milliliter) in supernatants of H/R-S–derived cell lines.
To check the validity of the immunohistochemical results, we also tested cell lines with different TIMP-1 production by ELISA using cytospin preparations for identical APAAP staining. In a blinded assay, a pathologist found that semiquantitative TIMP-1 staining strength directly correlated with TMP-1 levels on ELISA analysis (data not shown). Furthermore, when stained with the TIMP-1–specific mAb, cytospin preparations of cell lines produced slightly stronger staining of the cytoplasm when the cell lines underwent serum starvation before harvesting (data not shown). In contrast, none of the cell lines produced detectable amounts of TIMP-2 (data not shown).
TIMP-1 levels in plasma from patients with HD and healthy controls on ELISA analysis
Next, we assayed serum samples from 46 HD patients with either DP (n = 18) or CR (n = 28) for the presence of TIMP-1 protein. As shown in Figure 6, TIMP-1 was present in various amounts in plasma from all patients (both histological subtypes). In patients with DP, there was a trend toward higher TIMP-1 levels compared with those in controls and those in patients with CR; however, the differences were not significant (Figure 6).
TIMP-1 concentration in plasma from patients with HD.
Values for patients with different HD states (CR indicates complete remission; and DP, active disease present) are shown in comparison with values for healthy controls (CO) as measured by ELISA. Each circle represents one person. Mann-Whitney testing showed no significant differences in values for the following comparisons: DP versus CR (P = .52), DP versus CO (P = .42), and CR versus CO (P = .22).
TIMP-1 concentration in plasma from patients with HD.
Values for patients with different HD states (CR indicates complete remission; and DP, active disease present) are shown in comparison with values for healthy controls (CO) as measured by ELISA. Each circle represents one person. Mann-Whitney testing showed no significant differences in values for the following comparisons: DP versus CR (P = .52), DP versus CO (P = .42), and CR versus CO (P = .22).
Cell proliferation under the influence of rhTIMP-1 and antibody against TIMP-1
We found that TIMP-1 and not TIMP-2 was the TIMP expressed and secreted by H/R-S cells. Furthermore, there was no correlation between expression of TIMP-1 and MMP-1, MMP-2, and MMP-3. Therefore, we tested the hypothesis that TIMP-1 is an autocrine and paracrine growth factor in vitro for cell lines derived for H/R-S cells. We assayed tritium-thymidine uptake in these cell lines with and without the presence of either rhTIMP-1 or antibody against TIMP-1. We found that neither rhTIMP-1 nor the TIMP-1 antibody had a significant effect on cell proliferation (Table 1).
Effect of rh TIMP-1 and TIMP-1 antibody (Ab; clone 7-6C1) on tritium-thymidine uptake in cell lines derived from Hodgkin/Reed-Sternberg cells
| Cell line . | Control . | rhTIMP-1 . | TIMP-1 Ab . |
|---|---|---|---|
| KMH2 | 1674 ± 254 | 1620 ± 581 | 1710 ± 240 |
| L591 | 138 ± 56 | 115 ± 20 | 133 ± 21 |
| Holden | 4844 ± 487 | 4478 ± 287 | 4280 ± 207 |
| Sup-HD | 7481 ± 812 | 7144 ± 619 | 7686 ± 566 |
| Cell line . | Control . | rhTIMP-1 . | TIMP-1 Ab . |
|---|---|---|---|
| KMH2 | 1674 ± 254 | 1620 ± 581 | 1710 ± 240 |
| L591 | 138 ± 56 | 115 ± 20 | 133 ± 21 |
| Holden | 4844 ± 487 | 4478 ± 287 | 4280 ± 207 |
| Sup-HD | 7481 ± 812 | 7144 ± 619 | 7686 ± 566 |
Values are mean ± SD counts per minute derived from 6-fold cultures. There were no significant differences between values for controls and values for either rhTIMP-1 (500 ng/mL) or TIMP-1 Ab (5 μg/mL).
Induced apoptosis of H/R-S cells in the presence of rhTIMP-1
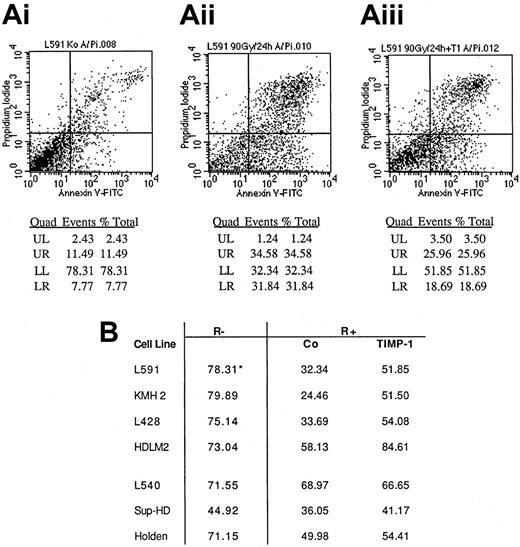
Because the higher production of TIMP-1 in the absence of serum indicated a stress-induced production of this molecule by the H/R-S–derived cell lines, we tested the influence of rhTIMP-1 on induced cell death in those cell lines. We found that induction of a high percentage of apoptosis in the cell lines by radiation required comparatively high doses of radiation. For the first series of experiments, the cell lines were incubated with or without rhTIMP-1 (500 ng/mL) for 15 minutes, irradiated (60-90 Gy), incubated for 4 to 24 hours, and subjected to annexin V and PI FACS analysis. We found that rhTIMP-1 inhibited or retarded radiation-induced apoptosis and diminished the numbers of dead cells in all the Hodgkin-derived cell lines in which increased annexin V and PI staining was induced by radiation (Figure 7). The death of radiation-resistant cell lines was less influenced by rhTIMP-1.
Effect of rhTIMP-1 on survival after radiation of Hodgkin-derived cell lines (FACS analysis).
(A) FACS analysis of apoptosis in the L591 cell line; shown are baseline levels (i), levels of radiation-induced apoptosis or cell death (ii), and levels of radiation-induced apoptosis or cell death with rhTIMP-1 present (iii). LL indicates viable cells with little PI and annexin V staining; LR, annexin V–positive cells; and UR, annexin V–positive and PI-positive cells. (B) Results for all cell lines tested. R− indicates no irradiation (left column); R+, after irradiation; Co, control cells irradiated in the absence of rhTIMP-1; TIMP-1, cells irradiated with rhTIMP-1 present; asterisk, viable cells expressed as the percentage with no annexin V or PI staining (ie, LL cells on FACS analysis).
Effect of rhTIMP-1 on survival after radiation of Hodgkin-derived cell lines (FACS analysis).
(A) FACS analysis of apoptosis in the L591 cell line; shown are baseline levels (i), levels of radiation-induced apoptosis or cell death (ii), and levels of radiation-induced apoptosis or cell death with rhTIMP-1 present (iii). LL indicates viable cells with little PI and annexin V staining; LR, annexin V–positive cells; and UR, annexin V–positive and PI-positive cells. (B) Results for all cell lines tested. R− indicates no irradiation (left column); R+, after irradiation; Co, control cells irradiated in the absence of rhTIMP-1; TIMP-1, cells irradiated with rhTIMP-1 present; asterisk, viable cells expressed as the percentage with no annexin V or PI staining (ie, LL cells on FACS analysis).
These results were confirmed in a subsequent series of experiments using detection of specific chromatin degradation by either nick end labeling with dUTP and by anti-BRDU FACS analysis or detection of tritium-thymidine–labeled DNA fragments in the supernatant and cytoplasm of apoptotic cells (Figure 8). In the DNA fragmentation assay, we also used H2O2 to induce apoptosis and cell death. As shown in Figure 8, rhTIMP-1 reduced H2O2-induced cell death. However, rather high concentrations of H2O2 were necessary because of the comparatively high resistance to apoptosis of the cell lines. With all 3 assessment methods used, inhibition of radiation-induced or H2O2-induced apoptosis by TIMP-1 was not complete but showed considerable variability (Figures 7 and 8).
Effect of rhTIMP-1 on survival of Hodgkin-derived cell line L591.
Effect of rhTIMP-1 on survival of the Hodgkin-derived cell line L591 after exposure to radiation (A,B) or H2O2 (B) as measured by detection of specific chromatin degradation by either nick-end labeling with dUTP and anti-BRDU and FACS analysis (A) or detection of tritium-thymidine–labeled DNA fragments in the supernatant and cytoplasm of apoptotic cells (B). (A) Shown are control conditions (i) compared with radiation conditions (ii) and rhTIMP-1 plus radiation conditions (iii). R1 indicates viable cells; and R2, apoptotic cells. Panel Aiv shows the results of an identical experiment in histogram form. (B) DNA fragmentation expressed as a percentage of values for controls in the absence of rhTIMP-1 and with use of H2O2 (left; 3 different experiments) or radiation (right) to induce apoptosis and cell death.
Effect of rhTIMP-1 on survival of Hodgkin-derived cell line L591.
Effect of rhTIMP-1 on survival of the Hodgkin-derived cell line L591 after exposure to radiation (A,B) or H2O2 (B) as measured by detection of specific chromatin degradation by either nick-end labeling with dUTP and anti-BRDU and FACS analysis (A) or detection of tritium-thymidine–labeled DNA fragments in the supernatant and cytoplasm of apoptotic cells (B). (A) Shown are control conditions (i) compared with radiation conditions (ii) and rhTIMP-1 plus radiation conditions (iii). R1 indicates viable cells; and R2, apoptotic cells. Panel Aiv shows the results of an identical experiment in histogram form. (B) DNA fragmentation expressed as a percentage of values for controls in the absence of rhTIMP-1 and with use of H2O2 (left; 3 different experiments) or radiation (right) to induce apoptosis and cell death.
Studies of the role of TIMP-1 as a candidate molecule for escape from the immune response in HD
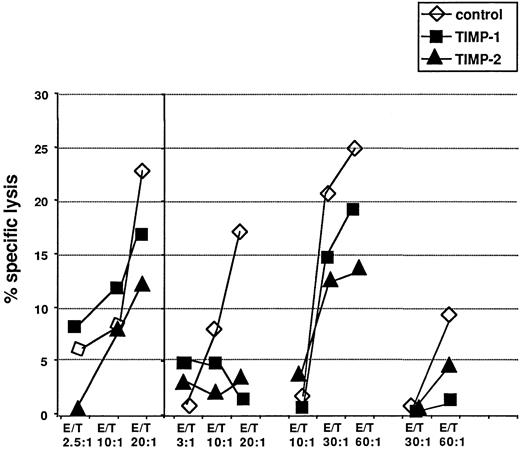
To investigate further the putative role of TIMP-1 in the mechanisms by which H/R-S cells can escape an effective immune response, we studied the effect of rhTIMP-1 and rhTIMP-2 on T-cell–mediated cytotoxicity in alloreactive MLC and antitumor T-cell cytotoxicity as in vitro model systems. In different sets of experiments, both rhTIMP-1 and rhTIMP-2 inhibited T-cell–mediated cytotoxicity against different target cells after only a few hours in the cultures (Figure 9). This effect was most pronounced at higher effector-to-target ratios; in several experiments, there was almost complete suppression of cytotoxicity by both TIMP molecules (Figure 9). However, inhibition of T-cell cytotoxicity by both TIMP molecules varied considerably among different experiments using different cell donors (Figure 9). At optimal effector-to-target ratios, there was a 20% to 90% inhibition of T-cell cytotoxicity by rhTIMP-1 throughout the complete series of experiments.
Effects of rhTIMP-1 and rhTIMP-2 on T-cell cytotoxicity.
Effects of rhTIMP-1 and rhTIMP-2 on T-cell cytotoxicity against autologous phytohemagglutinin-stimulated lymphocytes pulsed with a melanoma-associated peptide, Ile-Met-Asp-Gln-Val-Pro-Phe-Ser-Val (one representative experiment, left panel) and in allogeneic mixed lymphocyte reactions (3 experiments, right panel). Open triangles indicate controls without TIMP; solid symbols represent assays with either rhTIMP-1 or rhTIMP-2 (triangle) present. Values are means from triplicate experiments. Values for TIMPs at the highest effector-to-target ratios were significantly different from control values.
Effects of rhTIMP-1 and rhTIMP-2 on T-cell cytotoxicity.
Effects of rhTIMP-1 and rhTIMP-2 on T-cell cytotoxicity against autologous phytohemagglutinin-stimulated lymphocytes pulsed with a melanoma-associated peptide, Ile-Met-Asp-Gln-Val-Pro-Phe-Ser-Val (one representative experiment, left panel) and in allogeneic mixed lymphocyte reactions (3 experiments, right panel). Open triangles indicate controls without TIMP; solid symbols represent assays with either rhTIMP-1 or rhTIMP-2 (triangle) present. Values are means from triplicate experiments. Values for TIMPs at the highest effector-to-target ratios were significantly different from control values.
Discussion
For this investigation, we originally hypothesized that RNA expression of MMPs and TIMPs would be different in samples from patients with the NS subtype of HD than in those from patients with the MC subtype and that this would help explain the development of fibrotic tissue in NS. However, we found no significant difference in RNA expression between the 2 histological subtypes. In particular, expression of TIMP-1 was independent of the histological type of disease (Figures 1 and 2). Thus, the primary biologic function of TIMP-1 in HD is not to influence ECM composition by changing matrix degradation.
We found expression of TIMP-1 RNA in all lymph node sections from patients with HD, regardless of whether they had the NS or the MC subtype (Figure 1 and 2). Expression was mainly in H/R-S cells. At the protein level, TIMP-1 immunoreactivity was diffusely distributed in most cases. However, in some cases in which there was a shorter period of storage of frozen material, staining was accentuated around clusters of cells containing H/R-S cells. These results were verified by CD30 staining on adjacent serial sections, indicating that TIMP-1 is translated and secreted by H/R-S cells (Figure 4). In contrast, TIMP-2 expression was essentially absent from H/R-S cells and present only in reactive lymphoid tissue (Figure 3). Furthermore, ELISA analyses detected TIMP-1 in supernatants of most of the H/R-S–derived cell lines (Figure 5), whereas TIMP-2 was again essentially absent. These findings were not paralleled by considerable expression of MMP, and there was no significant difference between the NS and MC subtypes in the expression level when different amounts of ECM were present in the lesion. Additionally, TIMP-1 protein was detected by ELISA in plasma from patients with HD (Figure 6). However, levels were in the same range as those in healthy controls. This may be explained by the fact that H/R-S cells constitute only a minority of nucleated cells in Hodgkin lesions and that only proportions of H/R-S cells expressed TIMP-1 at notable levels.
Only a few studies of the expression and function of TIMPs in lymphoma have been reported, and none investigated HD. TIMP-1 is expressed at elevated levels in patients with malignant NHL, and such expression correlates with the clinical aggressiveness of the disease.39 In NHL, expression of TIMP-1 is regulated by cytokines such as IL-6.40 Interestingly, TIMP-1 expression was found to be restricted to the B-cell lineage and, only at low levels, to peripheral blood T cells, but was absent from neoplastic T cells. Expression of TIMP-2 was shown to be restricted to cells of the T-cell lineage, with high levels observed in neoplastic T cells.41 This distinction might not be absolute because, in our experiments, cell lines also described as expressing some T-cell markers, such as the Holden and HDLM2 lines,51 were found to produce and secrete TIMP-1. Thus, our findings do not unequivocally support the hypothesis that H/R-S cells have a B-cell origin. In this context, it is interesting that elevated levels of TIMP-1 in TIMP-1 transgenic mice inhibit growth and metastasis of T-cell lymphoma.62 This effect, however, was thought to be not restricted to this histological type but to result from metalloproteinase inhibition by TIMPs and antiangiogenic properties of TIMPs.
In accordance with studies describing cytokinelike activities of TIMPs in a wide variety of cell types,32,42-48 Guedez et al49,50 observed that TIMP-1 was a survival factor for B cells and induced differentiation in those cells. In our experiments assaying tritium-thymidine incorporation of H/R-S–derived cell lines, neither TIMP-1 nor TIMP-1 antibodies produced a major modulation (Table1). Thus, on the basis of our results, we cannot suggest a direct cytokinelike role of TIMP-1 in the proliferation of H/R-S cells. We also examined whether TIMP-1 affected radiation-induced and H2O2-induced apoptosis and cell death. We found that H/R-S–derived cell lines were extremely resistant to induction of apoptosis, thus necessitating use of high doses of radiation and high concentrations of H2O2. Several mechanisms explaining the relative resistance to apoptosis of H/R-S cells have been described in various studies,63-65 including microarray studies64,65; these mechanisms include constitutive nuclear factor κB activation63 64 and differential expression of a variety of apoptosis-influencing genes. Furthermore, Hodgkin-derived cell lines were often derived from patients with late-stage disease resistant to chemotherapy and radiation. However, we found that radiation-induced and H2O2-induced apoptosis of H/R-S–derived cell lines could be significantly inhibited or retarded and the numbers of dead cells diminished by rhTIMP-1 in vitro (Figures 7 and 8). Thus, in accordance with the findings of Guedez et al in B cells, our observation of inhibition of apoptosis by TIMP-1, together with the TIMP-1 plasma levels measured in patients with HD and healthy controls, indicate that TIMP-1 is a survival factor for H/R-S cells, with activity restricted to the autocrine and paracrine area.
In HD, with the extensive infiltration of lymph nodes by cells of the immunosurveillance system, immune-escape mechanisms are important in explaining disease progression. Most lymphocytes in HD are CD4+ and have a Th2 cytokine-production profile.10 Several factors supporting this phenotype of lymphocyte composition have been discussed.64 H/R-S cells express several members of the TNF receptor family, such as the FAS ligand (CD95L), that may induce apoptosis of activated FAS-positive, CD8+ T cells and NK cells.10 Furthermore, H/R-S cells produce TGF-β and IL-10, which have been found to down-modulate Th1 response.10 In this study, we used MLC as a model system for studying cellular cytotoxicity. Surprisingly, we were able to characterize TIMP-1 as a molecule produced by H/R-S cells that can inhibit T-cell cytotoxicity in alloreactive MLC and against tumor-associated antigen-presenting cells in vitro after being present in the assay for only a few hours (Figure 9). T-cell cytotoxicity in such reactions is exerted largely by CD8+cells.66 However, CD4+ T cells are reactive in autologous mixed lymphocyte reactions in HD samples,67 and CD4+ T cells are also involved in cytotoxicity with B-cell lymphoma cells belonging to the susceptible target-cell types.66 Thus, suppression of cellular immunity could be envisaged as a function of TIMP-1 in Hodgkin lymphoma. However, in our experiments, we have shown inhibition of T-cell cytotoxicity by TIMPs only by using lymphocytes from healthy volunteers, and it is not known whether this effect also occurs in tumor-infiltrating lymphocytes from patients with HD.
The mechanisms leading to inhibition of T-cell cytotoxicity by TIMP-1 must be further elucidated. In addition to this inhibition by TIMP-1, we found strong inhibition of T-cell cytotoxicity by TIMP-2 (Figure 9). In this context, it is interesting that induction of apoptosis in activated T-cells by TIMP-2 has been described.68 This was not found when TIMP-2 fragments lacking the MMP binding domain were used. Many cell-surface proteins are processed and shed by MMP-type proteases, and down-regulation of FAS ligand by shedding has been reported.69 Soluble FAS ligand inhibits cytotoxicity of membrane-bound FAS ligand.69 Inhibition of protease-induced soluble FAS ligand may explain induction of apoptosis by TIMPs through a phenomenon described as fratricide.70On the other hand, the cytotoxic lymphocyte serpin proteinase inhibitor 9 was shown to protect against granzyme B–mediated apoptosis without disturbing the FAS pathway,71 and this may be a mechanism controlling misdirected fratricide apart from the FAS pathway. Additional studies are needed to determine whether there is induction of apoptosis of T-cells by TIMP-1 or interference between TIMP-1 and granzyme B–mediated cytotoxicity in our experimental system. Interestingly, shedding inhibition of TNF-α receptors by TIMP-2, but not by TIMP-1, was observed; thus, shedding inhibition of molecules important for cellular cytotoxicity is specific for single TIMP forms.72
Viruses produce caspase inhibitors possibly to delay inflammatory processes and retard quick apoptosis of infected cells.73One of these molecules, cytokine response modifier A, belongs to the serpin proteinase inhibitor family.73 It is interesting that a major retroviral gag core protein is structurally related to TIMP-174 and that Tax proteins of human T-cell leukemia viruses induce expression of TIMP-1.75 Additional studies of the relation between proteins of viruses important for HD development and protease inhibitors would be of great interest.
Impaired specific T-cell cytotoxicity in HD testing targets, such as Epstein-Barr virus (EBV), has been observed in patients with EBV-positive HD.76,77 Our results provide an explanation for this phenomenon. However, aspects of immune escape in HD other than TIMP-1 might be important. Some could include cellular cooperation. It was reported that H/R-S cells produce a thymus and activation-regulated chemokine that is active in recruitment of CD4+ Th2 cells to be dominant in HD.10,78CD4+ cells were found to inhibit CD8+ cytotoxic T-lymphocyte immunosurveillance against experimental tumors, including B-cell lymphomas.79,80 Immunotoxin depletion of regulatory cell populations producing immune-escape molecules such as IL-10 has been tested as a method for augmenting antitumor cytotoxic T cells.81 Additional studies of the use of this technique in HD, including the possible role of MMPs and TIMPs, would be interesting.
Our ELISA analyses of plasma TIMP-1 levels in patients with different states of HD and in healthy controls revealed only a trend toward higher plasma levels in patients with DP, thus suggesting that the major role of TIMP-1 in HD is as an autocrine and paracrine molecule. This finding is somewhat in contrast to reports of comparatively high levels of TIMP-1 in blood samples from patients with advanced solid tumors.82 However, elevated IL-10 levels in the serum of patients with HD have been found to be associated with inferior disease-free survival.83 Thus, the prognostic importance of other immunomodulating molecules, including TIMP-1, in HD should be studied. Strategies for overcoming immune escape might be developed to improve the prognosis or increase the efficacy of immunotherapy.
Finally, because TIMP-1 has direct effects on cells changing their phenotype,32,42-50 the existence of specific receptors can be postulated. However, only limited information on TIMP-1 binding sites is available. TIMP-2 binds to membrane type 1 metalloproteinase,84,85 as does TIMP-1, although with a lower affinity.86 Because integrins can interact with metalloproteinases,87 integrin signaling may play a role in these effects of TIMP-1 on cells. However, this and other possibilities require further study.
In conclusion, this study showed that the proteinase inhibitor TIMP-1 is expressed by H/R-S cells in situ and in vitro. We also found that TIMP-1, besides having a role in the proteinase equilibrium, is an autocrine and paracrine survival factor for H/R-S cells and one of several immunosuppressive molecules expressed by H/R-S cells in situ and in vitro.
We thank U. Tank and G. Krull for technical assistance. Experiments were performed by E.O. as part of MD thesis.
Supported by a grant from Amgen-Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elisabeth Oelmann or Wolfgang E. Berdel, Department of Medicine (Hematology/Oncology), University Hospital, Westfaelische Wilhelms Universitaet Muenster, Albert-Schweitzer Street 33, D-48149, Muenster, Germany; e-mail:elisao@uni-muenster.de.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal