Children with Down syndrome (DS) have an increased risk for leukemia. The prognosis for DS acute myeloid leukemia (AML) is better than for non-DS AML, but the clinical outcome of DS acute lymphoblastic leukemia (ALL) is equal to that of non-DS ALL. Differences in prognosis may reflect differences in cellular drug resistance. In vitro drug resistance profiles were successfully investigated on leukemic cells from 13 patients with DS AML and 9 patients with DS ALL and were compared with reference data from 151 non-DS AML and 430 non-DS B-cell precursor (BCP) ALL. DS AML cells were significantly more sensitive to cytarabine (median, 12-fold), the anthracyclines (2-7–fold), mitoxantrone (9-fold), amsacrine (16-fold), etoposide (20-fold), 6-thioguanine (3-fold), busulfan (5-fold), vincristine (23-fold), and prednisolone (more than 1.1-fold), than non-DS AML cells. Compared with DS ALL, DS AML cells were significantly more sensitive to cytarabine only (21-fold). After short-term exposure to methotrexate, DS AML cells were 21-fold more resistant than non-DS AML cells, but no difference was observed after continuous exposure. DS ALL cells and non-DS BCP-ALL cells were equally sensitive to all drugs, including methotrexate. Normal peripheral blood mononuclear cells from DS and non-DS children without leukemia showed highly resistant drug profiles. It was concluded that the better prognosis of DS AML might, at least partially, be explained by a specific, relatively sensitive drug-resistance profile, reflecting the unique biology of this disease.
Introduction
Children with Down syndrome (DS) have an elevated risk for acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).1 Before the age of 5 years, the risk for AML is 4 times higher than the risk for ALL.1 When treated with chemotherapy, children with DS also experience more side effects (such as infections and mucositis) from treatment than children without DS.2-6 Remarkably, children with DS AML have a better prognosis than children with non-DS AML.2,7-9 In addition, their clinical characteristics differ from those of non-DS AML children: younger age, lower white blood cell count (WBC), and high incidence of French-American-British (FAB) M7.2,8-11Children with DS ALL have a similar clinical outcome when compared with children with non-DS ALL.4,5,12,13 Some studies report a lower frequency of T-ALL in DS; others do not confirm this.3-5
Differences in prognosis may reflect differences in cellular drug resistance, pharmacokinetics, or regrowth potential of residual disease. We have shown previously that drug-resistance testing provides clinically relevant information on resistance profiles from specific subgroups of leukemia patients.14-16 In addition, cellular resistance has been shown to predict treatment outcome, independent of other prognostic factors.17-19
In the current study, we analyzed differences in cellular drug resistance between samples from children with DS AML and DS ALL, and we compared the results with reference values of non-DS AML and non-DS B-cell precursor (BCP) ALL. Normal peripheral blood mononuclear cells (PBMCs) from nonleukemic children with and without DS were also compared for differences in chemosensitivity. This might reflect the greater sensitivity of DS patients for the side effects of chemotherapy.
Patients, materials, and methods
Patient samples
We tested bone marrow or peripheral blood samples from children (newborn to 18 years of age) given diagnoses of de novo AML or ALL, with and without Down syndrome (DS). Three different collaborative groups provided us with patient samples: the AML-BFM Study Group (Münster, Germany), the CoALL Study Group (Hamburg, Germany), and the Dutch Childhood Leukemia Study Group (DCLSG, Den Haag, The Netherlands). Central review of diagnoses, clinical data, and characteristics of disease by immunophenotype (ALL) or FAB classification (AML) was performed by reference laboratories of these groups.
We also tested normal, nonleukemic PBMCs from 9 children with DS and 12 children without DS. Children with inflammation or infection were excluded from this study. PBMCs were collected in hospitals in ‘s-Hertogenbosch (EV) and Amsterdam after informed consent was obtained, according to the Institutional Review Board guidelines.
In this study 2 reference groups were used: non-DS children with AML and non-DS children with BCP-ALL. The non-DS AML group consisted of 151 patients (78 boys, 73 girls) with a median age of 8.4 years. Median WBC was 35.5 × 109/L, and FAB classification showed the following: 5 patients with M0, 20 patients with M1, 36 patients with M2, 8 patients with M3, 44 patients with M4, 30 patients with M5, 1 patient with M6, 1 patient with M7, and 6 patients unclassified. The non-DS B-cell precursor ALL group consisted of 430 patients (238 boys, 192 girls). Median age was 4.4 years, and median WBC was 13.5 × 109/L.
Cells
Mononuclear cells were isolated by density-gradient centrifugation with Ficoll Isopaque. In ALL samples with a low (less than 90%) blast percentage (determined morphologically on May-Grünwald-Giemsa (MGG) cytospin slides), we used monoclonal antibodies linked to magnetic beads to remove nonleukemic cells, as described before.20 In AML samples, a low blast percentage (less than 80%) might have been caused by the presence of mature granulocytes (more than 10%) or lymphocytes (more than 10%). To eliminate granulocytes we performed freezing, in liquid nitrogen, and thawing.16 Lymphocytes were removed using immunomagnetic beads at room temperature to prevent phagocytosis of the beads by leukemic cells. After these enrichment procedures, the percentage of blasts was determined morphologically (MGG cytospin slides) and was confirmed with immunocytochemistry if morphologic results were inconclusive. The cutoff level to actually perform the 3-(4,5-dimethylthiazol-2,5-diphenyl) tetrazolium bromide (MTT) assay was set at 80% leukemic cells.
FAB classification was performed according to the criteria of Bennett et al,21 22 including the modifications to diagnose FAB M7. Immunophenotype of ALL cells was determined by flow cytometry or by immunocytochemistry. Patients with DS ALL appeared to have BCP-ALL, defined as sIg−/TdT+/HLA-Dr+/CD19+and either CD10+ or cμ+or both.
MTT assay
In vitro drug resistance of leukemia and PBMC samples was measured using a 4-day cell culture assay, based on the principle that only viable cells are able to reduce MTT to a colored formazan product that can be determined spectrophotometrically at 562 nm.23Optical density (OD) is linearly related to the number of viable cells.23 Drugs were tested in 6 different concentrations and in duplicate. Six control wells containing leukemic cells with culture medium, but no drugs, were used to determine the control cell survival (CCS) after 4 days of culture. Four wells with culture medium only were used as blank. Cytotoxicity was calculated at each drug concentration by the following equation after correction for the background OD of the blank wells: OD treated well/mean OD control wells × 100%. Results were considered evaluable only if the control wells contained 70% or more leukemic cells (determined by morphology after MGG staining) after 4 days of culture and if the mean control OD, after correction for the background, at day 4 exceeded 0.05 arbitrary units. The LC50 value, which is the drug concentration needed to kill 50% of the leukemia cells, was used as a measure of resistance. Sample source (bone marrow or peripheral blood) and cryopreservation did not influence the results obtained by cellular resistance testing.24 The following drugs were tested (range of concentration): amsacrine (0.006-20 μg/mL, Amsidine, Parke-Davis, Hoofddorp, The Netherlands); busulfan (1.23-300 μg/mL, Myleran, Glaxo-Wellcome, Zeist, The Netherlands); 2-chlorodeoxyadenosine (0.0004-40 μg/mL, Leustatin, Ortho-Biotech, Raritan, NJ); cytarabine (0.002-2.5 μg/mL, Cytosar, Pharmacia & Upjohn, Woerden, The Netherlands); daunorubicin (0.002-2 μg/mL, Cerubidine, Rhône-Poulenc Rorer, Amstelveen, The Netherlands); dexamethasone disodium phosphate (0.0002-6 μg/mL, Bufa Pharmaceutical Products, Uitgeest, The Netherlands); doxorubicin (0.008-8 μg/mL, Adriablastina, Pharmacia & Upjohn); etoposide (0.05-50 μg/mL, Vepesid, Bristol-Myers Squibb, Woerden, The Netherlands); idarubicin (0.002-2 μg/mL, Zavedos, Pharmacia & Upjohn); 4-hydroperoxy-ifosfamide (0.1-100 μg/mL, active metabolite of ifosfamide, Asta-Medica, Diemen, The Netherlands), L-asparaginase (0.003-10 IU/mL, Paronal, Christiaens, Breda, The Netherlands); 6-mercaptopurine (15.6-500 μg/mL, Puri-Nethol, Glaxo-Wellcome); mitoxantrone (0.001-1 μg/mL, Novantrone, AHP Pharma Wyeth Lederle, Hoofddorp, The Netherlands); prednisolone disodium phosphate (0.008-250 μg/mL, Bufa Pharmaceutical Products); 6-thioguanine (1.56-50 μg/mL, Lanvis, Glaxo-Wellcome); vincristine (0.05-50 μg/mL, Oncovin, Eli Lilly, Nieuwegein, The Netherlands). These concentration ranges were determined empirically to obtain the largest number of evaluable dose-response curves in non-DS ALL and AML, allowing the calculation of LC50 values in most, but not all, samples.
Thymidylate synthase inhibition assay
Thymidylate synthase (TS) inhibition assay (TSIA) is based on determination of the inhibition of the TS-catalyzed conversion of3H-dUMP to dTMP and 3H2O, after the exposure of intact cells to methotrexate (MTX).25 Briefly, 0.1 × 106 cells were incubated in 150 μL culture medium with and without MTX. After 4 hours, [5-3H]-2′-deoxycytidine (final concentration, 1 μM) was added as precursor for 3H-dUMP. Two conditions were studied: a short MTX exposure of 3 hours followed by an 18-hour drug-free period and a continuous MTX exposure of 21 hours. When the control TS activity was lower than 500 dpm, the results were considered not evaluable. Data were expressed as the concentration of MTX (μM) needed to inhibit 50% of the TS activity after short-term exposure (TSI50short) or continuous exposure (TSI50cont). PBMCs could not be studied with the TSIA because the TS levels of PBMCs were too low. When MTX is not retained in the cell because of decreased accumulation or increased elimination, a high TSI50short will be observed. After continuous exposure this resistance can be bypassed.
Statistics
Differences in the distribution of LC50 values were analyzed using the Mann-Whitney U test. For statistical comparisons of categorical variables, χ2 analysis was used, and Fisher exact test was used for small numbers of patients. P ≤ .01 was considered statistically significant (2-tailed test). If the number of samples in a specific subgroup was less than 4, statistical analysis was not performed and resistance ratios (differences in median LC50 values for a specific drug between 2 groups of patients) were not calculated.
For childhood ALL, we previously defined LC50 threshold levels for prednisolone sensitivity because these values showed a skewed distribution: highly sensitive, less than 0.1 μg/mL (μM); intermediately sensitive, 0.1-150 μg/mL (μM); and resistant, more than 150 μg/mL (μM).17
Results
Patient characteristics
Samples from 22 patients with DS AML and 20 with DS ALL were tested. Two children with transient myeloproliferative disease were included in the DS AML group. MTT assay was successful in 13 DS AML and 9 DS ALL samples. Their patient characteristics are given in Table 1. We also tested normal PBMCs from 9 children with DS and 12 without DS, all without leukemia.
Characteristics of patients with DS AML and DS ALL
| . | DS AML . | DS ALL . |
|---|---|---|
| Number | 13 | 9 |
| Median age, y | 1.7 | 4.5 |
| Range | 0.0-2.5 | 0.0-15.4 |
| Sex, male:female | 7:6 | 5:4 |
| Median WBC, ×109/L | 18.6 | 22.3 |
| Range | 1.9-201.0 | 4.3-116.2 |
| FAB classification | ||
| M0 | 2 | — |
| M2 | 0 | — |
| M5 | 0 | — |
| M6 | 1 | — |
| M7 | 8 | — |
| TMD | 2 | — |
| Unclassified | 0 | — |
| Immunophenotype | B-cell precursor ALL | |
| n = 9 |
| . | DS AML . | DS ALL . |
|---|---|---|
| Number | 13 | 9 |
| Median age, y | 1.7 | 4.5 |
| Range | 0.0-2.5 | 0.0-15.4 |
| Sex, male:female | 7:6 | 5:4 |
| Median WBC, ×109/L | 18.6 | 22.3 |
| Range | 1.9-201.0 | 4.3-116.2 |
| FAB classification | ||
| M0 | 2 | — |
| M2 | 0 | — |
| M5 | 0 | — |
| M6 | 1 | — |
| M7 | 8 | — |
| TMD | 2 | — |
| Unclassified | 0 | — |
| Immunophenotype | B-cell precursor ALL | |
| n = 9 |
Data are shown only for patients with successful MTT assay.
TMD indicates transient myeloproliferative disease.
Patients with DS AML were significantly younger (P < .001) than patients with non-DS AML. WBC (P = .17) and sex distribution (P = .9) were not different. Only 1 of the non-DS patients was classified as FAB M7, whereas 64% of the patients with DS AML were classified as such (P < .001).
Patients with DS ALL did not differ significantly from patients with non-DS BCP-ALL for age (P = .72), sex (P = .93), or WBC (P = .47). All 20 patients with DS-ALL had BCP-ALL.
Cellular drug resistance
MTT assay.
In DS AML, 59% of samples were tested successfully for at least 1 drug. Reasons for assay failure were too few cells or too low blast count at day 0 (n = 6) or too low blast count after 4 days of culture (n = 3). The 2 patients with transient myeloproliferative disease showed a drug resistance pattern similar to that of patients with DS AML. Patients with successful assays had higher WBCs than patients with nonsuccessful assays (P = .02; median, 10.6 vs 18.6 × 109/L). There were no significant differences for sex, age, or FAB type distribution.
In DS ALL, the success rate of the MTT assay was 45%. Reasons for failure were too few cells or too low blast count at day 0 (n = 3), too low blast count at day 4 (n = 6), or too low OD (n = 2). Patients with successful assays had higher WBCs than patients with nonsuccessful assays (P = .02; median, 7.1 vs 22.3 × 109/L). There were no significant differences for sex and age.
There were no significant differences between DS AML (median, 108%; range, 15%-268%) and DS ALL (median, 56%; range, 28%-263%) samples considering CCS after 4 days of culture (P = .72). The OD/105 cells (metabolic capacity of viable cells to convert MTT to formazan) was significantly (P = .001) higher in patients with DS AML (median, 0.63; range, 0.37-1.11) than in patients with DS ALL (median, 0.28; range, 0.13-0.65). Patients with DS AML and DS ALL did not differ significantly from those with non-DS AML and non-DS ALL, respectively, in either CCS or OD/105cells. For normal nonleukemic PBMCs, the OD/105 cell median was 0.36 (range, 0.18-0.53) and the CCS median after 4 days of culture was 77% (range, 22%-131%). There were no significant differences in OD/105 cells or in CCS between DS PBMCs and non-DS PBMCs, nor was there a significant correlation between the percentage of monocytes in a PBMC sample and the OD/105 cells of that particular sample (Spearman rank correlation ρ = 0.2;P = .48). DS PBMCs and non-DS PBMCs did not differ in the percentage of monocytes (P = .67).
We found marked differences in drug resistance between patients. Dose-response curves could be obtained for most drugs. However, in DS and non-DS AML, even the highest concentration of prednisolone was unable to kill more than 50% of the leukemia cells in a significant proportion of samples. For 6-thioguanine and vincristine, even the lowest concentration of drug already killed more than 50% of the leukemic cells in a sensitive subset of DS-AML samples.
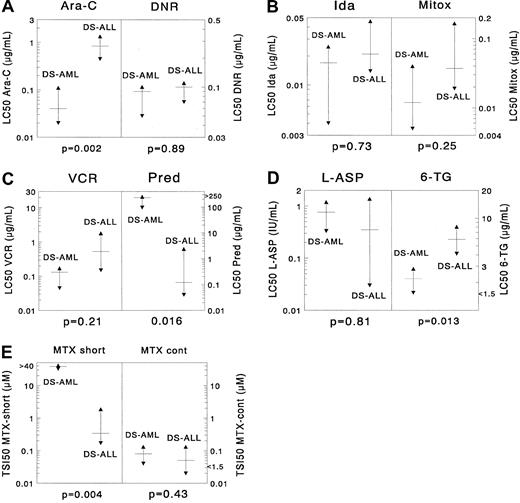
DS AML versus DS ALL.
Sample sizes for 9 drugs in the DS AML and DS ALL groups were large enough for statistical comparison. DS AML was significantly more sensitive than DS ALL only for cytarabine (median, 21-fold;P = .002). For 6-thioguanine there was a trend suggesting that DS AML samples were 2.7-fold more sensitive than DS ALL samples (P = .013). DS AML cells were significantly more resistant than DS ALL cells after short-term exposure to MTX (113-fold;P = .004); however, this was not the case after continuous MTX exposure (P = .43). For the other drugs no significant differences were found, though there was a trend suggesting that DS AML was more resistant to prednisolone than DS ALL (P = .016). When using the criteria for prednisolone sensitivity mentioned in “Patients, materials, and methods,” none of the DS AML samples was highly prednisolone sensitive, and 4 of 10 samples showed intermediate sensitivity. In DS ALL, 44% of the samples were in the highly prednisolone-sensitive group (χ2 analysis,P = .018). Median LC50 values are given in Table 2 for DS AML and in Table3 for DS ALL. Results are depicted in Figure 1.
Cellular drug resistance in patients with DS versus non-DS AML
| Drug . | DS AML . | Non-DS AML . | RR . | P . | ||
|---|---|---|---|---|---|---|
| N . | Median (P25-P75) . | N . | Median (P25-P75) . | |||
| Cytarabine | 13 | 0.04 | 143 | 0.46 | 11.5 | <.001 |
| (0.02-0.11) | (0.24-1.21) | |||||
| Daunorubicin | 12 | 0.09 | 130 | 0.20 | 2.2 | .005 |
| (0.05-0.10) | (0.09-0.38) | |||||
| Idarubicin | 8 | 0.017 | 94 | 0.122 | 7.2 | .002 |
| (0.004-0.025) | (0.039-0.265) | |||||
| Mitoxantrone | 10 | 0.012 | 113 | 0.11 | 9.2 | .002 |
| (0.005-0.04) | (0.03-0.35) | |||||
| Etoposide | 12 | 0.37 | 132 | 7.42 | 20.1 | <.001 |
| (0.16-0.60) | (1.92-19.41) | |||||
| 6-Thioguanine | 12 | 2.20 | 140 | 6.03 | 2.7 | <.001 |
| (<1.56-2.81) | (4.05-10.54) | |||||
| Amsacrine | 8 | 0.03 | 115 | 0.48 | 16.0 | <.001 |
| (0.02-0.08) | (0.14-1.32) | |||||
| 2-Chloro-deoxyadenosine | 6 | 0.016 | 103 | 0.020 | 1.3 | .42 |
| (0.004-0.023) | (0.04-0.031) | |||||
| Prednisolone | 10 | 231.6 | 146 | >250 | >1.1 | .006 |
| (95.9->250) | (>250->250) | |||||
| Vincristine | 11 | 0.13 | 135 | 2.99 | 23.0 | <.001 |
| (<0.05-0.17) | (0.75-23.92) | |||||
| L-Asparaginase | 10 | 0.76 | 123 | 0.88 | 1.2 | .83 |
| (0.33-1.19) | (0.23-1.48) | |||||
| Busulfan | 6 | 8.2 | 93 | 37.78 | 4.6 | <.001 |
| (6.1-13.7) | (25.59-59.82) | |||||
| 4-hydroperoxy-ifosfamide | 8 | 10.12 | 116 | 11.93 | 1.2 | .47 |
| (4.69-12.31) | (5.79-14.78) | |||||
| Methotrexate (TSI50 short) | 11 | 38.32 | 28 | 1.82 | 0.05 | .003 |
| (32.62->40) | (0.63-18.38) | |||||
| Methotrexate (TSI50 cont) | 9 | 0.08 | 27 | 0.05 | 0.6 | .22 |
| (0.04-0.13) | (0.03-0.06) | |||||
| Drug . | DS AML . | Non-DS AML . | RR . | P . | ||
|---|---|---|---|---|---|---|
| N . | Median (P25-P75) . | N . | Median (P25-P75) . | |||
| Cytarabine | 13 | 0.04 | 143 | 0.46 | 11.5 | <.001 |
| (0.02-0.11) | (0.24-1.21) | |||||
| Daunorubicin | 12 | 0.09 | 130 | 0.20 | 2.2 | .005 |
| (0.05-0.10) | (0.09-0.38) | |||||
| Idarubicin | 8 | 0.017 | 94 | 0.122 | 7.2 | .002 |
| (0.004-0.025) | (0.039-0.265) | |||||
| Mitoxantrone | 10 | 0.012 | 113 | 0.11 | 9.2 | .002 |
| (0.005-0.04) | (0.03-0.35) | |||||
| Etoposide | 12 | 0.37 | 132 | 7.42 | 20.1 | <.001 |
| (0.16-0.60) | (1.92-19.41) | |||||
| 6-Thioguanine | 12 | 2.20 | 140 | 6.03 | 2.7 | <.001 |
| (<1.56-2.81) | (4.05-10.54) | |||||
| Amsacrine | 8 | 0.03 | 115 | 0.48 | 16.0 | <.001 |
| (0.02-0.08) | (0.14-1.32) | |||||
| 2-Chloro-deoxyadenosine | 6 | 0.016 | 103 | 0.020 | 1.3 | .42 |
| (0.004-0.023) | (0.04-0.031) | |||||
| Prednisolone | 10 | 231.6 | 146 | >250 | >1.1 | .006 |
| (95.9->250) | (>250->250) | |||||
| Vincristine | 11 | 0.13 | 135 | 2.99 | 23.0 | <.001 |
| (<0.05-0.17) | (0.75-23.92) | |||||
| L-Asparaginase | 10 | 0.76 | 123 | 0.88 | 1.2 | .83 |
| (0.33-1.19) | (0.23-1.48) | |||||
| Busulfan | 6 | 8.2 | 93 | 37.78 | 4.6 | <.001 |
| (6.1-13.7) | (25.59-59.82) | |||||
| 4-hydroperoxy-ifosfamide | 8 | 10.12 | 116 | 11.93 | 1.2 | .47 |
| (4.69-12.31) | (5.79-14.78) | |||||
| Methotrexate (TSI50 short) | 11 | 38.32 | 28 | 1.82 | 0.05 | .003 |
| (32.62->40) | (0.63-18.38) | |||||
| Methotrexate (TSI50 cont) | 9 | 0.08 | 27 | 0.05 | 0.6 | .22 |
| (0.04-0.13) | (0.03-0.06) | |||||
LC50 values are given in μg/mL. For MTX, the TSI50 values (μM) are given after short-term (TSI50 short) or long-term (TSI50 cont) exposure.
RR, the median LC50 value (for MTX, TSI50) for non-DS AML divided by the median LC50 value (for MTX, TSI50) for DS AML.
P value derived from 2-tailed Mann-Whitney Utest.
L-asparaginase is given in IU/mL, not in μg/mL.
N indicates number of samples tested; P25-P75, 25th and 75th percentiles.
Cellular drug resistance in DS ALL versus non-DS BCP-ALL
| Drug . | DS ALL . | Non-DS BCP-ALL . | RR . | P . | ||
|---|---|---|---|---|---|---|
| N . | Median (P25-P75) . | N . | Median (P25-P75) . | |||
| Cytarabine | 7 | 0.84 | 288 | 0.48 | 0.6 | .43 |
| (0.45-1.34) | (0.25-1.19) | |||||
| Daunorubicin | 9 | 0.10 | 387 | 0.09 | 0.9 | .94 |
| (0.07-0.11) | (0.06-0.15) | |||||
| Idarubicin | 4 | 0.021 | 145 | 0.035 | 1.7 | .29 |
| (0.014-0.046) | (0.022-0.086) | |||||
| Doxorubicin | 7 | 0.26 | 193 | 0.27 | 1.0 | .68 |
| (0.22-0.28) | (0.12-0.37) | |||||
| Mitoxantrone | 6 | 0.037 | 197 | 0.045 | 1.2 | .85 |
| (0.019-0.166) | (0.015-0.102) | |||||
| 6-Thioguanine | 7 | 5.88 | 297 | 5.92 | 1.0 | .78 |
| (4.12-8.03) | (3.83-8.60) | |||||
| 6-Mercaptopurine | 4 | 158.8 | 278 | 97.9 | 0.6 | .53 |
| (<15.0-302.6) | (50.6-240.0) | |||||
| Prednisolone | 9 | 0.12 | 352 | 0.46 | 3.8 | .12 |
| (0.04-2.37) | (0.19-19.7) | |||||
| Dexamethasone | 4 | 0.07 | 239 | 0.06 | 0.9 | .77 |
| (0.05-1.59) | (0.01-0.47) | |||||
| Vincristine | 8 | 0.53 | 371 | 0.68 | 1.3 | .53 |
| (0.15-1.79) | (0.24-2.50) | |||||
| L-Asparaginase | 9 | 0.35 | 352 | 0.08 | 0.2 | .40 |
| (0.03-1.37) | (0.01-1.04) | |||||
| Methotrexate | 10 | 0.34 | 88 | 0.42 | 1.2 | .69 |
| (TSI50short) | (0.17-1.85) | (0.16-1.43) | ||||
| Methotrexate | 11 | 0.05 | 94 | 0.09 | 1.8 | .16 |
| (TSI50cont) | (0.02-0.13) | (0.04-0.19) | ||||
| Drug . | DS ALL . | Non-DS BCP-ALL . | RR . | P . | ||
|---|---|---|---|---|---|---|
| N . | Median (P25-P75) . | N . | Median (P25-P75) . | |||
| Cytarabine | 7 | 0.84 | 288 | 0.48 | 0.6 | .43 |
| (0.45-1.34) | (0.25-1.19) | |||||
| Daunorubicin | 9 | 0.10 | 387 | 0.09 | 0.9 | .94 |
| (0.07-0.11) | (0.06-0.15) | |||||
| Idarubicin | 4 | 0.021 | 145 | 0.035 | 1.7 | .29 |
| (0.014-0.046) | (0.022-0.086) | |||||
| Doxorubicin | 7 | 0.26 | 193 | 0.27 | 1.0 | .68 |
| (0.22-0.28) | (0.12-0.37) | |||||
| Mitoxantrone | 6 | 0.037 | 197 | 0.045 | 1.2 | .85 |
| (0.019-0.166) | (0.015-0.102) | |||||
| 6-Thioguanine | 7 | 5.88 | 297 | 5.92 | 1.0 | .78 |
| (4.12-8.03) | (3.83-8.60) | |||||
| 6-Mercaptopurine | 4 | 158.8 | 278 | 97.9 | 0.6 | .53 |
| (<15.0-302.6) | (50.6-240.0) | |||||
| Prednisolone | 9 | 0.12 | 352 | 0.46 | 3.8 | .12 |
| (0.04-2.37) | (0.19-19.7) | |||||
| Dexamethasone | 4 | 0.07 | 239 | 0.06 | 0.9 | .77 |
| (0.05-1.59) | (0.01-0.47) | |||||
| Vincristine | 8 | 0.53 | 371 | 0.68 | 1.3 | .53 |
| (0.15-1.79) | (0.24-2.50) | |||||
| L-Asparaginase | 9 | 0.35 | 352 | 0.08 | 0.2 | .40 |
| (0.03-1.37) | (0.01-1.04) | |||||
| Methotrexate | 10 | 0.34 | 88 | 0.42 | 1.2 | .69 |
| (TSI50short) | (0.17-1.85) | (0.16-1.43) | ||||
| Methotrexate | 11 | 0.05 | 94 | 0.09 | 1.8 | .16 |
| (TSI50cont) | (0.02-0.13) | (0.04-0.19) | ||||
LC50 values are given in μg/mL. For MTX, the TSI50 values (μM) are given after short-term (TSI50 short) or long-term (TSI50 cont) exposure.
RR, the median LC50 value (for MTX, TSI50) for non-DS ALL divided by the median LC50 value (for MTX, TSI50) for DS ALL.
P value derived from 2-tailed Mann-Whitney Utest.
L-asparaginase is given in IU/mL, not in μg/mL.
N indicates number of samples tested; P25-P75, 25th and 75th percentiles.
Differences in cellular resistance between DS AML and DS ALL for (A) cytarabine (Ara-C) and daunorubicin (DNR), (B) idarubicin (Ida) and mitoxantrone (Mitox), (C) vincristine (VCR) and prednisolone (Pred), (D) L-asparaginase (L-ASP) and 6-thioguanine (6-TG), and (E) MTX (after short-term exposure and after continuous exposure).
The median LC50 value is depicted as a horizontal solid line, and the 25th and 75th percentiles are depicted as triangles. DS AML cells are significantly more sensitive to cytarabine (median, 21-fold) than DS ALL cells. For 6-thioguanine there was a trend suggesting that DS AML was more sensitive than DS ALL (median, 2.7-fold), and for prednisolone there was a trend suggesting that DS ALL cells are more sensitive than DS AML cells (median, almost 2000-fold). DS AML cells were significantly more resistant than DS ALL cells after short-term exposure to methotrexate (median, 113-fold); however, this was not the case after continuous methotrexate exposure. For the other drugs, no significant differences were found.
Differences in cellular resistance between DS AML and DS ALL for (A) cytarabine (Ara-C) and daunorubicin (DNR), (B) idarubicin (Ida) and mitoxantrone (Mitox), (C) vincristine (VCR) and prednisolone (Pred), (D) L-asparaginase (L-ASP) and 6-thioguanine (6-TG), and (E) MTX (after short-term exposure and after continuous exposure).
The median LC50 value is depicted as a horizontal solid line, and the 25th and 75th percentiles are depicted as triangles. DS AML cells are significantly more sensitive to cytarabine (median, 21-fold) than DS ALL cells. For 6-thioguanine there was a trend suggesting that DS AML was more sensitive than DS ALL (median, 2.7-fold), and for prednisolone there was a trend suggesting that DS ALL cells are more sensitive than DS AML cells (median, almost 2000-fold). DS AML cells were significantly more resistant than DS ALL cells after short-term exposure to methotrexate (median, 113-fold); however, this was not the case after continuous methotrexate exposure. For the other drugs, no significant differences were found.
DS AML versus non-DS AML.
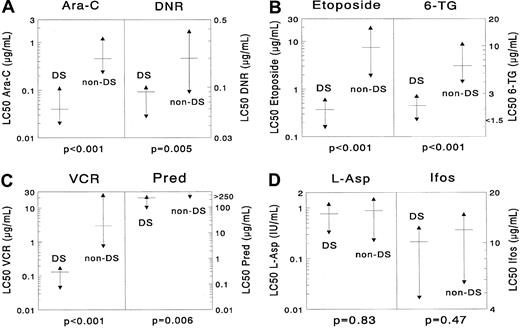
DS AML was significantly more sensitive than non-DS AML for the following drugs: cytarabine (median, 11.5-fold;P < .001), daunorubicin (2.2-fold;P = .005), idarubicin (7.2-fold; P = .002), mitoxantrone (9.2-fold; P = .002), etoposide (20.1-fold;P < .001), 6-thioguanine (2.7-fold;P < .001), amsacrine (16.0-fold; P < .001), prednisolone (more than 1.1-fold; P = .006), vincristine (23.0-fold; P < .001), and busulfan (4.6-fold;P < .001). Cells from patients with DS AML were 21.1-fold (median) more resistant to MTX after short-term exposure than cells from patients with non-DS AML (P = .003). However, they were equally sensitive after continuous MTX exposure (P = .22). No significant differences were found for L-asparaginase (P = .83), 2-chlorodeoxyadenosine (P = .42), and 4-hydroperoxy-ifosfamide (P = .47). Data are shown in Table 2 and Figure2.
Differences in cellular resistance between DS AML and non-DS AML for (A) cytarabine (Ara-C) and daunorubicin (DNR), (B) etoposide and 6-thioguanine (6-TG), (C) vincristine (VCR) and prednisolone (Pred), (D) L-asparaginase (L-ASP) and ifosfamide (Ifos).
DS AML is significantly more sensitive to cytarabine (median, 11.5-fold), daunorubicin (2.2-fold), etoposide (20.1-fold), 6-thioguanine (2.7-fold), vincristine (23.0-fold), and prednisolone (more than 1.1-fold). However, the differences for L-asparaginase and 4-hydroperoxy ifosfamide were not significant. The median LC50 value is depicted as a horizontal solid line, and the 25th and 75th percentiles are depicted as triangles. More results are shown in Table 2.
Differences in cellular resistance between DS AML and non-DS AML for (A) cytarabine (Ara-C) and daunorubicin (DNR), (B) etoposide and 6-thioguanine (6-TG), (C) vincristine (VCR) and prednisolone (Pred), (D) L-asparaginase (L-ASP) and ifosfamide (Ifos).
DS AML is significantly more sensitive to cytarabine (median, 11.5-fold), daunorubicin (2.2-fold), etoposide (20.1-fold), 6-thioguanine (2.7-fold), vincristine (23.0-fold), and prednisolone (more than 1.1-fold). However, the differences for L-asparaginase and 4-hydroperoxy ifosfamide were not significant. The median LC50 value is depicted as a horizontal solid line, and the 25th and 75th percentiles are depicted as triangles. More results are shown in Table 2.
DS ALL versus non-DS BCP-ALL.
There were no statistically significant differences in drug resistance between cells from patients with DS ALL and those with non-DS BCP-ALL, including methotrexate, after short- and long-term MTX exposure. Data are shown in Table 3.
Peripheral blood mononuclear cells.
We found normal nonleukemic PBMCs to be resistant to most tested drugs, with the exception of 2-chlorodeoxyadenosine in cells from patients without DS. For cytarabine, daunorubicin, etoposide, vincristine, 6-thioguanine, and 2-chlorodeoxyadenosine (the latter only in DS PBMCs), the highest concentration of drug was unable to kill more than 50% of the cells in a large proportion of samples. For prednisolone and L-asparaginase, this was so in all samples. Dose-response curves were obtained in all patients for 4-hydroperoxy-ifosfamide and amsacrine only. No significant differences were found between DS and non-DS PBMCs. Data are shown in Table4.
Cellular drug resistance of normal, nonleukemic PBMCs of children with and without Down syndrome
| Drug . | DS PBMC . | Non-DS PBMC . | RR . | P . | ||
|---|---|---|---|---|---|---|
| N . | Median LC50(P25-P75) . | N . | Median LC50 (P25-P75) . | |||
| Cytarabine | 8 | >2.5 | 11 | 0.97 | >2.6 | .03 |
| (2.29->2.5) | (0.56-2.45) | |||||
| Daunorubicin | 9 | 1.54 | 12 | 0.39 | 3.9 | .10 |
| (0.43->2.0) | (0.32-1.29) | |||||
| Etoposide | 9 | >50 | 12 | 35.0 | >1.4 | .68 |
| (22.2->50) | (25.3->50) | |||||
| 6-Thioguanine | 8 | >50 | 11 | >50 | 4-150 | .45 |
| (49.3->50) | (33.1->50) | |||||
| 2-Chloro- | 4 | >40 | 12 | 0.032 | >1250 | .22 |
| deoxyadenosine | (30.76->40) | (0.03-0.06) | ||||
| Prednisolone | 8 | >250 | 12 | >250 | 4-150 | .41 |
| (>250->250) | (>250->250) | |||||
| Vincristine | 9 | 41.03 | 11 | 32.47 | 1.3 | .30 |
| (34.33->50) | (18.31-47.79) | |||||
| L-Asparaginase | 8 | >10 | 12 | >10 | 4-150 | .41 |
| (>10->10) | (>10->10) | |||||
| 4-Hydroperoxy- | 6 | 4.00 | 12 | 1.49 | 2.7 | .13 |
| ifosfamide | (3.19-4.40) | (1.05-4.11) | ||||
| Amsacrine | 8 | 2.05 | 10 | 2.47 | 0.8 | .53 |
| (1.69-2.5) | (1.59-2.52) | |||||
| Drug . | DS PBMC . | Non-DS PBMC . | RR . | P . | ||
|---|---|---|---|---|---|---|
| N . | Median LC50(P25-P75) . | N . | Median LC50 (P25-P75) . | |||
| Cytarabine | 8 | >2.5 | 11 | 0.97 | >2.6 | .03 |
| (2.29->2.5) | (0.56-2.45) | |||||
| Daunorubicin | 9 | 1.54 | 12 | 0.39 | 3.9 | .10 |
| (0.43->2.0) | (0.32-1.29) | |||||
| Etoposide | 9 | >50 | 12 | 35.0 | >1.4 | .68 |
| (22.2->50) | (25.3->50) | |||||
| 6-Thioguanine | 8 | >50 | 11 | >50 | 4-150 | .45 |
| (49.3->50) | (33.1->50) | |||||
| 2-Chloro- | 4 | >40 | 12 | 0.032 | >1250 | .22 |
| deoxyadenosine | (30.76->40) | (0.03-0.06) | ||||
| Prednisolone | 8 | >250 | 12 | >250 | 4-150 | .41 |
| (>250->250) | (>250->250) | |||||
| Vincristine | 9 | 41.03 | 11 | 32.47 | 1.3 | .30 |
| (34.33->50) | (18.31-47.79) | |||||
| L-Asparaginase | 8 | >10 | 12 | >10 | 4-150 | .41 |
| (>10->10) | (>10->10) | |||||
| 4-Hydroperoxy- | 6 | 4.00 | 12 | 1.49 | 2.7 | .13 |
| ifosfamide | (3.19-4.40) | (1.05-4.11) | ||||
| Amsacrine | 8 | 2.05 | 10 | 2.47 | 0.8 | .53 |
| (1.69-2.5) | (1.59-2.52) | |||||
LC50 values are given in μg/mL.
RR, the median LC50 value for DS PBMC, divided by the median LC50 value for non-DS PBMC.
P value derived from 2-tailed Mann-Whitney Utest.
L-asparaginase is given in IU/mL (IU/L), not in μg/mL (μM).
N indicates number of samples tested; P25-P75, 25th and 75th percentiles.
RR could not be calculated because both median LC50 values exceeded the maximum concentration.
Discussion
Children with DS AML have a better prognosis with current chemotherapy than patients with non-DS AML.2,7-9Differences in prognosis may reflect differences in cellular drug resistance. It can be postulated, therefore, that the presence of an extra copy of chromosome 21 results in enhanced sensitivity to chemotherapeutic drugs.26 However, DS ALL does not carry a better prognosis than non-DS ALL.4,5,12,13 In the current study, in vitro cellular drug-resistance profiles of DS AML and DS ALL samples were studied to determine whether DS AML and ALL have different resistance patterns than their non-DS counterparts. In addition, PBMCs of nonleukemic children with and without DS were tested; it was speculated that DS PBMCs would be more chemosensitive than non-DS PBMCs, possibly reflecting the higher incidence of chemotherapy-related side effects in children with DS.3 4
DS AML cells were significantly more sensitive to many drugs regularly used in AML treatment than non-DS AML cells. In addition, significant sensitivity to 2 so-called typical ALL drugs was observed—vincristine and prednisolone. The latter may not be clinically relevant, however, because the median LC50 value in DS AML is still approximately 500 times higher than in non-DS ALL. Moreover, none of the DS AML samples was highly sensitive to prednisolone, as defined by our cutoff levels in ALL.17 DS ALL cells showed drug-resistance profiles that were equally as sensitive as non-DS BCP-ALL cells. BCP-ALL cells, however, are relatively sensitive compared with other immunophenotypes in ALL, such as CD10/cμ-negative BCP- and T-cell ALL.15 These results are in agreement with clinical data showing that patients with DS ALL have prognoses similar to those of patients with non-DS non–high-risk ALL. Both DS and non-DS PBMCs showed resistant drug profiles (except for 2-CdA in non-DS PBMCs). Therefore, testing PBMC sensitivity does not seem an adequate model to predict side effects of anticancer agents.
Two other groups have published data on in vitro drug sensitivity of DS leukemic cells. Taub et al27 showed that DS AML cells were significantly more sensitive to cytarabine and daunorubicin, the 2 drugs tested in that study. Frost et al28 reported a study on 5 patients with DS AML and 5 with DS ALL. DS AML cells were significantly more sensitive than non-DS AML cells to cytarabine, doxorubicin, amsacrine, and dexamethasone. For etoposide and 6-thioguanine, a trend for sensitivity of DS AML cells was found. DS ALL was significantly more resistant to dexamethasone and L-asparaginase than non-DS ALL, but numbers were small. Additional studies by Taub et al26,29 regarding cytarabine sensitivity showed higher Ara-CTP levels and 12-fold higher cysthationine-β-synthase (CBS, localized to chromosome 21q22.3) transcript levels in DS versus non-DS AML cells. Moreover, transfection of CBS, in CBS-deficient CCRF-CEM leukemic cells, resulted in enhanced cytarabine sensitivity and increased dCK enzyme activity.30 In contrast, carbonyl reductase (localized to 21q22.1), which catalyzes the reduction of daunorubicin to daunorubicinol, did not show higher transcript levels in DS myeloblasts when compared with non-DS AML cells.26 Consequently, the differences between DS and non-DS AML cells did not reflect the predicted 1.5-fold gene-dosage effect. Therefore, other selective regulatory factors of chromosome 21 gene expression appear to be involved in Down syndrome leukemic cells.26 This might also be disease- or even cell line-related, which is supported by our results on DS PBMCs. These were as resistant as normal non-DS PBMCs. Furthermore, DS AML cells were 21-fold more sensitive to cytarabine than DS ALL cells, whereas non-DS AML and ALL do not differ in cytarabine resistance.16
For the other drugs, no significant differences were found between DS AML and DS ALL, whereas in an earlier study we found non-DS AML to be relatively resistant when compared with non-DS ALL for a panel of drugs.16 Because of this general pattern of drug sensitivity in DS AML, we suggest that a general mechanism, rather than a specific mechanism for each separate drug, is responsible for drug sensitivity in this particular disease.26 Several of these potential general mechanisms are suggested in the literature: (1) superoxide dismutase overexpression (SOD), localized to 21q22.1, shows 3.8-fold higher transcript levels in DS than in non-DS myeloblasts.26 SOD primarily protects against oxygen radicals, but it also induces the production of hydrogen peroxide, which may lead to enhanced cell death from oxidative damage.31,32 (2) enhanced apoptosis with p53 and CD95 has been detected in the brains of patients with DS, but no studies have been reported on DS leukemic cells.33 In addition, enhanced apoptosis of DS granulocytes has been described.34 (3) defective DNA repair after in vitro mutagen exposure has been reported, and it may be related to the increased cancer susceptibility of DS patients.35
Taken together, these findings may, at least partially, explain the good clinical outcome of children with DS AML, making us question the need to develop separate treatment protocols for DS AML. The first example of a specific DS AML clinical trial was recently reported by Kojima et al,36 and it shows that treatment modification toward less intensive therapy is possible without affecting clinical outcome. Patients with DS AML included in the AML93 protocol of the BFM-AML Study Group (n = 41) were also treated with a less dose-intensive schedule (dose reduction for anthracyclines and no stem cell transplantation), resulting in a 5-year event-free survival rate of 67% ± 8% (U. Creutzig, personal communication, 2001). It has been noted that the better survival of DS patients coincided with the introduction of the high-dose cytarabine-containing regimens,26 but this might also reflect such factors as improvements in supportive care and willingness to treat patients with DS AML. Based on our in vitro data and the promising results with dose reductions in clinical trials mentioned above, one might question the need for high-dose regimens with the associated side effects in the DS population.
MTX cannot be evaluated by MTT assay, but we recently described the TSIA to measure MTX sensitivity.25 The reduced folate carrier (RFC, localized to chromosome 21q22.2-q22.3) transports MTX into the cell, but at higher concentrations passive diffusion also occurs.37 Studies in hyperdiploid ALL have shown that the high accumulation of MTX polyglutamates was related to RFC overexpression and was significantly related to chromosome 21 copy number.37 In a study by Zhang et al,38however, the RFCs of 2 patients with DS ALL appeared to be comparable to nonhyperdiploid ALL, and Taub et al26 reported no differences in RFC transcript levels between DS and non-DS AML. We could not demonstrate significant differences in TS-inhibition (short-term exposure) between DS and non-DS ALL. DS AML cells were even significantly more MTX resistant (short-term exposure) than non-DS AML cells, which is in disagreement with one report on increased MTX polyglutamylation in 2 patients with DS AML (comparable to ALL).39 In non-DS AML samples, we reported earlier that polyglutamylation was low, despite similar sensitivity to non-DS ALL after continuous exposure to MTX.40 It has also been reported that CBS influences MTX metabolism, but transfection of CBS does not lead to enhanced MTX sensitivity.30 We showed here that long-term MTX exposure results in similar TSI50values for DS-AML and DS ALL and their non-DS counterparts. This suggests that long-term infusion might overcome MTX resistance in DS AML, as we previously reported for non-DS AML.41 One study addressed MTX pharmacokinetics and reported that median MTX plasma concentrations were significantly higher in DS patients than in matched controls with non-DS ALL.6
Our results in DS and non-DS ALL might suggest that the mere presence of trisomy 21 (gene dosage effect) does not account for chemosensitivity and that, subsequently, the better prognosis for DS AML is attributed to the distinct and unique biology of this particular disease. However, the clinical outcome of non-DS ALL is better than for non-DS AML, and chromosome 21 is involved in approximately 50% of the non-DS ALL cases, such as the t(12;21)/TEL-AML gene rearrangement, and in nearly all hyperdiploid ALL cases, where it may contribute to improved clinical outcome.42,43 These abnormalities are also associated with specific, relatively sensitive drug-resistance profiles.14 44 Consequently, it is still possible that abnormalities of chromosome 21 contribute to relatively sensitive drug-resistance profiles in DS AML, DS ALL, and non-DS ALL.
The failure rate of the MTT assay in DS leukemia is higher than we previously reported for non-DS AML or ALL.16,17 In DS AML, failures are mainly caused by a low blast percentage in the bone marrow or by a lack of cells, which can be explained by the myelofibrosis or myelodysplasia, often accompanying DS AML.2,11 In DS ALL, however, the main cause of assay failure was not surviving the 4-day cell culture. In previous studies, we also described this for hyperdiploid ALL and hypothesized that this might have resulted from a higher propensity for leukemic cells to undergo spontaneous apoptosis.14 Therefore, the results we present here on DS ALL may overestimate drug resistance, based on biased selection of samples surviving the 4-day cell culture.
One drawback of this study is that we were unable to compare children with and without DS based on FAB M7 classification because of the lack of FAB M7 samples in the children without DS. Non-DS FAB M7 is heterogeneous and can be further subdivided into at least 2 groups—children (often infants) with t(1;22) and older children without t(1;22).45 Both groups have poorer prognoses than do children with DS AML.45 46
In conclusion, DS AML has a distinct drug sensitivity profile, with relative sensitivity to drugs frequently used in AML but also to the classic ALL-drug vincristine. This may, at least partially, explain its favorable prognosis over non-DS AML. On the contrary, DS ALL does not show enhanced in vitro drug sensitivity when compared with non-DS BCP-ALL, which is in agreement with clinical studies showing a similar prognosis for these 2 groups of patients.
We thank all the hospital personnel and clinicians participating in the German AML-BFM Study Group, the German COALL Study Group, and the DCLSG. We also thank the technicians of the Laboratory of Pediatric Oncology in Amsterdam for performing the drug-resistance testing. Board members of the AML-BFM Study Group are C. Bender-Götze, F. Berthold, J. Boos, U. Creutzig, A. Feldges, H. Gadner, N. Graf, G. Henze, J. Hermann, H. Jürgens, H. Kabisch, D. Körholz, T. Klingebiel, C. M. Niemeyer, A. Reiter, J. Ritter, and J. Stary. Board members of the DCLSG are H. van den Berg, J. P. M. Bökkerink, S. S. N. de Graaf, B. Granzen, P. M. Hoogerbrugge, W. A. Kamps, F. A. E. Nabben, R. Pieters, J. A. Rammeloo, T. Révész, and A. J. P. Veerman. Finally, we thank Mrs. A. Heus for excellent secretarial support.
Supported in part by the Landelijke Vereniging van Crematoria (Eindhoven, The Netherlands).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Christian M. Zwaan, Dept of Pediatric Hematology/Oncology, Vrije Universiteit Medical Center, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; e-mail: cm.zwaan@vumc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal