Human T-cell leukemia–lymphoma virus (HTLV) type-2 can induce the survival and proliferation of CD34+ TF-1 cells deprived of interleukin (IL)-3. This effect did not require productive infection and occurred when HTLV-2 was produced from T cells (CMo), but not from B cells (BMo), unless the latter virus was complexed with anti–HLA-DR monoclonal antibodies (mAbs). Cellular and molecular mechanisms triggered by HTLV-2 interaction with TF-1 cells were here investigated. Activation of signal transducer and activator of transcription (STAT) 5 protein occurred in TF-1 cells incubated either with IL-3 or with HTLV-2/CMo; in addition the virus, but not IL-3, activated STAT1. The effect of HTLV-2 required several hours, suggesting dependence on the induction of cellular factors. By screening a panel of secreted factors, granulocyte macrophage–colony-stimulating factor (GM-CSF), interferon (IFN)-γ, and stem cell factor (SCF) were found induced by HTLV-2 in TF-1 cells. Of note is the fact that these molecules induce a variety of biologic effects through the activation of STAT proteins, including STAT1 and STAT5. Neutralization experiments indicated that GM-CSF and IFN-γ, but not SCF, were responsible for HTLV-2–induced STAT activation, whereas anti–GM-CSF antibodies greatly inhibited TF-1 cell proliferation. Finally, incubation of BMo virus with anti–HLA-DR mAb rescued TF-1 cell survival in the absence of IL-3. Thus, HTLV-2 interaction with CD34+ precursor cells may lead to the expression of cytokines that, by inducing autocrine activation of STATs, may influence the host's regenerative capacity and immune response to HTLV-2 and to other infectious agents.
Introduction
Human T-cell leukemia–lymphoma virus (HTLV) type-2, together with HTLV-1, belongs to the HTLV–bovine leukemia virus group of the Oncovirinae family; this virus was first identified in a T-cell line derived from a patient with hairy cell leukemia.1 In spite of the fact that HTLV-1 and HTLV-2 are highly related, they differ with regard to pathogenicity and cellular tropism. In a considerable percentage of patients, HTLV-1 is the etiologic agent of acute T-cell leukemia and lymphoma, and infection has been associated with the development of tropical spastic paraparesis and myelopathy.2,3 In contrast, the correlation between HTLV-2 infection and hematopoietic malignancies is still controversial.4-7 However, HTLV-2 infection of T cells in vitro results in spontaneous cell proliferation and transformation.6,8-11 In addition, HTLV-2 can be found in some patients with neurodegenerative and lymphoproliferative disorders and in a significant fraction (up to 10%) of intravenous drug users, frequently co-infected with human immunodeficiency virus (HIV)–1.12,13 HTLV-1 enters CD4+ and CD8+ T cells,14 whereas HTLV-2 infects preferentially CD8+ T cells, though in patients with high proviral load both viruses can also integrate in monocytes and B cells.15-18 The entry receptors used by these viruses have not yet been identified; experimental evidence suggests that the same receptor molecule (whose gene has been localized on chromosome 17, region q) may be shared by the 2 viruses.19 20
T lymphocytes transformed in vitro by HTLV-121 and leukemic cells from patients with acute T-cell leukemia and lymphoma exhibit constitutive hyperactivation of signal transducer and activator of transcription (STAT) proteins,22 a family of transcription factors essential for cytokine-regulated processes such as cellular proliferation, differentiation, and survival by the activation of downstream genes.23-25 The STATs are usually activated by the Janus kinases (JAK), a family of receptor-associated enzymes catalyzing the phosphorylation of tyrosine residues.25 It has been shown that JAK3, STAT5A, and STAT5B are essential for normal T-lymphocyte proliferation.26,27 In addition to HTLV-1, we have previously shown that HIV-1 infection is often associated in vivo and in vitro with the constitutive activation of STAT1 and of a truncated isoform of STAT5.28 In contrast to HTLV-1– and HIV-1–infected cells, human T lymphocytes transformed in vitro by HTLV-2 did not show evidence of STAT activation.6Furthermore, no information is available on the activation state of STAT proteins in patients infected with HTLV-2.
TF-1 is a CD34+ cell line responsive to several hematopoietic growth factors such as interleukin (IL)-3, erythropoietin (EPO), stem cell factor (SCF), and granulocyte macrophage–colony-stimulating factor (GM-CSF) in terms of survival and proliferation.29 All these factors mediate their pleiotropic effects through the activation of the JAK/STAT pathway. In particular, IL-3, EPO, and GM-CSF activate STAT5, whereas interferon (IFN)-γ and SCF have been shown to activate STAT1.25,30We have previously reported that the Mo strain of HTLV-2, when derived from T cells (CMo), unlike when derived from B cells (BMo), rescued TF-1 and bone marrow–derived CD34+ primary cells from apoptosis induced by IL-3 deprivation.31 Pretreatment of BMo virus with specific monoclonal antibodies (mAbs) against a subset of HLA/DR antigens, which are more abundantly expressed on B rather than T cells, functionally converted BMo to a CMo-like virus in its antiapoptotic ability.31
We have here investigated whether CMo-dependent rescue of IL-3–deprived TF-1 cells from apoptosis and cell proliferation could be attributed to a functional mimicry of IL-3 by HTLV-2 CMo, with particular focus on the ability of CMo and BMo to activate the JAK/STAT pathway and on the underlying mechanisms of this activation.
Materials and methods
Cells and HTLV-2 strains
The TF-1 cell line, established from human bone marrow of an erythroleukemic patient,32 was cultivated in RPMI 1640 (Gibco, Grand Island, NY) supplemented with 10% fetal calf serum (Gibco), and 0.11 nM (2 ng/mL) human recombinant IL-3 (Genzyme, Boston, MA). The T-cell line C344 harboring the HTLV-2 Mo provirus and the Epstein-Barr virus (EBV)–negative B-cell line BJAB infected with HTLV-2 Mo strain were grown in RPMI 1640 plus 10% fetal calf serum. C344 and BJAB cells were used as producers for the virus isolates CMo and BMo, respectively.
CD34+ hematopoietic precursor cells were isolated from cord blood after Ficoll-Hypaque gradient by the indirect magnetic labeling system CD34 progenitor cell isolation kit (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. The enriched CD34+ cell population was more than 95% pure at FACScan analysis staining with a specific anti-CD34 mouse mAb (HPCA-2; Becton Dickinson, Mountain View, CA) and were cultivated in RPMI 1640 supplemented with 10% fetal calf serum.
Purified virions from supernatants were obtained as described previously.31 Briefly, supernatants were clarified at low-speed centrifugation (1000g for 10 minutes) and were passaged through 0.45-μm filters. To obtain concentrated virus preparations, the clarified supernatants were centrifuged at 50 000g, and the sedimented particles were then purified by ultracentrifugation on sucrose gradient (25% to 60%). The 1.16 to 1.18 g/mL density fractions were pooled, dialyzed, and pelleted by centrifugation. To preserve envelope glycoprotein integrity, the virion concentrates were also purified on Sepharose CL-4B (Amersham Pharmacia Biotech Italy, Milan) chromatography column, and the fractions containing virus were pooled and centrifuged in microfuge for 90 minutes. Virus particles were resuspended in medium at 0.1 the original volume. Viral titers of the concentrated virus preparations are defined by levels of p19 antigen in fluids (Retro-tek HTLV p19 Gag antigen ELISA; ZeptoMetrix, Buffalo, NY). For inoculation with HTLV-2, TF-1 cells, after 24 hours of IL-3 deprivation, were incubated with clarified supernatants of either C344 or BJAB cells containing 0.5 to 1 ng/mL HTLV-2 p19 Gag antigen equivalent, as previously described.31
Proliferation assay
The proliferative capacity of TF-1 cells after incubation with HTLV-2 in the absence of IL-3 was evaluated by [3H]-thymidine incorporation at different time points. Briefly, TF-1 cells were deprived of IL-3 for 24 hours and then seeded at a concentration of 2.5 × 105 cells/well in 300 μL culture medium and incubated with 0.037 MBq/mL (1 μCi/mL) [3H]-thymidine (22.2 × 1010 Bq/mmol [6 Ci/mmol]) for 6 hours at different times after virus treatment.
Antibodies
Rabbit antiserum raised against C-terminal epitopes of STAT5A (PA-ST5A) and STAT5B (PA-ST5B)—neutralizing antibodies against IL-3 (MAB203), IL-3Rα chain (MAB301), SCF (AF-255-NA), and GM-CSF (MAB215)—were obtained from R&D Systems (Minneapolis, MN); anti–phospho-STAT5A/B (Y694/Y699) rabbit polyclonal antibody (06-798) was purchased from Upstate Biotechnology (Lake Placid, NY); affinity-purified rabbit polyclonal antibodies raised against an N-terminal (residues 5-24) (sc-836) epitope of STAT5 and anti-STAT1α/β rabbit polyclonal antibody (E-23, sc-346) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); phospho-STAT1 (Tyr 701) was purchased from New England BioLabs (Beverly, MA). Rabbit polyclonal antibodies against 2 different epitopes of the IFN-γR2 chain, γR99 and γR38, were kindly donated by Dr F. Novelli (University of Turin, Italy). An anti–HLA-DR mAb, D1.12,33 was kindly provided by Dr R. S. Accolla (University of Insubria, Varese, Italy).
Whole-cell extracts and electrophoretic mobility shift assay
Whole-cell extracts were prepared by repeated cycles of cell freezing and thawing. Frozen TF-1 cell pellets were resuspended in a high-salt buffer (buffer C, 20 mM HEPES pH 7.9, 400 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 10% [vol/vol] glycerol) supplemented with a cocktail of protease inhibitors that included leupeptin (20 μM (10 μg/mL)), pepstatin A (14 μM (10 μg/mL)), aprotinin (3.3 μM (33 μg/mL)), E-64 (28 μM (10 μg/mL)), AEBSF (1 mM), diisopropyl fluorophosphate (3 mM), and the phosphatase inhibitors sodium vanadate (Na3VO4) (1 mM), sodium fluoride (50 mM), and 0.5% Nonidet P-40. Cell disruption was achieved by 3 freeze-and-thaw cycles in dry ice. Insoluble material was removed by centrifugation at 12 000g for 15 minutes at 4°C, and the resultant supernatants were aliquoted and stored at −80°C before use. Protein concentration was evaluated by a protein assay kit based on the Bradford method (Bio-Rad, Hercules, CA).
Electrophoretic magnetic shift assay (EMSA) was performed by incubating whole-cell extracts (WCEs) with [γ-32P]ATP end-labeled, double-stranded oligonucleotides corresponding to the prolactin-responsive element (PRE) located within the promoter of the β-casein promoter,34 and the DNA-protein complexes were resolved as previously described.35
Immunoblot analyses
Cellular proteins were denatured by the addition of an equal volume of sample buffer 2× (50 mM Tris-base, pH 6,8, 4% sodium dodecyl sulfate [SDS], 10% 2-β-mercaptoethanol, 20% glycerol) and heated for 3 minutes at 100°C before electrophoretic separation on 7.5% SDS–polyacrylamide gel electrophoresis and subsequent transfer to nitrocellulose membrane Hybond enhanced chemiluminescence (Amersham, Little Chalfont, United Kingdom) by electroblotting. Membranes were blocked in 5% low-fat dry milk, 20 mM Tris, pH 7.6, 137 mM NaCl, and 0.2% Tween 20 for 1 hour at room temperature and were further incubated (overnight at 4°C) with the desired primary antibody. Anti-STAT5A and anti-STAT5B antibodies and anti–phospho-STAT5A/B antibody were diluted 1:2000, whereas anti–phospho-STAT1 and anti-STAT1 were diluted 1:1000, as recommended by the manufacturer. Antibody binding was visualized by using the horseradish peroxidase–conjugated secondary antibody (anti-rabbit antibody, diluted 1:10 000). The signal was revealed by the enhanced chemiluminescence system (Amersham) according to the manufacturer's instructions.
Enzyme-linked immunosorbent assay
Cell-free culture medium was assayed for cytokine and hormone content by commercially available enzyme-linked immunosorbent assay (ELISA) kits recognizing only bioactive proteins. Colorimetric ELISA for IL-2 (sensitivity less than 7 pg/mL), IL-3 (sensitivity less than 7.4 pg/mL), IL-6 (sensitivity less than 0.7 pg/mL), IL-15 (sensitivity less than 2 pg/mL), SCF (sensitivity less than 9 pg/mL) from R&D Systems, IL-5 (sensitivity less than 2 pg/mL), IL-10 (sensitivity less than 3 pg/mL), GM-CSF (sensitivity less than 2 pg/mL), tumor necrosis factor-α (sensitivity less than 5 pg/mL), IFN-α (sensitivity less than 3 pg/mL), IFN-γ (sensitivity less than 2 pg/mL) from Endogen (Woburn, MA), chemiluminescent ELISA for GH (sensitivity less than 0.13 IU/L [mIU/mL]), and PRL (sensitivity less than 0.5 ng/mL from Diagnostic Products (Los Angeles, CA) was performed according to manufacturers' instructions.
Results
Incubation of TF-1 cells with CMo, but not with BMo, HTLV-2 induces the activation of STAT1 and STAT5
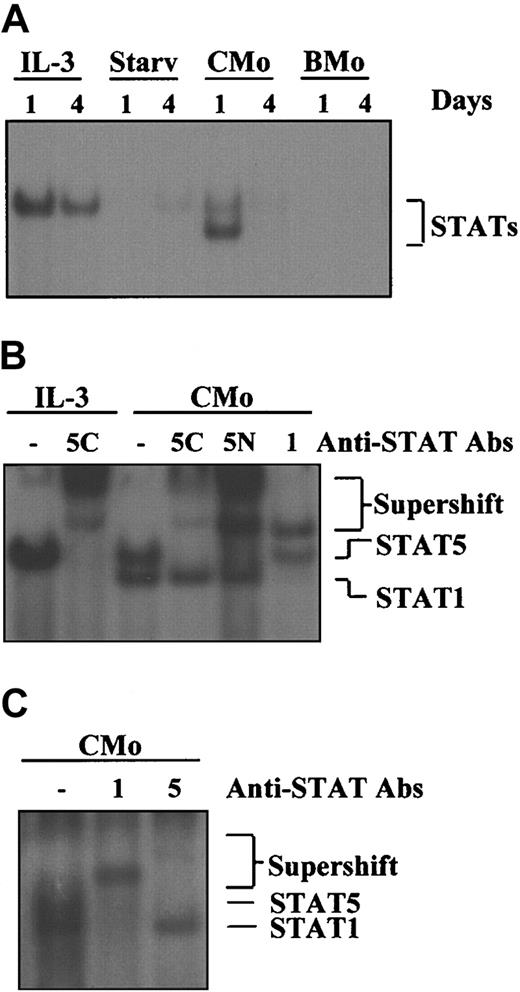
We have previously shown that contact between TF-1 cells and HTLV-2 CMo, but not BMo, triggers a mitogenic effect comparable to that produced by IL-3.31 To investigate whether CMo mimicked IL-3 at the molecular level, namely in the activation of the JAK/STAT pathway, we performed EMSA experiments using the PRE probe corresponding to a STAT-specific DNA element and WCEs derived from TF-1 cells either grown in IL-3, starved from IL-3, or exposed to CMo or BMo HTLV-2 strains for 1 and 4 days (Figure1A). Of note, TF-1 cells subjected to CMo for 24 hours (Figure 1, lanes 5-6) showed 2 DNA-binding complexes that disappeared after 4 days, whereas cells inoculated with BMo were negative for STAT activation at both time points (lanes 7-8). These results demonstrated that CMo, like IL-3, activated the JAK/STAT pathway; in addition, CMo activated a different complex and showed a more transient effect on STAT activation than IL-3.
CMo, but not the BMo HTLV-2 strain, induces STAT1 and STAT5 activation in TF-1 cells.
(A) EMSA using the PRE probe and 8 μg WCE from TF-1 cells either grown in IL-3 (lanes 1-2) or starved from IL-3 (lanes 3-4) or incubated in IL-3–deprived medium with CMo or BMo HTLV-2 strains for 1 and 4 days (lanes 5-8), respectively. STAT/DNA-binding activity was positive (lanes 1-2) and negative (lanes 3-4) in TF-1 cells grown in the presence or in the absence of IL-3, respectively. (B) Supershift analysis using the PRE probe; 8 μg WCE from TF-1 cells grown in IL-3 (lanes 1-2) or incubated with CMo virus in IL-3–deprived medium for 1 day and anti-STAT1 (1), anti-STAT5 antibodies raised against a C-terminal (5C) or an N-terminal (5N) epitope (lanes 3-6). (C) Cord blood–derived CD34+ cells were incubated with CMo for 24 hours and STAT1 (1), and STAT5 (5) activation was determined by supershift analysis using the PRE probe on WCE (8 μg). Starv indicates starved from IL-3.
CMo, but not the BMo HTLV-2 strain, induces STAT1 and STAT5 activation in TF-1 cells.
(A) EMSA using the PRE probe and 8 μg WCE from TF-1 cells either grown in IL-3 (lanes 1-2) or starved from IL-3 (lanes 3-4) or incubated in IL-3–deprived medium with CMo or BMo HTLV-2 strains for 1 and 4 days (lanes 5-8), respectively. STAT/DNA-binding activity was positive (lanes 1-2) and negative (lanes 3-4) in TF-1 cells grown in the presence or in the absence of IL-3, respectively. (B) Supershift analysis using the PRE probe; 8 μg WCE from TF-1 cells grown in IL-3 (lanes 1-2) or incubated with CMo virus in IL-3–deprived medium for 1 day and anti-STAT1 (1), anti-STAT5 antibodies raised against a C-terminal (5C) or an N-terminal (5N) epitope (lanes 3-6). (C) Cord blood–derived CD34+ cells were incubated with CMo for 24 hours and STAT1 (1), and STAT5 (5) activation was determined by supershift analysis using the PRE probe on WCE (8 μg). Starv indicates starved from IL-3.
To demonstrate which members of the STAT family were activated by CMo, antibody-mediated supershift experiments were performed using the PRE probe and WCEs obtained from cells maintained in the presence of IL-3 or exposed to CMo for 24 hours in IL-3–deprived medium. As expected, IL-3 induced the activation of STAT5 (Figure 1B, lanes 1-2), whereas CMo activated STAT5, corresponding to the upper migrating band eliminated by 2 different anti-STAT5 antibodies (lanes 3-5), and STAT1, corresponding to the lower migrating band supershifted by the anti-STAT1 antibody (lane 6).
Furthermore, we analyzed whether STAT5 or STAT1 was activated in cord blood–derived CD34+ cells after 24-hour incubation with CMo. As shown in Figure 1C, both STAT proteins were supershifted by the specific antibodies, indicating that the CMo-induced activation of the STATs is a feature not restricted to the TF-1 cell line model, but it can be extended to primary cells.
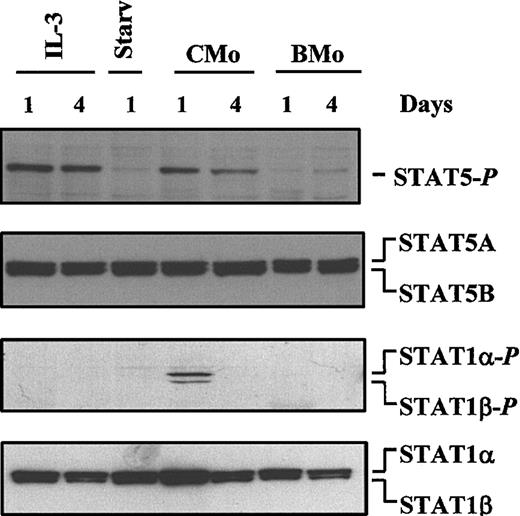
HTLV-2 CMo, but not BMo, induces phosphorylation of STAT1 and STAT5 on tyrosine residues in TF-1 cells
To further evaluate the activation state of STAT1 and STAT5, WCEs were analyzed by Western blot assay for tyrosine phosphorylation using either anti–phospho-STAT5– or anti–phospho-STAT1–specific antibodies. In agreement with the EMSA results, stimulation of TF-1 cells with IL-3 induced the phosphorylation of tyrosine residues of only STAT5 (Figure 2, first panel from the top, lanes 1-2), whereas CMo, but not BMo, induced Tyr phosphorylation of STAT1α/β and STAT5 (Figure 2, first and third panels, lanes 4-7). In partial contrast to the EMSA data, STAT5 remained activated up to day 4, whereas the duration of STAT1 activation paralleled that observed in EMSA. To demonstrate that equal amounts of proteins were loaded on the gel, the filters were stripped and reprobed with either a mixture of anti-STAT5A and anti-STAT5B (second panel) or anti-STAT1α/β–specific antibodies (Figure 2, last panel).
CMo-dependent tyrosine phosphorylation of STAT1 and STAT5.
Immunoblot analysis of WCEs (15 μg) of TF-1 cells either grown in IL-3 for 1 and 4 days (lanes 1-2) or starved of IL-3 for 1 day (lane 3) or incubated in IL-3–deprived medium with CMo or BMo HTLV-2 strains for 1 and 4 days (lanes 4-7), respectively. In the first panel from the top, the filter was hybridized with anti–phospho-STAT5. In the second panel the filter, after stripping, was hybridized with a mixture of anti-STAT5A and anti-STAT5B antibodies. In the third panel, a new filter containing the same samples shown in the other panels was probed with anti–phospho-STAT1 and, after stripping, with anti-STAT1 antibody (last panel).
CMo-dependent tyrosine phosphorylation of STAT1 and STAT5.
Immunoblot analysis of WCEs (15 μg) of TF-1 cells either grown in IL-3 for 1 and 4 days (lanes 1-2) or starved of IL-3 for 1 day (lane 3) or incubated in IL-3–deprived medium with CMo or BMo HTLV-2 strains for 1 and 4 days (lanes 4-7), respectively. In the first panel from the top, the filter was hybridized with anti–phospho-STAT5. In the second panel the filter, after stripping, was hybridized with a mixture of anti-STAT5A and anti-STAT5B antibodies. In the third panel, a new filter containing the same samples shown in the other panels was probed with anti–phospho-STAT1 and, after stripping, with anti-STAT1 antibody (last panel).
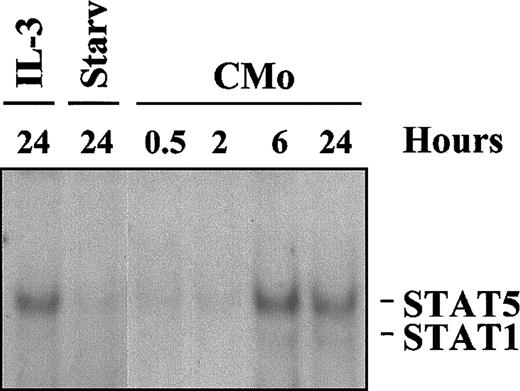
STAT activation is a late event
To investigate whether the observed activation of STAT proteins resulted from the direct interaction of CMo with cell surface receptors, TF-1 cells were incubated for different times with Cmo, and WCEs were subjected to EMSA for STATs. As shown in Figure3, STAT5 binding to PRE appeared, in this experiment, only after 6 hours of incubation with CMo (lane 5), whereas the band corresponding to STAT1 was barely detectable only after 24 hours (lane 6). These results suggest that CMo-induced STAT activation did not result from direct contact between the virus and cell surface receptor(s) but that it was dependent on secondary events, such as the secretion of some cytokine(s) or growth factor(s) capable of inducing the JAK/STAT pathway.
Kinetics of CMo-dependent STAT activation.
EMSA using the PRE probe and 8 μg WCE from TF-1 cells grown in or starved of IL-3 for 24 hours (lanes 1-2) or incubated, in IL-3–deprived medium, with CMo for 0.5, 2, 6, and 24 hours (lanes 3-6).
Kinetics of CMo-dependent STAT activation.
EMSA using the PRE probe and 8 μg WCE from TF-1 cells grown in or starved of IL-3 for 24 hours (lanes 1-2) or incubated, in IL-3–deprived medium, with CMo for 0.5, 2, 6, and 24 hours (lanes 3-6).
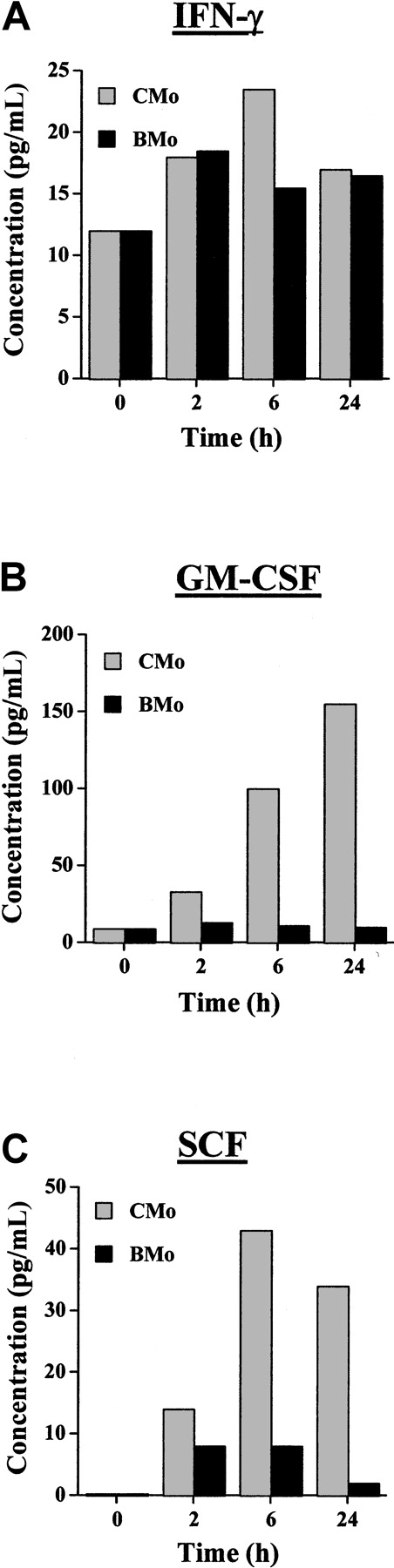
Incubation of TF-1 cells with HTLV-2 CMo promotes cytokine secretion
To investigate whether secreted factor(s) were released from TF-1 cells on incubation with CMo, we evaluated by ELISA the content of a panel of cytokines and growth factors to which TF-1 are known to be responsive in the cell culture supernatants (Table1). TF-1 cells grown in IL-3 did not produce detectable levels of any tested cytokine. Only a minimal release of IFN-α (10 pg/mL) and IFN-γ (2 pg/mL) was observed in cells starved for 24 hours in the absence of IL-3. The substantial amount of IL-3 (40 pg/mL) detected in starved cells was likely actively produced by TF-1 cells, though we cannot formally exclude the possibility of an incomplete washout of the cytokine from the cell culture. When TF-1 cells were cultivated for 24 hours in the presence of CMo, high levels of GM-CSF (up to 1040 pg/mL) were produced. In addition, low levels of IFN-γ (19 pg/mL) and SCF (77 pg/mL) were detected. We also estimated the production of GM-CSF, IFN-γ, and SCF by TF-1 cells at time 0 (ie, after 24 hours of starvation from IL-3) or inoculated for 2, 6, and 24 hours with CMo in comparison to that by cells inoculated for the same time points with BMo. In agreement with the results shown in Table 1, the levels of IFN-γ were higher than those of GM-CSF and SCF at time 0 (Figure 4). IFN-γ secretion was induced at low level by BMo and CMo viruses after 2 hours of exposure, but only CMo virus was capable of increasing the production of IFN-γ after 6 hours. In contrast, GM-CSF and SCF were secreted only after CMo exposure.
Cytokine production in supernatants of HTLV-2/CMo-treated TF-1 cultures
| Cytokine . | TF-1 + IL-3 . | TF-1 − IL-3 . | TF-1 − IL-3 + CMo . |
|---|---|---|---|
| IL-2 | Neg | Neg | Neg |
| IL-3 | 1300 | 40 | Neg |
| IL-5 | Neg | Neg | Neg |
| IL-6 | Neg | Neg | Neg |
| IL-10 | Neg | Neg | Neg |
| IL-15 | Neg | Neg | Neg |
| TNF-α | Neg | Neg | Neg |
| IFN-α | Neg | 10 | Neg |
| IFN-γ | Neg | 2 | 19 |
| GM-CSF | Neg | Neg | 1040 |
| SCF | Neg | Neg | 77 |
| GH | Neg | Neg | Neg |
| PRL | Neg | Neg | Neg |
| Cytokine . | TF-1 + IL-3 . | TF-1 − IL-3 . | TF-1 − IL-3 + CMo . |
|---|---|---|---|
| IL-2 | Neg | Neg | Neg |
| IL-3 | 1300 | 40 | Neg |
| IL-5 | Neg | Neg | Neg |
| IL-6 | Neg | Neg | Neg |
| IL-10 | Neg | Neg | Neg |
| IL-15 | Neg | Neg | Neg |
| TNF-α | Neg | Neg | Neg |
| IFN-α | Neg | 10 | Neg |
| IFN-γ | Neg | 2 | 19 |
| GM-CSF | Neg | Neg | 1040 |
| SCF | Neg | Neg | 77 |
| GH | Neg | Neg | Neg |
| PRL | Neg | Neg | Neg |
Values are expressed in pM (pg/mL). Cytokine secretion was determined by ELISA in supernatants of TF-1 cells grown in IL-3, deprived of IL-3, or incubated after 24 hours of IL-3 starvation with HTLV-2 CMo for 24 hours.
TNF, tumor necrosis factor; GH, growth hormone; PRL, prolactin.
Cytokine production from TF-1 cells incubated with CMo or BMo HTLV-2 strains.
(A) IFN-γ. (B) GM-CSF. (C) SCF production measured by ELISA in culture supernatants of TF-1 cells at time 0 (cells starved of IL-3 for 24 hours) or exposed for 2, 6, or 24 hours to either CMo (gray bars) or BMo (black bars). Estimated values of the cytokine content in the culture supernatants were the result of cytokine accumulation after 2, 6, and 24 hours after the addition of the viruses.
Cytokine production from TF-1 cells incubated with CMo or BMo HTLV-2 strains.
(A) IFN-γ. (B) GM-CSF. (C) SCF production measured by ELISA in culture supernatants of TF-1 cells at time 0 (cells starved of IL-3 for 24 hours) or exposed for 2, 6, or 24 hours to either CMo (gray bars) or BMo (black bars). Estimated values of the cytokine content in the culture supernatants were the result of cytokine accumulation after 2, 6, and 24 hours after the addition of the viruses.
HTLV-2 CMo-dependent STAT5 activation is mediated by the secretion of GM-CSF
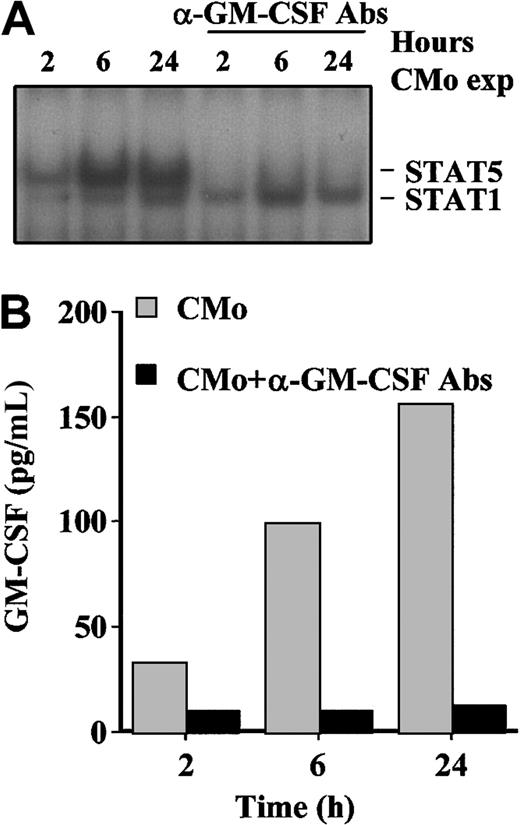
Because GM-CSF is an inducer of STAT5 phosphorylation, we hypothesized that this cytokine was responsible of the observed activation of STAT5 after CMo incubation. To verify this hypothesis, TF-1 cells were cultivated in the presence of CMo for 2, 6, and 24 hours in the absence or in the presence of anti–GM-CSF–neutralizing antibody (570 (pg/mL)). EMSA experiments revealed that the anti–GM-CSF antibody indeed abolished the formation of the DNA-binding complex corresponding to STAT5 at all time points, whereas it had no effect on the PRE–STAT1 complex (Figure 5A). To verify that GM-CSF was neutralized from the culture supernatants, we measured the level of GM-CSF detectable in the absence or in the presence of anti–GM-CSF antibody. As shown in Figure 5B, the antibody fully sequestered the secreted cytokine at all time points. Of note, the trend of GM-CSF production (with a peak at 24 hours) paralleled the intensity of the STAT5-DNA binding complex in EMSA (Figure5A).
GM-CSF secreted from TF-1 cells on CMo incubation activates STAT5.
(A) EMSA using the PRE probe and 8 μg WCE from TF-1 cells inoculated with CMo for 2, 6, and 24 hours in the absence (lanes 1-3) or in the presence (lanes 4-6) of neutralizing anti–GM-CSF antibodies. (B) GM-CSF production measured by ELISA in culture supernatants of TF-1 cells incubated for different times with CMo in the absence (gray bars) or in the presence (black bars) of neutralizing anti–GM-CSF antibodies. Exp indicates exposed.
GM-CSF secreted from TF-1 cells on CMo incubation activates STAT5.
(A) EMSA using the PRE probe and 8 μg WCE from TF-1 cells inoculated with CMo for 2, 6, and 24 hours in the absence (lanes 1-3) or in the presence (lanes 4-6) of neutralizing anti–GM-CSF antibodies. (B) GM-CSF production measured by ELISA in culture supernatants of TF-1 cells incubated for different times with CMo in the absence (gray bars) or in the presence (black bars) of neutralizing anti–GM-CSF antibodies. Exp indicates exposed.
HTLV-2 CMo-dependent STAT1 activation is mediated by the secretion of IFN-γ
In addition to GM-CSF, SCF and IFN-γ were secreted by TF-1 after incubation with CMo exposure (Table 1). IFN-γ is a strong and specific inducer of STAT1, whereas SCF has been shown to activate the JAK/STAT pathway.30 Therefore, we performed EMSA analysis with WCEs from TF-1 cells incubated with CMo for 6 hours (time corresponding to the peak of secretion of IFNγ and SCF as shown in Figure 4) in the presence of anti-SCF (25 (pg/mL)) or of anti–IFN-γR2 (3.7 (pg/mL))–neutralizing antibodies. Surprisingly enough, though the amount of SCF in the culture supernatants was greater than that of IFN-γ, the complete neutralization of SCF (confirmed by ELISA) did not prevent the activation of STAT1 (data not shown). In contrast, the mixture of 2 specific antibodies recognizing different epitopes of the IFN-γR2 chain completely hampered, as expected, both IFN-γ–dependent (Figure6, lanes 1-2) and CMo-dependent activation of STAT1 (Figure 6, lanes 3-5). In conclusion, these results demonstrate that the activation of STAT5 and STAT1 by CMo is sustained by the expression and release of GM-CSF and IFN-γ, respectively. This was further demonstrated by the absence of STAT5 and STAT1 when the anti–GM-CSF and anti–IFN-γR2 antibodies were used in combination (Figure 6, lane 5).
IFN-γ secreted from TF-1 cells on CMo incubation activates STAT1.
EMSA using the PRE probe and 8 μg WCE from TF-1 cells stimulated with IFN-γ (10 nM [ng/mL]) (lane 1) or incubated with CMo (lane 3) for 6 hours in the absence (lanes 1, 3) or in the presence of a mixture of 2 anti-IFN-γR2 antibodies alone (each one at 10 μM (μg/mL)) (lane 4) or in combination with anti–GM-CSF–neutralizing antibodies (8 μM (μg/mL)) (lane 5).
IFN-γ secreted from TF-1 cells on CMo incubation activates STAT1.
EMSA using the PRE probe and 8 μg WCE from TF-1 cells stimulated with IFN-γ (10 nM [ng/mL]) (lane 1) or incubated with CMo (lane 3) for 6 hours in the absence (lanes 1, 3) or in the presence of a mixture of 2 anti-IFN-γR2 antibodies alone (each one at 10 μM (μg/mL)) (lane 4) or in combination with anti–GM-CSF–neutralizing antibodies (8 μM (μg/mL)) (lane 5).
Anti–HLA-DR mAbs functionally convert HTLV-2 BMo into a STAT-activating virus
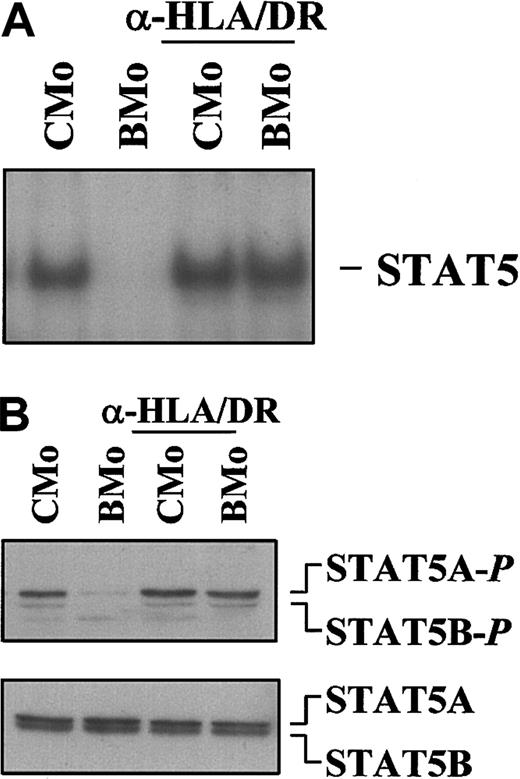
We have previously reported that incubation of HTLV-2 BMo with mAb recognizing the DR subset of class II antigens conferred the ability to the virus of rescuing TF-1 cells from apoptosis induced by IL-3 deprivation, as observed with the CMo strain.31 Therefore, we analyzed whether pretreatment of BMo with anti–DR-specific mAb could also restore the ability to induce the JAK/STAT pathway. TF-1 cells exposed for 24 hours to anti–HLA-DR-BMo virus indeed showed a strong activation of STAT5 as shown in Figure7A (EMSA) and Figure 7B (Western blot).
Specific mAbs against HLA-DR antigens functionally convert BMo to a CMo-like virus.
CMo- and BMo-derived virions were incubated with saturating concentration of anti-HLA class II DR subset mAb D1.12 for 2 hours at 4°C and for 2 hours at 37°C before contact with the IL-3–deprived TF-1 cells. (A) EMSA using the PRE probe and 8 μg WCE from TF-1 cells exposed to either CMo or BMo viruses that were pretreated (lanes 3-4) or left untreated (lanes 1-2) with anti-HLA-II/DR specific mAbs D1.12. (B) Immunoblot analysis using the same WCEs (15 μg) of the EMSA of panel A and anti–phospho-STAT5 antibodies (first panel). In the second panel the filter, after stripping, was hybridized with a mixture of anti-STAT5A and anti-STAT5B antibodies.
Specific mAbs against HLA-DR antigens functionally convert BMo to a CMo-like virus.
CMo- and BMo-derived virions were incubated with saturating concentration of anti-HLA class II DR subset mAb D1.12 for 2 hours at 4°C and for 2 hours at 37°C before contact with the IL-3–deprived TF-1 cells. (A) EMSA using the PRE probe and 8 μg WCE from TF-1 cells exposed to either CMo or BMo viruses that were pretreated (lanes 3-4) or left untreated (lanes 1-2) with anti-HLA-II/DR specific mAbs D1.12. (B) Immunoblot analysis using the same WCEs (15 μg) of the EMSA of panel A and anti–phospho-STAT5 antibodies (first panel). In the second panel the filter, after stripping, was hybridized with a mixture of anti-STAT5A and anti-STAT5B antibodies.
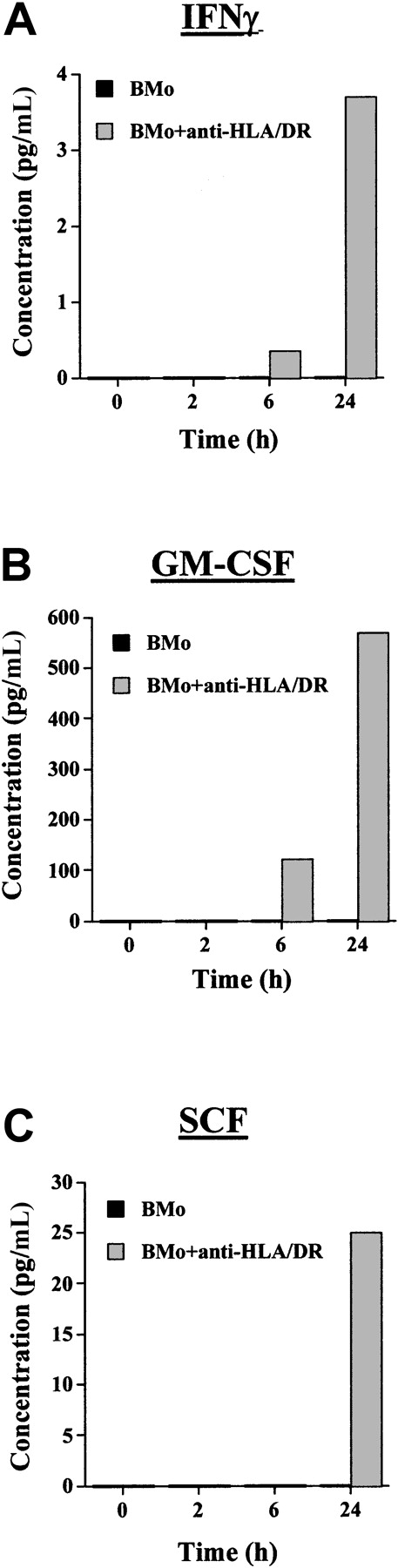
Anti–HLA-DR mAbs functionally convert HTLV-2 BMo to a cytokine-inducing virus
To evaluate whether the activation of STAT5 by anti–HLA-DR-BMo virus was caused by the secretion of the same cytokines induced by CMo, the kinetics of cytokine secretion similar to that shown in Figure 4was demonstrated. In agreement with the gel shift and Western blot results (Figure 7), the only cytokine produced in considerable amount after 24 hours of incubation with anti–HLA-DR-BMo was GM-CSF (570 pM [pg/mL]), whereas the levels of IFNγ and the level of SCF were low (3.7 and 25 pM [pg/mL], respectively) (Figure8). In conclusion, the anti–HLA-DR-BMo virus preferentially promoted the secretion of GM-CSF, which, in turn, activated STAT5, whereas the anti–HLA-DR-BMo–dependent release of IFNγ was not sufficient to stimulate detectable activation of STAT1.
Cytokine production from TF-1 cells incubated with BMo or anti–HLA-DR mAb-pretreated BMo HTLV-2 strains.
IFN-γ (A) GM-CSF, (B) SCF, and (C) production were measured by ELISA in culture supernatants of TF-1 cells at time 0 or were exposed for 2, 6, or 24 hours to BMo (black bars) or BMo pretreated with anti–HLA-DR–specific mAbs D1.12 (gray bars).
Cytokine production from TF-1 cells incubated with BMo or anti–HLA-DR mAb-pretreated BMo HTLV-2 strains.
IFN-γ (A) GM-CSF, (B) SCF, and (C) production were measured by ELISA in culture supernatants of TF-1 cells at time 0 or were exposed for 2, 6, or 24 hours to BMo (black bars) or BMo pretreated with anti–HLA-DR–specific mAbs D1.12 (gray bars).
GM-CSF is responsible for cell survival and proliferation of TF-1 cells exposed to CMo
To verify whether the mitogenic effect induced by the virus in IL-3–deprived cells was sustained by the secretion of GM-CSF, the incorporation of [3H]-thymidine into DNA of TF-1 cells exposed to different stimuli was evaluated. TF-1 cells deprived of IL-3 and incubated for 24 hours with either CMo or anti–HLA-DR-BMo viruses proliferated almost comparably, whereas cells exposed to BMo lost their proliferative capacity over time (Figure9A). Of interest, when an anti–GM-CSF antibody was added to the culture of CMo- and anti–HLA-DR-BMo–exposed TF-1 cells, a rapid decrease in [3H]-thymidine incorporation was observed. The efficacy of the anti–GM-CSF antibody was tested in culture of TF-1 cells cultivated with GM-CSF (Figure 9B). These results demonstrate that the proliferative ability of TF-1 cells exposed to HTLV-2 was indeed dependent on the secretion of GM-CSF.
TF-1 cell proliferation after incubation with HTLV-2 virus.
TF-1 cells were deprived of IL-3 for 24 hours and then (A) incubated for 24 hours with CMo, Bmo, or anti–DR-BMo in the absence or in the presence of anti–GM-CSF antibodies (8 μM (μg/mL)), (B) supplemented with GM-CSF (1 nM [ng/mL]) in the absence or in the presence of anti-GM-CSF antibodies.
TF-1 cell proliferation after incubation with HTLV-2 virus.
TF-1 cells were deprived of IL-3 for 24 hours and then (A) incubated for 24 hours with CMo, Bmo, or anti–DR-BMo in the absence or in the presence of anti–GM-CSF antibodies (8 μM (μg/mL)), (B) supplemented with GM-CSF (1 nM [ng/mL]) in the absence or in the presence of anti-GM-CSF antibodies.
Discussion
In this study we have shown that HTLV-2 induced survival and proliferation of the CD34+ TF-1 cell line in the absence of IL-3 or other growth factors. These effects were observed only when HTLV-2 was derived from T cells (CMo), but not from B cells (BMo).31 Given that CMo exposure to TF-1 cells and to CD34+ primary cells promoted the activation of STAT5, which is activated by IL-3, we initially hypothesized that CMo could activate STAT5 by either direct binding to IL-3R or through the secretion of IL-3. Several findings excluded this hypothesis. First, when TF-1 cells were incubated with CMo in the presence of either anti–IL-3R or neutralizing anti–IL-3 antibody, STAT activation still occurred (data not shown). Second, IL-3 was not secreted by TF-1 on exposure to CMo (Table 1). Third, CMo induced the phosphorylation not only of STAT5 but also of STAT1, which is not usually activated by IL-3 (Figure 1). Together these results suggested that an alternative mechanism had to be invoked to explain the CMo-mediated survival of TF-1 cells. CMo did not induce the JAK/STAT pathway directly; rather, it induced it through the secretion of GM-CSF and IFN-γ, which in turn activated STAT5 and STAT1, respectively. In this regard, the production of IFN-γ and GM-CSF by T cells infected by HTLV-1 and -2 has been reported,36 and the activation of both genes was shown to be dependent on the trans-acting function of HTLV-1 and -2 Tax proteins.37,38 In contrast, we here show that the HTLV-2–dependent secretion of these 2 cytokines occurred in cell types other than T lymphocytes—ie, CD34+ cells—and it was independent of productive viral infection. Indeed, TF-1 cells are not susceptible to HTLV-2 infection, as previously shown by the lack of polymerase chain reaction amplification of a fragment of thetax31 or pol (data not shown) genes from TF-1 cells exposed to HTLV-2 CMo strain after 3 and 7 days of culture. In support of these findings, the incubation of TF-1 cells with CMo strain in the presence of the reverse transcriptase inhibitor 3′-azido-3′deoxythymidine did not prevent STAT activation (data not shown).
The observation that anti–HLA-DR mAbs restored the ability of BMo to induce the JAK/STAT pathway (Figure 7) suggests that virus cell recognition is required. This event is likely impeded by the presence of HLA-DR antigens on the surfaces of the viral Env. These antigens could interact either in cis with the HTLV-2 Env or intrans with the cellular receptor for HTLV-2 and could, therefore, interfere with the Env-receptor binding process. Noteworthy, unlike CMo, the tax gene was amplified by polymerase chain reaction in TF-1 cells exposed to HTLV-2 BMo strain after 7 days of culture,31 indicating that BMo can enter and integrate these cells though it fails to spread productively thereafter. Based on this observation, we speculate that the cell surface receptor(s) engaged by HTLV-2 might be several and that viral entry and induction of cytokine secretion are regulated by distinct cell surface receptors. Similar results were obtained by Martin and Southern,39who reported that co-cultivation of HTLV-2–infected T cells, but not B cells, with resting peripheral blood mononuclear cells (PBMCs) induced their proliferation; the authors concluded that the induction of proliferation was dependent on a T-cell–specific signal.39 In our model system, the ability of CMo to induce cell proliferation was not determined by a specific T-cell signal because BMo mimicked CMo in the presence of anti–HLA-DR mAbs, indicating that the cytokine–survival inductive stimulus did not derive from molecule(s) selectively present on the surfaces of T cells and entrapped in the HTLV-2 envelope during the budding process.
It has been reported that in vitro infection of T cells with HTLV-1,21 or the spontaneous proliferation of cells derived from patients with adult T-cell leukemia,22 is associated with the constitutive activation of the JAK/STAT pathway. In particular, it has been shown that the transition from an IL-2–dependent to an IL-2–independent cell proliferation corresponded to the activation of JAK1, JAK3, STAT3, and STAT5 in cord blood–derived T lymphocytes, suggesting that the JAK/STAT pathway could participate in the HTLV-1–mediated T-cell transformation.21 In contrast, HTLV-2 infection was shown to transform T cells independently of JAK/STAT activation.6 Here, we demonstrate that contact between CD34+ TF-1 cells with HTLV-2 can induce, though indirectly, the JAK/STAT pathway. We can speculate that the discrepancy between our findings and those reported by Mulloy et al6 may be explained by the fact that only transformed cells chronically infected with HTLV-2 had been studied. In our system, the cells were directly incubated with the virus and analyzed for STAT activation thereafter. In addition, we have observed that activation of the JAK/STAT pathway occurs by in vitro cultivation of PBMCs derived from HTLV-2–infected patients (Bovolenta et al, manuscript in preparation).
TF-1 cells are known to proliferate in response to a large panel of cytokines and growth factors, among them IL-3, IFN-γ, SCF, and GM-CSF.29 IFN-γ is a potent Th1 cytokine endowed with antiviral activity40; however, conflicting results have been reported regarding its role in hematopoiesis.41-44Strong evidence of a positive effect of IFN-γ, alone or in synergy with other factors such as SCF and GM-CSF, on the survival and growth of hematopoietic precursors in human and murine systems has been reported.44 45 In our model, IFN-γ–dependent activation of STAT1 was more variable than GM-CSF–induced STAT5 activation, suggesting that the induction of IFN-γ by CMo may be influenced by multiple factors. Furthermore, when BMo was functionally converted to a CMo-like virus, it did not induce activation of STAT1, only STAT5, because only GM-CSF, but not IFN-γ, was secreted at substantial levels (Figure 8).
Although SCF can activate STAT1 in the human MO7e cell line and in normal precursor cells,46 neutralization of SCF by specific antibodies did not prevent the activation of STAT1 by CMo exposure, indicating that SCF was not involved. The proliferative role of GM-CSF, irrespective of the presence of other growth factors, in TF-1 and in primary CD34+ cells is well documented.29 GM-CSF is conventionally administered for the mobilization of CD34+ cells in the peripheral blood in autologous transplantation.47
The evidence that a relative high percentage of intravenous drug users infected with HIV-1 are also HTLV-2 positive12 raises the question of a potential reciprocal influence of HTLV-2 and HIV-1 infections. We have previously shown in co-infected patients that HTLV-2 infection of PBMCs induced the production of the HIV-suppressive CC-chemokines, and particularly of MIP-1α, responsible for the inhibitory effect of HTLV-2 on HIV-1 replication ex vivo.48 The current findings may further support the hypothesis that HTLV-2 may influence HIV-1 infection by inducing the secretion of GM-CSF and IFN-γ. These 2 important cytokines may contribute to slower HIV-1 disease progression observed in several co-infected patients. In a recent clinical trial conducted on AIDS patients, it has been demonstrated that GM-CSF decreases viral replication and increases the number of circulating CD4+ T cells, the primary target of HIV-1.49 Thus, the ability of HTLV-2 to induce the secretion of antiapoptotic and proliferative cytokines may increase the number of hematopoietic precursors and expand CD4+ T lymphocytes depleted by HIV infection.
Supported in part by the II National Project of Research against AIDS of the Istituto Superiore di Sanità (G.P., U.B.) and by the AIRC Program 2000 (U.B.) (Rome, Italy). The Universities of Parma and Verona participated in the HERN concerted action supported by the BIOMED Program of the European Commission.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Chiara Bovolenta, GenEra S.p.A., via Olgettina n. 58, 20132 Milano, Italy; e-mail: c.bovolenta@hsr.it.

![Fig. 6. IFN-γ secreted from TF-1 cells on CMo incubation activates STAT1. / EMSA using the PRE probe and 8 μg WCE from TF-1 cells stimulated with IFN-γ (10 nM [ng/mL]) (lane 1) or incubated with CMo (lane 3) for 6 hours in the absence (lanes 1, 3) or in the presence of a mixture of 2 anti-IFN-γR2 antibodies alone (each one at 10 μM (μg/mL)) (lane 4) or in combination with anti–GM-CSF–neutralizing antibodies (8 μM (μg/mL)) (lane 5).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/1/10.1182_blood.v99.1.224/6/m_h80121939006.jpeg?Expires=1767737666&Signature=dbt0YXcwt3akQeXURHNFkOgP4ObNmGiq1B9JqBZfjWBIfFClQIml0m4VnxFw4IPGx9oSDRNOSjrn7vZO7z6PJWT0ZxGxF7l0NJS5QE0WoiiMizNdpKSJgi7lK2fsKSw7LW9AP8KjSCQsCPmIB4ca-wAW7UX4acRuWiwTo~Fyo~U1g-6cpKAp-9rD428D8eRypg5FthmTPRC-jQ8wNVk553T0fnAO9vlCuM5HWOzWIb0Du-7OM1CxdUq6Y4kgYHnv9w8-qPNQjCyboIPgP56D49G456F1th8A7CYJP5ky2RduSatYOAHVcd01hpO9gf91zMGWZU~NmA2bfIHf4GR9Kw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 9. TF-1 cell proliferation after incubation with HTLV-2 virus. / TF-1 cells were deprived of IL-3 for 24 hours and then (A) incubated for 24 hours with CMo, Bmo, or anti–DR-BMo in the absence or in the presence of anti–GM-CSF antibodies (8 μM (μg/mL)), (B) supplemented with GM-CSF (1 nM [ng/mL]) in the absence or in the presence of anti-GM-CSF antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/1/10.1182_blood.v99.1.224/6/m_h80121939009.jpeg?Expires=1767737666&Signature=FzujtFYRhVMHNZZcXXLfO4cmTwcYZipE8DQnNYtcXsxzxK573YptOyV36vxZl~wlNJ5x0h47RuwKF2n-qiJX2s3NbgfHfGilEiSA2Tf4ECWvAhJ6HCUzUC1R6eBA4tMOsscHjKewnkTIMEXutwKCXSrglyynJPzBFa730spTxHl-wUg6smfy2IKlWfbfg~xxTZeDj10iY6CkuVSDLeAh7almrJw507e2H4FbZL-NHylosIRcdMjVy~f5vXncwdRXLDJ00Yatc8NRvSn9DwPrX2-g6E72~saG34oce7eDiXahgrN-OlXVUEsFxTLOXHDyesRM8wAvpEZMqKDTPtDXiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal