Abstract
Immunohistochemistry (IHC) was performed on archived bone marrow (BM) with a phosphospecific anti-AKT antibody. IHC on 26 BM biopsies from patients with multiple myeloma (MM) demonstrated phospho-AKT staining of malignant plasma cells in a cell membrane–specific pattern, whereas nonmalignant hematopoietic cells did not stain. Preabsorption of the antibody with phosphorylated AKT peptide, but not nonphosphorylated peptide, abrogated staining. Frequency of plasma cell staining in BMs of patients with stage I or smoldering MM was significantly less than that of stage III MM marrows. Plasma cells in 10 patients with monoclonal gammopathy of undetermined significance were not stained by the antibody. To investigate the significance of AKT activation, 2 cell lines initiated from cultures of primary MM cells were also studied. Both demonstrated constitutive AKT activation. Interruption of AKT activation and activity, achieved by either exposure to wortmannin or by ectopic expression of a dominant negative AKT mutant, resulted in inhibition of MM cell growth in vitro. These results indicate that activation of the AKT kinase is a characteristic of MM cells and suggest that AKT activity is important for MM cell expansion.
Introduction
In multiple myeloma (MM), enhanced proliferation and resistance to apoptosis account for expansion of the malignant clone.1,2 Previous investigations have demonstrated alteration of the ERK,3 jun kinase,4STAT,5 and AKT kinase6 signaling cascades in MM cells and implicated the pathways in clonal expansion. These studies, however, were mainly investigations in cell lines, and it is unclear whether the observations can be extrapolated to tumors in patients. We therefore used immunohistochemical (IHC) staining to test activation of AKT in situ in MM cells of patients. AKT is activated downstream of phosphatidylinositol 3-kinase (PI3-kinase) by translocation to the cell membrane7 and phosphorylation at Ser and Thr residues.8 Its activity is inhibited by the tumor suppressor PTEN phosphatase. Our IHC assay with a phosphospecific anti-AKT antibody confirmed frequent AKT activation in MM cells of patients with stage III disease. Further evaluation of 2 MM cell cultures, recently explanted from patients, indicated that AKT activation may play a role in MM cell expansion.
Study design
Myeloma cells
Bone marrow (BM) cells from one MM patient and peripheral blood from a second with plasma cell leukemia were separated by an immunoabsorption column to isolate high CD38-expressing cells (as described by Tu et al6). The separated cells consisted of more than 98% plasma cells by light microscopy. These cells were cultured in vitro without growth factors in complete RPMI media. After 1 week, the cultures were composed of 100% malignant plasma cells that began to slowly proliferate and have been maintained in culture for at least 4 months. These cells morphologically resemble plasma cells and express high levels of membrane CD38 and monoclonal cytoplasmic light chain. Their doubling time is approximately 60 hours.
Transient transfections and flow cytometry
The kinase-inactive AKT construct, HA-AKT(K179M), functions as a dominant negative inhibitor of endogenous AKT.9 It was cloned into the enhanced green fluorescence protein (EGFP)–expressing vector, pEGFP-C2 (Clontech) atHindIII/SmaI sites. Myeloma cells were transiently transfected with HA-AKT(K179M) or empty pEGFP-C2 vector by electroporation (250 V for 25 ms). At 24 hours after transfection, viable cells were stained with the DNA dye Hoechst at 2.5 μg/mL for 20 minutes and cell cycle distribution was determined by first gating on EGFP-fluorescing cells. EGFP fluorescence demonstrated 8% to 10% transfection efficiency for both HA-AKT(K179M) and empty vector.
Patient population
Archival BM biopsies obtained from patients at Kaiser Permanente Hospital (Woodland Hills, CA) were immunostained for phosphorylated AKT. Biopsies were obtained at the time of diagnosis between 1990 and 1999 and clinically staged by the Durie-Salmon system. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM were diagnosed in patients as previously described.10 11
IHC staining
Immunohistochemical staining was performed on Bouin-fixed biopsies as previously reported,12 using a diaminobenzadine-based detection method. The phosphospecific anti-AKT antibody, obtained from Bioscience (catalog no. 44-622; Princeton, NJ), which recognizes phosphorylated AKT, was used as the primary antibody. Negative controls, where the primary anti-AKT antibody was omitted, were uniformly negative for immunostaining. In preabsorption studies, the phosphospecific anti-AKT antibody was preadsorbed with 1 to 2 μg/mL of either phosphorylated AKT peptide (sequence = Ac-C(Ahx)KHFPQF(pS)YSAS-NH2 or nonphosphorylated peptide (sequence = Ac-C(Ahx)KHFPQFSYSAS-NH2). (Single-letter amino acid codes used.) Frequency of phospho-AKT expression was determined by counting at least 250 plasma cells from 3 different areas in BMs from patients with myeloma. For patients with MGUS, only 50 to 100 plasma cells were enumerated.
Western blot analysis
Western blot analysis was performed as previously described.13 Densitometric analysis was used to determine the median effective dose (ED50) for wortmannin (drug dose inhibiting phosphorylation by 50%).
Statistics
The t test was used to determine significance.
Results and discussion
AKT activation in myeloma BM biopsies
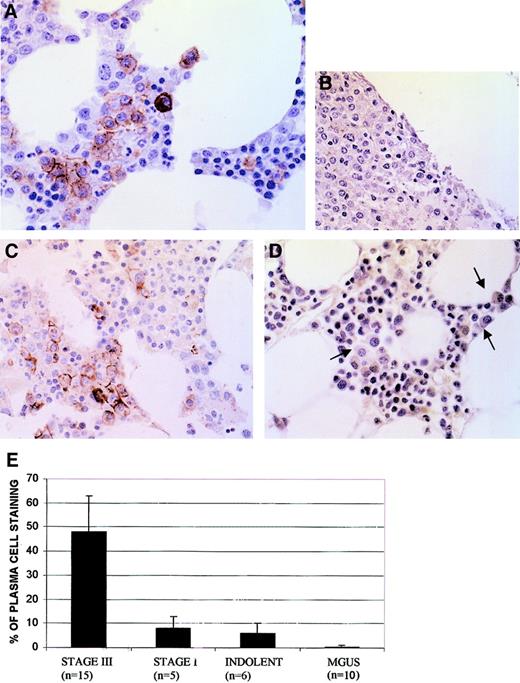
The AKT kinase is activated by phosphorylation at Ser473. We thus tested activation of AKT in myeloma marrow by IHC using a phosphospecific anti-AKT antibody that only detects AKT when it is phosphorylated at Ser473. As shown in Figure1, the phospho-AKT antibody readily stained myeloma plasma cells. Immunostaining was present in a cell membrane–specific pattern, consistent with the known subcellular locale when AKT becomes activated. Of note, staining was specific for myeloma tumor cells because nonmalignant hematopoietic cells in these same biopsies were negative. In 3 of the BM samples, immunostaining of MM cells was abrogated when the antibody was absorbed with phosphorylated, but not nonphosphorylated, AKT peptide, attesting to the specificity of immunostaining. Examples of peptide preabsorption in one BM sample are shown in panels B and C of Figure 1.
Immunohistochemistry of BM stained with phosphospecific anti-AKT antibody.
(A) Myeloma marrow with immunostained plasma cells in a membrane-specific pattern. (B) Myeloma marrow stained with antibody after antibody was first preabsorbed with phosphorylated AKT peptide. (C) Myeloma marrow (same as used in panel B) stained with the same antibody after antibody was preabsorbed with nonphosphorylated AKT peptide. (D) MGUS marrow stained with phosphospecific AKT antibody. Three plasma cells are shown with arrows; original magnification × 400. (E) Frequency (mean ± SD) of plasma cells immunostaining in patient marrows.
Immunohistochemistry of BM stained with phosphospecific anti-AKT antibody.
(A) Myeloma marrow with immunostained plasma cells in a membrane-specific pattern. (B) Myeloma marrow stained with antibody after antibody was first preabsorbed with phosphorylated AKT peptide. (C) Myeloma marrow (same as used in panel B) stained with the same antibody after antibody was preabsorbed with nonphosphorylated AKT peptide. (D) MGUS marrow stained with phosphospecific AKT antibody. Three plasma cells are shown with arrows; original magnification × 400. (E) Frequency (mean ± SD) of plasma cells immunostaining in patient marrows.
The percent of malignant plasma cells positively stained in these biopsies was significantly (P < .05) higher in Durie-Salmon stage III patients than in stage I (Figure 1E). Frequency of staining in stage I disease was comparable to that of indolent MM. No plasma cell immunostaining was detected in marrows from 10 patients with MGUS (Figure 1E). Although the degree of plasma cell infiltration of these MGUS marrows was low (mean 2%), the plasma cells were easily identified and were clearly nonreactive with the antibody (Figure 1D, arrows).
Inhibition of AKT activation inhibits MM cell growth
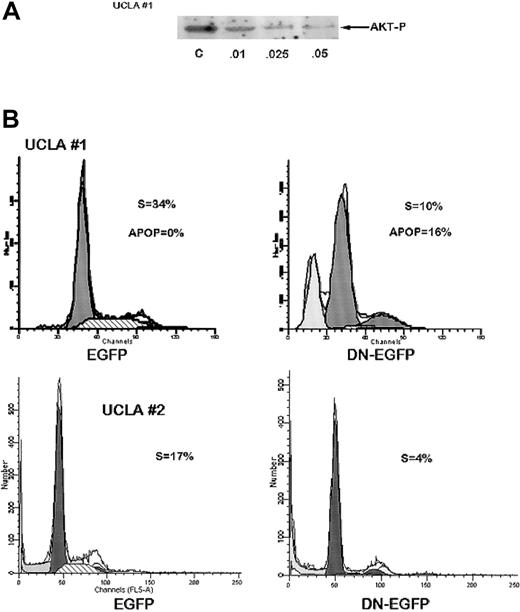
We next studied MM cell populations from 2 patients; the cells were successfully cultured over a 4-month period. Immunoblotting demonstrated constitutive phosphorylation of AKT which, in one case (UCLA no. 1), was sensitive to the PI3-kinase inhibitor, wortmannin (Figure 2A). Wortmannin also induced a cytoreductive effect on these MM cells and this correlated with its ability to inhibit AKT phosphorylation. The concentration required for 50% inhibition of cell recovery (LD50) after 72 hours was 0.02 μM for UCLA no. 1 cells, whereas the ED50 for inhibition of AKT activation was 0.01 μM. Constitutive AKT phosphorylation in the UCLA no. 2 cell line was more resistant to wortmannin with an inhibition detected only at wortmannin concentrations more than 0.1 μM. We also transiently transfected these MM cells with a plasmid expressing a dominant negativeAKT gene (K179M) fused to the EGFP gene, or (as a control) the empty vector expressing only EGFP, and then performed flow cytometry for cell cycle analysis (Hoechst staining) on EGFP-gated cells. The expression of EGFP from both plasmids in both MM cell populations was comparable (7%-10%) after transfection. As shown in representative experiments (Figure 2B), expression of the dominant negative K179M in UCLA no. 1 MM cells resulted in a decreased number of cells in S phase (10% versus 34% in control cells) and increase in apoptosis (16% versus 0%) as shown by a sub-G1 peak. In UCLA no. 2 cells, expression of the dominant negative AKT resulted in a decrease in S-phase distribution (4% versus 17%). Cell cycle distribution in cells transfected with the control EGFP plasmid were not altered compared to nontransfected cells (not shown). The 2 experiments shown in Figure 2B were repeated twice with identical results.
Inhibition of AKT activity in MM cells curtails cell growth and S-phase distribution.
(A) UCLA no. 1 cells were treated with increasing concentrations of wortmannin (shown below protein bands in μM) for 2 hours and Western blot then performed with phosphospecific AKT antibody. Immunoblot for total AKT (not shown) showed no differences in expression of total AKT in all groups. Additional cells treated identically were cultured for 72 hours with the same concentrations of wortmannin and then viable cell recovery was recorded. Mean results of 4 independent experiments were used to determine LD50 as described. (B) UCLA no. 1 MM cells (top panels) and UCLA no. 2 cells (bottom panels) were transiently transfected with control EGFP vector (left panels) or EGFP vector expressing DN AKT (DN-EGFP, right panels). Twenty-four hours after transfection, cells were stained with Hoechst dye and cell cycle analysis performed by gating on EGFP+ cells. Percent of cells in S phase or apoptotic (APOP) is shown.
Inhibition of AKT activity in MM cells curtails cell growth and S-phase distribution.
(A) UCLA no. 1 cells were treated with increasing concentrations of wortmannin (shown below protein bands in μM) for 2 hours and Western blot then performed with phosphospecific AKT antibody. Immunoblot for total AKT (not shown) showed no differences in expression of total AKT in all groups. Additional cells treated identically were cultured for 72 hours with the same concentrations of wortmannin and then viable cell recovery was recorded. Mean results of 4 independent experiments were used to determine LD50 as described. (B) UCLA no. 1 MM cells (top panels) and UCLA no. 2 cells (bottom panels) were transiently transfected with control EGFP vector (left panels) or EGFP vector expressing DN AKT (DN-EGFP, right panels). Twenty-four hours after transfection, cells were stained with Hoechst dye and cell cycle analysis performed by gating on EGFP+ cells. Percent of cells in S phase or apoptotic (APOP) is shown.
In this study, AKT was frequently activated in MM cells and the frequency of activation correlated with disease activity, being significantly greater in stage III disease compared to stage I or indolent MM and being undetected in MGUS. We6 and others14 have shown that interleukin 6 and insulinlike growth factor 1 can activate AKT in myeloma cells. Thus, the detected AKT activation in MM marrow may have been due to cytokine stimulation in situ. However, as shown in 2 MM cell populations in vitro, constitutive activation may exist. This could be due to autocrine cytokine stimulation, loss-of-function PTEN mutations, or to gain-of-function mutations in AKT or PI3-kinase. Hyun et al14 found PTEN mutations in some human myeloma cell lines. Thus, PTEN mutations may occur in patients with myeloma and could explain heightened AKT activation.
When the AKT pathway was paralyzed by transient transfection with a dominant negative AKT construct, a decrease in S-phase distribution (and increase in apoptosis in one case) was found in Hoechst-stained cells. This is consistent with the work of Hyun and coworkers14 who described an inhibitory effect of PTEN transfection in MM cells concurrent with a decrease in AKT activation.
In summary, these results indicate that activation of AKT occurs in MM plasma cells. Because of its central location, activating diverse downstream proliferative and antiapoptotic pathways, AKT is a promising target for future molecular-based therapy. In addition, the frequent activation in myeloma tumor cells compared to nonmalignant cells suggests a therapeutic window may exist in patients.
The authors thank the UCLA flow cytometry core lab of the Jonsson Comprehensive Cancer Center for assistance and Dr Jay Persselin for his continued support.
Supported by research funds of the Veterans Administration, including the Research Enhancement Awards Program entitled “Cancer Gene Medicine,” a year 2000 Senior Research Award from the Multiple Myeloma Research Foundation, grant CDA DAMD 17-0-1-0214 awarded by the United States Army, and grants NS3621 and CA69381 awarded by the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alan Lichtenstein, Hematology-Oncology, VA West LA Hospital, W111H, 11301 Wiltshire Blvd, Los Angeles, CA 90073; e-mail: alichten@ucla.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal