Abstract
Macrophage colony-stimulating factor (M-CSF) is one of several hematologic growth factors capable of regulating the survival, proliferation, and differentiation of macrophages, but its role in modulation of the accumulation and function of alveolar macrophages (AMs) in vivo is not well defined. Osteopetrotic (Op/Op) mice have no detectable M-CSF and show variable tissue-specific reductions in macrophage numbers. It was hypothesized that AMs would be decreased in number and have altered function in Op/Op mice because of the absence of M-CSF. Lung macrophages identified by Mac-3 staining in lung sections were decreased in number in 20-day-old Op/Op mice (P < .001) but not Op/Op mice older than 4 months (P = .68) compared with findings in age-matched littermate controls. The numbers of AMs recovered by bronchoalveolar lavage (BAL) were also reduced in young but not adult Op/Op mice compared with controls. Expression of interleukin-3 (IL-3) was increased in the lungs of Op/Op mice compared with controls as determined by quantification of IL-3 cytokine levels (P = .04), bioactivity (P = .02), and messenger RNA transcript levels. AMs of Op/Op mice spontaneously released higher levels of matrix metalloproteinases (MMPs) than AMs of controls as determined by immunohistochemical staining of AMs and zymographic assessment of BAL fluid and AM lysates. Consistent with an increased release of MMP, Op/Op mice had abnormal elastin deposition and spontaneously developed emphysema in the absence of molecular or cellular evidence of lung inflammation. These data show that the AM deficiency observed in young Op/Op mice is spontaneously corrected with age and is associated with increased lung levels of IL-3, spontaneous MMP expression by AMs, and destruction of lung tissue.
Introduction
Alveolar macrophages (AMs) are pulmonary residents of the bone marrow–derived mononuclear phagocyte system that play a critical role in several diverse lung functions and in lung host defense.1 AMs arise from circulating blood monocytes that enter the lungs and other tissues2,3 and undergo terminal differentiation into tissue macrophage populations that are heterogeneous in their level of accumulation,4,5functional activity,4 and expression of cell-surface molecules.6,7 The mechanisms regulating the abundance and heterogeneity of AMs and other tissue macrophage populations are not well defined. However, exposure of macrophages to different tissue microenvironments varying in the local amount or form of hematologic growth factors capable of regulating macrophage proliferation has been proposed.5
Macrophage colony-stimulating factor (M-CSF, also known as CSF-1) is one of several factors known to regulate the survival, proliferation, and differentiation of mononuclear phagocytic lineage cells.8 M-CSF is abundant in blood, present in most tissues (though in lower and various levels depending on the type of tissue), and expressed as 3 biologically active forms: a circulating secreted glycoprotein isoform, a proteoglycan isoform, and a cell-surface isoform produced locally in tissues.8,9 Recombinant M-CSF was shown to promote accumulation of murine AMs in vivo10 and survival of human AMs in vitro,11 and M-CSF levels are increased in the lungs of smokers in association with significant increases in numbers of AMs.12 Granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 3 (IL-3) are also capable of regulating macrophage proliferation.5,13AM numbers are increased markedly in transgenic mice expressing GM-CSF in the lung from a lung-specific transgene.14 However, AM numbers are not reduced in GM-CSF knockout mice lacking GM-CSF,15 16suggesting that GM-CSF is not essential for constitutive accumulation of AMs.
Osteopetrotic mice are homozygous for a naturally occurring recessive frameshift mutation (Csf-1op or Op) in the M-CSF gene17 and consequently have no M-CSF.18 They thus provide a good model for studying the role of M-CSF in vivo. In Op/Op mice, various M-CSF–dependent macrophage populations are decreased in number and show developmental anomalies in a tissue-specific manner.5,19,20 For example, bone marrow monocytes/macrophages, which usually increase progressively with age in normal mice, are reduced in number in young Op/Op mice.5However, bone marrow macrophages still increase in number with age in these mice in parallel with spontaneous correction of osteopetrosis.21 Macrophage populations that increase transiently in number at 2 weeks of age in normal mice are reduced in number, with or without preservation of the transient increase (eg, in the intestine or periosteum, respectively), or are absent (eg, in kidneys) in Op/Op mice.5 AMs and pleural macrophages in Op/Op mice are reduced to 30% and 16%, respectively, of levels in littermate controls at 2 weeks of age20; however, changes in AM accumulation with age have not been studied. Administration of exogenous GM-CSF, IL-3, or both corrects deficiencies in macrophage accumulation in some but not all tissues in Op/Op mice, thereby suggesting that one of these factors might have a compensatory role.22,23 Mice genetically deficient in both M-CSF and GM-CSF (Op/Op mice with targeted ablation of the GM-CSF locus) still undergo spontaneous correction of osteopetrosis and hematopoietic deficiencies, thus demonstrating that GM-CSF is not the compensatory factor mediating age-dependent changes in macrophage accumulation in the blood–bone marrow compartment.24
M-CSF and GM-CSF regulate a large number of genes in myeloid cells,25 including matrix metalloproteinases (MMPs), a family of proteins whose various functions include tissue remodeling26,27 and antibacterial host defense.28 MMP gene expression in macrophages may be controlled by the relative levels of M-CSF and GM-CSF to which the cells are exposed because in vitro studies found that GM-CSF stimulates expression of MMP-12 whereas M-CSF represses MMP-12 expression in murine macrophages.29 Importantly, increased secretion of MMP-12 by lung macrophages was also shown to be a critical component of the mechanism of cigarette-smoke–induced emphysema in mice.30
The current study was undertaken to define the role of M-CSF in the regulation of accumulation and function of AMs in the lung in vivo. Young and adult Op/Op mice were studied to assess age-related effects on AMs. Because the lung is particularly sensitive to proteases, the consequences of M-CSF deficiency on MMP expression in AMs were also evaluated, as were the effects on lung structure.
Methods and materials
Mice
Genotypes are designated as follows. Mice homozygous for theCsf-1op allele (B6C3Fe-a/a–Csf-1op/Csf-1op) are designated as Op/Op, heterozygotes (B6C3Fe-a/a–Csf-1op/Csf-1+) as Op/+, and wild-type (B6C3Fe-a/a–Csf-1+/Csf-1+) as +/+. Mice were bred from heterozygous Op/+ pairs (Jackson Laboratory, Bar Harbor, ME) and caged with the lactating mother. Op/Op mice were identified by the absence of tooth eruption at 10 to 14 days of age and subsequently by genotyping using polymerase chain reaction (PCR).31 Op/+ and +/+ mice are phenotypically normal20 and served as age-matched littermate controls. Litters were thinned at 3 to 4 weeks, with 2 to 4 littermate controls kept for each Op/Op mouse. Pups were fed powdered mouse-food pellets, and all other aspects of routine care and housing of mice in a barrier facility were as described previously.32 All experiments involving animals were conducted under protocols approved by the Institutional Animal Care and Use Committee in the animal facility of the Children's Hospital Research Foundation.
Quantification of lung macrophages
Op/Op or control mice aged 20 to 120 days were killed, and the lungs were inflation-fixed, removed, and embedded in paraffin as described previously.33 Serial 5-μm sections were cut through the length of each lobe, placed on polylysine-coated slides, deparaffinized, and subjected to an antigen retrieval step (microwave irradiation for 15 minutes at full power; [Sharp Electronics, Mahwah, NJ] in 0.01 M citrate buffer [pH 6.0]).34 Slides were then incubated with primary antibody (rat anti–mouse Mac-3 monoclonal antibody [BD Pharmingen, San Diego, CA]; 1:3000 dilution in phosphate-buffered saline [PBS]; 4°C for 16 hours), secondary antibody (biotinylated anti–rat IgG [Vector Laboratories, Burlingame, CA]; 1:200 dilution in PBS; 25°C for 30 minutes), and then avidin-conjugated horseradish peroxidase.30 Color was developed using diaminobenzene, and cells were counterstained with nuclear Fast Red. Slides were viewed under a Nikon Microphot (Garden City, NY) FXA photomicroscope equipped with a digital image-analysis system, and lung macrophages were enumerated by morphometric analysis of tissue sections using Metamorph software (Universal Imaging, West Chester, PA). AMs were quantified using bronchoalveolar lavage (BAL) as described previously.35
Quantification of specific proteins in lungs by enzyme-linked immunosorbent assay
Expression of hematologic growth factors in the lungs was assessed in lung homogenates of Op/Op and age-matched littermate controls as described previously, with minor modifications.36 Briefly, lungs were homogenized in 5 mL PBS containing 1% Triton X-100 (Sigma, St Louis, MO) and 1 tablet Complete Mini EDTA Free protease inhibitor mixture (Roche Molecular Biochemicals, Indianapolis, IN). Lysates were cleared of debris by low-speed centrifugation and concentrated 10-fold by ultrafiltration (Centricon 10; Amicon, Bedford, MA). GM-CSF, IL-3, tumor necrosis factor α (TNF-α), and interleukin 6 (IL-6) were quantified by using Quantikine enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) in aliquots of lung homogenate containing 80 μg total protein. M-CSF and G-CSF were also evaluated by ELISAs established in our laboratory under conditions described in the GM-CSF Quantikine kit. Capture antibodies were anti–murine M-CSF (Oncogene Research Products, San Diego, CA) and anti–murine G-CSF (R&D Systems) antibodies; the detection antibody was a biotinylated, polyclonal antimurine antibody (R&D Systems). Color development used avidin-peroxidase complex (Sigma) with 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid and hydrogen peroxide, and quantification was done at 405 nm. Under the assay conditions used, M-CSF, GM-CSF, G-CSF, IL-3, TNF-α, and IL-6 were detected reproducibly at levels of 6, 1, 4, 1 to 2, 5, and 3 pg/mL, respectively, in lung homogenates. Surfactant protein D (SP-D) levels were measured in lung homogenates by using ELISAs as described previously.37
Quantification of IL-3 messenger RNA transcript levels
Total-lung RNA was purified from the lungs of Op/Op mice and littermate controls and subjected to reverse transcriptase (RT)–PCR amplification using oligonucleotide primers specific for the IL-3 gene as described previously.35
Quantification of IL-3 bioactivity
Op/Op mice and age-matched controls were killed at ages ranging from 3 to 6 months, and the lungs were lavaged with 5 1-mL aliquots of PBS. IL-3 bioactivity was then assessed in lysates using IL-3–growth responsive M-NFS-60 cells (R&D Systems) as described in the product literature for the anti–mouse IL-3 neutralizing antibody (R&D systems). Briefly, cells were plated as described and incubated for 24 hours in medium containing BAL (50 μL) preincubated with 1 μg anti–murine M-CSF antibody (Oncogene Research Products) to neutralize any M-CSF (present in control lysates). Cell growth was assessed by tritium-thymidine incorporation into DNA.
AM morphologic studies
AMs were recovered from Op/Op mice and control mice, washed, and evaluated by cytocentrifugation and Diff-Quick staining as described above. AM cell size was determined from digital photomicrographs by measuring the area of each cell with use of the “total area” and “equivalent radius” functions of the Metamorph software. More than 100 cells were evaluated for each determination.
Expression of MMPs
AMs were obtained by BAL and evaluated for MMP expression by immunohistochemical staining as described previously33 by using goat anti–mouse primary antibodies to MMP-2, MMP-9, or MMP-12 (Santa Cruz Biotechnology, Santa Cruz, CA; diluted 1:500, 1:500, and 1:400, respectively).
MMP enzyme activity levels in BAL fluid and BAL cell lysates were evaluated by zymography as described previously.38Briefly, BAL was collected from 2-month-old Op/Op or control mice, cells were recovered by centrifugation (500g for 7 minutes), and BAL fluid was concentrated 10-fold by using Centricon 10 filters. BAL fluid (10 μL) or cells (1.5 × 105 cells/mouse) were mixed with 10 μL Laemmli sample buffer (Bio-Rad, Hercules, CA) and electrophoresed on 1-mm–thick zymogram gels (Novex, San Diego, CA; 10% gelatin for BAL and 12% casein for cells) under nonreducing conditions. Gels were then washed in 2.5% Triton X-100 for 1 hour with gentle agitation on an orbital shaker; incubated (24 hours for BAL and 72 hours for cells) in 40 mM Tris-hydrochloric acid (pH 7.5), 10 mM calcium chloride, and 1 μM zinc chloride to permit digestion of gelatin and casein at the positions of the electrophoretic bands of proteolytic activity in the gel; stained for 3 hours using a colloidal blue staining kit (Novex); and destained with distilled water. Molecular weights were determined from prestained standards (Novex).
To determine whether IL-3 stimulates MMP release from AMs in the lung in vivo, recombinant IL-3 (R&D Systems) or PBS (control) was administered (100 ng/mouse) endotracheally into the lungs of wild-type mice. Forty-eight hours later, BAL was recovered and evaluated for the presence of MMP by zymography as described above. To determine whether the effect of IL-3 on macrophage MMP expression was direct or indirect, murine RAW264.7 macrophages were cultured in Dulbecco modified Eagle medium in the absence or presence of IL-3 (100 ng/mL) with or without cycloheximide (10 μg/mL) for 3 hours. Total RNA was then extracted and evaluated for MMP-2, MMP-9, and MMP-2 messenger mRNA (mRNA) transcripts using RT-PCR amplification as described above and the following oligonucleotide primers: 5′-CCACGCTGTGGTGTCCCAGACGTG-3′ and 5′-GAACTCGTGCGCTGCCACCAGGAA-3′ for MMP-2; 5′-TTGAGTCCGGCAGAC AATCCTTGC-3′ and 5′-CCTTATCCACGCGAATGACGCTCT-3′ for MMP-9; and 5′-TCGATGTGGAGTGCCCGATGTACA-3′ and 5′-ACGTATGTCATCAGCAGAGAGGCG-3′ for MMP-12.
Lung histopathological studies
Op/Op and control mice were killed at various ages ranging from 20 to 210 days, and lung histologic features were evaluated by light microscopy as described previously.35 Separate slides were stained with Masson trichrome or orcein stain and examined to assess the content and pattern of collagen or elastin deposition, respectively.33 The degree of pulmonary airway enlargement was quantified by morphometric analysis as described previously.33
Statistical analysis
Numeric data are presented as means ± standard error of the mean. Statistical comparisons were made using Student ttests. Statistical calculations were done with Sigma Plot software (version 4.0; SPSS, Chicago, IL).
Results
AMs are deficient in young but not adult Op/Op mice
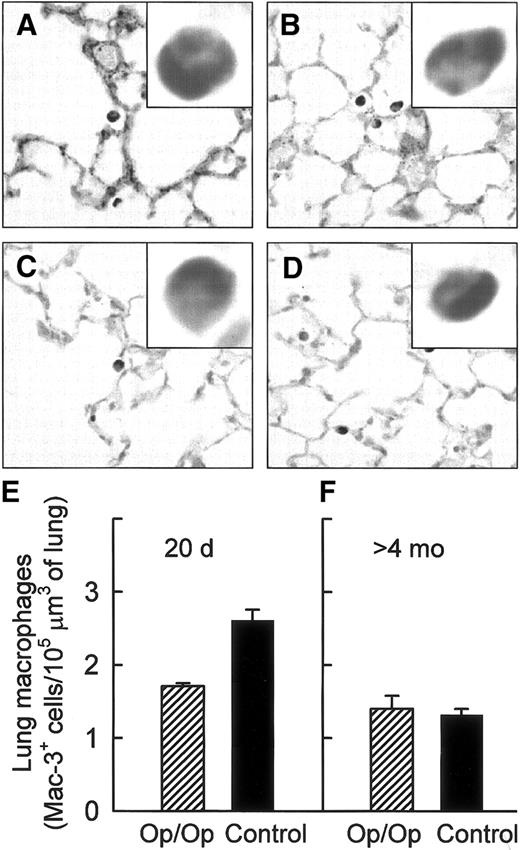
The intensity of Mac-3 surface antigen staining of lung macrophages was homogenous and similar in positive cells from both Op/Op and control mice of all ages (Figure 1A-D). Morphometric quantification of Mac-3–positive (Mac-3+) cells showed that lung macrophages were significantly reduced in young Op/Op mice compared with littermate controls (Figure 1E). In contrast, the numbers of Mac-3+cells were similar in adult Op/Op and control mice (Figure 1F). Although the number of Mac-3+ cells in the lungs of control mice declined significantly with increasing age, no such age-related decrease occurred in Op/Op mice.
Lung macrophages are relatively deficient in young but not adult Op/Op mice.
Lung macrophages were visualized in young and adult Op/Op and control mice by using immunohistochemical staining for Mac-3 antigen (A-D) and quantified by morphometric analysis (E-F). Mac-3 staining of lung macrophages was strong and uniform in Op/Op mice aged 20 days (A) and 120 days (C) and in age-matched littermate controls (B,D). (E) Morphometric analysis showed a relative deficiency of lung macrophages in young Op/Op mice compared with controls (n = 6/group; age, 20 days; P < .001). (F) In contrast, numbers of lung macrophages were similar in adult Op/Op mice and controls (n = 11/group; mean age, 165.2 ± 10.3 versus 161.5 ± 9.1 days; P = 0.68; F). In control mice, Mac-3+cell numbers declined with age (P < .0001), whereas in Op/Op mice, they did not (P > .21) (× 100; insets, × 700).
Lung macrophages are relatively deficient in young but not adult Op/Op mice.
Lung macrophages were visualized in young and adult Op/Op and control mice by using immunohistochemical staining for Mac-3 antigen (A-D) and quantified by morphometric analysis (E-F). Mac-3 staining of lung macrophages was strong and uniform in Op/Op mice aged 20 days (A) and 120 days (C) and in age-matched littermate controls (B,D). (E) Morphometric analysis showed a relative deficiency of lung macrophages in young Op/Op mice compared with controls (n = 6/group; age, 20 days; P < .001). (F) In contrast, numbers of lung macrophages were similar in adult Op/Op mice and controls (n = 11/group; mean age, 165.2 ± 10.3 versus 161.5 ± 9.1 days; P = 0.68; F). In control mice, Mac-3+cell numbers declined with age (P < .0001), whereas in Op/Op mice, they did not (P > .21) (× 100; insets, × 700).
Evaluation of cells recovered by BAL from Op/Op mice and age-matched littermate controls showed that AMs were relatively deficient in young but not adult Op/Op mice (Figure 2). Regression analysis of data from Op/Op mice aged 53 to 158 days showed little change in AM numbers with age (Figure 2A), a finding consistent with immunohistochemical data (Figure 1). Similar analysis of AMs in control mice found a significant reduction in AM numbers in adult compared with young mice (Figure 2B). Extrapolation of these regression equations (with data shown in Figure 2) to 20 days showed an AM deficiency in young Op/Op mice compared with littermate controls (6.06 versus 13.6 × 104 AMs/mouse, respectively), in agreement with previously reported data20 and the Mac-3 staining data (Figure 1).
AMs are relatively deficient in young but not adult Op/Op mice.
The numbers of AMs in BAL fluid recovered from Op/Op mice and littermate controls (aged 53-210 days) were determined from BAL total cell counts and differential cell staining. (A-B) AMnumbers were similar in adult Op/Op and age-matched littermate controls (n = 6/group; mean age, 149 ± 3.9 days [all mice older than 130 days]; P = .95). (A) Linear regression showed thatAM numbers in Op/Op mice did not change significantly with age (regression line and 95% confidence limits shown; R = 0.166; slope not significantly different from zero; P = .5). (B) A similar analysis showed that AM numbers in littermate control mice decreased significantly with age (slope, −515 ± 241 cells/mouse per day; R = 0.45; P = .046).
AMs are relatively deficient in young but not adult Op/Op mice.
The numbers of AMs in BAL fluid recovered from Op/Op mice and littermate controls (aged 53-210 days) were determined from BAL total cell counts and differential cell staining. (A-B) AMnumbers were similar in adult Op/Op and age-matched littermate controls (n = 6/group; mean age, 149 ± 3.9 days [all mice older than 130 days]; P = .95). (A) Linear regression showed thatAM numbers in Op/Op mice did not change significantly with age (regression line and 95% confidence limits shown; R = 0.166; slope not significantly different from zero; P = .5). (B) A similar analysis showed that AM numbers in littermate control mice decreased significantly with age (slope, −515 ± 241 cells/mouse per day; R = 0.45; P = .046).
Lung expression of colony-stimulating factors is altered in Op/Op mice
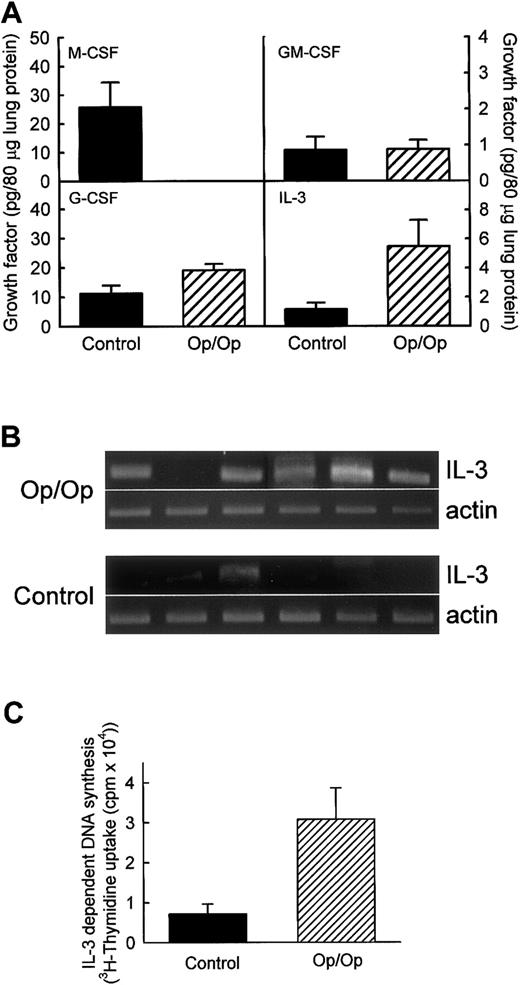
To evaluate possible compensatory expression of an alternative macrophage growth factor in the lungs of Op/Op mice, levels of GM-CSF, G-CSF, IL-3, and as a control, M-CSF were measured in lung homogenates of Op/Op mice and age-matched littermate controls (Figure3A). As expected, M-CSF was not present in the lungs of Op/Op mice but was easily detected in control mice. GM-CSF was detected in both but at similarly low levels in Op/Op mice and controls. G-CSF was detected in the lungs of both Op/Op and control mice, with slightly higher levels in Op/Op mice. IL-3 levels were markedly higher in the lungs of Op/Op mice than in those of controls. To confirm increased IL-3 expression in the lungs in Op/Op mice, RT-PCR was used to evaluate IL-3 mRNA transcript levels in total-lung RNA. IL-3 mRNA transcripts were easily detected in 5 of 6 Op/Op mice but were detected in only 1 of 6 littermate controls (Figure 3B). To evaluate further the possible compensatory role of IL-3 in Op/Op mice, IL-3 bioactivity was also quantified. IL-3 bioactivity was found to be increased significantly in BAL of Op/Op mice compared with homogenates of littermate controls (Figure 3C).
Expression of colony-stimulating factors is altered in lungs of Op/Op mice.
Expression of other CSFs potentially able to compensate for the M-CSF deficiency in Op/Op mice was evaluated in lung homogenates from Op/Op mice and age-matched littermate controls (n = 6/group for each assay; age, 127.1 ± 22.4 days). (A) Evaluation of cytokine protein levels. M-CSF was readily detected in lungs of control but not Op/Op mice. GM-CSF levels were similar in Op/Op and control mice (P = .97). G-CSF levels were slightly increased in Op/Op mice compared with controls (P = .05). IL-3 levels were significantly higher in Op/Op mice (P = .04). (B) Evaluation of IL-3 mRNA levels. Total-lung RNA was purified and evaluated by using IL-3–specific and β-actin–specific oligonucleotide primers. IL-3 mRNA was detected in the lungs of 5 of 6 Op/Op mice but in only 1 of 6 control mice. In contrast, β-actin mRNA levels were similar in all mice. (C) IL-3 bioactivity. Levels of functional IL-3 in BAL of Op/Op and control mice (n = 6/group) were assessed by stimulation of growth of the IL-3–sensitive M-NSF-60 cell line. Cell growth detected by tritium-thymidine incorporation was increased in Op/Op mice compared with controls when M-NFS-60 cells were incubated with BAL from the animals (P < .02).
Expression of colony-stimulating factors is altered in lungs of Op/Op mice.
Expression of other CSFs potentially able to compensate for the M-CSF deficiency in Op/Op mice was evaluated in lung homogenates from Op/Op mice and age-matched littermate controls (n = 6/group for each assay; age, 127.1 ± 22.4 days). (A) Evaluation of cytokine protein levels. M-CSF was readily detected in lungs of control but not Op/Op mice. GM-CSF levels were similar in Op/Op and control mice (P = .97). G-CSF levels were slightly increased in Op/Op mice compared with controls (P = .05). IL-3 levels were significantly higher in Op/Op mice (P = .04). (B) Evaluation of IL-3 mRNA levels. Total-lung RNA was purified and evaluated by using IL-3–specific and β-actin–specific oligonucleotide primers. IL-3 mRNA was detected in the lungs of 5 of 6 Op/Op mice but in only 1 of 6 control mice. In contrast, β-actin mRNA levels were similar in all mice. (C) IL-3 bioactivity. Levels of functional IL-3 in BAL of Op/Op and control mice (n = 6/group) were assessed by stimulation of growth of the IL-3–sensitive M-NSF-60 cell line. Cell growth detected by tritium-thymidine incorporation was increased in Op/Op mice compared with controls when M-NFS-60 cells were incubated with BAL from the animals (P < .02).
AM morphologic features
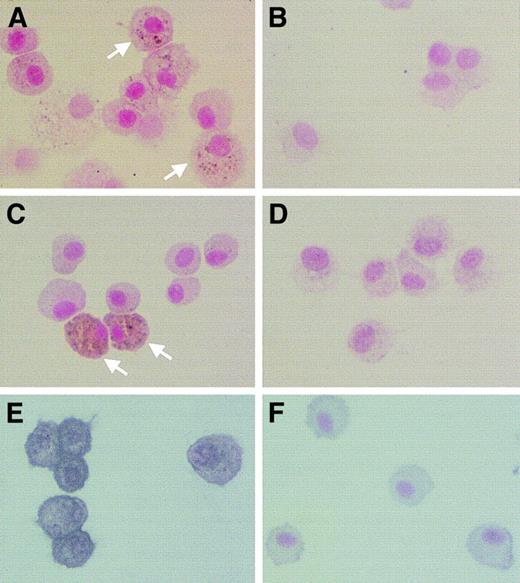
Light-microscopic evaluation of BAL cells stained with Diff-Quick revealed no differences in the proportion of macrophages in young or adult Op/Op and control mice (> 95% in all cases; Figure 4A-D). However, AMs of young Op/Op mice were significantly smaller, appeared less mature, and had less cytoplasm (Figure 4A) than those of age-matched littermate controls (Figure 4B). Morphometric analysis confirmed this finding, showing AM cell diameters of 14.2 ± 0.31 μm in young Op/Op mice and 16.0 ± 0.27 μm in control mice (Figure 4E). There were no differences in AM cell diameters in adult Op/Op and adult control mice (Figure 4F), although the mean diameter in both was larger than that in young mice. Standard transmission electron microscopy revealed no ultrastructural differences in AMs of adult Op/Op and control mice (data not shown).
Morphologic abnormalities in AMs of young Op/Op mice normalize with age.
(A-D) AMs from Op/Op mice or littermate controls were obtained by BAL, formed into sediments by cytocentrifugation, stained with Diff-Quick, and evaluated by light microscopy. (E-F) AM cell diameter was measured using Metamorph software. BAL cells consisted of more than 95% AMs in all mice, and they uniformly and strongly stained for Mac-3 antigen in samples from Op/Op mice (aged 20 ± 0 or 122 ± 14 days [A,C]) and littermate controls (aged 20 ± 0 or 120 ± 16 days [B,D]). In young mice, AM cell diameter was smaller in Op/Op mice than in littermate controls (105 106 cells counted in each group; P < .0001). In older mice,AM cell diameter in Op/Op and control mice was similar (107 118 cells counted in each group; P > .22) (× 100; insets, × 700).
Morphologic abnormalities in AMs of young Op/Op mice normalize with age.
(A-D) AMs from Op/Op mice or littermate controls were obtained by BAL, formed into sediments by cytocentrifugation, stained with Diff-Quick, and evaluated by light microscopy. (E-F) AM cell diameter was measured using Metamorph software. BAL cells consisted of more than 95% AMs in all mice, and they uniformly and strongly stained for Mac-3 antigen in samples from Op/Op mice (aged 20 ± 0 or 122 ± 14 days [A,C]) and littermate controls (aged 20 ± 0 or 120 ± 16 days [B,D]). In young mice, AM cell diameter was smaller in Op/Op mice than in littermate controls (105 106 cells counted in each group; P < .0001). In older mice,AM cell diameter in Op/Op and control mice was similar (107 118 cells counted in each group; P > .22) (× 100; insets, × 700).
AMs in Op/Op mice spontaneously express MMPs in vivo
We hypothesized that MMP expression may be increased in AMs of Op/Op mice because in vitro studies showed that expression of MMP-2, MMP-9, and MMP-12 genes are regulated by the relative levels of GM-CSF and M-CSF29 and because Op/Op mice express GM-CSF normally in the lung but do not express detectable levels of M-CSF (Figure 3). AMs of Op/Op but not control mice showed positive cytoplasmic staining for several MMPs (Figure5). Some AMs in Op/Op mice stained strongly for MMP-2 (gelatinase A), MMP-9 (gelatinase B), or MMP-12 (macrophage metalloelastase). In contrast, no AMs from control mice stained strongly for any MMP, only a few cells showed any staining at all, and that staining was minimal.
AMs in Op/Op mice spontaneously express MMPs.
AMs from Op/Op mice showed positive staining for MMP-2 (A), MMP-9 (C) and MMP-12 (E). Some of the cells showed particularly dark intracytoplasmic staining for MMP-2 and MMP-9 (white arrows). In contrast, few cells from control mice showed positive staining for these enzymes (B,D,F) (× 450).
AMs in Op/Op mice spontaneously express MMPs.
AMs from Op/Op mice showed positive staining for MMP-2 (A), MMP-9 (C) and MMP-12 (E). Some of the cells showed particularly dark intracytoplasmic staining for MMP-2 and MMP-9 (white arrows). In contrast, few cells from control mice showed positive staining for these enzymes (B,D,F) (× 450).
To assess functional MMP activity in the lung, BAL fluid from Op/Op and control mice was evaluated by using gelatin zymography (Figure6A). A strong band of proteolytic activity at 72 kd, corresponding in size to gelatinase A, was observed in Op/Op mice and was especially prominent in some animals. Although a band of similar size was detected in BAL fluid of control mice, the activity was reduced markedly. A band of proteolytic activity at 92 kd, corresponding in size to gelatinase B, was present in BAL fluid of all 4 Op/Op mice but no BAL fluid of controls. A third proteolytic activity at 22 kd, corresponding in size to MMP-12, was observed in BAL fluid of 3 of 4 Op/Op mice but was not detected in any BAL sample from controls. Casein zymograms confirmed the presence of the 22-kd proteolytic activity in AMs of Op/Op mice at levels clearly higher than in AMs of controls (Figure 6B). All these proteolytic activities were inhibited by EDTA, as expected for MMP enzyme activities (data not shown).
MMP enzyme activities are elevated in AMs and BAL fluid from Op/Op mice.
BAL fluid and cells were obtained from Op/Op and control mice at 2 months of age and evaluated by zymography. (A) Gelatin zymogram done by using BAL fluid from Op/Op (lanes 1-4) or control (lanes 5-8) mice. Proteolytic activity was elevated in BAL fluid of Op/Op mice at positions corresponding to molecular weights of 92 kd, 72 kd, and 22 kd, which correspond in size to MMP-9, MMP-2, and MMP-12, respectively. All proteolytic activity was inhibited completely by EDTA treatment of gels (not shown). (B) Casein zymogram done by using BAL cell lysates pooled from 4 Op/Op mice (lane 9) or 4 control mice (lane 10) mice. As in the experiment described above, proteolytic activity was inhibited completely by EDTA treatment of gels (not shown). (C) Gelatin zymogram done by using BAL fluid from control mice that received IL-3 or PBS 48 hours before collection. The analyses were done as described above for panel A. Increased proteolytic activity at 92 kd and 72 kd occurred in mice exposed to IL-3 but not in those exposed to PBS. (D) IL-3 stimulation of MMP mRNA transcript levels in murine RAW264.7 macrophages in vitro. Cells were cultured in the absence (lane 15) or presence of IL-3 (100 ng/mL; lane 16) or in the presence of IL-3 and cycloheximide (lane 17) for 3 hours, after which total RNA was isolated and evaluated by RT-PCR using MMP-sequence–specific primers.
MMP enzyme activities are elevated in AMs and BAL fluid from Op/Op mice.
BAL fluid and cells were obtained from Op/Op and control mice at 2 months of age and evaluated by zymography. (A) Gelatin zymogram done by using BAL fluid from Op/Op (lanes 1-4) or control (lanes 5-8) mice. Proteolytic activity was elevated in BAL fluid of Op/Op mice at positions corresponding to molecular weights of 92 kd, 72 kd, and 22 kd, which correspond in size to MMP-9, MMP-2, and MMP-12, respectively. All proteolytic activity was inhibited completely by EDTA treatment of gels (not shown). (B) Casein zymogram done by using BAL cell lysates pooled from 4 Op/Op mice (lane 9) or 4 control mice (lane 10) mice. As in the experiment described above, proteolytic activity was inhibited completely by EDTA treatment of gels (not shown). (C) Gelatin zymogram done by using BAL fluid from control mice that received IL-3 or PBS 48 hours before collection. The analyses were done as described above for panel A. Increased proteolytic activity at 92 kd and 72 kd occurred in mice exposed to IL-3 but not in those exposed to PBS. (D) IL-3 stimulation of MMP mRNA transcript levels in murine RAW264.7 macrophages in vitro. Cells were cultured in the absence (lane 15) or presence of IL-3 (100 ng/mL; lane 16) or in the presence of IL-3 and cycloheximide (lane 17) for 3 hours, after which total RNA was isolated and evaluated by RT-PCR using MMP-sequence–specific primers.
To determine the possible role of increased IL-3 levels in stimulation of MMP expression by AMs in vivo in Op/Op mice, IL-3 or PBS (control) was administered into the lungs of wild-type mice and MMP expression was evaluated 48 hours later. Zymography showed easily detected bands at 92 kd and 72 kd in mice exposed to IL-3 but not in those exposed to PBS (Figure 6C). Exposure of murine RAW264.7 macrophages to similar concentrations of IL-3 in vitro stimulated mRNA transcript levels for MMP-12 and, to a lesser extent, MMP-2 and MMP-9 (Figure 6D). Cycloheximide only partly reduced the IL-3–stimulated increase in MMP-12 mRNA transcripts and had minimal effect on MMP-2 and MMP-9 mRNA. Together, these results suggest that the stimulation of MMP expression is a direct effect of IL-3.
Op/Op mice spontaneously develop pulmonary emphysema
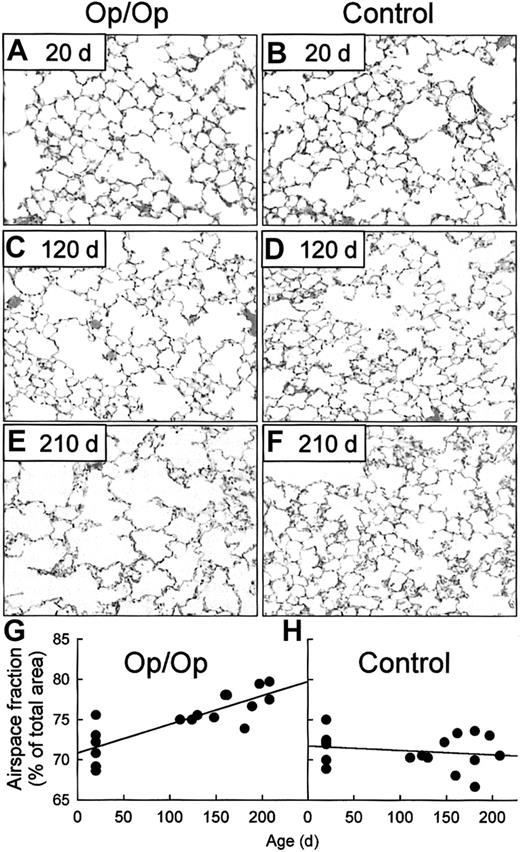
To evaluate the biochemical and functional consequences of spontaneous MMP release in the lungs of Op/Op mice, lung tissues were evaluated by using routine histologic studies, special stains to assess abnormalities in elastin and collagen deposition, and morphometric analysis to quantify derangements of sensitive lung-tissue structures. No histopathological abnormalities were detected in Op/Op mice or controls that were 20 days of age (Figure 7A-B). However, at 120 days of age, Op/Op but not control mice had airspace enlargement typical of panlobular emphysema (Figure 7C-D). Emphysema in Op/Op mice had worsened by 210 days while the lungs of age-matched controls remained normal (Figure 7E-F). Morphometric analysis showed similar values for the fractional area of airspace in young Op/Op and control mice (Figure 7G-H). However, linear regression analysis showed that the fractional area of airspace increased progressively and significantly with age in Op/Op mice, at a rate of 3.6 ± 0.6 × 10−2 %/day (Figure 7G). In contrast, no such change was observed in age-matched control mice and the slope of the regression line was not significantly different from zero (Figure 7H). Orcein histochemical staining showed that elastin deposition was normal in the lungs of young (20-day-old) Op/Op and control mice but was disordered in the lungs of adult Op/Op mice (aged 120 and 210 days; data not shown). Evaluation of deposition of collagen in the lungs by using Masson trichrome staining revealed no alterations in the pattern of collagen fibrils in the lungs of Op/Op or control mice at any age (data not shown). Histologic evaluation of the lungs of Op/Op mice of all ages did not detect an inflammatory cell infiltrate (Figure 7A-F), thereby suggesting that nonspecific inflammation was not an etiologic factor in the development of emphysema.
Op/Op mice develop progressive, age-dependent pulmonary emphysema.
In the histologic evaluation, lungs of Op/Op and control mice of various ages were obtained and stained with hematoxylin and eosin. Representative photomicrographs of lung parenchyma are shown for Op/Op (A,C,E) and control mice (B,D,F) aged 20 days (A-B), 120 days (C-D). and 210 days (E-F). Progressive emphysema with enlarging air spaces occurred in lungs of Op/Op mice but not control mice (× 40). In the morphometric evaluation, digital images of lung tissues from Op/Op and control mice were captured during microscopy (above) and assessed by quantitative morphometry to determine the fractional area of airspace (airspace fraction). In young mice (n = 6/group; age, 20 ± 0 days), the corresponding values for airspace fraction were similar in Op/Op and control mice (P = .89; G,H). In marked contrast, in adult mice (n = 11/group; mean age, 165.3 ± 10.3 days in Op/Op mice and 161.5 ± 9.1 days in controls), the airspace fraction in Op/Op mice was increased significantly compared with that in littermate controls (P < .001). (G) The airspace fraction in adult Op/Op mice was also increased compared with that in young Op/Op mice (P < .0001). (H) In control mice, the airspace fraction did not change with age (P = .38). Linear regression defined the progressive increase in the airspace fraction as 3.6 ± 0.6 × 10−2 %/day in Op/Op mice (R = 0.82; slope significantly different from zero;P = .0001), whereas there was no significant change in controls (−5.4 ± 7.3 × 10−3 %/day; R = 0.18; slope not different from zero; P = .48).
Op/Op mice develop progressive, age-dependent pulmonary emphysema.
In the histologic evaluation, lungs of Op/Op and control mice of various ages were obtained and stained with hematoxylin and eosin. Representative photomicrographs of lung parenchyma are shown for Op/Op (A,C,E) and control mice (B,D,F) aged 20 days (A-B), 120 days (C-D). and 210 days (E-F). Progressive emphysema with enlarging air spaces occurred in lungs of Op/Op mice but not control mice (× 40). In the morphometric evaluation, digital images of lung tissues from Op/Op and control mice were captured during microscopy (above) and assessed by quantitative morphometry to determine the fractional area of airspace (airspace fraction). In young mice (n = 6/group; age, 20 ± 0 days), the corresponding values for airspace fraction were similar in Op/Op and control mice (P = .89; G,H). In marked contrast, in adult mice (n = 11/group; mean age, 165.3 ± 10.3 days in Op/Op mice and 161.5 ± 9.1 days in controls), the airspace fraction in Op/Op mice was increased significantly compared with that in littermate controls (P < .001). (G) The airspace fraction in adult Op/Op mice was also increased compared with that in young Op/Op mice (P < .0001). (H) In control mice, the airspace fraction did not change with age (P = .38). Linear regression defined the progressive increase in the airspace fraction as 3.6 ± 0.6 × 10−2 %/day in Op/Op mice (R = 0.82; slope significantly different from zero;P = .0001), whereas there was no significant change in controls (−5.4 ± 7.3 × 10−3 %/day; R = 0.18; slope not different from zero; P = .48).
To explore further the possibility that occult inflammation was present, lung homogenates of Op/Op and control mice were evaluated by ELISA for proinflammatory cytokines TNF-α and IL-6. TNF-α levels were similar in Op/Op mice and controls (2.66 ± 0.87 versus 2.68 ± 1.20 pg/mL of lung homogenate; P = .99), as were levels of IL-6 (17.10 ± 4.41 versus 18.05 ± 6.16 pg/mL of lung homogenate; P = .90) Thus, nonspecific inflammation was not the cause of the emphysema. Because SP-D deficiency was previously identified as a cause of pulmonary emphysema in mice with ablation of the SP-D gene,33 we also measured SP-D levels in Op/Op and control mice. The concentration of SP-D in BAL fluid was similar in Op/Op and control mice (458.1 ± 63.5 versus 438.3 ± 48.6 ng/mL; P = .96), thus excluding altered SP-D expression as a cause of emphysema in the Op/Op mice.
Discussion
In this study, we sought information on the in vivo role of M-CSF in constitutive AM accumulation and function in the lungs by using the M-CSF–deficient Op/Op mouse model. Using several approaches, we found that AMs were relatively deficient in young Op/Op mice but normal in adult Op/Op mice. The small size and ultrastructural abnormalities previously described in AMs of young Op/Op mice39 were not present in AMs of adult Op/Op mice. Although GM-CSF levels in Op/Op and control mice were not different, IL-3 expression was greater in the lungs of Op/Op mice than in those of controls as determined by quantification of IL-3 cytokine levels, mRNA transcripts, and bioactivity. MMP-2, MMP-9, and MMP-12 levels were abnormally elevated in AMs and BAL of Op/Op mice and were associated with spontaneous development of pulmonary emphysema. Taken together, these data are consistent with the idea that IL-3 may compensate for the M-CSF deficiency in the lungs of Op/Op mice, resulting in preservation of AM numbers, but that this compensatory role may be associated with stimulation of MMP expression that contributes to structural damage in the lungs.
A major focus of this study was to determine the role of endogenous M-CSF expression in regulation of constitutive accumulation of AMs in the lung. The observation of a relative deficiency of AMs in Op/Op mice (∼ 65% of levels in littermate controls) aged 20 days is supported by studies showing that AM numbers are reduced to about 30% of control values at 14 days of age20 and that lung F4/80-positive cells in Op/Op mice are reduced to 8% of control values at 7 days of age.40 The observation that AM numbers are normal in adult Op/Op mice is novel but is consistent with previous findings of a spontaneous, age-dependent correction in hematologic abnormalities, numbers of macrophages and osteoclasts in bone marrow, and osteopetrosis of Op/Op mice.21 Thus, our results and previously reported data show that AM accumulation in Op/Op mice is most severely affected in young mice and that this abnormality is not present in adult mice. These data can be interpreted in 2 different ways. First, M-CSF may have a relatively greater importance in the perinatal and neonatal period than in adulthood. Interestingly, the period of relative deficiency coincides with postnatal alveolarization of the lung in mice.41 This suggests a possible role for M-CSF in stimulation of lung macrophage functions during alveolarization, for example, clearance of cells and macromolecular debris left over from tissue remodeling. Alternatively, M-CSF may be important for normal AM function throughout life. In either case, factors in addition to M-CSF are responsible for constitutive accumulation of AMs in the lungs, suggesting a functional overlap of M-CSF with one or more other factors that can compensate for the absence of M-CSF in Op/ Op mice.
Several lines of evidence support the idea that increased expression of IL-3 in the lungs may compensate for the M-CSF deficiency and stimulateAM accumulation in Op/Op mice. First, levels of IL-3 protein, mRNA, and bioactivity were significantly higher in the lungs of Op/Op mice than in those of littermate controls. Second, IL-3 is capable of stimulating macrophage accumulation in vivo.22Third, administration of exogenous IL-3 to Op/Op mice corrects some of the macrophage abnormalities in those mice.22 Although local expression of GM-CSF can increase AM accumulation in the lung,14 GM-CSF levels were not higher in the lungs of Op/Op mice than in those of controls. Furthermore, GM-CSF expression is not responsible for correction of the hematologic abnormalities in Op/Op mice because M-CSF–deficient Op/Op mice that are also deficient in GM-CSF as a result of targeted gene ablation still undergo age-dependent correction of hematologic abnormalities.24 Although G-CSF was slightly elevated in Op/Op mice, neutrophil accumulation was not observed, suggesting that the differences were not functionally important.
Significant, spontaneous expression of MMPs by AMs in Op/Op mice was an important finding of this study and was supported by results obtained by using several independent experimental approaches. These results were (1) increased MMP levels in AMs on immunohistochemical staining, (2) increased MMP activities in BAL fluid on zymography, (3) derangement of elastin fiber deposition in lung tissue, (4) development of emphysema confirmed by histopathological evaluation and morphometric analysis showing progressive loss of lung parenchyma and increase in the fractional area of airspace with age, and (5) IL-3–stimulated increases in MMP levels in the lungs of normal mice and higher IL-3 levels in Op/Op mice than in controls. Development of emphysema in the Op/Op mice did not appear to be due to nonspecific inflammation because neither cellular nor molecular evidence of parenchymal inflammation was observed. Although starvation is associated with emphysema in rats,42 there was no decrease in elastin or collagen in the Op/Op mice in our study, as would be expected with this cause of emphysema.43 Moreover, although the Op/Op mice were smaller than controls, they gained weight as they grew older.
The idea that increased expression of IL-3 in the lungs of Op/Op mice might constitute the molecular explanation for stimulation of MMP release by AMs is supported by the finding that IL-3 stimulates MMP-2 and MMP-9 expression in other hematopoietic cells (eg, CD34+ blood mononuclear cells).44Alternatively, it is possible that an imbalance of M-CSF and GM-CSF levels to which AMs are exposed in the lungs of Op/Op mice is predominantly responsible for increased MMP expression, since M-CSF is absent and GM-CSF levels are normal in the lungs of such mice (Figure3). This hypothesis is consistent with data showing that GM-CSF stimulates and M-CSF suppresses MMP gene expression in peritoneal macrophages.29 Finally, it is possible that both these molecular mechanisms are operating in the lungs of Op/Op mice. Regardless of whether one or both are responsible, the development of emphysema shows that MMP release is functionally important, since the lung is very sensitive to protease-antiprotease balance and expression of MMP-12 in macrophages is a requirement for the development of smoking-induced emphysema in mice.30
We thank Susan Wert for help with the morphometric analysis and William Hull for help with the SP-D ELISA analysis.
Supported by the Children's Hospital Research Foundation, Cincinnati, OH.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bruce C. Trapnell, Children's Hospital Medical Center, Division of Pulmonary Biology, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: bruce.trapnell@chmcc.org.

![Fig. 2. AMs are relatively deficient in young but not adult Op/Op mice. / The numbers of AMs in BAL fluid recovered from Op/Op mice and littermate controls (aged 53-210 days) were determined from BAL total cell counts and differential cell staining. (A-B) AMnumbers were similar in adult Op/Op and age-matched littermate controls (n = 6/group; mean age, 149 ± 3.9 days [all mice older than 130 days]; P = .95). (A) Linear regression showed thatAM numbers in Op/Op mice did not change significantly with age (regression line and 95% confidence limits shown; R = 0.166; slope not significantly different from zero; P = .5). (B) A similar analysis showed that AM numbers in littermate control mice decreased significantly with age (slope, −515 ± 241 cells/mouse per day; R = 0.45; P = .046).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/9/10.1182_blood.v98.9.2845/4/m_h82111718002.jpeg?Expires=1767895343&Signature=q5UMej5JtvaCFOBGbWPNInow6abRqKfR1Qw4~sNrKiDDRUM7dSR~-NNvF1iq-uHkSB7Of0aDstkckoz1gq~ZwgqwM6K38jX0Oee3FlLI9BWBBDHxvwFWtZ9QKvIoofic3GNYvyZFadQTwAjMnkBCRFG-aGd1pMlYwTTeAzalZBLdLM1oaHAwP1C9W8vyX5Mr9plLWcDbLaMrkB~hIGyFvCwH0ILkwMABWzLTuajZT8CNSn9megQbSmtFhtZx2aogslgbhmQTb5CFB~YYKuYtmOi3Azh-7FioT07p8cjrXkiaRdcew8PuxTVrh3P2eIxs4fFalXBgiO1JpsPGbB9ecg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Morphologic abnormalities in AMs of young Op/Op mice normalize with age. / (A-D) AMs from Op/Op mice or littermate controls were obtained by BAL, formed into sediments by cytocentrifugation, stained with Diff-Quick, and evaluated by light microscopy. (E-F) AM cell diameter was measured using Metamorph software. BAL cells consisted of more than 95% AMs in all mice, and they uniformly and strongly stained for Mac-3 antigen in samples from Op/Op mice (aged 20 ± 0 or 122 ± 14 days [A,C]) and littermate controls (aged 20 ± 0 or 120 ± 16 days [B,D]). In young mice, AM cell diameter was smaller in Op/Op mice than in littermate controls (105 106 cells counted in each group; P < .0001). In older mice,AM cell diameter in Op/Op and control mice was similar (107 118 cells counted in each group; P > .22) (× 100; insets, × 700).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/9/10.1182_blood.v98.9.2845/4/m_h82111718004.jpeg?Expires=1767895343&Signature=csrzvaRyoeXzx9IChpuWZX3GertBFbyNiAStoyMZZndCxTO1VmYSYbixMfIbwArfcF3qHlQ0WLO6JdGXCJaJGJ~EFVJToSWmwMidGlgApmHQ5doVEC4FH1NSxB0AhOSjiZ-LHmxJMTXA8YUKmsxOuS2VbcLIevSD9jUpkgmUkmYlRpkkAfwkb0JortbVWEDNk~D4Btrs6x4CQTyifwX1~POwvb2RspfXD69v0xqdPb~qRkdtZcLtiLRU3MqkVLeN4NM3N6Gq~43rod2~pYMuneEe4E4hDaTXK5yTUK2USo1n00HvRT8BpaYQjOfDmvfx2hFHeBMRWO~I-c1KKCjT0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal