Abstract
Despite thymic deletion of cells with specificity for self-antigens, autoreactive T cells are readily detectable in the normal T-cell repertoire. In recent years, a population of CD4+ T cells that constitutively express the interleukin-2 receptor-α chain, CD25, has been shown to play a pivotal role in the maintenance of self-tolerance in rodent models. This study investigated whether such a regulatory population exists in humans. A population of CD4+CD25+ T cells, taken from the peripheral blood of healthy individuals and phenotypically distinct from recently activated CD4+ T cells, was characterized. These cells were hyporesponsive to conventional T-cell stimuli and capable of suppressing the responses of CD4+CD25− T cells in vitro. Addition of exogenous interleukin-2 abrogated the hyporesponsiveness and suppressive effects of CD4+CD25+ cells. Suppression required cell-to-cell contact but did not appear to be via the inhibition of antigen-presenting cells. In addition, there were marked changes in the expression of Notch pathway molecules and their downstream signaling products at the transcriptional level, specifically in CD4+CD25+ cells, suggesting that this family of molecules plays a role in the regulatory function of CD4+CD25+ cells. Cells with similar phenotype and function were detected in umbilical venous blood from healthy newborn infants. These results suggest that CD4+CD25+ cells represent a population of regulatory T cells that arise during fetal life. Comparison with rodent CD4+CD25+ cells suggests that this population may play a key role in the prevention of autoimmune diseases in humans.
Introduction
Immune tolerance to self is essential to prevent autoimmunity. Clonal elimination of autoreactive T cells in the thymus is effective but incomplete and, consequently, peripheral mechanisms of tolerance such as anergy, immune deviation, deletion, and regulation/suppression are important.
A role for immunoregulatory T cells in the maintenance of self-tolerance was first suggested in the 1970s, but a succession of in vitro artifacts cast doubts upon the experimental data. However, more recent evidence from rodent models has implicated regulatory T cells in transplantation tolerance1-4 and the prevention of autoimmune disease.5-11 The phenotype and mechanisms of action of such cells are only partially defined. Two major cell types have been described: cytokine-secreting Th2, Th3, and Tr1 cells, which predominantly release interleukin-4 (IL-4), IL-10, and transforming growth factor β (TGF-β)12-16; and anergic T cells, which appear to require cell-to-cell contact to mediate their suppressive function.17-20
Spontaneously occurring populations of T cells that regulate autoimmune inflammation have been described in animals. One such population is the CD4+CD25+ subset found in normal mice.8,10 These cells resemble anergic cells in vitro and can suppress the responses of CD4+CD25− cells in coculture in a cell contact–dependent manner.18 Neonatally thymectomized mice, which are deficient in this population of T cells, develop multiorgan autoimmune disease. Adoptive transfer of CD4+CD25+ cells from normal mice can protect these mice from autoimmune diseases.9,10 16
The existence of immunoregulatory cells in human self-tolerance, particularly in the pathogenesis of autoimmune diseases, is poorly understood. This study examined the possibility that a spontaneously arising population of T cells with regulatory properties existed in man. Such a population was found in both adults and newborn infants, and the phenotype, function, and mechanism of action of these cells were explored.
Materials and methods
Subjects
Peripheral blood samples were obtained from healthy volunteers or from routine venesection from patients with primary polycythemia with no known white cell disorder. Following a normal pregnancy, umbilical cord blood was obtained from the placenta of healthy full-term infants, who were delivered by an elective cesarean section. These investigations were approved by the Hammersmith Hospital Research Ethics Committee.
Culture media, reagents, and antibodies
In all in vitro assays, RPMI 1640 medium supplemented with L-glutamine (2 mM/L), penicillin/streptomycin (100 IU/mL and 100 μg/mL, respectively), amphotericin (500 ng/mL), all from Gibco, Paisley, United Kingdom; and gentamicin (2 μg/mL, Sigma, Poole, United Kingdom), referred to as supplemented RPMI, with 10% (final concentration) added human AB serum (Harlan Sera-Lab, Loughborough, United Kingdom), was used. All cells were incubated at 37°C with 5% CO2 and 95% air. Phytohemagglutinin (PHA) was purchased from Sigma. Anti-CD3 monoclonal antibodies (UCHT-1 and OKT3) were purified from supernatants of hybridomas. Biotinylated anti-CD25 (7G7) was purchased from Ancell (Nottingham, United Kingdom). Phycoerythrin (PE)– and fluorescein isothiocyanate (FITC)–conjugated anti-CD25 (3G10, Caltag, Silverstone, United Kingdom); Quantum Red (QR)–conjugated anti-CD4 (Q4120, Sigma); FITC-conjugated anti-69 (CH/4), FITC-conjugated anti-CD45RO (UCHL1), from Serotec, Oxford, United Kingdom; FITC-conjugated anti-CD62L (Dreg56), FITC-conjugated anti-CD40L (TRAP1), PE-conjugated anti–CTLA-4 (BN13), FITC-conjugated anti-CD40 (catalog no. 33074X), from Pharmingen (San Diego, CA); FITC-conjugated anti-CD80 (DAL-1, Caltag); FITC-conjugated anti-CD86 (BU63, Caltag); FITC-conjugated anti-CD11c (KB90, Dako, Ely, United Kingdom); and FITC-conjugated anti–HLA-DR (catalog no. 347363, Becton Dickinson, Oxford, United Kingdom) were used in flow cytometry. Flow cytometry was acquired and analyzed using Cell Quest software (Becton Dickinson). Anti–human TGF-β1 (9016.2, R & D Systems, Abingdon, United Kingdom) and anti–human IL-10 (9D7) were used in proliferation assays as neutralizing antibodies and enzyme-linked immunosorbent assay (ELISA) as capture antibodies.
CTLL-2 cells
CTLL-2 cells are a murine cell line that responds to murine IL-2 and IL-4 but only to human IL-2. Cells were maintained in culture in supplemented RPMI medium with 10% fetal calf serum (Biowhittaker, Wokingham, United Kingdom) and human recombinant (rh)–IL-2 (10 U/mL, Boehringer Mannheim, Mannheim, Germany). They were subcultured every 2 to 3 days. Cells were rested in medium without IL-2 overnight prior to use in assays.
CD4+CD25+ and CD4+CD25− cells
Peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood samples by density gradient centrifugation over Lymphoprep (Nycomed, Birmingham, United Kingdom). For isolation of CD4+ T cells, PBMCs were incubated in medium supplemented with 2% fetal calf serum at 37°C on tissue culture dishes twice for 45 minutes each to remove adherent cells. Nonadherent cells were collected and washed. Non-CD4+ cells were depleted by incubation with a cocktail of antibodies (anti-CD8 [OKT8], anti-CD19 [BU12], anti–class II [L243], collected from supernatants of hybridomas, and anti-CD56) followed by magnetic bead (Dynal, Wirral, United Kingdom) separation. The purified CD4+ cells were incubated with biotinylated anti-CD25 antibodies, washed, and then incubated with streptavidin microbeads (MS+ Column, Miltenyi Biotec, Gladbach, Germany). CD4+CD25−cells were obtained by negative selection (flow through), and CD4+CD25+ cells were positively selected (eluted column-retained cells). In some experiments, CD4+cells were purified by flow cytometry using biotinylated anti-CD25 (7G7) antibodies followed by streptavidin-PE and FITC-conjugated anti-CD4 monoclonal antibodies (catalog No. 340133, Becton Dickinson) on a FACStar cell sorter (Becton Dickinson). The purity of the cell population was measured by flow cytometry and was between 90% to 95% for CD4+CD25− cells and between 80% to 90% for CD4+CD25+ cells. Cord blood mononuclear cells were isolated by density gradient centrifugation over Lymphoprep. Red blood cells and reticulocytes were removed by incubating cord blood mononuclear cells with mouse anti–human glycophorin A followed by incubation with goat anti–mouse immunoglobulin G magnetic particles (BioMag, Polysciences, Warrington, United Kingdom). Cord blood CD4+ T cells and CD4+CD25− cells were isolated as described above.
Accessory cells
Irradiated (30 Gy) T-cell–depleted PBMCs were used as accessory cells (ACs). PBMCs were incubated with anti-CD3 antibodies (OKT3) followed by magnetic bead separation (Dynal) to remove T cells.
Dendritic cells
Human monocyte-derived dendritic cells were generated by culturing adherent cells from PBMCs in the presence of GM-CSF and IL-4. After 7 days, dendritic cells were harvested and were used in coculture experiments with CD4+CD25+ or CD4+CD25− cells. After coculture, dendritic cells were isolated by removing CD4+ cells using magnetic beads (Dynal).
Mixed lymphocyte reactions
About 1 × 104 responder cells were cocultured with 5 × 104 stimulator cells per well in 96-well plates (final volume 200 μL) in the presence or absence of 30 units rh–IL-2, for 5 days. 3H-thymidine was added in the last 12 hours of the culture. The cells were harvested at the end of the culture, and 3H-thymidine incorporation was assessed by liquid scintillation spectrometry. All experiments were performed in triplicate.
Proliferation assays
About 1 × 104 to 2 × 104 responder cells (CD4+CD25− or CD4+CD25− cells) were cocultured with 1 × 104 (or specified otherwise) ACs per well in 96-well plates (final volume 200 μL/well). PHA, at a final concentration of 2 μg/mL, was added with or without 10 U/mL rh–IL-2. After 60 hours of culture, 3H-thymidine was added and the cells were cultured for a further 12 hours before 3H-thymidine incorporation was assessed.
Transwell assays
Transwells of pore size 0.4 μm were used (Costar, High Wycombe, United Kingdom); 1.5 × 105CD4+CD25− cells per well were cultured in 24-well plates in the presence of ACs (1.5 × 105cells/well) with 2 μg/mL PHA (referred to as lower wells). Equal numbers of CD4+CD25+ or CD4+CD25− cells were added into either the Transwells (referred to as upper wells) or the lower wells directly. The Transwells were inserted onto the 24-well plate. ACs (1.5 × 105 cells/well) were present in all upper wells. After 60 hours of culture, the cells in the lower and upper wells were harvested separately and transferred to 96-well plates.3H-thymidine was added, and the cells were cultured for a further 12 hours before the incorporation of 3H-thymidine was measured.
Limiting dilution analyses
Serial dilutions of responder cells in 24 replicates were cocultured with 5 × 104 irradiated stimulator cells per well in 96-well plates for 5 days. The exact number of responder cells depended on the number of responder cells obtained from each individual, with top dilutions ranging from 2 × 104 to 8 × 104 cells per well. Wells were scored positive if the counts were above 3 SD of the average count of the control (wells containing only irradiated stimulator cells). The frequency, confidence interval, and χ2 value for each assay were calculated by the maximum likelihood method using GLIM software (NAG, Oxford, United Kingdom). For all data, a probability estimate of the data conforming to single-hit kinetics was calculated.
Cytokine ELISA
Supernatants were taken from cultures at 72 hours. Antibodies from clones 9D7, 9016.2, and 43-11 (Immunokontact, Witney, United Kingdom) were used as capture antibodies for detection of IL-10, TGF-β1, and interferon γ (IFN-γ), respectively. The corresponding detection antibodies used in the assays are 12G8 (Pharmingen), anti–TGF-β chicken immunoglobulin (R & D Systems), and 45-15 (Immunokontact).
Real-time polymerase chain reaction quantitation of mRNA
CD4+CD25+ and CD4+CD25− cells were purified as mentioned above. Cells were solubilized in a guanidinium buffer (6 M guanidinium thiocyanate, 0.1 mM citrate, 1% [vol/vol] sarcosyl, 0.4% [vol/vol] β-mercaptoethanol, 0.1 mM sodium acetate). Lysates were subjected to a protein extraction using phenol, and RNA was recovered by precipitation with isopropanol. Total RNA (1 μg) extracted from control or activated T cells was incubated with 1.5 μg oligo-d(T)15 primer at 95°C for 15 minutes and then cooled on ice for 5 minutes. A first-strand complementary DNA synthesis was performed by adding 400 units Superscript II reverse transcriptase (Gibco) containing RT buffer (25 mM Tris-HCl, pH 8.3; 37.5 mM KCl; 1.5 mM MgCl2), 10 mM dithiothreitol, and deoxyribonucleoside triphosphate mixture (50 μM each) and incubating at 42°C for 90 minutes. The reaction was stopped, and secondary structures denatured, by incubation at 75°C for 10 minutes. Real-time polymerase chain reaction (PCR) was performed using an ABI7700 sequence detection system (PE Applied Biosystems, London, United Kingdom) in the presence of Sybro-green. This fluorochrome incorporates stoichiometrically into the amplification product, providing real-time quantification of the double-stranded DNA PCR product. Primers for each gene of interest were designed for use under real-time PCR conditions, to amplify an 80– to 100–base pair fragment with 59°C annealing temperature (Primer Express, PE Applied Biosystems). Because of the high homology between members of each family, sequences targeted for primer design were restricted to the lowest homolog regions. The optimization of the real-time PCR reaction was then performed according to the manufacturer's instructions. For each analysis, transcription of the gene of interest was compared with transcription of the housekeeping gene GAPDH, which was amplified in parallel.
Results
A subset of CD4+ T cells constitutively expressing IL-2 receptor-α (CD25) can be isolated from peripheral blood
We studied the expression of CD25 on CD4+ T cells obtained from peripheral blood samples using flow cytometry and found that the proportion of CD4+ T cells that express CD25 ranged from 15% to 30% (data not shown). These CD4+CD25+ T cells can be isolated using magnetic beads or flow cytometric sorting with the resultant purity of 90% to 95% for CD4+CD25− T cells and 80% to 90% for CD4+CD25+ T cells (Figure1A). In this study, we used peripheral samples from adult healthy volunteers and routine venesection samples from primary polycythemic individuals who were screened to exclude white cell disorders.
Naturally occurring human CD4+CD25+ cells can be found in healthy individuals and are phenotypically distinct from recently activated CD4+ cells.
(A) CD4+CD25+ cells constitute a significant proportion of CD4+ T cells in peripheral blood of healthy individuals. Purified CD4+ T cells were stained with anti-CD4 FITC and anti-CD25 PE. The resultant purity of CD4+CD25+ and CD4+CD25− cells isolated using magnetic beads from a typical experiment is shown. (B) Phenotype of CD4+CD25+ cells from peripheral blood of healthy individuals. In the top panel, 100 μL peripheral blood was stained with anti-CD4–QR, anti-CD25− PE, and FITC-conjugated monoclonal antibodies as indicated. The dot plots were gated on CD4+ lymphocytes. In the bottom panel, 100 μL peripheral blood was stained with anti-CD4–QR, anti-CD25−FITC, and anti–CTLA-4–PE (thin solid lines) or isotype-matched control (thick solid lines). The histogram plot was gated on a CD4+CD25+ or CD4+CD25−lymphocyte population. (C) The phenotype of recently activated CD4+ cells that express CD25 is distinct from “constitutive” CD4+CD25+ cells. PBMCs from the same healthy individuals were stimulated with PHA (2 μg/mL) for 2 days. The cells were harvested and stained with anti-CD4–QR, anti-CD25− PE, and the indicated FITC-conjugated monoclonal antibodies. The dot plots were gated on CD4+lymphocytes.
Naturally occurring human CD4+CD25+ cells can be found in healthy individuals and are phenotypically distinct from recently activated CD4+ cells.
(A) CD4+CD25+ cells constitute a significant proportion of CD4+ T cells in peripheral blood of healthy individuals. Purified CD4+ T cells were stained with anti-CD4 FITC and anti-CD25 PE. The resultant purity of CD4+CD25+ and CD4+CD25− cells isolated using magnetic beads from a typical experiment is shown. (B) Phenotype of CD4+CD25+ cells from peripheral blood of healthy individuals. In the top panel, 100 μL peripheral blood was stained with anti-CD4–QR, anti-CD25− PE, and FITC-conjugated monoclonal antibodies as indicated. The dot plots were gated on CD4+ lymphocytes. In the bottom panel, 100 μL peripheral blood was stained with anti-CD4–QR, anti-CD25−FITC, and anti–CTLA-4–PE (thin solid lines) or isotype-matched control (thick solid lines). The histogram plot was gated on a CD4+CD25+ or CD4+CD25−lymphocyte population. (C) The phenotype of recently activated CD4+ cells that express CD25 is distinct from “constitutive” CD4+CD25+ cells. PBMCs from the same healthy individuals were stimulated with PHA (2 μg/mL) for 2 days. The cells were harvested and stained with anti-CD4–QR, anti-CD25− PE, and the indicated FITC-conjugated monoclonal antibodies. The dot plots were gated on CD4+lymphocytes.
CD4+CD25+ T cells are phenotypically distinct from recently activated CD4+ T cells that express CD25
Because CD25 is up-regulated during T-cell activation, it was important to determine whether the CD4+CD25+cells isolated from peripheral blood of healthy individuals simply represented recently activated cells. We compared the surface expression of activation makers on CD4+CD25+ cells from peripheral blood with CD4+CD25+ cells obtained from PHA-stimulated PBMCs. We found that most CD4+CD25+ cells from peripheral blood expressed CD45RO, CD62 ligand (CD64L), and cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) but not CD69, CD40 ligand (CD40L) (Figure 1B). In contrast, CD4+ cells in which CD25 expression was induced by PHA stimulation exhibited high levels of CD69, and a much higher proportion of these cells were CD45RO−, whereas the level of CD62L expression was similar (Figure 1C).
CD4+CD25+ T cells are hyporesponsive to polyclonal T-cell stimuli in vitro
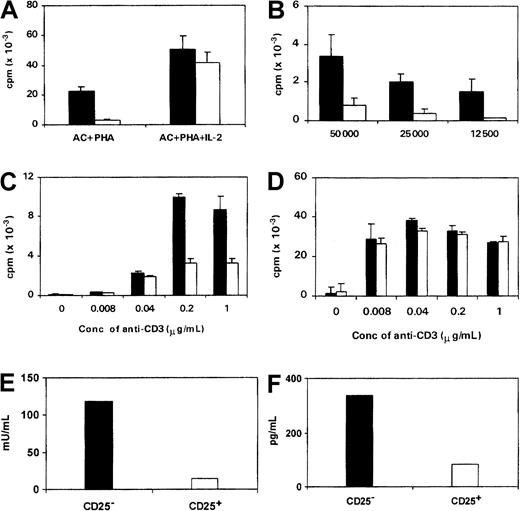
Despite the constitutive expression of CD25, the proliferative responses of CD4+CD25+ T cells to PHA or soluble anti-CD3 in the presence of autologous ACs and to HLA-mismatched stimulator cells were considerably lower than those of CD4+CD25− T cells (Figure2A-C). The CD4+CD25+ cells also produced less IL-2 and IFN-γ in response to these stimuli (Figure 2E,F). However, when IL-2 was added into the culture, the responsiveness of CD4+CD25+ cells was restored (Figure2A,D).
CD4+CD25+ cells are hyporesponsive to polyclonal T-cell stimuli.
(A-D) The proliferative responses of CD4+CD25+(■) and CD4+CD25− (▪) cells to various stimuli are shown. In each experiment, 1 × 104 to 2 × 104 responder cells were cultured with PHA (2 μg/mL) plus 1 × 104 irradiated autologous ACs with or without IL-2 (10 U/mL, A); irradiated allogeneic PBMCs (B); soluble anti-CD3 with ACs (C); and soluble anti-CD3 with IL-2 (10 U/mL) in the presence of ACs (D). Conc indicates concentration. (E-F) Production of IL-2 (E) and IFN-γ (F) in the supernatants from the culture of CD4+CD25+ or CD4+CD25−(2 × 104) cells stimulated with PHA for 3 days in the presence of autologous ACs.
CD4+CD25+ cells are hyporesponsive to polyclonal T-cell stimuli.
(A-D) The proliferative responses of CD4+CD25+(■) and CD4+CD25− (▪) cells to various stimuli are shown. In each experiment, 1 × 104 to 2 × 104 responder cells were cultured with PHA (2 μg/mL) plus 1 × 104 irradiated autologous ACs with or without IL-2 (10 U/mL, A); irradiated allogeneic PBMCs (B); soluble anti-CD3 with ACs (C); and soluble anti-CD3 with IL-2 (10 U/mL) in the presence of ACs (D). Conc indicates concentration. (E-F) Production of IL-2 (E) and IFN-γ (F) in the supernatants from the culture of CD4+CD25+ or CD4+CD25−(2 × 104) cells stimulated with PHA for 3 days in the presence of autologous ACs.
CD4+CD25+ T cells suppress the responses of CD4+CD25− T cells in coculture
When CD4+CD25+ cells were cocultured with CD4+CD25− cells, the responses to PHA or soluble anti-CD3 in the presence of autologous ACs were significantly suppressed (Figure 3A,B) and the production of IL-2 and IFN-γ was profoundly inhibited (Figure 3C,D). The suppression occurred in a dose-dependent manner, and 50% inhibition was observed at a ratio of 1:1 CD4+CD25+:CD4+CD25−cells (Figure 3E). Addition of IL-2 to the coculture abrogated this suppression (Figure 3A).
CD4+CD25+ cells suppress the responses of CD4+CD25− cells in coculture.
(A-B) CD4+CD25− cells (▪) or CD4+CD25+ (■) (1 × 104cells/well) or coculture of both CD4+CD25+ and CD4+CD25− cells (▨) (total 2 × 104 cells/well) were stimulated in the presence of irradiated autologous ACs (1 × 104 cells/well) with PHA (2 μg/mL, A) in the absence or presence of IL-2 (10 U/mL); soluble anti-CD3 for 3 days (B). Proliferation was measured by3H-thymidine incorporation.(C,D) Production of IL-2 (C) and IFN-γ (D) in coculture of CD4+CD25−(1 × 104) and CD4+CD25− cells (░) or CD4+CD25+ (1 × 104) cells (▨) in response to PHA (2 μg/mL) in the presence of autologous ACs (2 × 104) for 72 hours. (E) The proliferative responses of CD4+CD25− cells (1 × 104) to PHA (2 μg/mL) and autologous ACs (2 × 104) in the presence of various numbers of CD4+CD25+ cells.
CD4+CD25+ cells suppress the responses of CD4+CD25− cells in coculture.
(A-B) CD4+CD25− cells (▪) or CD4+CD25+ (■) (1 × 104cells/well) or coculture of both CD4+CD25+ and CD4+CD25− cells (▨) (total 2 × 104 cells/well) were stimulated in the presence of irradiated autologous ACs (1 × 104 cells/well) with PHA (2 μg/mL, A) in the absence or presence of IL-2 (10 U/mL); soluble anti-CD3 for 3 days (B). Proliferation was measured by3H-thymidine incorporation.(C,D) Production of IL-2 (C) and IFN-γ (D) in coculture of CD4+CD25−(1 × 104) and CD4+CD25− cells (░) or CD4+CD25+ (1 × 104) cells (▨) in response to PHA (2 μg/mL) in the presence of autologous ACs (2 × 104) for 72 hours. (E) The proliferative responses of CD4+CD25− cells (1 × 104) to PHA (2 μg/mL) and autologous ACs (2 × 104) in the presence of various numbers of CD4+CD25+ cells.
The suppressive function of CD4+CD25+ cells cannot be explained by passive IL-2 consumption by these cells
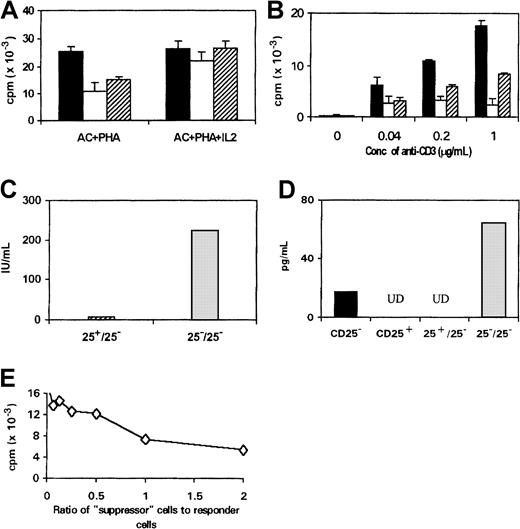
One mundane explanation for the suppressive property of CD4+CD25+ cells was that these cells competed with CD4+CD25− cells for growth factors such as IL-2. To investigate this possibility, we measured the proliferation of the IL-2–dependent murine CTLL-2 cells to a titration of recombinant human IL-2 in the presence or absence of either CD4+CD25+ or CD4+CD25−cells. Because neither CD4+CD25+ nor CD4+CD25− cells proliferated in response to rh–IL-2 alone, the proliferation measured in the assay can be considered as solely contributed by the proliferation of CTLL-2 cells. The proliferation of the CTLL-2 cells was unaffected by the presence of CD4+CD25+ or CD4+CD25−cells (Figure 4A), suggesting that neither cell population consumed a significant amount of IL-2. The suppressive property of CD4+CD25+ cells is thus unlikely to be the result of passive consumption of IL-2 by these cells.
Suppressive property of CD4+CD25+ cells is not dependent on suppressive cytokines or other soluble factors and cannot be explained by IL-2 consumption by these cells.
(A) 5000 CTLL-2 cells (▪) were cultured with various concentrations (Conc) of rh–IL-2 in the presence or absence of either CD4+CD25+ (⋄) or CD4+CD25− (○) cells (2 × 104cells/well) for 36 hours with 3H-thymidine added during the last 12 hours of culture. The proliferation of CD4+CD25+ or CD4+CD25−cells to IL-2 alone is negligible (mean cpm = 69 and 95, respectively) compared with that of the CTLL-2 cells in the presence of IL-2. (B) Supernatants from cultures of either CD4+CD25+or CD4+CD25− (1 × 104) cells with ACs (2 × 104) and PHA (2 μg/mL) were measured for the production of IL-10 and TGF-β in 3 separate experiments. (C,D) CD4+CD25− cells (1.5 × 105cells/well) were cultured in 24-well plates in the presence of ACs (1.5 × 105) and PHA (2 μg/mL). CD4+CD25+ or CD4+CD25−cells (1.5 × 105) cells were added to the upper or lower chamber as indicated (ACs were present in both the lower and upper chambers). The proliferative responses were measured separately from the cells harvested from the lower wells (C) or upper wells (D) after 3 days culture. (E) CD4+CD25+ and CD4+CD25− (1 × 104) cells were cultured in various combinations with ACs (1 × 104) plus PHA (2 μg/mL) and the indicated antibody or IL-2.
Suppressive property of CD4+CD25+ cells is not dependent on suppressive cytokines or other soluble factors and cannot be explained by IL-2 consumption by these cells.
(A) 5000 CTLL-2 cells (▪) were cultured with various concentrations (Conc) of rh–IL-2 in the presence or absence of either CD4+CD25+ (⋄) or CD4+CD25− (○) cells (2 × 104cells/well) for 36 hours with 3H-thymidine added during the last 12 hours of culture. The proliferation of CD4+CD25+ or CD4+CD25−cells to IL-2 alone is negligible (mean cpm = 69 and 95, respectively) compared with that of the CTLL-2 cells in the presence of IL-2. (B) Supernatants from cultures of either CD4+CD25+or CD4+CD25− (1 × 104) cells with ACs (2 × 104) and PHA (2 μg/mL) were measured for the production of IL-10 and TGF-β in 3 separate experiments. (C,D) CD4+CD25− cells (1.5 × 105cells/well) were cultured in 24-well plates in the presence of ACs (1.5 × 105) and PHA (2 μg/mL). CD4+CD25+ or CD4+CD25−cells (1.5 × 105) cells were added to the upper or lower chamber as indicated (ACs were present in both the lower and upper chambers). The proliferative responses were measured separately from the cells harvested from the lower wells (C) or upper wells (D) after 3 days culture. (E) CD4+CD25+ and CD4+CD25− (1 × 104) cells were cultured in various combinations with ACs (1 × 104) plus PHA (2 μg/mL) and the indicated antibody or IL-2.
The suppressive function of CD4+CD25+ cells does not appear to be mediated by soluble factors
To determine whether the suppressive function of CD4+CD25+ cells is mediated by secretion of soluble factors, a Transwell system was used. As shown in Figure 4C, if CD4+CD25+ cells were mixed with CD4+CD25− cells in the lower well, inhibition of proliferation was observed. However, if the CD4+CD25+ cells were placed in the upper well, which also contained ACs, and were thus physically separated from the CD4+CD25− cells by a semipermeable membrane, no inhibition was observed, although the CD4+CD25+ cells remained hyporesponsive (Figure4D). These observations suggest that soluble factors do not play an essential role in mediating the suppressive function of CD4+CD25+ cells and that the suppression requires cell-to-cell contact. Moreover, these data argue further against IL-2 consumption being the mechanism of suppression.
The suppressive function of CD4+CD25+ cells is not mediated by IL-10 or TGF-β1
To determine whether regulatory cytokines, such as IL-10 and TGF-β, were responsible for the suppressive effects of this T-cell population, the levels of these cytokines were measured in the supernatants of stimulated cultures. In only 1 of 3 experiments were these cytokines detected and then only at very low concentrations (Figure 4B). To exclude the possibility that the ELISA assay was insufficiently sensitive to detect biologically active concentrations of TGF-β, purified TGF-β was titrated into cultures of mitogen-stimulated CD4+CD25+ T cells to determine the concentration required to reproduce the inhibition caused by the CD25+ cells. This revealed that a concentration in excess of 80 pg/mL was needed to see significant inhibition of T-cell proliferation; the ELISA assay was able to detect concentrations as low as 1.0 pg/mL (data not shown). Thus, it is unlikely that functionally significant amounts of TGF-β were secreted by the CD25+population. Furthermore, addition of neutralizing antibodies against these 2 cytokines failed to reverse the suppression caused by the CD25+ cells (Figure 4E) or reverse the hyporesponsiveness (data not shown).
CD4+CD25+ T cells do not inhibit APC function
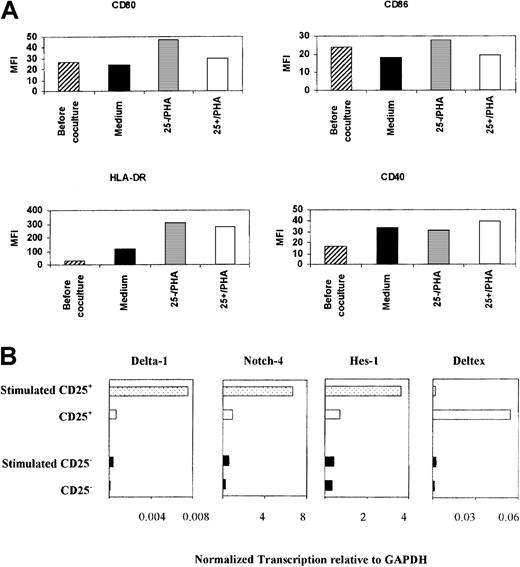
One possible target of the suppressive effects of the CD4+CD25+ cells is the antigen-presenting cell (APC), as we have shown previously in the regulation mediated by anergic T cells.19 To address this possibility, we cultured premature monocyte-derived dendritic cells with either CD4+CD25+ cells or CD4+CD25− cells in the presence of PHA for 48 hours. Although dendritic cells that had been cocultured with CD4+CD25+ cells expressed a lower level of CD80 and CD86 than those cocultured with CD4+CD25−cells, the levels of these molecules were similar to dendritic cells that had been cocultured with medium only (Figure5A). The expression of CD40 and HLA-DR was comparable. Furthermore, dendritic cells that had been precultured with CD4+CD25+ T cells were fully competent in functional assays (data not shown). By exclusion, these findings suggest that these regulatory cells act directly on neighboring T cells.
CD4+CD25+ cells did not inhibit APC function.
(A) Immature monocyte-derived dendritic cells (1 × 106) were cocultured with either CD4+CD25− or CD4+CD25+ (1 × 106) cells or medium for 48 hours. The cells were stained with FITC-conjugated monoclonal antibodies. The mean florescence intensities were measured. Dendritic cells were gated according to the forward and side scatter characteristics. (B) Notch signaling was specifically increased in CD4+CD25+ cells upon stimulation. Transcription of Notch-4, Delta-1, Hes-1, and deltex in CD4+CD25+ and CD4+CD25− cells, before and after activation with anti-CD3 and anti-CD28. Transcription of each gene is normalized to that of GAPDH.
CD4+CD25+ cells did not inhibit APC function.
(A) Immature monocyte-derived dendritic cells (1 × 106) were cocultured with either CD4+CD25− or CD4+CD25+ (1 × 106) cells or medium for 48 hours. The cells were stained with FITC-conjugated monoclonal antibodies. The mean florescence intensities were measured. Dendritic cells were gated according to the forward and side scatter characteristics. (B) Notch signaling was specifically increased in CD4+CD25+ cells upon stimulation. Transcription of Notch-4, Delta-1, Hes-1, and deltex in CD4+CD25+ and CD4+CD25− cells, before and after activation with anti-CD3 and anti-CD28. Transcription of each gene is normalized to that of GAPDH.
A member of the Notch family and one of its ligands are overexpressed on CD4+CD25+ cells
One candidate set of molecules in T-cell–mediated regulation is the Notch family of receptors and its ligands, which belong to the Jagged and Delta molecular families. Thus, overexpression of Jagged-1 in antigen-pulsed APCs induced tolerance in naive T cells, which could then be adoptively transferred.21 Additionally, certain family members were differentially transcribed when a human T-cell clone was anergized or activated (F.P. and J.I., unpublished observations, 2000). There are no staining reagents that reliably detect or block the various family members on living cells, and we therefore used real-time PCR quantitation of the corresponding transcripts in CD4+CD25+ and CD4+CD25− cells. This revealed that deltex, a positive regulator of the Notch signaling pathway,22,23was highly up-regulated in CD4+CD25+ cells compared with CD4+CD25− cells. Furthermore, upon stimulation, the transcription of Notch-4 and Delta-1 dramatically increased only in CD4+CD25+ cells, concurrent with a rise in Hes-1 transcript (Figure 5B). The expression of Notch-1, Jagged-1, and Jagged-2 was similar among CD4+CD25+ and CD4+CD25− cells (data not shown). Hes-1 is a downstream mediator of Notch pathway signaling,24 and these data suggest that stimulation of CD4+CD25+ cells effects a change in Notch signaling, possibly via modulation of Notch-4. Additionally, the rise in Delta-1 transcription raises the possibility that stimulated CD4+CD25+ T cells become capable of Notch-mediated T cell–to–T cell communication. Thus, when antigen-specific T-cells were transfected with Delta-1, they were able to inhibit the responses of antigen-experienced cells and became capable of linked suppression.25 These observations suggest that Notch signaling contributes to the regulatory effects of these cells.
The presence of CD4+CD25+ T cells is responsible for nonlinear kinetics in limiting dilution analyses of alloresponses
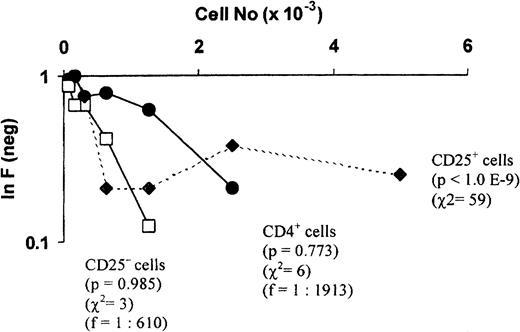
We used limiting dilution assays to detect subpopulations of cells exhibiting multihit kinetics consistent with suppressive activity in coculture. Figure 6 shows a limiting dilution analysis plot of a mixed lymphocyte reaction using unfractionated CD4+, CD4+CD25−, or CD4+CD25+ cells as responders. A significant deviation from single-hit kinetics was observed with unfractionated CD4+ cells, whereas depletion of the CD4+CD25+ population led to restoration of single-hit kinetics and a significant increase in frequency. When enriched CD4+CD25+ cells were used, multihit kinetics was observed. These results suggest that the CD4+CD25+ subpopulation contains cells with a suppressive or regulatory function.
CD4+CD25+ cells are responsible for the multihit kinetics observed in limiting dilution analysis in alloresponses.
Limiting dilution analyses using CD4+, CD4+CD25−, or CD4+CD25+ cells as responder cells. Serial dilutions of responder cells were cocultured with allogeneic irradiated PBMCs (5 × 104) for 5 days. Control wells contained stimulator cells only. The precursor frequency (f), χ2value (χ2), and a probability estimate of the data conforming to single-hit kinetics (p) for each responder subset were indicated. Calculation of the precursor frequency in the CD4+CD25+ subset was not meaningful because of the highly significant deviation of the data from single-hit kinetics.
CD4+CD25+ cells are responsible for the multihit kinetics observed in limiting dilution analysis in alloresponses.
Limiting dilution analyses using CD4+, CD4+CD25−, or CD4+CD25+ cells as responder cells. Serial dilutions of responder cells were cocultured with allogeneic irradiated PBMCs (5 × 104) for 5 days. Control wells contained stimulator cells only. The precursor frequency (f), χ2value (χ2), and a probability estimate of the data conforming to single-hit kinetics (p) for each responder subset were indicated. Calculation of the precursor frequency in the CD4+CD25+ subset was not meaningful because of the highly significant deviation of the data from single-hit kinetics.
Cells with similar phenotype and function to adult CD4+CD25+ cells are found in umbilical venous blood from healthy newborn infants
We observed that a proportion of CD4+ T cells from umbilical venous blood in newborn infants express CD25 (P.J.D. et al, unpublished observation, 2000). We tested whether this subpopulation of CD4+CD25+ cells showed similar phenotype and behavior to their adult counterparts. About 5% to 15% of CD4+ cells expressed CD25, and these had a similar phenotype to adult CD4+CD25+ cells: neonatal CD4+CD25+ cells expressed CD62L and CTLA4 but not CD69, CD40L. However, most neonatal cells expressed CD45RA, with a small percentage expressing CD45RO. Indeed, the CD4+CD45RO+ cells were confined to the CD4+CD25+ population (Figure7A). Because of the relatively small volume of umbilical venous blood that could be collected, it was not possible to obtain sufficient numbers of CD4+CD25+ cells for detailed functional analysis. However, by examining the kinetics of limiting dilution analysis against allogeneic stimulator cells using either CD4+CD25− cells or unfractionated CD4+ cells as responders, we again found that the unfractionated CD4+ population exhibited significant deviation from single-hit kinetics, and depletion of CD4+CD25+ cells restored the single-hit kinetics and led to an increase in frequency (Figure 7B). These findings suggest that the CD4+CD25+ cells from cord blood also possess regulatory function.
CD4+CD25+ cells from umbilical cord blood of normal-term infants are phenotypically similar to those from adult peripheral blood.
(A) Cord blood mononuclear cells were isolated and stained with anti-CD4–QR, anti-CD25− PE, and FITC-conjugated monoclonal antibodies as indicated (middle panel). The dot plots were gated on CD4+ lymphocytes as shown in the top panel. Peripheral mononuclear cells from cord blood were stained with anti-CD4–QR, anti-CD25− FITC, and anti–CTLA-4–PE (thin solid lines) or isotype-matched control (thick solid lines). The histogram plot was gated on CD4+CD25+ or CD4+CD25− lymphocytes (bottom panel). (B) Limiting dilution analyses using CD4+ (♦) or CD4+CD25− (■) cells as responder cells. Serial dilutions of responder cells were cocultured with allogeneic irradiated PBMCs (5 × 104) for 5 days. Control wells contained stimulator cells only.
CD4+CD25+ cells from umbilical cord blood of normal-term infants are phenotypically similar to those from adult peripheral blood.
(A) Cord blood mononuclear cells were isolated and stained with anti-CD4–QR, anti-CD25− PE, and FITC-conjugated monoclonal antibodies as indicated (middle panel). The dot plots were gated on CD4+ lymphocytes as shown in the top panel. Peripheral mononuclear cells from cord blood were stained with anti-CD4–QR, anti-CD25− FITC, and anti–CTLA-4–PE (thin solid lines) or isotype-matched control (thick solid lines). The histogram plot was gated on CD4+CD25+ or CD4+CD25− lymphocytes (bottom panel). (B) Limiting dilution analyses using CD4+ (♦) or CD4+CD25− (■) cells as responder cells. Serial dilutions of responder cells were cocultured with allogeneic irradiated PBMCs (5 × 104) for 5 days. Control wells contained stimulator cells only.
CD4+CD25+ cells retain their suppressive properties after in vitro expansion
As described in Figures 2 and 3, the CD4+CD25+ cells could be driven to divide by stimulation in the presence of exogenous IL-2. Given that cell division can lead to recovery of responsiveness by T cells rendered anergic in vitro, we tested the suppressive activity of the CD4+CD25+ cells after 2 days of stimulation with coimmobilized anti-CD3 and anti-CD28 antibodies, followed by 2 days in rh–IL-2. As shown in Figure 8, the CD25+ cells retained their suppressive properties after in vitro expansion. In contrast, CD4+CD25−cells treated in exactly the same manner were not suppressive. These data indicate that the CD4+CD25+ cell population is a stable cell lineage that can be expanded while retaining its regulatory function.
CD4+CD25+ cells can be expanded in vitro but retain their suppressive phenotype.
(A) CD4+CD25+ or CD4+CD25− cells were stimulated with immobilized anti-CD3 (1 μg/mL) and anti-CD28 (10 μg/mL) for 3 days, and the cells were washed and rested in medium containing 30 U/mL rh–IL-2 for 2 days. The cells were then stained with FITC- or PE-conjugated monoclonal antibodies as indicated. (B) CD4+CD25+ or CD4+CD25−(1 × 104) cells generated as in panel A were cocultured with autologous unstimulated CD4+CD25−(1 × 104) cells in the presence of autologus ACs (1 × 104) and PHA (2 μg/mL) for 3 days.
CD4+CD25+ cells can be expanded in vitro but retain their suppressive phenotype.
(A) CD4+CD25+ or CD4+CD25− cells were stimulated with immobilized anti-CD3 (1 μg/mL) and anti-CD28 (10 μg/mL) for 3 days, and the cells were washed and rested in medium containing 30 U/mL rh–IL-2 for 2 days. The cells were then stained with FITC- or PE-conjugated monoclonal antibodies as indicated. (B) CD4+CD25+ or CD4+CD25−(1 × 104) cells generated as in panel A were cocultured with autologous unstimulated CD4+CD25−(1 × 104) cells in the presence of autologus ACs (1 × 104) and PHA (2 μg/mL) for 3 days.
Discussion
In this study, CD4+CD25+ T cells were detected in peripheral blood samples from adult volunteers and in umbilical venous blood taken from newborn infants. These cells had a distinctive phenotype, expressing a mixture of markers of memory and naive T cells, and caused substantial inhibition of proliferation and cytokine secretion by CD4+CD25− T cells. Mechanistically, the regulation effected by these cells appears to require cell-to-cell contact, is not mediated by known cytokines, does not lead to inhibition of APC function, and may involve signaling through the Notch receptors.
The results cannot be attributed to the recent activation of CD4+ T cells in vivo inducing expression of CD25. Indeed, the similarity of results between healthy volunteers, individuals with primary polycythemia, and newborns argues against an inflammatory origin in any one of these groups. Individuals with primary polycythemia were screened to exclude white cell disorders, and neonatal samples were derived from infants delivered by elective cesarean section, making it unlikely that parturition was responsible for inducing this population of cells. Equally, these findings are unlikely to be the result of an in vitro artifact because of the use of anti-CD25 monoclonal antibody in the positive selection process. The clone of antibody that we employed has been shown not to inhibit anti-CD3–stimulated proliferation or IL-2 binding of T cells.26 27 Furthermore, we have confirmed that the addition of this antibody in concentrations up to 10 μg/mL did not affect the proliferation of CD4+ T cells in response to PHA in the presence of autologous ACs (data not shown). In addition, our observation that PHA-stimulated CD4+ cells (that had up-regulated CD25 expression) were phenotypically distinct from naturally occurring CD4+CD25+ cells argues further against these being recently activated CD4+ cells. Indeed, in vitro stimulation and expansion of CD4+CD25+ cells did not abolish their suppressive phenotype, while similar treatment to CD4+CD25− cells failed to generate “suppressor” cells. CD4+CD25+ thus seem to be a spontaneously arising population of cells with novel phenotype and function.
In the mouse, regulatory CD4+CD25+ cells appear to arise in the thymus in that CD4+CD25+thymocytes behave similarly to mature peripheral CD4+CD25+ T cells.28 In this respect, it is interesting that 5% to 15% of the CD4+ T cells from umbilical venous blood samples of normal-term infants express CD25. Neonatal CD4+CD25+ T cells have a similar phenotype to their adult counterparts except that they more frequently express CD45RA. However, the functional implication of this differential expression of CD45 isoforms is unclear. In mice, both CD45RBlo (“memory”) and CD45RBhi(“naive”) subsets of CD4+CD25+ cells inhibit the proliferation of CD4+CD25− cells equally.29 Neonatal CD4+CD25+cells also appear to possess regulatory function because depletion of these cells converted multihit kinetics in limiting dilution analysis into single-hit kinetics and revealed a higher precursor frequency. The presence of CD3+CD25+ cells has been described in healthy fetuses as early as 16 weeks' gestation,30raising the possibility that this population arises soon after thymic ontogeny commences. The presence of significant numbers of CD4+CD25+ T cells in healthy individuals from the neonatal period into the adult life argues for a significant and persistent role in the immune system.
T-cell anergy has been implicated in the maintenance of peripheral tolerance. Although anergic human T-cell clones have been generated by a variety of methods in vitro, most of the in vivo evidence has come from rodent models, and naturally occurring anergic T cells have not previously been described in man. In this study, the behavior of CD4+CD25+ cells isolated from peripheral blood of healthy individuals was characteristic of anergic cells. To our knowledge, this is the first set of data indicative of the existence of naturally occurring anergic T cells in vivo. We have previously demonstrated that human and murine anergic T-cell clones could inhibit antigen-specific and allospecific T-cell proliferation17,19and prolong skin allograft survival in vivo.31 Similarly, CD4+CD25+ cells were also capable of inhibiting the responses of CD4+CD25− T cells. Taken together, these observations suggest that anergic T cells are not simply immunologically inert cells but may play an active regulatory role in the maintenance of immunologic tolerance.
The mechanism(s) of regulation effected by these cells remains to be elucidated fully. From the results obtained here, it does not involve known regulatory cytokines or passive consumption of IL-2 and appears to require cell-to-cell contact. We have demonstrated previously that anergic murine T-cell clones inhibit the responses of responsive T-cell clones via inhibition of the APCs in a cognate manner.19More recently, Cederbom and colleagues32 demonstrated that murine CD4+CD25+ T cells down-regulate the expression of CD80 and CD86 on dendritic cells. However, in this study, although CD4+CD25+ cells were less efficient in inducing the up-regulation of costimulation molecules CD80 and CD86 on monocyte-derived dendritic cells compared with CD4+CD25− cells, we did not observe any significant down-regulation of these costimulatory molecules or their functional capacity. These data suggest that CD4+CD25+ cells may directly regulate the function of responsive T cells.
It has been suggested recently that Notch and its ligands may be important in the induction and maintenance of tolerance. Delta-1 expression is increased on peripheral T cells during the induction of tolerance with high-dose peptide delivered intranasally. Furthermore, antigen-specific CD4+ T cells transfected with Delta-1 inhibited the response of antigen-primed T cells and induced linked suppression.25 Additionally, overexpression of human Jagged-1, a Notch ligand, on murine APCs induces naive peripheral CD4+ T cells to become regulatory cells and transfer antigen-specific tolerance to recipient mice.21 We have also observed the up-regulation of these family member genes in an anergic human T-cell clone (F.P. and J.I, unpublished observations, 2000). In this respect, it is interesting that we found differential transcription of deltex, which facilitates Notch signaling in CD4+CD25+ T cells. Furthermore, following stimulation with anti-CD3 and anti-CD28, Notch-4 and Delta-1 transcriptions were up-regulated, along with Hes-1, a downstream mediator of Notch signaling. These data imply a role of Notch and its ligands in the development or maintenance of the regulatory function of CD4+CD25+ cells. Currently, blocking studies are not possible in that no antibodies are available to detect or inhibit the interactions between Notch:Notch ligand family members, possibly reflecting the high degree of conservation of these protein between species. Another molecule that is expressed on CD4+CD25+ cells, but not CD4+CD25− cells, is CTLA-4. It is possible that the high-affinity interaction between CTLA-4 and B7 molecules expressed on APCs prevents or diminishes the delivery of costimulatory signals by APC to CD4+CD25− cells.
Some of the most persuasive evidence for the existence of “suppressor” cells has come from limiting dilution analysis.33-35 A zigzag appearance of the semilogarithmic plot has been attributed to the presence of more than one responder cell type, one of which has regulatory effects. More recently, Dozmorov and colleagues36,37 proposed a 2-cell subtype model to explain these observations. The first subtype (LPC1) exhibited single-hit kinetics and was responsive; the second subset (LPC2) exhibited multihit kinetics and had the ability to inhibit the responses of the first subset. In their studies using murine CD4+ T cells, they found that the “suppressor” subset was contained within the “memory” CD4+ T-cell population and addition of IL-2 or neutralizing antibodies to IL-10 restored single-hit kinetics.38 In keeping with these findings, we showed that the human CD4+CD25+cells exhibited multihit kinetics in response to alloantigens. In contrast, the CD4+CD25− cells conformed to single-hit kinetics but the unfractionated CD4+ population did not. These observations are consistent with the suggestion that CD4+CD25+ cells may contain the LPC2 cells described by Dozmorov and colleagues. The multihit kinetics displayed by the CD4+CD25+ cell population may be attributable to the heterogeneity of the population. It is possible that improving the purity of the CD4+CD25+cells would yield single-hit kinetics with very low or undetectable frequencies in limiting dilution analysis against alloantigens. However, even with flow cytometric sorting, we have not been able to obtain a CD4+CD25+ cell population with purity greater than 90% without severely compromising the yield.
Taken together, our results demonstrate that a proportion of CD4+ T cells from adult peripheral blood and neonatal umbilical venous blood constitutively express CD25 and are distinct from recently activated CD4+ T cells. This subset of CD4+ T cells resembles anergic cells and possesses suppressive function in vitro. The suppression appears to be cell-to-cell contact dependent and may involve the Notch signaling between neighboring T cells. This subpopulation of CD4+ T cells has similar characteristics to CD4+CD25+in mice, which are critical in the maintenance of self-tolerance and the prevention of autoimmune disease. Human CD4+CD25+ cells may have an equally important role in the regulation of autoimmunity and transplant tolerance.
We thank Drs Andrew George and Hans Stauss for critical review of the manuscript, Drs Ragnar Lindstedt, Silvia Vendetti, and Juo-Guan Chai for helpful discussions, Sue Douglas and Gary Warns for technical assistance. We also thank the nursing staff on the Haematology Day Unit of Hammersmith Hospital for arranging the collection of blood samples. We are grateful for the financial support of the Wellcome Trust, Garfield Weston Foundation, and Action Research and Wellbeing.
Supported by the Wellcome Trust, Garfield Weston Foundation, and Action Research and Wellbeing. W.F.N. is a recipient of a Wellcome Trust training fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert I. Lechler, Dept of Immunology, Hammersmith Campus, Imperial College School of Medicine, Du Cane Rd, London W12 0NN, United Kingdom; e-mail: r.lechler@ic.ac.uk.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal