Abstract
Tryptases are serine proteases primarily expressed in mast cells. Normal blood basophils express only trace amounts of the enzyme. However, recent immunohistochemical studies have raised the possibility that neoplastic basophils express significant amounts of tryptase. In this study, tryptase expression was analyzed in normal and neoplastic basophils by immunoelectron microscopy using antitryptase monoclonal antibody G3. Basophils were obtained from patients with chronic myeloid leukemia (CML), idiopathic myelofibrosis (IMF), and myelodysplastic syndrome (MDS), and from healthy donors. Tryptase-immunoreactive material was detected in cytoplasmic granules of basophils in CML, IMF, and MDS. By contrast, normal basophils did not contain significant amounts of tryptase by immunoelectron microscopy. As assessed by reverse transcription-polymerase chain reaction, neoplastic basophils contained messenger RNA (mRNA) for α-tryptase, but no β-tryptase mRNA. In summary, these data provide evidence that neoplastic basophils in CML, IMF, and MDS can express detectable amounts of tryptase. Therefore, tryptase should not be regarded as specific for mast cells when neoplastic myeloid cells are analyzed.
Introduction
Basophils and mast cells (MCs) are effector cells of allergic reactions.1-3 They express high-affinity IgE-binding sites and various granular mediators.2,4,5Both cells derive from CD34+ hemopoietic progenitor cells.1,2,6 However, basophils differ from MCs in their ultrastructure, expression of surface antigens, and response to growth factors.7-10 Likewise, interleukin-3 (IL-3) is a potent differentiation factor for human basophils but not for human MCs.9,10 MCs, in turn, grow from CD34+ cells in response to stem cell factor (SCF).11 12
Tryptases are serine proteases specifically expressed in MCs.13,14 Two major subtypes, α- and β-tryptases, have been identified and cloned.15,16 MCs contain both types of the enzyme. Other hemopoietic cells do not express significant amounts of tryptases; in fact, only trace amounts are detectable in normal blood basophils, and other myeloid cells appear to be tryptase-negative.14 Recently, however, significant amounts of tryptases were detected in immature basophillike cell lines.17,18 Moreover, it was found that in patients with chronic myeloid leukemia (CML), immature granulated cells with characteristics of basophils react with antibodies against tryptase.19 However, it could not be clarified whether these cells are indeed basophils, belong to the MC lineage, or would represent an “intermediate cell.”
Study design
Isolation and culture of cells
Peripheral blood (PB) was obtained from 12 healthy volunteers, 6 patients with CML (chronic phase), 3 patients with myelodysplastic syndrome (MDS; 1 with refractory anemia with ring sideroblasts [RARS], and 2 with refractory anemia with excess blasts [RAEB]), and 8 patients with idiopathic myelofibrosis (IMF). All patients gave informed consent. Mononuclear cells (MNCs) were isolated using Ficoll. The percentage of basophils in PB-MNCs was 1% to 7% in controls, 2.7% to 30% in CML, 2% to 27% in MDS, and 0.5% to 23% in IMF samples.
Cord blood (CB) was obtained from 2 full-term deliveries after informed consent was given by the mothers. CB-MNCs were enriched for CD34+ cells using monoclonal antibody QBEND/10 and magnetic beads. CD34+ cells were cultured in RPMI-1640 medium with 10% fetal calf serum (FCS), and either recombinant human stem cell factor (rhSCF; 100 ng/mL) or rhIL-3 (100 U/mL) (Promocell, Heidelberg, Germany) at 37°C for 28 days.
Levels of total tryptase (β-tryptase + α-protryptase) were measured in cell lysates by a commercial fluoroimmunoenzyme assay23 (FIA; Pharmacia, Uppsala, Sweden). Tryptase levels per basophil were calculated from total cell numbers and percentage counts.
Electron microscopy and immunoelectron microscopy
Electron microscopy and immunoelectron microscopy were performed as reported20-22 using PB-MNCs (CML, n = 2; RAEB, n = 1; IMF, n = 1; controls, n = 3) and CD34+CB-MNCs (on day 0 and after culture in IL-3 or SCF). Cells were fixed in 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4). Then, cells were washed, resuspended in 2% agar, and centrifuged. Pellets were postfixed in 1.3% OsO4, stained en bloc with 2% uranyl acetate in sodium maleate buffer (pH 4.4), dehydrated, and embedded in EPON 812. Ultrathin sections were cut and placed on gold grids. Postembedding immunogold labeling was performed on osmium-fixed, epoxy resin-embedded sections as described previously22 using antitryptase monoclonal antibody G3 (Chemicon, Temecula, CA). After incubation with G3 (4 hours), grids were washed and incubated with goat antimouse antibody conjugated with 10 nM gold particles. Sections were contrasted in uranyl acetate and lead citrate.
Reverse transcription–polymerase chain reaction
Total RNA was extracted from PB-MNCs (CML, n = 2; IMF, n = 1) and reverse-transcribed into complementary DNA (cDNA) as described.24 The cDNA aliquots (6 μL) were used for polymerase chain reaction (PCR) amplification in 50 μL volume containing PCR buffer, 1.25 U Taq polymerase, 25 μM of upstream and downstream primers (MWG Biotech, Ebersberg, Germany) specific for tryptase (5′ primer: 5′ GAGGCCCCCAGGAGCAAGTG 3′; 3′ primer: 5′ ACATCGCCCCAGCCAGTGAC 3′) or β-actin. Primers were selected to be complementary to identical regions in α- and β-tryptase cDNAs and to display restriction site differences (only β-tryptase–specific PCR products contained a DraIII restriction site). Samples were amplified in 32 cycles at 94°C (1 minute), annealing for 1 minute (63°C), and extension at 72°C (1 minute) after initial denaturation (95°C, 2 minutes). PCR products were subjected to restriction fragment length polymorphism using endonuclease DraIII (Boehringer Mannheim, Mannheim, Germany).
Results and discussion
Detection of tryptase in neoplastic basophils
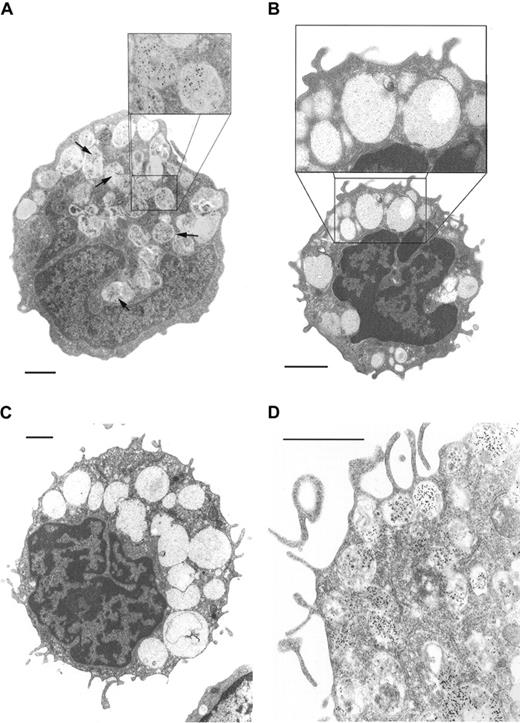
Basophils from 2 patients with CML, 1 with IMF, and 1 with MDS were examined by electron microscopy. In all samples, basophils were easily identified as round cells containing cytoplasmic granules including so-called particulate granules. In immature basophils nuclei appeared to be round or bilobed. Mature basophils showed segmented nuclei. As assessed by immunoelectron microscopy, a proportion of neoplastic basophils (10%-50%) contained tryptase in their cytoplasmic granules (Figure 1). However, not all granules in a given cell appeared to be labeled. Also, the intensity of labeling in basophils varied from donor to donor. In basophils from healthy donors (n = 3) granules appeared to be tryptase negative.
Tryptase immunoelectron microscopy.
(A) Blood basophil from a patient with chronic phase CML. Tryptase-immunoreactive material is localized to a subset of granules (arrows; original magnification × 5000; inset, × 10 000). (B) Basophil of a healthy donor shows no tryptase expression by immunoelectron microscopy (original magnification × 6500; inset × 13 000). (C) Tryptase immunoelectron microscopy of a CB culture–derived basophil (IL-3, day 13); no significant amounts of tryptase could be detected (original magnification × 4000). (D) Tryptase immunoelectron microscopy of a CB culture–derived MC (SCF, day 28). In this cell tryptase-reactive material could be detected. Note surface projections and granular staining pattern (original magnification × 12 500). 1 bar = 1 μm.
Tryptase immunoelectron microscopy.
(A) Blood basophil from a patient with chronic phase CML. Tryptase-immunoreactive material is localized to a subset of granules (arrows; original magnification × 5000; inset, × 10 000). (B) Basophil of a healthy donor shows no tryptase expression by immunoelectron microscopy (original magnification × 6500; inset × 13 000). (C) Tryptase immunoelectron microscopy of a CB culture–derived basophil (IL-3, day 13); no significant amounts of tryptase could be detected (original magnification × 4000). (D) Tryptase immunoelectron microscopy of a CB culture–derived MC (SCF, day 28). In this cell tryptase-reactive material could be detected. Note surface projections and granular staining pattern (original magnification × 12 500). 1 bar = 1 μm.
Evaluation of tryptase expression in cultured cells
As assessed by immunoelectron microscopy, isolated CD34+ CB-MNCs (blasts, day 0) were tryptase negative. At all times investigated (days 13, 21, 28), SCF-cultured MCs expressed tryptase in their granules, whereas cultured basophils (day 13) did not contain significant amounts of tryptase (Figure 1).
Measurement of cellular tryptase
As assessed by FIA, the levels of total tryptase (β-tryptase + α-protryptase) in basophils (pg/cell) were significantly higher in CML (median: 0.09 pg/cell; mean ± SD = 0.11 ± 0.09) compared with healthy controls (median: 0.03; mean ± SD = 0.03 ± 0.02; P < .05). A higher median tryptase level in basophils was also recorded in MDS, but not in IMF (Table 1).
Patient characteristics and results
| Variable . | CML* . | MDS* . | IMF* . | Controls . |
|---|---|---|---|---|
| n | 6 | 3 | 8 | 12 |
| Age (median) | 53 | 80 | 78 | 26 |
| WBC/μL (median) | 24 000 | 5810 | 8440 | ND |
| Hemoglobin, g/dL (median) | 12.8 | 8.2 | 10.1 | ND |
| Percent blood basophils (median) | 4 | 3 | 3 | ND |
| Percent basophils in MNCs (median) | 7 | 4.6 | 4 | 3.3 |
| Tryptase, pg/basophil (median) | 0.09† | 0.55 | 0.01 | 0.03† |
| Variable . | CML* . | MDS* . | IMF* . | Controls . |
|---|---|---|---|---|
| n | 6 | 3 | 8 | 12 |
| Age (median) | 53 | 80 | 78 | 26 |
| WBC/μL (median) | 24 000 | 5810 | 8440 | ND |
| Hemoglobin, g/dL (median) | 12.8 | 8.2 | 10.1 | ND |
| Percent blood basophils (median) | 4 | 3 | 3 | ND |
| Percent basophils in MNCs (median) | 7 | 4.6 | 4 | 3.3 |
| Tryptase, pg/basophil (median) | 0.09† | 0.55 | 0.01 | 0.03† |
WBC indicates white blood cell; ND, not done.
A subset of patients was treated with cytoreductive drugs. In CML, 3 patients were untreated, 1 received hydroxyurea, and 2 a combination of hydroxyurea and interferon-α. Of the MDS patients, 1 received hydroxyurea, and 2 were untreated. In the IMF group, 3 patients received hydroxyurea and 1 interferon-α; the remaining patients were not treated.
As assessed by Mann-Whitney U test, the median tryptase levels in basophils were significantly higher in CML patients compared to the control group (P < .05). In the MDS group and IMF group the levels of tryptase per basophil varied greatly from donor to donor, and overall no significant deviation from the control group was found (P > .05).
Tryptase messenger RNA expression
A PCR product of 383 base pairs was generated from cDNA obtained from PB-MNCs of patients with CML (n = 2) and IMF (n = 1). Restriction enzyme digestion (DraIII) resulted in partial degradation of PCR products in a mast cell line (HMC-1) indicating expression of both α- and β-tryptase messenger RNA (mRNA). By contrast, no digestion occurred with the patients' PCR products, suggesting that basophils contained only α-tryptase mRNA, but not β-tryptase mRNA.
Interpretation of data
Recent observations have raised the possibility that in contrast to normal basophils, immature neoplastic basophils can express significant amounts of tryptase.17-19 Notably, in patients with CML, immature “basophillike” cells were found to react with antitryptase monoclonal antibody.19 However, it could not be clarified whether the labeled cells were indeed basophils or MC-lineage cells. Our immunoelectron microscopy experiments clearly show that tryptase is expressed in the granule compartment of basophils in patients with CML, MDS, and IMF. By contrast, no significant amounts of tryptase were detected in normal basophils.
Interestingly, the so-called particulate granules contained tryptase-immunoreactive material. This granule type is a typical ultrastructural feature of basophils.2,3 7 However, there was a significant variability in expression of granular tryptase when basophils from different donors were compared. These observations suggest that tryptase production, release, or degradation in basophils varies among donors.
Two types of tryptases, α and β types, have been identified and cloned.15 16 In the present study, the α type of tryptase was found to be the predominant type expressed in neoplastic basophils at the mRNA level. The abnormal expression ofα-tryptase gene products in basophils in myeloid malignancies may have several explanations. One could be that the transformation of myeloid progenitors is associated with increased enzyme production. The possibility that tryptase is selectively expressed at a certain (immature) stage of basophil maturation seems unlikely; in fact, cultured immature basophils did not express substantial amounts of tryptase.
So far, tryptase has been widely used as a specific marker to identify MCs in patients with mastocytosis and other hematologic disorders.25 The results of our study suggest that in various myeloid neoplasms, tryptase may also be detectable in basophils. Therefore, tryptase should not be regarded as MC specific in such patients. Whether basophil tryptase can be used as a marker to monitor patients with CML or other myeloid neoplasms is currently under investigation.
Supported by Fonds zur Förderung der Wissenschaftlichen Forschung in Österreich-FWF, grant P-12517 and grant P-14031.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter Valent, Department of Internal Medicine I, Division of Hematology and Hemostaseology, Währinger Gürtel 18-20, A-1090 Vienna, Austria; e-mail: peter.valent@akh-wien.ac.at.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal