Abstract
Bispecific antibodies (bsAbs) can efficiently mediate tumor cell killing by redirecting preactivated or costimulated T cells to disseminated tumor cells, especially in a minimal residual disease situation. This study demonstrates that the trifunctional bsAb BiLu is able to kill tumor cells very efficiently without any additional costimulation of effector cells in vitro and in vivo. Remarkably, this bsAb also induces a long-lasting protective immunity against the targeted syngeneic mouse tumors (B16 melanoma and A20 B-cell lymphoma, respectively). A strong correlation was observed between the induction of a humoral immune response with tumor-reactive antibodies and the survival of mice. This humoral response was at least in part tumor specific as shown in the A20 model by the detection of induced anti-idiotype antibodies. Both the survival of mice and antitumor titers were significantly diminished when F(ab′)2 fragments of the same bsAb were applied, demonstrating the importance of the Fc region in this process. With the use of T-cell depletion, a contribution of a cellular antitumor response could be demonstrated. These results reveal the necessity of the Fc region of the bsAb with its potent immunoglobulin subclass combination mouse immunoglobulin G2a (IgG2a) and rat IgG2b. The antigen-presenting system seems to be crucial for achieving an efficient tumor cell killing and induction of long-lasting antitumor immunity. Hereby, the recruitment and activation of accessory cells by the intact bsAb is essential.
Introduction
Bispecific antibodies (bsAbs) are regarded as powerful tools for the treatment of malignant cells in a minimal residual disease situation, as single disseminated tumor cells are most easily targeted by an immunologic attack. However, the bsAbs described to date normally activate only a single class of effector cell, ie, either T cells,1 natural killer cells,2Fcγ-receptor type I+ cells,3 or Fcα-receptor type I+ cells.4 Here, we introduce a new quality with an intact bsAb, consisting of the 2 evolutionary-related isotypes—mouse immunoglobulin G2a (IgG2a) × rat IgG2b—that are both potent in terms of immunologic effector functions. This intact bsAb is able to simultaneously redirect and activate T cells (via one arm) and accessory cells (via the Fc region) in the vicinity of tumor cells.5 We demonstrate here that such a trifunctional bsAb can also induce effective immune responses in 2 different syngeneic mouse tumor models. Furthermore, this isotype combination leads to a high production yield of bsAbs with a simple, 1-step purification method.6
The successful induction of antitumor immunity was observed in various murine tumor models by using gene therapy,7 vaccination strategies,8,9 or antibody-mediated immunotherapy,10,11 demonstrating the feasibility of new concepts in cancer therapy. However, the transfer of these encouraging approaches into the clinic is still hampered by certain disadvantages. One obstacle is the insufficient gene transfer in gene therapy approaches. Moreover, recent studies elucidate the complexity of T-cell regulation, revealing that, in addition to CD28, other costimulatory molecules and cytokines are necessary for appropriate T-cell activation. In this context, Renner et al12 demonstrated the need for adhesion molecules such as LFA-1 and CD2 as costimulatory signaling molecules, rather than as pure cellular contact mediators in CD3 and CD28 bsAb-stimulated T lymphocytes. Therefore, the gene transfer of single costimulatory molecules or cytokines into tumor cells will probably not be sufficient to achieve physiologic T-cell activation at the tumor site and induction of tumor-specific memory T cells. This handicap may be overcome by fusing tumor cells with dendritic cells (DCs)8 13 or by transferring genes encoding tumor antigens into DCs; however, both approaches are technically complex and therefore may be unsuitable for routine clinical application.
In our study, we tried to mimic the natural situation by using intact bsAbs to redirect not only T cells but also accessory cells to the tumor site, allowing a simultaneous activation of the antigen-presenting system. In contrast to other approaches, this process could be accomplished without the need for complicated gene transfer or cell fusion techniques. The trifunctional bsAb binds and activates the T cell by the CD3 molecule so that the activation signal 1 is delivered to the T cell. The activation of accessory cells is initiated by the binding to the Fc region of the bsAb by Fcγ-receptor type I14 and the simultaneous interaction with costimulatory molecules of the T cell such as CD40L. Vice versa activated accessory cells deliver all necessary costimulatory signals to the T cell in the postulated tri-cell complex.5 After activation by trifunctional bsAbs tumor material is phagocytosed by accessory cells as has already been demonstrated.14 We now show the induction of a cellular and humoral antitumor immunity after application of the trifunctional bsAb, BiLu, in vivo. Thereby, the immune response could be directed against the tumor-specific immunoglobulin idiotype (Id) that was not targeted by the bsAb in the A20 lymphoma model. Moreover, the induced antitumor immunity was protective as demonstrated in rechallenge experiments with untransfected A20 wild-type cells that lack the human anchor protein epithelial cell adhesion molecule (EpCAM)15 recognized by the bsAb BiLu. These data support the tri-cell complex hypothesis and make trifunctional bsAb a promising tool, especially in the treatment of malignant lymphomas.
Materials and methods
Mice and cell lines
C57BL/6 (H-2b) and BALB/c (H-2d) mice, 7 to 8 weeks of age, were purchased from Bomholtgaard (Ny, Denmark). The B16 cell line transfected with human EpCAM (B16-EpCAM) was kindly provided by M. Dohlsten.16 The human EpCAM-transfected cell line 293Ep and the corresponding vector control 293Δ were kindly provided by Markus Münz (HNO Klinik Grosshadern, Munich, Germany). Stable EpCAM transfection of the A20 cell line (A20-EpCAM) was made by using the expression vector pCEP4 (Invitrogen, NV Leek, Netherlands) deleted of the ori P and containing the complementary DNA of human EpCAM (kindly provided by R. Zeidler, HNO Klinik Grosshadern). B16 (CRL-6322), A20 wild-type (TIB-208), and HCT-8 (CCL-244) tumor cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). All cell cultures were maintained in RPMI 1640 media (Life Technologies, Paisley, Scotland) supplemented with 5% fetal calf serum (FCS), 50 μM β-mercaptoethanol, 2 mM glutamine, 1 × nonessential amino acids, and 100 U/mL penicillin and streptomycin (complete media). Additionally, Geneticin G418 was added to B16-EpCAM cells (0.5 mg/mL), and A20-EpCAM cells were grown in complete media containing Hygromycin B (0.5 mg/mL).
Production and purification of bsAb BiLu and bsF(ab′)2
The bsAb BiLu consists of the 2 parental antibodies 17A217 specific for murine CD3 and C215 directed to human EpCAM (kindly provided by M. Dohlsten, Pharmacia Upjohn, Uppsala, Sweden). It is an intact bsAb with the IgG subclass combination rat IgG2b × mouse IgG2a and was produced by using the quadroma technology.18 A single-step purification with protein A was performed as described.6 BsF(ab′)2 was obtained by enzymatic digestion of the purified intact bsAb with pepsin (Merck, Darmstadt, Germany). Hereby, 1 mg BiLu (0.4 mg/mL) was digested with 50 μg pepsin (10 Fip-U/mg) at 37°C and at pH 4.1 in 0.1 M acetate buffer for 7 hours. The reaction was stopped by adding 1 M Tris until pH 8 was reached. Residual intact bsAbs as well as digested Fc portions were separated from bsF(ab′)2 on FPLC MonoS cation exchange chromatography (Pharmacia Upjohn, Sweden). Purity and biological binding activity were confirmed by sodium dodecyl sulfate (SDS) gel electrophoresis, enzyme-linked immunosorbent assay (ELISA), flow cytometry, and cytotoxicity assays.
In vitro cytotoxicity assay
BsAb-mediated tumor cell killing was measured by a colorimetric MTT-based assay as described previously.19 Briefly, B16 tumor cells transfected with the human EpCAM gene or wild-type B16 tumor cells were coincubated with spleen cells of naive C57BL/6 mice for 24 to 48 hours in 96-well flat-bottom plates at the indicated ratios and in the presence of 50 ng/mL bsAb. Alternatively the antibody concentration was titrated from 50 to 0.05 ng/mL at a fixed E/T ratio of 20:1. To increase T-lymphocyte frequency, spleen cells were reduced of B lymphocytes by panning with anti–IgG+M (Dianova, Hamburg, Germany). As measured by flow-activated cell sorter (FACS) the effector cell population consisted of about 50% CD4+ T cells, 35% CD8+ T cells, 5% macrophages, and 10% remaining B cells (not shown). Then, after removal of effector cells by washing, viable adherent B16 cells were stained with MTT solution (0.5 mg/mL; Sigma, Deisenhofen, Germany) for 4 hours. The MTT solution was removed, and blue crystals of formazan were dissolved in dimethyl sulfoxide. Absorbance was measured with a spectrophotometer at 540 nm. Results were calculated as follows: the percentage of cell death = 100 × (C − E)/(C − B), where C is the optical density reading of target cells without effectors (control), B is the background without any cell population, and E is the optical density reading of adherent tumor cells remaining in the wells after coincubation with effector cells. In all cases at least triplicates were performed, and SD was less than 15%.
FACS analysis
Target cells (2-4 × 105) were incubated with the primary antibody for 30 minutes on ice in FACS buffer (phosphate-buffered saline with 5% FCS and 0.1% NaN3). After washing, cells were stained with a second fluorescein isothiocyanate (FITC)– or phycoerythrin-labeled antibody, washed, and suspended in FACS buffer with propidium iodide. Flow cytometry was performed by using a FACSCalibur cytometer and the CellQuest analysis program (Becton Dickinson, Heidelberg, Germany). For detection of bsAbs binding on murine CD3, EL-4 lymphoma or spleen cells from mice were used as targets. Binding to human EpCAM was assessed with the transfected cell lines A20-EpCAM, B16-EpCAM, 293Ep, or with human HCT-8 cells. Polyclonal antimouse IgG, antirat IgG (Dianova, Hamburg, Germany), or monoclonal anti–rat IgG2b (ATCC/Tib174) and antimouse IgG2a (R19-15, Pharmingen, Hamburg, Germany) were used as secondary detection antibodies.
Assessment for tumor-reactive antibodies
The presence of tumor-reactive antibodies in mice sera was determined by flow cytometry. For this purpose mice sera were diluted 1:30 in FACS buffer and incubated with 2 to 4 × 105 B16 or A20 target cells. After washing antibodies bound to tumor cells were detected either by FITC-conjugated polyclonal rat anti–mouse IgG or with IgG subclass-specific antibodies against mouse IgG1 and IgG2a (Pharmingen, Hamburg, Germany). Reactivity was calculated as the percentage of positively stained tumor cells. The nonparametric Mann-Whitney U test was used to evaluate statistical differences between serologic reactions.
Detection of EpCAM-specific antibodies
Preimmune and postimmune sera of mice were pooled in groups and titrated against human EpCAM-transfected 293Ep cells. Cell-bound antibodies were detected by flow cytometry using a FITC-conjugated goat antimouse IgG1 antibody (Southern Biotechnology Associates, Birmingham, AL). EpCAM specificity of detected antibodies was verified by serum titration against the 293Δ cell line that does not express the EpCAM antigen (vector control).
Anti-idiotypic ELISA
Antibodies against the A20 Id were measured as follows: ELISA plates were coated with A20 IgG2a purified from culture supernatants, incubated with serially diluted preimmune or immune sera, followed by biotin-labeled goat antimouse IgG1 (Amersham Life Science, Buckinghamshire, United Kingdom), and developed with avidin-peroxidase. Reactivity of the sera with the constant domains of the A20 immunoglobulin was excluded by a similar ELISA using the irrelevant BALB/c-derived IgG2a monoclonal antibody TPA02 (TRION Pharma GmbH, Munich, Germany) as the capturing antigen. Quantification of the anti-Id levels was performed by using the 6D4 (H.L., unpublished data, July 1993) mIgG1 anti-Id antibody against the mIgG2a 7D620 antibody as a standard.
In vivo therapy with bsAb
C57BL/6 mice received 5 × 103 B16-EpCAM melanoma cells intraperitoneally on day 0. BsAb treatment was started with 2.5 μg BiLu on day 2 and continued with 1 μg each on days 4 and 7. Alternatively, 10 μg bsAb was given on day 2 and another 5 μg on days 4 and 7. The parental group was treated with a combination of the 2 monospecific antibodies C215 and 17A2 by using equivalent doses for each antibody. Control groups received no antibody. Surviving mice were rechallenged with a reduced but still lethal amount of 750 B16-EpCAM cells. Mice were killed after apparent intraperitoneal tumor growth (abdominal swelling) that was confirmed by postmortem dissection. BALB/c mice were challenged with 2 × 106 A20-EpCAM cells intravenously followed by intraperitoneal injection of 4 μg bsAb BiLu or bsF(ab′)2 3 hours later. Again the control with parental antibodies was performed. In all cases at least 2 independent experiments were carried out with a minimum of 6 mice per group. Statistical analysis of survival data was performed by using the log-rank test.
Vaccinations and adoptive serum transfer
Female BALB/c mice 10 to 12 weeks old were immunized against the syngeneic A20 tumor line by intraperitoneal injection of 5 × 104 irradiated (50 Gy) A20-EpCAM or A20 wild-type cells 2 hours after intraperitoneal donation of 4 μg bsAb BiLu or bsF(ab′)2 (priming immunization). Four weeks later mice received an identical booster immunization. To evaluate the role of CD4+ T-helper (TH) cells in the immunization process a group of mice was depleted of CD4+ T cells by injection of 400 to 750 μg CD4.2 antibody21 every 3 days before priming and booster immunization. CD4+ or CD8+ T-cell depletion in the effector phase was performed with 500 μg CD4.2 or CD8.2 antibody21 4 days before the tumor challenge. For control, mice were treated only with irradiated tumor cells without bsAb. The final challenge consisted of 7 × 105 viable, untransfected wild-type A20 cells and was performed (intraperitoneally) 6 weeks after the priming immunization. Alternatively, 4 × 105 A20 cells were given intravenously. Blood samples were collected from the tail vein before any treatment (control) and 2 days before the challenge. For adoptive transfer experiments sera of bsAb-immunized mice were pooled, and 300 μL serum was injected intravenously together with 3 × 105 A20 wild-type cells into naive BALB/c mice. Control mice received serum of naive nonimmunized animals in combination with tumor cells. Each experiment has been repeated at least once. All animal groups comprised 6 mice. Statistical analysis of survival curves was performed by using the log-rank test.
Results
Intact bsAb BiLu reveals high antitumor efficacy in vitro and in vivo
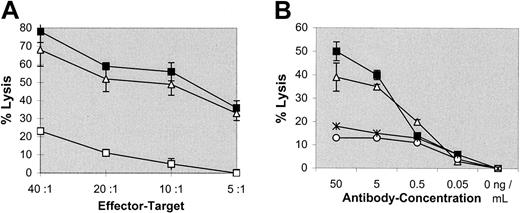
To evaluate the efficacy of the intact bsAb, BiLu, with specificities anti-CD3 × antihuman EpCAM in redirected lysis of tumor cells, cytotoxicity experiments were carried out in vitro. Spleen cells of naive C57BL/6 mice with an increased T-cell frequency of about 85% were obtained by removing B cells by anti–IgG+M panning. Different than described by others,22,23 thus obtained T cells were not preactivated by interleukin 2 (IL-2) or other stimulatory molecules. Nevertheless, the melanoma line B16-EpCAM was efficiently killed by these effector cells in the presence of bsAb BiLu (Figure 1A). Furthermore, bsAb-mediated lysis was much greater than that achieved by using the parental antibodies at equimolar amounts and was observed over a wide range of bsAb concentrations. Activity was still detected at 5 ng/mL (Figure1B). Also bsF(ab′)2 fragments efficiently induced tumor cell killing, indicating comparable biological activity (Figure 1A,B). Finally, the cell-mediated lysis was mainly antigen specific because B16 wild-type cells that do not express the target antigen EpCAM were only weakly killed (Figure 1A). However, there was a tumor growth inhibition of B16 cells especially at higher E/T ratios. Although we do not know the exact mechanism of this inhibition, bystander effects such as the release of cytokines like tumor necrosis factor α or interferon γ might be responsible for this observation.24 In summary, the bsAb BiLu revealed high and predominant antigen-specific lytic capacity for syngeneic tumor cells in vitro.
BsAb-mediated cytotoxicity in vitro.
Tumor cell killing was measured by a colorimetric MTT-based assay. (A) Varying E/T ratios were carried out with a constant amount of 50 ng/mL bsAb BiLu (▪) or bsF(ab′)2 (▵) targeted against transfected B16-EpCAM cells. To differentiate antigen-independent cell lysis by the bsAb, nontransfected B16 target cells were used (bsAb wild-type, ■). (B) At an E/T ratio of 20:1, the bsAb BiLu (▪) and bsF(ab′)2 (▵) were titrated from 50 to 0.05 ng/mL. Controls with the parental antibodies were performed at equimolar amounts (17A2+C215, *; and C215, ○). B16-EpCAM cells were used as targets. Effector cell-induced background lysis in the absence of antibody was subtracted.
BsAb-mediated cytotoxicity in vitro.
Tumor cell killing was measured by a colorimetric MTT-based assay. (A) Varying E/T ratios were carried out with a constant amount of 50 ng/mL bsAb BiLu (▪) or bsF(ab′)2 (▵) targeted against transfected B16-EpCAM cells. To differentiate antigen-independent cell lysis by the bsAb, nontransfected B16 target cells were used (bsAb wild-type, ■). (B) At an E/T ratio of 20:1, the bsAb BiLu (▪) and bsF(ab′)2 (▵) were titrated from 50 to 0.05 ng/mL. Controls with the parental antibodies were performed at equimolar amounts (17A2+C215, *; and C215, ○). B16-EpCAM cells were used as targets. Effector cell-induced background lysis in the absence of antibody was subtracted.
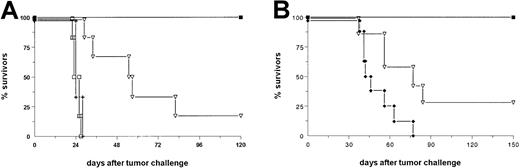
As a next step, we analyzed the antitumor efficiency of the bsAb BiLu in vivo. In a therapeutic approach we injected C57BL/6 mice with a lethal dose of B16-EpCAM melanoma cells intraperitoneally and initiated bsAb therapy 2 days later. A total dose of 4.5 μg (2.5/1/1 [2.5 μg on day 2, 1 μg on day 4, and 1 μg on day 7 after tumor challenge]) was sufficient to cure 100% of the animals, whereas all control mice died within 28 days (Figure2A). In addition, the therapeutic outcome of the parental group, which received 4.5 μg of each parental antibody, was significantly worse (P < .006) as compared with the bsAb group. In contrast to the observed tumor growth inhibition of untransfected B16 cells in vitro such an effect was not seen in vivo: the growth of untransfected B16 wild-type tumor could not be inhibited or delayed by the bsAb (Figure 2A). This finding clearly demonstrates the high specificity of bsAb BiLu-induced tumor cell killing and underlines the importance of retargeted cytotoxicity. To further evaluate whether these results would be similar with other tumors, we repeated this experiment in a B-cell lymphoma model with EpCAM-transfected A20 cells. A single dose of 4 μg bsAb BiLu was sufficient to inhibit tumor growth with 100% survivors, whereas an equimolar amount of both parental antibodies led to a significantly worse inhibition of tumor growth, with 29% survivors (P = .0009; Figure 2B). These results clearly demonstrated the benefit of the redirection principle by this bsAb in the B16 melanoma and A20 lymphoma models. Hence, our data are in good accordance with the findings of other groups that observed the same superiority of bsAb compared with parental, monospecific antibodies in different lymphoma models.25-28
The bsAb BiLu reveals high antitumor activity in vivo in 2 different syngeneic tumor models.
(A) C57BL/6 mice (n = 6) received 5 × 103B16-EpCAM cells intraperitoneally on day 0. BsAb treatment was started with 2.5 μg on day 2 and continued with 1 μg each on days 4 and 7 (▪). The group that received parental antibodies (▿) (n = 6) was treated with a combination of the 2 monospecific antibodies C215 (antihuman EpCAM) and 17A2 (antimurine CD3). The control group (+) (n = 6) received no antibody. To clarify the antigen dependency of the treatment one group of mice (■) (n = 6) was challenged with 5 × 103 untransfected B16 cells and injected with bsAb as indicated above. Experiments were repeated 3 times with similar results. (B) BALB/c mice were challenged with 2 × 106A20-EpCAM cells intravenously followed by intraperitoneal injection of 4 μg bsAb (▪) (n = 12) or bsF(ab′)2 (♦) (n = 8) 3 hours later. Again, a control group with parental antibodies (▿) (n = 7) was included, and data were confirmed by another independent experiment.
The bsAb BiLu reveals high antitumor activity in vivo in 2 different syngeneic tumor models.
(A) C57BL/6 mice (n = 6) received 5 × 103B16-EpCAM cells intraperitoneally on day 0. BsAb treatment was started with 2.5 μg on day 2 and continued with 1 μg each on days 4 and 7 (▪). The group that received parental antibodies (▿) (n = 6) was treated with a combination of the 2 monospecific antibodies C215 (antihuman EpCAM) and 17A2 (antimurine CD3). The control group (+) (n = 6) received no antibody. To clarify the antigen dependency of the treatment one group of mice (■) (n = 6) was challenged with 5 × 103 untransfected B16 cells and injected with bsAb as indicated above. Experiments were repeated 3 times with similar results. (B) BALB/c mice were challenged with 2 × 106A20-EpCAM cells intravenously followed by intraperitoneal injection of 4 μg bsAb (▪) (n = 12) or bsF(ab′)2 (♦) (n = 8) 3 hours later. Again, a control group with parental antibodies (▿) (n = 7) was included, and data were confirmed by another independent experiment.
BsAb-treated mice develop tumor-reactive antibodies and long-lasting antitumor protection
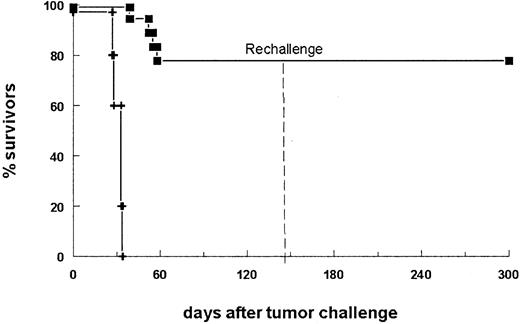
In a second therapy experiment, 14 of 18 bsAb-treated mice survived the primary B16-EpCAM tumor challenge (Figure3). To analyze differences in immune responses between mice that succumbed to the tumor and those that successfully rejected it, we assessed the sera for tumor-reactive antibodies. Indeed, we found a strong humoral response specific for the tumor in all surviving animals. In contrast, sera of mice that did not survive displayed only a weak reaction (Table1, mice 15-18). Analysis of the immunoglobulin subclass composition revealed a dominant IgG2a response, whereas no IgG1 antitumor antibodies could be detected. To determine whether antitumor protection was also generated in the surviving animals, we challenged the mice a second time with a lethal number of tumor cells in the absence of bsAb. All animals survived the tumor rechallenge (Figure 3). Consequently, the initial treatment of the tumor with this bsAb led not only to total tumor eradication but also to the induction of immune protection. Notably, all the mice rechallenged on day 144 after the primary challenge were still able to reject the tumor, indicating the high efficacy and long duration of the antitumor response.
BsAb-treated mice develop long-lasting antitumor protection.
The survival of mice is shown after donation of 5 × 103B16-EpCAM cells intraperitoneally on day 0 followed by the application of 10 μg bsAb BiLu (▪) on day 2 and another 5 μg on days 4 and 7. The control group received no antibody (+). Mice surviving the first tumor challenge (14 of 18 mice) were rechallenged on day 144 with a minimal lethal dose of 750 B16-EpCAM cells intraperitoneally. This time no bsAb treatment was performed. Whereas all control mice (n = 5) developed a tumor (not shown), mice pretreated with the bsAb BiLu successfully rejected the second tumor challenge. The experiment was repeated twice with similar results.
BsAb-treated mice develop long-lasting antitumor protection.
The survival of mice is shown after donation of 5 × 103B16-EpCAM cells intraperitoneally on day 0 followed by the application of 10 μg bsAb BiLu (▪) on day 2 and another 5 μg on days 4 and 7. The control group received no antibody (+). Mice surviving the first tumor challenge (14 of 18 mice) were rechallenged on day 144 with a minimal lethal dose of 750 B16-EpCAM cells intraperitoneally. This time no bsAb treatment was performed. Whereas all control mice (n = 5) developed a tumor (not shown), mice pretreated with the bsAb BiLu successfully rejected the second tumor challenge. The experiment was repeated twice with similar results.
Detection of tumor-reactive antibodies in the B16 melanoma model
| Mice . | % reactivity with B16-EpCAM cells (sera on day 0) . | % reactivity with B16-EpCAM cells (sera immediately before death) . | % reactivity with B16-EpCAM cells (sera on day 143)* . | mIgG2a† . | mIgG1† . |
|---|---|---|---|---|---|
| 1 | 5 | 87 | + | − | |
| 2 | 6 | 42 | + | − | |
| 3 | 5 | 70 | + | − | |
| 4 | 4 | 73 | + | − | |
| 5 | 5 | 30 | + | − | |
| 6 | 5 | 66 | + | − | |
| 7 | 5 | 57 | + | − | |
| 8 | 6 | 71 | + | − | |
| 9 | 5 | 47 | + | − | |
| 10 | 5 | 49 | + | − | |
| 11 | 6 | 69 | + | − | |
| 12 | 5 | 40 | + | − | |
| 13 | 5 | 54 | + | − | |
| 14 | ND | ND | ND | ND | |
| 15 | 4 | 8 | − | − | |
| 16 | 5 | 14 | − | − | |
| 17 | 6 | 10 | − | − | |
| 18 | 5 | 18 | + | − | |
| Control serum | 3 | 4 | 2 | − | − |
| Mice . | % reactivity with B16-EpCAM cells (sera on day 0) . | % reactivity with B16-EpCAM cells (sera immediately before death) . | % reactivity with B16-EpCAM cells (sera on day 143)* . | mIgG2a† . | mIgG1† . |
|---|---|---|---|---|---|
| 1 | 5 | 87 | + | − | |
| 2 | 6 | 42 | + | − | |
| 3 | 5 | 70 | + | − | |
| 4 | 4 | 73 | + | − | |
| 5 | 5 | 30 | + | − | |
| 6 | 5 | 66 | + | − | |
| 7 | 5 | 57 | + | − | |
| 8 | 6 | 71 | + | − | |
| 9 | 5 | 47 | + | − | |
| 10 | 5 | 49 | + | − | |
| 11 | 6 | 69 | + | − | |
| 12 | 5 | 40 | + | − | |
| 13 | 5 | 54 | + | − | |
| 14 | ND | ND | ND | ND | |
| 15 | 4 | 8 | − | − | |
| 16 | 5 | 14 | − | − | |
| 17 | 6 | 10 | − | − | |
| 18 | 5 | 18 | + | − | |
| Control serum | 3 | 4 | 2 | − | − |
EpCAM indicates epithelial cell adhesion molecule; IgG, immunoglobulin G; ND, not done.
Measurements were performed by flow cytometry. Tumor-reactive antibodies were detected with polyclonal fluorescein isothiocyanate-conjugated rat antimouse IgG antibody. Numbers represent percentage of positively stained cells.
Measurements of tumor-reactive sera were done as described in above footnote, but detection followed with IgG subclass-specific antibodies against mouse IgG2a and mouse IgG1.
BsAb BiLu induces antitumor immunity in the A20 lymphoma model
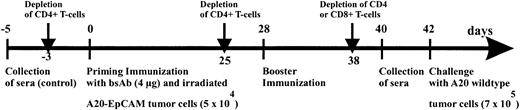
Next, we investigated the exact contribution of humoral and cellular immune responses to the observed antitumor immunization. Having established that the combination of BiLu and vital tumor cells induce an antitumor response, it was important to adapt the immunization protocol to the clinical situation of tumor patients. Therefore, we designed a vaccination strategy with a defined dose of irradiated, proliferation-incompetent tumor cells. We also switched from the aggressively growing B16 melanoma model to the more moderate proliferating A20 lymphoma model. In these experiments BALB/c mice were immunized with irradiated A20-EpCAM cells and bsAb BiLu. Finally, we challenged the mice deliberately with untransfected A20 wild-type cells to see whether an immune response against the whole tumor cell independent of the target antigen EpCAM could be achieved (Figure4). Again, prior to challenge tumor-reactive antibodies were detectable in the sera of BiLu-treated mice but not in control mice treated only with irradiated tumor cells (Table 2). These data indicated that the intact bsAb was essential for the generation of the humoral antitumor response. Moreover, the presence of induced antibodies against the tumor correlated with the survival of mice. All control mice died, whereas animals treated with intact bsAb and developing tumor-reactive antibodies survived the tumor challenge (Figure5).
Immunization scheme of BALB/c mice.
Mice were immunized with 4 μg bsAb BiLu followed by 5 × 104 irradiated A20-EpCAM cells given intraperitoneally 2 hours later. Then, after 4 weeks an identical booster immunization was performed, and finally mice were intraperitoneally challenged another 2 weeks later with 7 × 105 A20 wild-type cells. The role of the T cells was investigated by antibody-induced depletion of CD4+ or CD8+ T cells with 400 to 750 μg CD4.2 antibody and 500 μg CD8.2 antibody, respectively, at indicated time points. From all mice blood samples were collected before any treatment (control sera) as well as 2 days before tumor challenge.
Immunization scheme of BALB/c mice.
Mice were immunized with 4 μg bsAb BiLu followed by 5 × 104 irradiated A20-EpCAM cells given intraperitoneally 2 hours later. Then, after 4 weeks an identical booster immunization was performed, and finally mice were intraperitoneally challenged another 2 weeks later with 7 × 105 A20 wild-type cells. The role of the T cells was investigated by antibody-induced depletion of CD4+ or CD8+ T cells with 400 to 750 μg CD4.2 antibody and 500 μg CD8.2 antibody, respectively, at indicated time points. From all mice blood samples were collected before any treatment (control sera) as well as 2 days before tumor challenge.
Detection of tumor-reactive antibodies in the A20 lymphoma model
| Group . | Mice . | % reactivity with A20 cells (preimmune sera) . | % reactivity with A20 cells (sera after vaccination on day 40) . | Vaccination treatment . |
|---|---|---|---|---|
| A | 1 | 1 | 40 | Application of intact bsAb BiLu and irradiated tumor cells |
| 2 | 1 | 46 | ||
| 3 | 1 | 18 | ||
| 4 | 1 | 29 | ||
| 5 | 2 | 28 | ||
| 6 | 1 | 30 | ||
| B | 7 | 2 | 4 | Application of intact bsAb BiLu and irradiated tumor cells; depletion of CD4+ T cells |
| 8 | 1 | 14 | ||
| 9 | 2 | 9 | ||
| 10 | 2 | 5 | ||
| 11 | 2 | 3 | ||
| 12 | 2 | 15 | ||
| C | 13 | 1 | 1 | Application of bsF(ab′)2 fragments and irradiated tumor cells |
| 14 | 2 | 1 | ||
| 15 | 2 | 1 | ||
| 16 | 2 | 1 | ||
| 17 | 2 | 2 | ||
| 18 | 1 | 2 | ||
| D | 19 | 1 | 2 | Control: application of irradiated tumor cells without antibody |
| 20 | 1 | 3 | ||
| 21 | 2 | 4 | ||
| 22 | 1 | 1 | ||
| 23 | 1 | 1 | ||
| 24 | 1 | 1 |
| Group . | Mice . | % reactivity with A20 cells (preimmune sera) . | % reactivity with A20 cells (sera after vaccination on day 40) . | Vaccination treatment . |
|---|---|---|---|---|
| A | 1 | 1 | 40 | Application of intact bsAb BiLu and irradiated tumor cells |
| 2 | 1 | 46 | ||
| 3 | 1 | 18 | ||
| 4 | 1 | 29 | ||
| 5 | 2 | 28 | ||
| 6 | 1 | 30 | ||
| B | 7 | 2 | 4 | Application of intact bsAb BiLu and irradiated tumor cells; depletion of CD4+ T cells |
| 8 | 1 | 14 | ||
| 9 | 2 | 9 | ||
| 10 | 2 | 5 | ||
| 11 | 2 | 3 | ||
| 12 | 2 | 15 | ||
| C | 13 | 1 | 1 | Application of bsF(ab′)2 fragments and irradiated tumor cells |
| 14 | 2 | 1 | ||
| 15 | 2 | 1 | ||
| 16 | 2 | 1 | ||
| 17 | 2 | 2 | ||
| 18 | 1 | 2 | ||
| D | 19 | 1 | 2 | Control: application of irradiated tumor cells without antibody |
| 20 | 1 | 3 | ||
| 21 | 2 | 4 | ||
| 22 | 1 | 1 | ||
| 23 | 1 | 1 | ||
| 24 | 1 | 1 |
bsAb indicates bispecific antibody.
Sera of immunized BALB/c mice were assessed for tumor-reactive antibodies by flow-activated cell sorter analysis as described in “Materials and methods.” The reaction is shown as the percentage of positively stained tumor cells. P < .0023 for the differences between group A and the other groups B-D, by the Mann-Whitney U test.
BALB/c mice immunized with intact bsAb BiLu and irradiated A20-EpCAM cells successfully reject A20 wild-type challenge.
Mice were immunized as indicated in Figure 4 with bsAb (▪) or bsF(ab′)2 fragments (♦) and irradiated A20-EpCAM cells. The control group was treated only with irradiated tumor cells without bsAb (+). The CD4 depletion group (■) was identically treated to the bsAb group but injected with CD4+ T-cell–depleting CD4.2 antibody. In 2 independent experiments similar data were obtained.
BALB/c mice immunized with intact bsAb BiLu and irradiated A20-EpCAM cells successfully reject A20 wild-type challenge.
Mice were immunized as indicated in Figure 4 with bsAb (▪) or bsF(ab′)2 fragments (♦) and irradiated A20-EpCAM cells. The control group was treated only with irradiated tumor cells without bsAb (+). The CD4 depletion group (■) was identically treated to the bsAb group but injected with CD4+ T-cell–depleting CD4.2 antibody. In 2 independent experiments similar data were obtained.
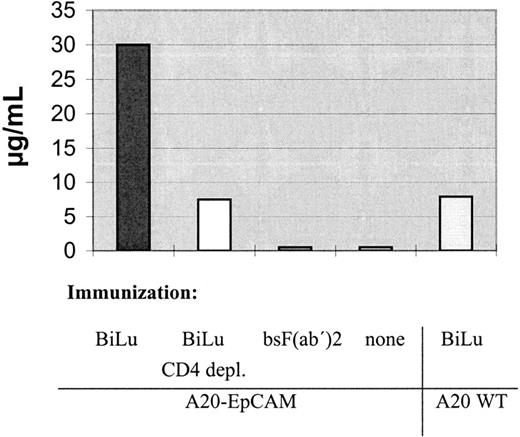
We further analyzed the mice sera for EpCAM-specific antibodies. Interestingly, the human antigen was not immunogenic per se. Only after injection of intact bsAb BiLu and A20-EpCAM cells, antibodies against human EpCAM were generated (Table 3). Thereby, the induction of an idiotypic network response can be excluded, because the donation of BiLu in combination with EpCAM− A20 wild-type cells did not lead to an immune response against the tumor antigen. Remarkably, mice developed a humoral response and were protected against wild-type A20 cells, although they were immunized with transfected A20-EpCAM cells. As a consequence, an antitumor response against antigens other than the target antigen EpCAM must have been induced. An absolutely tumor-specific antigen of B-cell lymphomas is the immunoglobulin Id. To investigate whether this antigen was targeted, we also looked for anti-Id antibodies. Indeed, we found significant titers against the A20 immunoglobulin Id after we immunized mice with bsAb BiLu and A20-EpCAM cells according to our vaccination protocol (Figure6). However, anti-Id antibodies were not detectable in the sera of control mice that had been immunized solely with irradiated A20-EpCAM cells in the absence of bsAb. However, the application of BiLu and A20 wild-type cells resulted in a weak anti-A20 Id response, too. Challenging these mice with A20 wild-type cells revealed a survival rate of 50% (Figure7). This result suggests that target-antigen–independent effects are also part of the bsAb-mediated antitumor immunization, but full tumor protection (100% survival) is only accomplished after specific immunization with bsAb-targeted A20-EpCAM cells (Figure 7). In summary, the injection of the trifunctional bsAb BiLu raised humoral responses against at least 2 different tumor-specific antigens, A20 Id and EpCAM.
Antihuman epithelial cell adhesion molecule response in treated mice
| Serum pool of immunized mice3-150 . | Reactivity . | |
|---|---|---|
| 293Ep cells . | 293Δ cells (vector control) . | |
| A20-EpCAM | 03-151 | 0 |
| A20-EpCAM + BiLu | 240 | 0 |
| A20-EpCAM + bsF(ab′)2 | 0 | 0 |
| A20WT + BiLu | 0 | 0 |
| Serum pool of immunized mice3-150 . | Reactivity . | |
|---|---|---|
| 293Ep cells . | 293Δ cells (vector control) . | |
| A20-EpCAM | 03-151 | 0 |
| A20-EpCAM + BiLu | 240 | 0 |
| A20-EpCAM + bsF(ab′)2 | 0 | 0 |
| A20WT + BiLu | 0 | 0 |
Mice were immunized according to Figure 4. Sera of mice were pooled in groups and titrated against human epithelial cell adhesion molecule (EpCAM), expressing 293Ep cells and nonexpressing 293Δ cells (vector control), respectively. Cell-bound antibodies were detected by flow cytometry using a fluorescein isothiocyanate-conjugated goat antimouse immunoglobulin G1 antibody.
Data reflect serum dilutions at which percentage of positively stained cells was still 3 times above background reaction with control sera (serum pool before immunization).
Antibodies directed against the A20 immunoglobulin Id could be detected in sera of BALB/c mice immunized with intact bsAb BiLu and A20-EpCAM cells.
This response was clearly diminished after depletion of CD4+ T cells in the immunization phase. The use of A20 wild-type cells and intact bsAb resulted in a weak reaction, too. For the anti-idiotypic ELISA pooled and serially diluted preimmune or immune sera were incubated on A20 Id-coated ELISA plates, followed by biotin-labeled goat antimouse IgG1 and developed with avidin-peroxidase. Preimmune sera revealed no reaction.
Antibodies directed against the A20 immunoglobulin Id could be detected in sera of BALB/c mice immunized with intact bsAb BiLu and A20-EpCAM cells.
This response was clearly diminished after depletion of CD4+ T cells in the immunization phase. The use of A20 wild-type cells and intact bsAb resulted in a weak reaction, too. For the anti-idiotypic ELISA pooled and serially diluted preimmune or immune sera were incubated on A20 Id-coated ELISA plates, followed by biotin-labeled goat antimouse IgG1 and developed with avidin-peroxidase. Preimmune sera revealed no reaction.
Specific versus nonspecific immunization.
To differentiate between target-antigen–dependent and –independent immunization effects induced by the bsAb BiLu, mice were immunized as outlined in Figure 4 with bsAb and irradiated A20-EpCAM cells (▪), or with bsAb and nontransfected A20 wild-type cells (■). Only the use of A20-EpCAM cells resulted in full tumor protection. Control mice (+) that received irradiated A20 wild-type cells without bsAb revealed no protection. Each group comprised 6 mice.
Specific versus nonspecific immunization.
To differentiate between target-antigen–dependent and –independent immunization effects induced by the bsAb BiLu, mice were immunized as outlined in Figure 4 with bsAb and irradiated A20-EpCAM cells (▪), or with bsAb and nontransfected A20 wild-type cells (■). Only the use of A20-EpCAM cells resulted in full tumor protection. Control mice (+) that received irradiated A20 wild-type cells without bsAb revealed no protection. Each group comprised 6 mice.
To determine whether this humoral antitumor response contributed to the observed protection against the A20 wild-type challenge, we performed adoptive transfer experiments. Sera of bsAb-immunized mice were pooled and transferred together with vital A20 cells into naive BALB/c mice. Although the protection effect was moderate, tumor growth in these mice was delayed significantly when compared with control mice that received A20 cells in sera of unimmunized animals (P = .018). This demonstrated that the obtained antitumor protection was mediated at least in part by the humoral reaction against the tumor (Figure 8).
Adoptive transfer of immune serum into naive BALB/c mice significantly delays tumor growth (P = .018).
Sera taken from mice 6 weeks after immunization with bsAb BiLu (▪) and irradiated tumor cells were pooled and transferred (300 μL) together with 3 × 105 viable A20 cells intravenously into naive BALB/c mice. The control group (+) received serum of naive, untreated mice in combination with tumor cells.
Adoptive transfer of immune serum into naive BALB/c mice significantly delays tumor growth (P = .018).
Sera taken from mice 6 weeks after immunization with bsAb BiLu (▪) and irradiated tumor cells were pooled and transferred (300 μL) together with 3 × 105 viable A20 cells intravenously into naive BALB/c mice. The control group (+) received serum of naive, untreated mice in combination with tumor cells.
Because the production of antibodies against tumor-specific or -associated antigens by B lymphocytes requires the support of CD4+ TH cells, we expected to suppress this reaction by the application of a depleting anti-CD4 antibody during the immunization phase. In fact, the generation of antibodies against A20 cells, in general, as well as specific anti-idiotypic antibodies was diminished after depletion of TH cells (Table 2, Figure 6), resulting also in a significantly reduced number of surviving animals (Figure 5 and Figure 9;P = .02). Therefore, TH cells were mandatory for bsAb-based induction of tumor-reactive antibodies as well as for tumor protection. Next, we evaluated the role of T cells in the effector phase. To this end, the depletion of CD8+ T cells caused a decrease of survival rate from 100% to 66% (Figure 9), whereas the depletion of CD4+ T cells had no effect (not shown). This finding indicated the participation of cytotoxic CD8+ T lymphocytes in the eradication of the tumor. Taken together, these results demonstrated the generation of humoral as well as cellular immunity against the A20 lymphoma induced in the presence of intact bsAb BiLu.
The depletion of CD8+ T cells in the effector phase reduces tumor protection.
Moreover, depletion of CD4+ T cells in the priming phase results in a significant loss of tumor rejection (P = .02). BALB/c mice (n = 6) were vaccinated according to the immunization protocol outlined in Figure 4. Depletion of T cells was performed with injections of CD8.2 antibody during the effector phase (■, CD8-E) or with CD4.2 antibody during the immunization phase (⋄, CD4-I). Control mice (+) were not pretreated. All mice received an intravenous tumor challenge of 4 × 105 A20 cells. ▪ indicates bsAb.
The depletion of CD8+ T cells in the effector phase reduces tumor protection.
Moreover, depletion of CD4+ T cells in the priming phase results in a significant loss of tumor rejection (P = .02). BALB/c mice (n = 6) were vaccinated according to the immunization protocol outlined in Figure 4. Depletion of T cells was performed with injections of CD8.2 antibody during the effector phase (■, CD8-E) or with CD4.2 antibody during the immunization phase (⋄, CD4-I). Control mice (+) were not pretreated. All mice received an intravenous tumor challenge of 4 × 105 A20 cells. ▪ indicates bsAb.
Fc region of the bsAb BiLu is obligatory for the induction of anti-A20 immunity and efficient tumor cell killing
To clarify the role of the Fc region of the bsAb for the induction of antitumor immunity, we evaluated the efficacy of F(ab′)2fragments of BiLu. Complete bsAb was digested with pepsin under limiting conditions and purified on FPLC ion exchange chromatography. FACS analysis and SDS gel electrophoresis proved that biologically active bsF(ab′)2 fragments of high purity were obtained (not shown). This finding was confirmed by in vitro cytotoxicity assays in which the mediated tumor cell killing proved to be comparable to intact bsAb (Figure 1). However, these bsF(ab′)2 fragments failed to induce an immune protection against A20 lymphoma cells in vivo. Neither tumor-reactive, EpCAM-specific, nor anti-A20 Id antibodies were detectable nor was fast performance liquid chromatography effective protection against the tumor challenge observed (Tables 2 and 3, Figures 5 and 6). These data underline the essential role of the Fc part for the development of protective immunity.
Weiner et al29 compared genetically constructed bsF(ab′)2 with intact bsAb redirecting CD3 T cells to the 38C13 lymphoma Id. They reported increased T-cell activation and significantly improved therapeutic outcome when complete bsAb was used. In this context, we were also interested in evaluating the direct tumor killing capacity of bsF(ab′)2 compared with the intact bsAb BiLu in a therapeutic setting. Therefore, we challenged BALB/c mice with A20-EpCAM cells and began with antibody treatment (4 μg) 3 hours later. Whereas the application of bsAb BiLu resulted in a 100% protection against the tumor, all mice in the bsF(ab′)2group succumbed to the lymphoma (Figure 2B; P < .0001). Even the control group receiving the mixture of parental antibodies had a better outcome in long-term survival. In summary, the Fc portion of the bsAb was of indispensable importance for the therapeutic efficacy as well as for the immunization potency in the A20 tumor model.
Discussion
In this study, we have used newly designed intact bsAbs6 that redirect not only T cells but also Fc receptor+ cells to the tumor site. On the basis of previous investigations,5 we argue that 2 mechanisms are mainly responsible for the high antitumor efficacy observed with this reagent. First, in addition to the recruited T cells, accessory cells are activated by an interaction between the Fc region of the intact bsAb and Fcγ receptors.14 The combination of the 2 potent isotypes, mouse IgG2a and rat IgG2b, seems to be crucial in this process. This activation of accessory cells leads to the secretion of cytokines such as IL-12, tumor necrosis factor α, and the DC-specific cytokine DC-CK1, as well as to the presentation of costimulatory molecules to the T cell.5 Thus, T cells are postulated to be activated via signal 1 by the anti-CD3 binding arm, and all necessary costimulatory signals can be delivered by the activated accessory cells (Figure 10). Secondly, the accessory cells contribute to the tumor cell killing by using different mechanisms, including phagocytosis.14 This concentrated attack of different immune cells leads to a significantly improved tumor cell elimination compared with the mixture of both parental antibodies in vitro (Figure 1B) and in vivo (Figure 2A,B). This holds true for the A20 B-cell lymphoma as well as for the solid-growing B16 melanoma. The essential role of the Fc region in this context could be demonstrated by the use of F(ab′)2fragments of the same bsAb. These bsF(ab′)2 fragments had comparable lytic capacity in vitro, proving their general biological activity, but were rather ineffective in vivo. One reason for this observation may be a nonspecific activation of immune cells caused by mechanical stress during spleen cell preparation, making an Fc-mediated activation of effector cells unnecessary. Otherwise, such effects do not exist in vivo. Another reason for the inefficiency of bsF(ab′)2 fragments in vivo might be their shorter half-life. But even the application of increased and repeated doses to compensate for this handicap did not lead to an effectiveness as observed with intact bsAb.29 Remarkably, after the addition of costimulatory agents such as IL-2 or staphylococcal enterotoxin B (SEB) superantigen, Weiner et al29were able to reach an improved killing efficacy with bsF(ab′)2 fragments. Recently, similar observations were described with the use of bispecific single-chain variable fragments.30 These results support the view that the presence of costimulatory signals is mandatory for full T-cell activation and efficient tumor cell killing and may explain why bsF(ab′)2 fragments alone are less therapeutic.
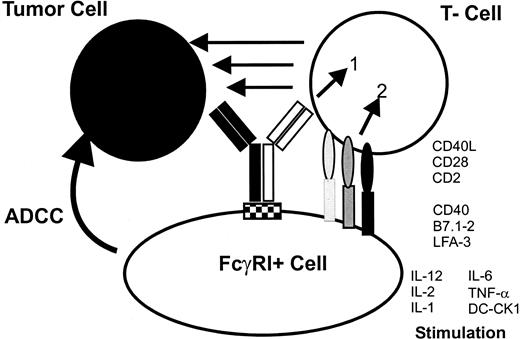
The postulated tri-cell complex model suggesting interactions that led to the induction of tumor immunity and improved tumor cell destruction by intact bsAb.
The use of mouse IgG2a × rat IgG2b intact bsAb leads to the simultaneous recruitment of tumor cells, T cells, and Fcγ-receptor+ accessory cells. The formation of this complex induces the activation of different classes of effector cells, resulting in excellent antitumor activity. The stimulation of accessory cells is demonstrated by the production of cytokines such as IL-1, IL-6, IL-12, and the DC-specific cytokine DC-CK1.5Activated accessory cells, particularly professional antigen-presenting cells such as DCs or activated macrophages mediate costimulatory signals, eg, via CD40-CD40L to T cells that are necessary to prevent T-cell anergy. Furthermore, tumor material is phagocytosed,14 processed, and presented by professional antigen-presenting cells after activation by bsAb—an important prerequisite for the induction of antitumor immunity. The tri-cell complex is only a model and should not implicate a 1:1:1 ratio of the 3 involved cell types.
The postulated tri-cell complex model suggesting interactions that led to the induction of tumor immunity and improved tumor cell destruction by intact bsAb.
The use of mouse IgG2a × rat IgG2b intact bsAb leads to the simultaneous recruitment of tumor cells, T cells, and Fcγ-receptor+ accessory cells. The formation of this complex induces the activation of different classes of effector cells, resulting in excellent antitumor activity. The stimulation of accessory cells is demonstrated by the production of cytokines such as IL-1, IL-6, IL-12, and the DC-specific cytokine DC-CK1.5Activated accessory cells, particularly professional antigen-presenting cells such as DCs or activated macrophages mediate costimulatory signals, eg, via CD40-CD40L to T cells that are necessary to prevent T-cell anergy. Furthermore, tumor material is phagocytosed,14 processed, and presented by professional antigen-presenting cells after activation by bsAb—an important prerequisite for the induction of antitumor immunity. The tri-cell complex is only a model and should not implicate a 1:1:1 ratio of the 3 involved cell types.
Obviously, the most interesting feature of this new intact bsAb format is its ability to induce a long-lasting tumor immunity as demonstrated by using 2 different syngeneic mouse tumor models. In the B16 melanoma model even 144 days after the first tumor contact and treatment with bsAb, a second challenge, without bsAb, was rejected in all mice, thereby demonstrating the long-lasting protection mechanism (Figure 3). In contrast, the combinatorial usage of 2 bsF(ab′)2fragments (CD3 × EpCAM + CD28 × EpCAM) in the identical tumor model only led to a marginal tumor elimination and poor induction of tumor immunity.31 This result further supports the hypothesis that the Fc portion of the bsAb is crucial for the killing capacity as well as for the induction of tumor immunity and suggests that single costimulatory signals via CD28 to the T cell are not sufficient to replace physiologic T-cell activation mediated by accessory cells.
To adapt the immunization protocol to the clinical situation, we designed a vaccination strategy with a defined dose of irradiated, proliferation-incompetent tumor cells (Figure 4). We also switched from the aggressively growing B16 melanoma model to the more moderate proliferating A20 lymphoma that is more similar to most courses of human cancer. However, in both tumor models a strong correlation was found between the generation of tumor-reactive antibodies after initial bsAb treatment and the survival of mice after rechallenge with tumor cells only.
A closer look at the induced humoral response revealed the presence of EpCAM-specific antibodies. The development of such antibodies was strictly restricted to mice vaccinated with intact bsAbs and was independent of an idiotypic network response (Table 3). To clarify that the immune protection was not based only on the artificially introduced human EpCAM antigen, we challenged the mice deliberately with untransfected A20 wild-type cells. In spite of this critical modification, all immunized mice survived as shown in Figure 5. Moreover, in the A20 B-cell lymphoma model we were able to raise an anti-Id response without targeting the tumor-specific Id. These results provide clear evidence that our vaccination strategy yielded immune responses against various antigens of the individual tumor, independent of the surrogate target antigen EpCAM recognized by the bsAb on the tumor cell. The significant loss of immune protection using bsF(ab′)2 fragments of the identical bsAb underlines the importance of the Fc region in this process (Figure 5). Importantly, this finding was recognized prior to the wild-type challenge by the failure to detect tumor-reactive antibodies. Therefore, assessment of antitumor antibodies served as a prognostic factor for evaluating the success of the vaccination strategy (Table 2 and Figure 6).
By using bsAb and A20 wild-type cells without the target antigen EpCAM, we detected a humoral anti-A20 Id response, although the bsAb could not interact with A20 wild-type cells directly (Figure 6). This immune response is likely due to nonspecific activation of T cells and antigen-presenting cells induced by the trifunctional bsAb. However, the observed antibody response raised by this unspecific activation was rather weak. Moreover, challenging these mice revealed only a partial protection against the tumor (Figure 7). Therefore, optimal immunization and full antitumor protection was only accomplished when trifunctional bsAb and target antigen-expressing cells were combined.
Depletion experiments were used to evaluate the role of T cells in both the induction and the effector phase. As shown in Table 2, the induction of the tumor-specific humoral response was clearly diminished (P < .0023) after CD4+ T-cell depletion in the priming phase. Moreover, a significant decrease of survival rate (P = .02) was observed in this group (Figure 9). These results indicated a clear contribution of a cellular immune response to the observed antitumor immunity. This finding could be further confirmed by the depletion of CD8+ T cells in the effector phase, which caused a markedly loss of tumor protection. However, adoptive serum transfer experiments revealed only a weak protection by the isolated use of tumor-reactive antibodies (Figure 8). Taken together, humoral as well as cellular immune responses were generated against A20 lymphoma cells, whereby cell-mediated immunity may be of major importance.
Bendandi et al32 recently described an anti-Id vaccination approach for follicular lymphoma in an adjuvant situation, resulting in molecular remissions. However, the encouraging results were hampered by the need to generate individual Id-producing hybridomas. Here, the use of intact bsAb in combination with sorted irradiated tumor cells could allow a simple application that is also capable of inducing an anti-Id response. Although we see no signs of toxicity in the preclinical mouse models, it will be essential to investigate possible side effects and toxicity of this potent immune activation in a clinical setting. In summary, the vaccination strategy described here achieves a long-lasting antitumor immunity without the complexity of gene transfer or cell-fusion techniques and therefore opens new perspectives for a broader clinical application.
We thank D. J. Schendel, M. Roskrow, E. Noessner, R. Zeidler, and R. Mocikat for suggestions and critically reading of the manuscript and P. Reitmeir for helping us with statistical analysis. Expert technical assistance from S. Erndl, S. Wosch, U. Bamberg, and J. Jasny is gratefully acknowledged.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Horst Lindhofer, GSF Institute of Clinical Molecular Biology and Tumor Genetics, Marchioninistr.25, 81377 Munich, Germany; e-mail: lindhofer@gsf.de.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal