Osteoprotegerin (OPG), the neutralizing decoy receptor for the osteoclast activator RANK ligand, was measured in serum taken from patients with multiple myeloma at the time of diagnosis. Median OPG was lower in the patients with myeloma (7.4 ng/mL; range, 2.6-80; n = 225) than in healthy age- and sex-matched controls (9.0 ng/mL; range 5.1-130; n = 40; P = .02). Importantly, OPG levels were associated with degree of radiographically assessed skeletal destruction (P = .01). The median OPG level in patients lacking osteolytic lesions was 9.1 ng/mL, as compared with 7.6 ng/mL and 7.0 ng/mL, respectively, in patients with minor or advanced osteolytic disease. Furthermore, OPG levels were associated with World Health Organization performance status (P = .003) and correlated to serum levels of carboxy-terminal propeptide of type I procollagen (PICP; P < .001) but not with clinical stage or survival. These findings suggest impaired OPG function in myeloma and give a rationale for OPG as a therapeutic agent against myeloma bone disease.
Introduction
Multiple myeloma is a plasma cell malignancy localized in the bone marrow and is strongly associated with bone destruction. It has been shown that the balance between expression of RANK ligand (RANKL) and osteoprotegerin (OPG) governs osteoclastogenesis as well as the activity of mature osteoclasts.1 In the presence of macrophage colony-stimulating factor (M-CSF), RANKL directly induces osteoclast differentiation by binding to its receptor RANK (receptor activator of NF-κB).1,2 OPG functions as a secreted inhibitor of bone resorption by binding to RANKL, thereby blocking the interaction between RANKL and RANK.1 Increased RANKL activity or decreased OPG activity could possibly contribute to the development of osteolytic lesions in patients with myeloma. The purpose of this study was to define the serum level of OPG in a large well-characterized population of patients with myeloma.
Study design
Patients
A total of 592 patients entered the Nordic Myeloma Study Group (NMSG) randomized α-interferon trial in the period from June 1990 to November 1992. Patients were randomized to receive melphalan and prednisolon with or without addition of low-dose α-interferon.3 For all patients the following parameters were registered at the time of diagnosis: age, sex, Durie and Salmon4 stage, World Health Organization (WHO) performance status, a grading of bone morbidity in 3 stages (based on radiographs of skull, vertebrae, pelvis, femora, and humeri), percentage of plasma cells in the bone marrow, urine immunoglobulin/24 hours, immunoglobulin class, and serum concentrations of the following factors: M-protein, albumin, calcium, creatinine, and β2-microglobulin. Approximately 400 sera were drawn at diagnosis, and later the following additional serum factors were analyzed: bone-specific alkaline phosphatase (bALP), interleukin-6 (IL-6), IL-6 receptor, C-reactive protein (CRP), osteocalcin, carboxy-terminal telopeptide of type I collagen (ICTP), hepatocyte growth factor (HGF), carboxy-terminal propeptide of type I procollagen (PICP), amino-terminal propeptide of type III procollagen (PIIINP), and syndecan-1.5-7
Remaining serum samples from 225 patients were available for OPG analysis. Mean patient age was 66.6 ± 9.3 years (± SD, range 32-87). Nine percent of patients were in stage I, 30% in stage II, and 61% in stage III disease. No significant differences were found between the present study population and the original patient material in regard to any of the studied parameters. The survival of the 225 patients did not differ significantly from the total study population. The NMSG study found no significant survival difference between the 2 arms of treatment,3 thus it was possible to pool data from the treatment arms in the evaluation of prognostic significance for the studied parameters. Control samples were obtained from 40 healthy age- and sex-matched individuals.
OPG measurements
Serum OPG was measured by enzyme-linked immunosorbent assay (ELISA). An OPG antibody pair (R&D Systems, Minneapolis, MN) was used according to the manufacturer's instructions. All samples were run in duplicate. The standard curve was linear between 0.2 and 2 ng/mL, and samples were diluted to concentrations within this range. The addition of soluble RANKL, heparin, or syndecan-1 did not interfere with the detection of OPG.
Statistical analyses
All statistical analyses were performed with the SPSSX/PC computer program (SPSS Inc., Chicago, IL). OPG levels were skewed and, thus, logarithmically transformed before entering analyses in which normality was required. Results were considered statistically significant when P values were < .05.
Results and discussion
Serum OPG levels are significantly reduced in patients with myeloma
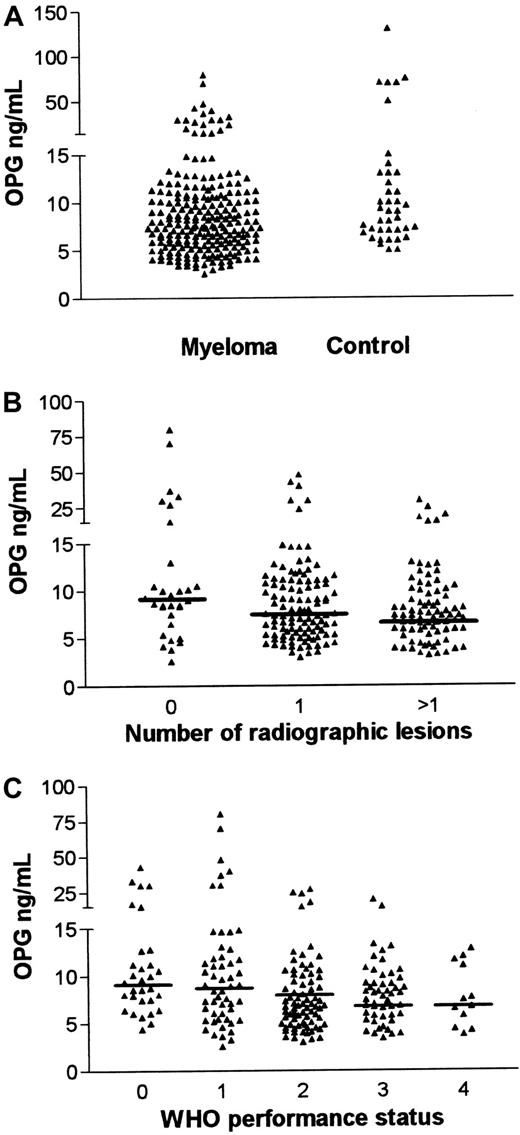
The OPG levels in our cohort of patients with myeloma at the time of diagnosis were 9.9 ± 9 ng/mL (mean ± SD; n = 225) and in controls 19 ± 27 ng/mL (n = 40). The median OPG concentrations in myeloma and control sera were 7.4 ng/mL (range, 2.6-80) and 9.0 ng /mL (range, 5.1-130), respectively (Figure1A). This difference was statistically significant (P = .02, Mann-Whitney U test). In 42 of the patients with myeloma (approximately 20%), OPG levels were below 5.1 ng/mL, the lowest level measured in the control group. Myeloma cells may produce osteoclast-activating cytokines or induce the production of such factors from neighboring stromal cells,8-10 and thereby induce increased expression of RANKL. However, RANKL activity is balanced by OPG activity, and our current data suggest that a decrease in osteoclast inhibitory capacity can add up with the stimulatory effects of osteoclast-activating factors in myeloma bone disease.
Serum OPG levels in myeloma.
(A) Serum OPG levels in patients with myeloma and in healthy controls. OPG in serum samples drawn at diagnosis from 225 patients with multiple myeloma and 40 healthy age- and sex-matched controls was analyzed by ELISA. The median OPG level in patients with myeloma was 7.4 ng/mL (range, 2.6-80) and 9 ng/mL (range, 5.1-130) in controls (P = .02). (B) Serum OPG levels in myeloma grouped by degree of skeletal involvement. Patients were grouped by degree of skeletal involvement of the disease as judged by radiography. Mean OPG levels were 15.5 ng/mL (no lesions), 9.5 ng/mL (one lesion), and 8.3 ng/mL (more than one lesion). Median OPG levels were 9.1 ng/mL (no lesions), 7.6 ng/mL (one lesion), and 7.0 ng/mL (more than one lesion), respectively. Horizontal bars indicate median values. (C) Serum OPG levels in myeloma grouped by WHO performance status. Patients were grouped by WHO performance status. Mean OPG levels were 12 ng/mL (WHO 0), 13.5 ng/mL (WHO 1), 8.0 ng/mL (WHO 2), 8.0 ng/mL (WHO 3), and 7.5 ng/mL (WHO 4). Median OPG levels were 9.3 ng/mL (WHO 0), 9.0 ng/mL (WHO 1), 8.0 ng/mL (WHO 2), 6.8 ng/mL (WHO 3), and 7.0 ng/mL (WHO 4). Horizontal bars indicate median values.
Serum OPG levels in myeloma.
(A) Serum OPG levels in patients with myeloma and in healthy controls. OPG in serum samples drawn at diagnosis from 225 patients with multiple myeloma and 40 healthy age- and sex-matched controls was analyzed by ELISA. The median OPG level in patients with myeloma was 7.4 ng/mL (range, 2.6-80) and 9 ng/mL (range, 5.1-130) in controls (P = .02). (B) Serum OPG levels in myeloma grouped by degree of skeletal involvement. Patients were grouped by degree of skeletal involvement of the disease as judged by radiography. Mean OPG levels were 15.5 ng/mL (no lesions), 9.5 ng/mL (one lesion), and 8.3 ng/mL (more than one lesion). Median OPG levels were 9.1 ng/mL (no lesions), 7.6 ng/mL (one lesion), and 7.0 ng/mL (more than one lesion), respectively. Horizontal bars indicate median values. (C) Serum OPG levels in myeloma grouped by WHO performance status. Patients were grouped by WHO performance status. Mean OPG levels were 12 ng/mL (WHO 0), 13.5 ng/mL (WHO 1), 8.0 ng/mL (WHO 2), 8.0 ng/mL (WHO 3), and 7.5 ng/mL (WHO 4). Median OPG levels were 9.3 ng/mL (WHO 0), 9.0 ng/mL (WHO 1), 8.0 ng/mL (WHO 2), 6.8 ng/mL (WHO 3), and 7.0 ng/mL (WHO 4). Horizontal bars indicate median values.
OPG levels were associated with WHO performance status and markers of bone remodeling
Radiographic examination provides a rough estimate of the bone disease. Nevertheless, as shown in Figure 1B, OPG levels were associated with the degree of radiographic skeletal involvement (P = .01, one-way analysis of variance). Others have shown that patients with myeloma without bone lesions have an increased osteoblast recruitment concomitant with an increase in bone resorption in this stage of the disease.11 A balanced enhancement of bone remodeling, in which osteoblasts are activated to counteract the increase in bone resorption, thus seems to be an early event in myeloma bone disease. Osteoblasts are probably an important, and maybe the principal, source of OPG.12 If elimination of OPG is not accelerated by the disease, one would have expected OPG levels to be elevated in early myeloma, much in the same way as is seen in women with postmenopausal osteoporosis.13 In the present study, OPG levels in patients without bone lesions did not differ significantly from those in healthy controls, suggesting an early impairment in the compensatory capacity of stromal/osteoblastic cells. OPG binds to heparan sulfate chains of syndecan-1 expressed on the surface of myeloma cells.14 Syndecan-1 is shown to be involved in internalization of heparan sulfate-binding molecules,15,16 but whether OPG is eliminated by this route is unknown. In later stages of the disease, bone formation and osteoblast function are impaired,17 which gives an explanation to the reduced OPG levels in patients with overt bone destruction.
Bone pain caused by osteolysis and pathological fractures decreases quality of life among patients with myeloma and makes a substantial effect on the WHO performance status. Low OPG levels appear to be a marker of bone disease. Our finding that low OPG levels are related to impaired performance status (P = .003, one-way analysis of variance), as illustrated in Figure 1C, is therefore not surprising.
OPG also correlated significantly with PICP (n = 67; Pearson correlation, 0.59; P < .001) and PIIINP (n = 67; Pearson correlation, 0.29; P = .02). PICP is cleaved off during type I collagen synthesis, and serum PICP is a marker of bone formation.18 19 Consequently, the positive correlation between OPG and PICP levels provides additional support to the concept of impaired osteoblast function during the development of myeloma bone disease. In a multivariate linear regression analysis involving all of the variables with significant correlation to OPG (n = 67; PICP, PIIINP, WHO performance status, and degree of skeletal involvement), PICP was the best predictor of OPG (r2 = 0.34). The model was marginally improved by the inclusion of WHO performance status (r2 = 0.38).
The OPG level did not predict response to treatment or survival. Neither did it correlate significantly with clinical stage, type, or concentration of M-component, age, percentage of plasma cells in the bone marrow, secretion of urinary M-component/24 hours, hemoglobin, nor with serum albumin, bALP, β2-microglobulin, calcium, creatinine, CRP, ICTP, HGF, IL-6, IL-6 receptor, osteocalcin, or syndecan-1.
Recently, blockage of skeletal destruction was reported after OPG was administrated to mice with sarcoma-induced osteolysis.20The decreased level of OPG in myeloma bone disease gives a rationale for OPG as a new treatment for multiple myeloma bone disease.
Supported by grants from the Norwegian Cancer Society, Rakel and Otto Kr. Bruun's legat and the Cancer Fund, Trondheim University Hospital for the osteoprotegerin work and by Schering Plough for the Nordic Myeloma Study Group trial.
C.S. and Ø.H. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Øyvind Hjertner, Institute of Cancer Research and Molecular Biology, Norwegian University of Science and Technology, MTFS, N-7489 Trondheim, Norway; e-mail:oyvind.hjertner@medisin.ntnu.no.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal