We recently reported that human dendritic cells release the leaderless secretory protein interleukin-1β (IL-1β) following specific interaction with alloreactive T lymphocytes. To clarify the molecular mechanism underlying this secretion, this study investigated the intracellular trafficking of IL-1β in dendritic cells and the signal(s) regulating its release. Results show that a fraction of the intracellular IL-1β precursor colocalizes with the hydrolase cathepsin D in endolysosomes of dendritic cells; secretion of both proteins is elicited by stimuli that induce intracellular calcium increases. Alloreactive CD8+ T lymphocytes generate a Ca++ influx in dendritic cells followed by enrichment in endolysosomes containing IL-1β and cathepsin D beneath the membrane in contact with T cells. These events result in polarized exocytosis of secretory lysosomes, mediated by microtubules, with release of IL-1β and cathepsin D toward the interacting CD8+ T cell.
Introduction
Regulated secretion is traditionally considered as a specialized process occurring in only a few polarized cell types, namely, exocrine, endocrine, and neuronal cells.1 However, former observations that, on binding to antigen-presenting B cells, T-helper cells release lymphokines preferentially over the membrane area where T-cell receptor cross-linking is occurring,2 3 led to the hypothesis that a regulated polarized secretion may exist also in nonpolarized cells.
It is now clear that many hemopoietic cells use regulated secretion; examples are mast cells and granulocytes, which degranulate in response to Fc receptor cross-linking,4 or platelets, which respond to vascular lesions by releasing small molecules and proteins from intracellular granules.5 Unlike conventional secretory cells, endowed with specific structures for storage and release, hemopoietic cells use secretory lysosomes, a mixture organelle between lysosomes and secretory granules.6 Interestingly, other cell types, including fibroblasts, are able to transform conventional lysosomes into a secretory organelle underlying inducible exocytosis7; thus, regulated secretion seems to be a more widespread phenomenon than previously thought. However, even if secretory lysosomes are quite ubiquitous, the ability of directing their content toward a given target, resulting in polarized secretion, has been described so far only for a few immune cells, namely, T lymphocytes or natural killer cells.8 9 This may depend on how exocytosis is induced. Indeed, in most cell types exocytosis is driven by stimuli triggering structures distributed uniformly on the plasma membrane (such as IgE receptors on mast cells); in contrast, in the case of T and natural killer cells, binding to target cells with engagement of a single or few receptor complexes results in local activation leading to polarized degranulation.
Although it is known that increases in intracellular free calcium concentration ([Ca++]i) and cytoskeleton rearrangement occur during the process,7 the molecular mechanisms underlying regulated lysosome exocytosis are still unclear.
We have recently demonstrated in monocytes an adenosine triphosphate (ATP)–dependent exocytosis of secretory lysosomes, resulting in release of lysosomal enzymes and of the mature form of interleukin-1β (IL-1β).10 The latter belongs to the family of leaderless secretory proteins, which despite their extracellular localization and function, lack a secretory signal peptide and are externalized through nonclassical pathways, avoiding the endoplasmic reticulum–Golgi exocytotic route.11,12 IL-1β is synthesized on free cytosolic ribosomes as a 35-kd precursor (pro–IL-1β), which undergoes processing by the IL-1β-converting enzyme (ICE)/caspase I to the mature form of 17 kd.13Unlike lysosomal enzymes, pro–IL-1β does not reach the lysosomal lumen from the biosynthetic pathway but rather through a partially defined mechanism, involving its translocation from the cytosol.10 Pro–IL-1β is also produced by dendritic cells (DCs),14 the professional antigen-presenting cells,15 and released in a number of ICE-dependent and ICE-independent bioactive molecular forms following contact with alloreactive CD8+ but not CD4+ T lymphocytes.16 Here we demonstrate the existence of calcium-dependent exocytosis of secretory lysosomes in DCs, induced by specific binding to CD8+ T cells, that results in polarized secretion of IL-1β and cathepsin D toward the interacting T lymphocyte.
Materials and methods
Derivation of DCs from adherent cells
The DCs were obtained by culturing adherent cells from peripheral blood mononuclear cells (PBMCs) from healthy donors for 8 days in RPMI 1640 medium (Biochrom, Berlin, Germany) supplemented with 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Biochrom), 10% heat-inactivated fetal calf serum (PAA Labour, Linz, Austria), 40 ng/mL recombinant granulocyte-macrophage colony-stimulating factor (Schering-Plough, Milan, Italy) and 1000 U/mL recombinant interleukin-4 as described.16,17 The cell population obtained was more than 95% CD14−, CD80+, CD86+, HLA-DR+, as reported.17 Media were endotoxin free as shown by the Limulus lysate colorimetric assay (PBI, Milan, Italy). Lipopolysaccharide (LPS, 1 μg/mL, Sigma Chemical, St Louis, MO) was added during the last 24 hours of culture to induce DC maturation.16 17
Establishment of mixed lymphocyte reaction, enrichment of CD4+ or CD8+ T cells
Purified T cells were obtained from PBMCs after 2 rounds of plastic adherence followed by immunodepletion of CD14+ and HLA-DR+ cells with immunomagnetic beads (Dynal, Milan, Italy). Allospecific T cells were obtained by coculturing for 1 week purified T cells with allogenic irradiated (4000 rad) PBMCs. The antigen specificity of allogeneic T cells was assessed as described.17 The percent of CD8+ and CD4+ T lymphocytes after 7 days of mixed lymphocyte reaction (MLR) was evaluated by fluorescence-activated cell sorter (FACS) analysis. CD8+ and CD4+ T lymphocytes were negatively selected from 7-day MLRs by immunodepletion with anti-CD4 or anti-CD8 monoclonal antibodies (mAbs) as described.16 After removal of CD8+ or CD4+ cells, respectively, the percent of CD8+or CD4+ T cells in the remaining cell population (evaluated by FACS analysis) varied from 85% to 95% in the different experiments.
Cytolytic assay and cytoplasmic immunofluorescence
Cytolytic activity of 7-day or, as a positive control, 18-day alloreactive T cells against DCs was tested in a 51Cr release assay as described.18 Briefly, DCs were loaded with 51Cr and cocultured for 4 hours with alloreactive T cells, used as effector cells at an effector/target ratio of 10:1. Results are expressed as percentage of cytotoxicity as described.18 T cells from 7-day MLRs were also tested for their content in intracellular perforin by cytoplasmic immunofluorescence using the antiperforin mAb δG9 (Pharmingen, San Diego, CA), before or after 6 hours of contact with DCs.
Culture conditions
The DCs, prepared as above, were incubated alone or with allospecific T cells from 7- day MLRs (whole T cells or CD8+ or CD4+ purified T cells) at a T cell/DC ratio of 10:1.16 Culture of DCs alone or cocultures with T cells were carried out for different periods of time, up to 6 hours. When indicated, ionomycin (1 mM, Sigma) or the L-type calcium channel19 agonist BayK 8644 (10 μM, Sigma) was added for the last 10 minutes, or thapsigargin (10 nM, Sigma) for the last 30 minutes. In other experiments, the L-type calcium channel blocker nifedipine (10 μM, Sigma) or the calcium chelator ethylene glycol-bis(β-aminoethyl ether)-N, N, N′, N′-tetraacetic acid (EGTA, 5 mM, Sigma) was present during the entire duration of coculture. At the end of the incubations, supernatants were concentrated by 10% trichloroacetic acid (TCA); cells were lysed in 1% Triton X-100 (Bio-Rad, Milan, Italy) containing buffer.16 In other experiments, DCs were pretreated with nocodazole (Sigma) for 1 hour at 30 μM and cocultured for 6 hours with alloreactive T cells at a T cell/DC ratio of 10:1, in the presence of 20 μM nocodazole. As a control, DCs were fixed for 10 minutes with 1% glutaraldehyde (Sigma) prior to 6 hours of coculture with T cells.
Subcellular fractionation by differential ultracentrifugation and Percoll density gradient
Subcellular fractionation was carried out as described by Pitt and coworkers20 with slight modifications.10Briefly, cells were washed, resuspended in homogenizing buffer (250 mM sucrose, 5 mM EGTA, 20 mM Hepes-KOH, pH 7.2) at 5 × 107/mL and broken in a Dounce homogenizer. Unbroken cells, debris, and nuclei were discharged by 3 cycles of centrifugation at 800, 1000, and 1200g, and the postnuclear supernatant (PNS) obtained was diluted 10-fold in homogenizing buffer and centrifuged at 35 000g for 1 minute. The pellet was kept as P1; the P1 supernatant was centrifuged at 50,000 g for 5 minutes, leading to a second pellet (P2). The 2 pellets were treated with 0.1 mg/mL proteinase K (Sigma) for 30 minutes on ice (to remove cytosolic pro–IL-1β possibly bound to the external membrane of the organelles) in the presence or absence of 0.1% Triton X-100 followed by addition of protease inhibitors (Sigma). The P2 supernatant was spun at 100,000g for 30 minutes, and the resulting supernatant was kept as cytosolic fraction and concentrated by 10% TCA precipitation. P1 and P2 pellets obtained as above were resuspended in 1 mL of a buffer containing 3 mM imidazole and 250 mM sucrose at pH 7, and mixed with 9 mL of the same buffer containing Percoll (Sigma) up to 25%.10,21,22 After centrifugation for 2 hours at 90 000g (Beckman TiSW41 rotor, 27 000 rpm), fractions were collected using a needle connected to a peristaltic pump (Amersham Pharmacia Biotech, Milan, Italy), membranes were lysed with 0.5% Triton X-100, diluted 5-fold in the same buffer, ultracentrifuged 30 minutes at 100 000g to remove Percoll,10,21 22and concentrated by TCA precipitation.
Western blot analysis
Aliquots of cell lysates corresponding to 0.5 × 105 DCs (or 100 μg proteins; protein dosage performed with the Bio-Rad kit based on the colorimetric Lowry method) and the correspondent TCA-concentrated supernatants, or aliquots of PNS, TCA-concentrated cytosol (100 μg proteins) and the corresponding whole P1, P2, or aliquots from the different Percoll gradient fractions were solubilized in reducing sample buffer and resolved on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions.10 Gels were electrotransferred onto nitrocellulose filters (Hybond ECL, Amersham Pharmacia Biotech), stained with Ponceau S (Sigma) to confirm equal protein loading (not shown), and destained prior to blocking overnight with 10% nonfat dry milk in phosphate-buffered saline (PBS). Filters were hybridized with the antihuman IL-1β mAb 3ZD (IgG1, provided by National Cancer Institute Biological Resources Branch, Frederick, MD) or the antihuman cathepsin D mAb (IgG2a, Calbiochem, Milan, Italy), followed by a horseradish peroxidase (HRP)–conjugated goat antimouse IgG (Dako, Milan, Italy), or with the rabbit antihuman Rab7 antibody (gift of S. Méresse, Marseille, France23), followed by an HRP-conjugated goat antirabbit IgG (Dako) and developed by ECL-Plus (Amersham Pharmacia Biotech) according to the manufacturer's instructions. When stated, densitometric analyses of the blots were performed.10
Conventional and immunoelectron microscopy
The P1 and P2 fractions were processed for postembedding immunocytochemistry as described.10,24 Briefly, fractions were fixed in 1.0% glutaraldehyde (Gibco Laboratories, Grand Island, NY) in PBS for 1 hour at room temperature, partially dehydrated in ethanol, and embedded in LR White resin (Electron Microscopy Sciences, Fort Washington, PA). Thin sections were collected on nickel grids and processed for double immunolabeling; sections were incubated first with anti–IL-1β mAb (IgG1) followed by 10 nM goat antimouse IgG gold–conjugated (British Biocell International, Carditt, United Kingdom), postfixed with 1.0% glutaraldehyde for 10 minutes to prevent interference among different antibodies and gold conjugates,25 and then incubated with rabbit anticathepsin D antibody (Upstate Biotechnology, Lake Placid, NY) followed by 18 nm protein-A gold prepared by the citrate method.26 Control experiments were performed using as primary antibodies an unrelated isotype-matched (IgG1) mAb and a rabbit preimmune serum (both kindly provided by Dr A. Santoni, Rome, Italy). All sections were finally stained with uranyl acetate and lead citrate. DCs interacting with CD4+ or CD8+ T cells for various periods of time were fixed in glutaraldehyde as above and were processed for conventional thin section electron microscopy as described.24 Thin sections were examined unstained and poststained with uranyl acetate and lead hydroxide. Quantitative evaluation of immunolabeling was performed by comparing the number of small (10 nm) and large (18 nm) gold particles present inside organelles displaying the ultrastructural appearance of late endosomes/lysosomes. Fifty images of each type of structure, randomly photographed from 3 different immunolabeling experiments, were analyzed.
Single cell analysis of calcium fluxes by video-microscopy and ratio-imaging
Single cell analysis of calcium fluxes was performed as described.27 CD4+ or CD8+alloreactive or nonspecific T cells were loaded 1 hour at 37°C with 1 μM FURA 2-am (Sigma) and added to DCs, similarly loaded with FURA 2-am, cultured on round coverslips, placed in a microincubator (Medical System, Greenvale, NY) on an inverted epifluorescence Axiovert 10 microscope (Zeiss, Oberkochen, Germany) and maintained at 37°C by a temperature controller (TC-202, Medical System). FURA 2-am was excited with a high-pressure 75 W xenon arc lamp fitted with appropriate filters on a shutter controlled by a Pentium 90 MHz computer. Excitation light was at 334 and 380 nm; emitted light was filtered at 510 nm. Two 334:380 ratios were taken each second and video images collected with an intensified charged coupled device camera (Atto Instruments, Rockville, MD) and recorded every 15 seconds using the image-processor program Attofluor RatioVision 6.08 (Atto Instruments). Results were stored as ratio of FURA 2-am fluorescence at 334 nm divided by the fluorescence at 380 nm excitation. The [Ca++]i was calculated according to Grynkiewicz and colleagues.28[Ca++]i increases were measured on interaction of DCs with CD4+ or CD8+ and nonspecific or specific T lymphocytes. The integrity of DCs after coincubation with T cells was controlled by evaluating the maintenance of FURA 2-am fluorescence.
Results
IL-1β secretion by DCs is paralleled by secretion of cathepsin D
We have previously shown that maturation stimuli or interaction with the CD40 ligand expressed by activated CD4+ T cells induce in DCs pro–IL-1β synthesis but not secretion. In contrast, CD8+ allospecific T lymphocytes do not induce per se pro–IL-1β synthesis, but drive processing and secretion of the bioactive cytokine by mature DCs.16
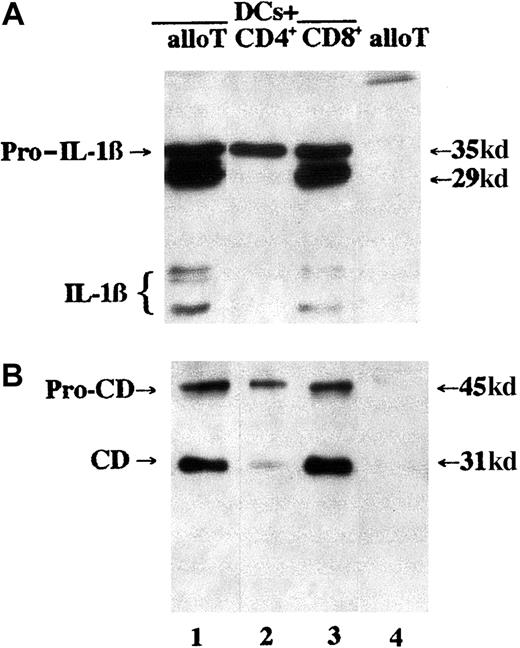
Noteworthy, IL-1β secretion induced in LPS-stimulated DCs by unfractionated alloreactive T lymphocytes (Figure1A, lane 1) or by purified CD8+ allospecific T cells (Figure 1A, lane 3) is accompanied by secretion of the lysosomal form of cathepsin D,29 running as a 31-kd band (Figure 1B, lanes 1 and 3). Conversely, secretion of cathepsin D (and of IL-1β) is barely detectable or undetectable after culture of DCs with CD4+alloreactive T lymphocytes (Figure 1A,B, lane 2). Interestingly, the secretion of the endosomal form of cathepsin D (procathepsin D, Mr 45 kd)29 is almost unaffected by interaction with alloreactive T cells. As a control, supernatants from alloreactive T cells cultured 6 hours without DCs contain no IL-1β and only barely detectable cathepsin D (lane 4). T-cell–induced IL-1β and cathepsin D release is an active process independent of DC damage; indeed, T lymphocytes from 7-day MLRs were not cytotoxic, as evaluated in a 51Cr release assay18 (< 5% of DC lysis at day 7 versus > 60% at day 18). In keeping with this, we failed to detect secreted granzyme in 7-day MLR supernatants, confirming the immature state of CD8+ T cells (not shown). Moreover, more than 90% of CD8+ T cells were still positive for intracellular perforin after contact with DCs, as evaluated by cytoplasmic immunofluorescence.
CD8+ alloreactive T lymphocytes induce DCs to secrete both IL-1β and cathepsin D.
DCs were cocultured with alloreactive unfractionated T lymphocytes (allo T, lane 1) or purified CD4+ (lane 2) or CD8+ (lane 3) allospecific T cells at a DC/T cell ratio of 1:10 for 6 hours. Lane 4 shows an equal number of alloreactive T cells that were cultured alone for 6 hours. At the end of the incubations, supernatants were collected and analyzed for their content in IL-1β (A) or cathepsin D (B) by Western blotting. CD indicates cathepsin D; pro-CD, procathepsin D. One representative experiment of 10 is shown.
CD8+ alloreactive T lymphocytes induce DCs to secrete both IL-1β and cathepsin D.
DCs were cocultured with alloreactive unfractionated T lymphocytes (allo T, lane 1) or purified CD4+ (lane 2) or CD8+ (lane 3) allospecific T cells at a DC/T cell ratio of 1:10 for 6 hours. Lane 4 shows an equal number of alloreactive T cells that were cultured alone for 6 hours. At the end of the incubations, supernatants were collected and analyzed for their content in IL-1β (A) or cathepsin D (B) by Western blotting. CD indicates cathepsin D; pro-CD, procathepsin D. One representative experiment of 10 is shown.
Pro–IL-1β is contained in endolysosomes
The concomitant secretion of IL-1β and cathepsin D prompted us to investigate whether the 2 proteins colocalize in organelles belonging to the endolysosomal compartment. LPS-activated DCs were cultured for 4 hours with or without alloreactive CD8+ T cells, and the PNSs obtained at the end of the culture period were subjected to 2 sequential ultracentrifugations, giving rise to 2 pellets, P1 and P2, enriched in lysosomes and endosomes, respectively.10,20 As shown in Figure 2A, DC pro–IL-1β is detected in both P1 and P2 (lanes 3 and 4). Comparison with the pro–IL-1β band present in the cytosolic fraction (lane 1) allowed the calculation that particulated pro–IL-1β ranged, in the different experiments, from 10% to 20% of the total cellular pro–IL-1β. Interestingly, the P1 fraction from DCs cultured with alloreactive T cells (Figure 2B, lane 3), contained also a 29-kd IL-1β band of intensity similar to the 35-kd pro–IL-1β band. This band, which is almost undetectable in the cytosolic fraction (lane 1), corresponds to the first product of ICE processing, is absent in cells treated with ICE inhibitors,16 30 and is also secreted in large amounts by DCs cultured with alloreactive T cells (Figure 1A). These observations suggest that pro–IL-1β present in P1 is the direct precursor of secreted IL-1β.
Pro–IL-1β is present in P1 and P2 fractions; processing on interaction with alloreactive T cells.
P1 and P2 fractions (lanes 3 and 4) were obtained by sequential ultracentrifugation of PNS from mature DCs cultured 5 hours alone (A) or with alloreactive CD8+ T cells (at a DC/T cell ratio of 1:10, panel B), treated with proteinase K and solubilized in sample buffer. Lane 1: cytosol (TCA-concentrated, 1/20 of total, 100 μg); lane 2: PNS (1/20 of total). Samples were blotted and hybridized with anti–IL-1β mAb. Note the appearance of the ICE-specific 29-kd IL-1β band, of approximately the same intensity as the 35-kd, in P1 from DCs cultured with alloreactive T cells (B, lane 3). The low-molecular-weight band (17 kd) in lanes 3B and 4B is erratic and probably due to nonspecific endoproteases activated during the preparation of the samples, as already reported.10 One representative experiment of 5 is shown.
Pro–IL-1β is present in P1 and P2 fractions; processing on interaction with alloreactive T cells.
P1 and P2 fractions (lanes 3 and 4) were obtained by sequential ultracentrifugation of PNS from mature DCs cultured 5 hours alone (A) or with alloreactive CD8+ T cells (at a DC/T cell ratio of 1:10, panel B), treated with proteinase K and solubilized in sample buffer. Lane 1: cytosol (TCA-concentrated, 1/20 of total, 100 μg); lane 2: PNS (1/20 of total). Samples were blotted and hybridized with anti–IL-1β mAb. Note the appearance of the ICE-specific 29-kd IL-1β band, of approximately the same intensity as the 35-kd, in P1 from DCs cultured with alloreactive T cells (B, lane 3). The low-molecular-weight band (17 kd) in lanes 3B and 4B is erratic and probably due to nonspecific endoproteases activated during the preparation of the samples, as already reported.10 One representative experiment of 5 is shown.
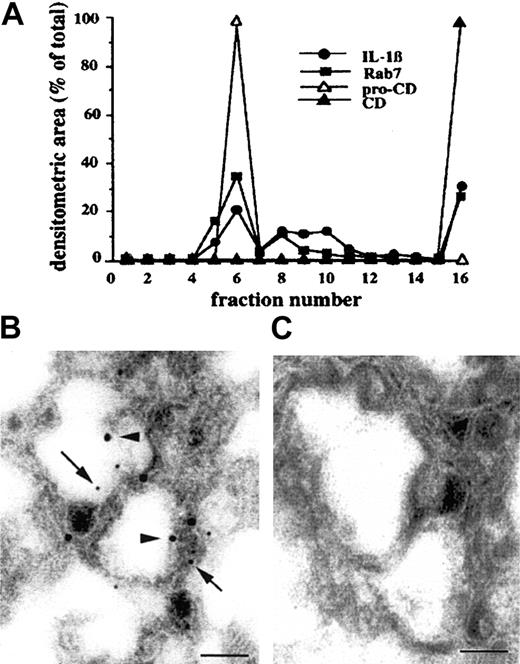
To clarify whether IL-1β–containing organelles belong to endosomes, late endosomes, or lysosomes, P1 and P2 pellets were pooled and subjected to a 25% Percoll density gradient; the fractions obtained were analyzed for their content in cathepsin D, IL-1β, or Rab7, a marker of late endosomal-lysosomal compartment.23Figure 3A shows that the endosomal procathepsin D is present in fraction 6, whereas the lysosomal cathepsin D accumulates at the bottom of the gradient, in fraction 16. Pro–IL-1β and Rab7 colocalize in 2 major peaks, the first cofractionating with procathepsin D and the second with cathepsin D. A third minor peak of pro–IL-1β and Rab7 was also detected in fractions 8 to 10. These data suggest that particulated pro–IL-1β is contained mostly in late endosomes and lysosomes. This was further confirmed by immunoelectron microscopy analysis of P1 and P2 pellets, showing the presence of organelles with the morphology of endolysosomal structures containing electron-dense material that stain with both anti–IL-1β mAb (small gold particles, arrows in Figure 3B) and anticathepsin D antibody (large gold particles, arrowheads in Figure3B). Quantitative evaluation of immunolabeling allowed the calculation that 65% to 70% of organelles with endolysosomal morphology were positive for either one or both of the 2 proteins; of these, 70% were double positive, whereas 10% stained for IL-1β only and the remaining 20% for cathepsin D only. When an isotype-matched (IgG1) mAb and a rabbit preimmune serum were used as control primary antibodies, no labeling was detected (Figure 3C).
Percoll density gradient and immunoelectron microscopic analysis of IL-1β–containing organelles.
(A) P1 and P2 pellets from PNS of DCs were pooled and subjected to a 25% Percoll density gradient. Fractions were collected from the top of the gradient and analyzed for their content in procathepsin D and mature cathepsin D (pro-CD and CD), IL-1β, or Rab7 by Western blotting and densitometry. One representative experiment of 3 is shown. Panels B and C show immunoelectron microscopic analysis of pooled P1 and P2 pellets. Sections were stained with anti–IL-1β IgG1 mAb (B) or an unrelated IgG1 mAb (C) followed by goat antimouse IgG gold–conjugated (small gold particles, arrows) and with rabbit anticathepsin D antiserum (B) or rabbit preimmune serum (C) followed by protein-A gold (large gold particles, arrowheads). Bars indicate 1 μm.
Percoll density gradient and immunoelectron microscopic analysis of IL-1β–containing organelles.
(A) P1 and P2 pellets from PNS of DCs were pooled and subjected to a 25% Percoll density gradient. Fractions were collected from the top of the gradient and analyzed for their content in procathepsin D and mature cathepsin D (pro-CD and CD), IL-1β, or Rab7 by Western blotting and densitometry. One representative experiment of 3 is shown. Panels B and C show immunoelectron microscopic analysis of pooled P1 and P2 pellets. Sections were stained with anti–IL-1β IgG1 mAb (B) or an unrelated IgG1 mAb (C) followed by goat antimouse IgG gold–conjugated (small gold particles, arrows) and with rabbit anticathepsin D antiserum (B) or rabbit preimmune serum (C) followed by protein-A gold (large gold particles, arrowheads). Bars indicate 1 μm.
Cathepsin D and IL-1β secretion by DCs is dependent on Ca++
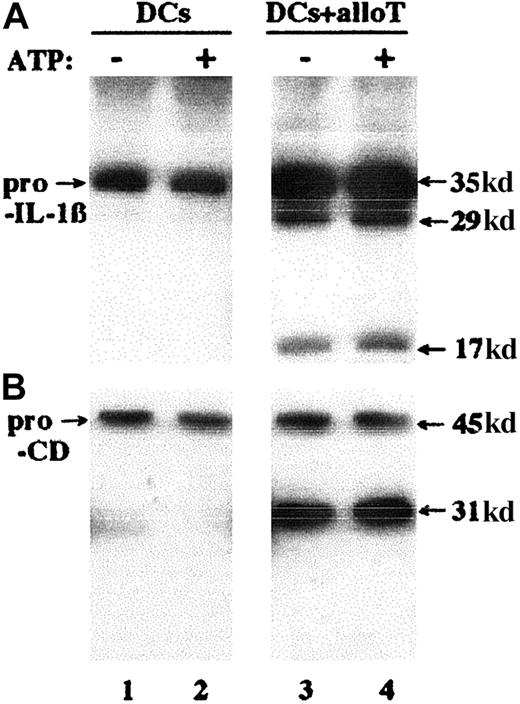
In monocytes, IL-1β secretion is triggered by extracellular ATP.10,31-33 In contrast, although DCs express purinergic receptors,14 ATP increased neither IL-1β (Figure4A) nor cathepsin D (Figure 4B) secretion by mature DCs cultured alone (Figure 4, lanes 1 and 2) or with alloreactive T cells (compare lanes 3 and 4). Because exocytosis of secretory lysosomes is a Ca++-regulated process,7,34,35 and secretion by DCs of a number of cytokines, including the leaderless secretory protein IL-18, is Ca++ dependent,27,36 we investigated whether IL-1β and cathepsin D secretion is induced in DCs by increases in [Ca++]i. As shown in Figure5A, 10-minute ionomycin stimulation of mature DCs results in secretion of both pro–IL-1β (lane 2) and lysosomal cathepsin D (lane 6). Of note, the calcium ionophore triggers the release of large quantities of IL-1β precursor (20%-40% of the total pro–IL-1β in the different experiments), but of only minute amounts of the 29-kd processed form (Figure 5A, lane 2, compare with secretion induced by T cells in lane 3). This observation indicates that the [Ca++]i increase itself is not able to promote an efficient pro–IL-1β processing. Ionomycin also potentiates the secretion of IL-1β (lane 4) and cathepsin D (lane 8) induced by alloreactive T cells. Interestingly, secretion of procathepsin D (lanes 5-8) is only slightly affected by alloreactive T cells and ionomycin, further supporting that pro–IL-1β-containing lysosomes, rather than endosomes, undergo exocytosis following interaction with T cells or the [Ca++]i rise. To evaluate the role of extra- and intracellular Ca++ on IL-1β and cathepsin D secretion, we compared the effects of EGTA, of the L-type Ca++ channel agonist Bay K8644, or of the L-type Ca++ channel blocker nifedipine with those of thapsigargin, which induces Ca++ release from internal stores (Figure 5B,C). Removal of extracellular Ca++ by EGTA prevents the secretion driven by ionomycin treatment (Figure 5B, EGTA + iono) and decreases that induced by alloreactive T cells (Figure 5C, EGTA). Furthermore, exposure to Bay K8644 results in enhancement of secretion, both basal (Figure 5B, BayK) and T-cell induced (Figure 5C, BayK), whereas nifedipine partially inhibits the secretion triggered by alloreactive T cells (Figure 5C, NFD). Treatment of DCs with thapsigargin (Figure 5B, thapsi) induces secretion, unaffected by EGTA (Figure 5B, EGTA + thapsi), even if at a lesser extent than Bay K8644. These data suggest that both intra- and extracellular Ca++ are involved in the secretion elicited in DCs by the specific interaction with CD8+ T cells; moreover, they confirm that calcium influx is mediated by L-type Ca++ channels, that are expressed and functional on DCs.27 Again, the rate of secretion of procathepsin D is poorly affected by [Ca++]i modifications, suggesting that a baseline of procathepsin D release occurs in LPS-activated DCs and is Ca++ insensitive. To rule out that secreted IL-1β and cathepsin D derive from T lymphocytes rather than from DCs, LPS-treated DCs were fixed with glutaraldehyde before coincubation with alloreactive T cells. In this case, negligible cathepsin D was found in supernatants at the end of the coculture (Figure 5C, GA-treated DCs + alloT, −) and only a small amount was induced by ionomycin (Figure 5C, GA-treated DCs + alloT, iono), corresponding to about 20% of that recovered after ionomycin-stimulation of the same cocultures with nonfixed DCs. Secreted IL-1β was barely detected or undetectable in all conditions.
Exogenous ATP does not induce IL-1β secretion by DCs.
Supernatants from LPS-treated DCs, cultured 6 hours without (lanes 1 and 2) or with (lanes 3 and 4) alloreactive T cells at a DC/T cell ratio of 1:10, unstimulated (lanes 1 and 3) or stimulated (lanes 2 and 4) with 1 mM ATP for the last 15 minutes were analyzed by Western blotting for their content in secreted IL-1β (A) and cathepsin D (B); pro-CD indicates procathepsin D. One representative experiment of 3 is shown.
Exogenous ATP does not induce IL-1β secretion by DCs.
Supernatants from LPS-treated DCs, cultured 6 hours without (lanes 1 and 2) or with (lanes 3 and 4) alloreactive T cells at a DC/T cell ratio of 1:10, unstimulated (lanes 1 and 3) or stimulated (lanes 2 and 4) with 1 mM ATP for the last 15 minutes were analyzed by Western blotting for their content in secreted IL-1β (A) and cathepsin D (B); pro-CD indicates procathepsin D. One representative experiment of 3 is shown.
Secretion of both cathepsin D and IL-1β by DCs is dependent on [Ca++]i increases.
(A) LPS-activated DCs cultured 6 hours without (lanes 1, 2, 5, and 6) or with (lanes 3, 4, 7, and 8) alloreactive T cells at a DC/T cell ratio of 1:10 were untreated (−, lanes 1, 3, 5, and 7) or stimulated for the last 10 minutes with 1 mM ionomycin (+, lanes 2, 4, 6, and 8). Supernatants were TCA concentrated and analyzed by Western blot for their content in IL-1β (lanes 1-4) and cathepsin D (lanes 5-8). Panels B and C show the densitometric analyses of IL-1β, procathepsin D, and cathepsin D present in supernatants of LPS-activated DCs, cultured 6 hours alone (B, DCs) or with alloreactive T cells (C, DCs + allo T) in the presence or absence of different substances for the times indicated: 5 mM EGTA (during the entire time of incubation), 10 μM nifedipine (NFD, 30 minutes of treatment, before coculture with T cells), 1 mM ionomycin (iono, for the last 10 minutes of incubation), 10 μM Bay K8644 (BayK, for the last 15 minutes of incubation), 10 nM thapsigargin (thapsi, for the last 30 minutes of incubation), or 10 nM thapsigargin and 5 mM EGTA (thapsi + EGTA, for the last 30 minutes of incubation). As a control, DCs were fixed for 10 minutes with 1% glutaraldehyde (C, GA-treated DCs + allo T) and secretion induced by T cells was evaluated with or without ionomycin stimulation. One representative experiment of 3 is shown.
Secretion of both cathepsin D and IL-1β by DCs is dependent on [Ca++]i increases.
(A) LPS-activated DCs cultured 6 hours without (lanes 1, 2, 5, and 6) or with (lanes 3, 4, 7, and 8) alloreactive T cells at a DC/T cell ratio of 1:10 were untreated (−, lanes 1, 3, 5, and 7) or stimulated for the last 10 minutes with 1 mM ionomycin (+, lanes 2, 4, 6, and 8). Supernatants were TCA concentrated and analyzed by Western blot for their content in IL-1β (lanes 1-4) and cathepsin D (lanes 5-8). Panels B and C show the densitometric analyses of IL-1β, procathepsin D, and cathepsin D present in supernatants of LPS-activated DCs, cultured 6 hours alone (B, DCs) or with alloreactive T cells (C, DCs + allo T) in the presence or absence of different substances for the times indicated: 5 mM EGTA (during the entire time of incubation), 10 μM nifedipine (NFD, 30 minutes of treatment, before coculture with T cells), 1 mM ionomycin (iono, for the last 10 minutes of incubation), 10 μM Bay K8644 (BayK, for the last 15 minutes of incubation), 10 nM thapsigargin (thapsi, for the last 30 minutes of incubation), or 10 nM thapsigargin and 5 mM EGTA (thapsi + EGTA, for the last 30 minutes of incubation). As a control, DCs were fixed for 10 minutes with 1% glutaraldehyde (C, GA-treated DCs + allo T) and secretion induced by T cells was evaluated with or without ionomycin stimulation. One representative experiment of 3 is shown.
Interaction with CD8+ T cells results in a Ca++ influx in DCs
The above observations point to the requirement of a [Ca++]i increase for lysosome exocytosis with release of IL-1β and cathepsin D. We then investigated whether the interaction between DCs and alloreactive CD8+ T cells results in a Ca++ influx in DCs. Enriched CD4+ or CD8+ alloreactive T cells were loaded with FURA 2-am and added to DCs, similarly loaded with the fluorescent probe, and the oscillations in [Ca++]i were monitored at the single cell level. Figure 6A shows that the formation of a contact zone between DCs and alloreactive CD8+ T lymphocytes is followed by Ca++ rises in DCs, but not in CD8+ T cells. Conversely, triggering of DCs by allospecific CD4+ T cells results in a Ca++response in T cells, but not in DCs (Figure 6B), in keeping with previous observations.37 Panels C and D in Figure 6 show the mean of Ca++ responses in 15 DCs interacting with T cells and confirm that a sustained Ca++ influx is induced in DCs by CD8+ but not CD4+ alloreactive T cells.
A rise in DC [Ca++]i follows interaction with CD8+ but not CD4+ T cells.
Enriched CD8+ (A) or CD4+ (B) alloreactive T cells loaded with FURA 2-am were added to FURA 2-am–loaded DCs and the oscillations in [Ca++]i were monitored at the single cell level. Pictures were taken at intervals of 10 minutes. Panels C and D show the mean of 15 Ca++ responses induced in DCs by CD8+ alloreactive T cells (C) and in CD4+alloreactive T cells by DCs (D). Results are expressed as ratio of the light emitted at 510 nm after excitation at 334 nm or 380 nm. One representative experiment of 3 is shown.
A rise in DC [Ca++]i follows interaction with CD8+ but not CD4+ T cells.
Enriched CD8+ (A) or CD4+ (B) alloreactive T cells loaded with FURA 2-am were added to FURA 2-am–loaded DCs and the oscillations in [Ca++]i were monitored at the single cell level. Pictures were taken at intervals of 10 minutes. Panels C and D show the mean of 15 Ca++ responses induced in DCs by CD8+ alloreactive T cells (C) and in CD4+alloreactive T cells by DCs (D). Results are expressed as ratio of the light emitted at 510 nm after excitation at 334 nm or 380 nm. One representative experiment of 3 is shown.
Polarization of DC endolysosomes toward the interacting CD8+ T cell
Ultrastructural analyses show that the interaction between DCs and CD8+ T cells after 5 hours of coculture is associated with recruitment of endolysosomes and mitochondria in the areas of contact among the cells (Figure7B). Quantitative analyses performed by examining 70 DC-CD8+ T-cell aggregates allowed the calculation that 71% of DCs interacting with CD8+ T lymphocytes displayed lysosome polarization toward the interacting T cells. The lysosome recruitment is already evident after a short coincubation (10-20 minutes) and increases with time (not shown). In contrast, polarization is not observed after binding of CD4+ T cells to DCs (Figure 7A; in 65% of the 70 DC-CD4+ T-cell aggregates examined, CD4+ T lymphocytes interacted with DCs in perinuclear areas displaying no sign of lysosome polarization). No sign of DC morphologic alteration is detectable, confirming the integrity of DCs after culture with T cells. Panels C and D in Figure 7 show a higher magnification of the contact areas among DCs and CD8+ T cells, with details of a tight contact between the plasma membranes of the interacting cells (arrowhead in panel C) and of fusion sites of endolysosomes with the DC plasma membrane (arrows in panels C and D). Immunoelectron microscopy analysis with anticathepsin D antibody (large gold, arrowheads) and anti–IL-1β mAb (small gold, arrows) confirmed the colocalization of the 2 proteins in the endolysosomes polarized toward the interacting T cell (Figure 7E and insets). Quantitative analyses revealed that of 70 T cells interacting with DCs examined, none displayed intracellular staining for IL-1β, confirming the absence of IL-1β production by alloreactive T cells.
Polarization of DC endolysosomes toward the interacting CD8+ T cells.
Ultrastructural analysis of the interaction of DCs with CD4+ (A) or CD8+ (B-D) allospecific T cells. Cells were cocultured for 5 hours and processed for conventional thin section electron microscopy. Interaction between DCs and CD4+ T cells occurs primarily in correspondence with the DC areas occupied by the nucleus and is not characterized by polarization of cellular organelles (A). In DCs interacting with CD8+ T lymphocytes, both mitochondria and endolysosomal structures are polarized toward the areas of contact at the opposite site of the cell portion containing the nucleus (B). Panels C and D show details of a tight contact between the plasma membranes of the interacting cells (arrowhead in panel C) and of fusion sites of endolysosomes with the DC plasma membrane (arrows in panels C and D). (E) Immunoelectron microscopic analysis of the localization of IL-1β and cathepsin D in DCs interacting with CD8+ T cells. Double immunogold labeling with anticathepsin D (large gold particles) and anti–IL-1β (small gold particles) antibodies shows the colocalization of the 2 proteins in endolysosomal structures in the DC cytoplasm oriented toward the cell contact with a CD8+ T lymphocyte (arrow). Insets with enlargement of the areas in dotted boxes show groups of endolysosomes double-labeled for IL-1β (arrows) and cathepsin D (arrowheads). PM indicates plasma membrane; Nu, nucleus; M, mitochondria; and G, Golgi complex. Bars in panels A and B are 2 μm; in panels C and D, 0.5 μm; in insets, 0.1 μm.
Polarization of DC endolysosomes toward the interacting CD8+ T cells.
Ultrastructural analysis of the interaction of DCs with CD4+ (A) or CD8+ (B-D) allospecific T cells. Cells were cocultured for 5 hours and processed for conventional thin section electron microscopy. Interaction between DCs and CD4+ T cells occurs primarily in correspondence with the DC areas occupied by the nucleus and is not characterized by polarization of cellular organelles (A). In DCs interacting with CD8+ T lymphocytes, both mitochondria and endolysosomal structures are polarized toward the areas of contact at the opposite site of the cell portion containing the nucleus (B). Panels C and D show details of a tight contact between the plasma membranes of the interacting cells (arrowhead in panel C) and of fusion sites of endolysosomes with the DC plasma membrane (arrows in panels C and D). (E) Immunoelectron microscopic analysis of the localization of IL-1β and cathepsin D in DCs interacting with CD8+ T cells. Double immunogold labeling with anticathepsin D (large gold particles) and anti–IL-1β (small gold particles) antibodies shows the colocalization of the 2 proteins in endolysosomal structures in the DC cytoplasm oriented toward the cell contact with a CD8+ T lymphocyte (arrow). Insets with enlargement of the areas in dotted boxes show groups of endolysosomes double-labeled for IL-1β (arrows) and cathepsin D (arrowheads). PM indicates plasma membrane; Nu, nucleus; M, mitochondria; and G, Golgi complex. Bars in panels A and B are 2 μm; in panels C and D, 0.5 μm; in insets, 0.1 μm.
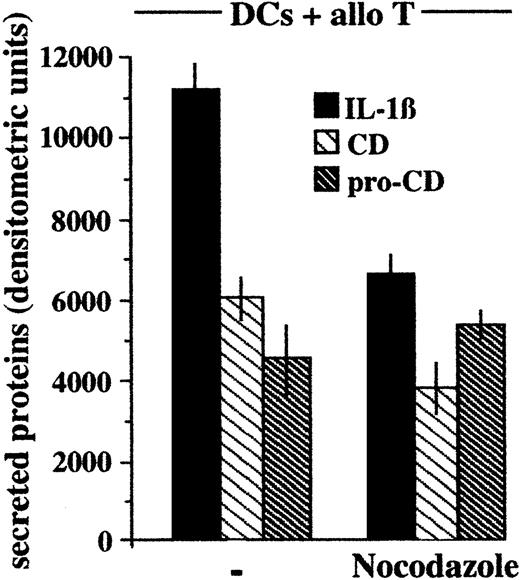
To investigate the mechanism of endolysosome polarization, T- cell–induced secretion of cathepsin D and IL-1β was analyzed after treatment of DCs with the microtubule poison nocodazole. As shown in Figure 8, treatment with nocodazole inhibits by about 40% the secretion of cathepsin D and IL-1β, while not affecting secretion of procathepsin D; this suggests the involvement of the microtubular cytoskeleton in endolysosome recruitment and exocytosis.
Nocodazole inhibits secretion of cathepsin D and IL-1β.
Densitometric analyses of IL-1β, procathepsin D, and cathepsin D present in supernatants of LPS-activated DCs after 6 hours of coculture with alloreactive T cells (DCs + allo T) in the presence or absence of nocodazole. CD indicates cathepsin D; pro-CD, procathepsin D. One representative experiment of 3 is shown.
Nocodazole inhibits secretion of cathepsin D and IL-1β.
Densitometric analyses of IL-1β, procathepsin D, and cathepsin D present in supernatants of LPS-activated DCs after 6 hours of coculture with alloreactive T cells (DCs + allo T) in the presence or absence of nocodazole. CD indicates cathepsin D; pro-CD, procathepsin D. One representative experiment of 3 is shown.
Discussion
In this paper we describe a novel mechanism of regulated secretion in DCs, mediated by Ca++-dependent exocytosis of endolysosomes, which is induced by CD8+ alloreactive T cells on interaction with the antigen-presenting cells. This mechanism of secretion allows the polarized release of IL-1β as well as of the lysosome hydrolase cathepsin D toward the target cell, namely, the same T cell that elicited the degranulation. These conclusions are based on the following evidence: (1) secretion of IL-1β is accompanied by secretion of mature, lysosomal cathepsin D; (2) a fraction of intracellular pro–IL-1β is contained in cathepsin D+organelles, belonging to the endolysosomal compartment; (3) particulated pro–IL-1β, but not cytosolic pro–IL-1β, undergoes ICE processing after coculture with alloreactive CD8+ T cells; and (4) binding to alloreactive CD8+ T cells induces in DCs a sustained Ca++ influx accompanied by a redistribution of IL-1β and cathepsin D+ endolysosomes toward the interacting T cell.
We have recently shown that IL-1β secretion by monocytes involves exocytosis of IL-1β–containing endolysosomal-related vesicles,10 suggesting that organelles belonging to the endolysosomal compartment may represent the vehicle to transport leaderless secretory proteins out of the cell. However, unlike lysosomal proteins, which are sorted to lysosomes from the biosynthetic pathway, leaderless secretory proteins must be transported to the endolysosomal lumen from the cytosol where their synthesis occurs. Interestingly, a pathway of translocation to lysosomes under stress conditions has been described for cytosolic proteins in mammalian cells38; furthermore, in Dictyostelium discoideum, a leaderless adhesion protein translocates from the cytosol to contractile vacuoles (acidic organelles similar to lysosomes) and is expressed on the cell surface by regulated exocytosis of these vacuoles.39
Unlike in monocytes, where exocytosis of IL-1β–containing lysosomes is induced by exogenous ATP,10 in DCs release of IL-1β and cathepsin D is triggered by increases in [Ca++]i. This difference may be due to the presence in DCs of secretory lysosomes equipped with sensors different from those of monocytes, responding to different stimuli. Of note, in DCs most of the organelles positive for IL-1β are also positive for cathepsin D, whereas in monocytes 3 types of endolysosomes were identified, one containing pro–IL-1β only, one containing cathepsin D only, and one containing both.10 It is possible that only the first type, absent in DCs, is responsive to ATP; in keeping with this, in monocytes ATP is more active in inducing IL-1β than cathepsin D secretion,10 whereas in DCs the release of the 2 proteins is similarly induced by Ca++. Because ATP activates ICE,40 the different sensitivities of the 2 cell types may also account for the different rates of ICE-processed 17-kd IL-1β recovered from monocytes (> 90%) and DCs (< 10%).
Alloreactive CD8+ T cells promote a Ca++ influx in DCs. Although it is well documented that antigen presentation results in increases in [Ca++]i in helper T cells,37 and a low Ca++ response has been recently shown in 30% of DCs interacting with a CD4+T-cell clone,41 this is the first report that documents the ability of CD8+ T lymphocytes to induce Ca++ mobilization in DCs. Interestingly, a specific interaction between CD8+ T cells and DCs is required, because nonspecific CD8+ T cells are able to induce in DCs neither IL-1β secretion16 nor Ca++ influx (not shown). Ca++ itself is not able to promote IL-1β maturation, as indicated by the finding that the IL-1β secreted following ionomycin or thapsigargin stimulation is mostly unprocessed. In contrast, the interaction of alloreactive CD8+ T cells with DCs results in generation and secretion also of the 29-kd ICE-dependent IL-1β form and of other IL-1β low-molecular-weight forms. This suggests that alloreactive T cells induce in DCs not only [Ca++]i increases but also the activation of mechanisms leading to pro–IL-1β processing.
In conclusion, these findings demonstrate the existence of cross-talk between DCs and CD8+ T lymphocytes. T cells induce the functional polarization of DCs; in turn, DCs degranulate toward the triggering T lymphocytes. Although it is conceivable that the polarized secretion of IL-1β may serve to activate locally the interacting T cell without spreading around the potentially dangerous cytokine, the meaning of the polarized secretion of cathepsin D is less evident. However, it has been proposed that antigen-presenting DCs are eliminated in the lymph node by the very same immune response they elicited42; this cytotoxic T-cell–mediated clearance of DCs would serve as a mechanism of negative feedback to limit the T-cell response. Obviously, this process of clearance must be tightly regulated to allow an efficient immune reaction. It is tempting to speculate that the polarized release of lysosomal hydrolases may represent a defense of DCs against an early death due to premature release of lytic enzymes by the interacting CD8+ T cell.
We thank Drs G. Angelini and R. Sitia for critically reading the manuscript, Drs S. Mèresse and A. Santoni and the NCI Biological Resources Branch for the generous gift of antibodies, and the Blood Centers of Gaslini Scientific Institute and Galliera Hospital for providing buffy coats.
Supported in part by grants from Associazione Italiana per le Ricerca sue Cancro and Consiglio Nazionale Ricerche, target project on Biotechnology. C.A. is supported by a fellowship from Fondazione Italiana per le Ricerca sue Cancro.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anna Rubartelli, National Cancer Research Institute, Largo Rosanna Benzi, 10, 16132 Genova, Italy; e-mail:annarub@hp380.ist.unige.it.


![Fig. 5. Secretion of both cathepsin D and IL-1β by DCs is dependent on [Ca++]i increases. / (A) LPS-activated DCs cultured 6 hours without (lanes 1, 2, 5, and 6) or with (lanes 3, 4, 7, and 8) alloreactive T cells at a DC/T cell ratio of 1:10 were untreated (−, lanes 1, 3, 5, and 7) or stimulated for the last 10 minutes with 1 mM ionomycin (+, lanes 2, 4, 6, and 8). Supernatants were TCA concentrated and analyzed by Western blot for their content in IL-1β (lanes 1-4) and cathepsin D (lanes 5-8). Panels B and C show the densitometric analyses of IL-1β, procathepsin D, and cathepsin D present in supernatants of LPS-activated DCs, cultured 6 hours alone (B, DCs) or with alloreactive T cells (C, DCs + allo T) in the presence or absence of different substances for the times indicated: 5 mM EGTA (during the entire time of incubation), 10 μM nifedipine (NFD, 30 minutes of treatment, before coculture with T cells), 1 mM ionomycin (iono, for the last 10 minutes of incubation), 10 μM Bay K8644 (BayK, for the last 15 minutes of incubation), 10 nM thapsigargin (thapsi, for the last 30 minutes of incubation), or 10 nM thapsigargin and 5 mM EGTA (thapsi + EGTA, for the last 30 minutes of incubation). As a control, DCs were fixed for 10 minutes with 1% glutaraldehyde (C, GA-treated DCs + allo T) and secretion induced by T cells was evaluated with or without ionomycin stimulation. One representative experiment of 3 is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2152/5/m_h81911583005.jpeg?Expires=1769094603&Signature=HZmA2a1qBONBGgEBjzGUpEroG7bi~3EiTb1onGpNlufYJb3PgiZprEfR7RHbAbvum7emF54DXPf1J1Sn3NiPkJv2QBpH956TIdsT3qRoCXGPODldVGvLkI91HuYJVOKpeQvmvLohVyimj45iMrMNYcoaF1yyTQN0CfWIju4loyXuV6F3OTp9DjDJ-0ImNQrLmDtBYNluktgIjsQ3zI39OldtazrpGTLo-aLipgC~LCgvu13IdsnIr4MAxm-7CAGI5AR9~xPEbd8ExjoNlJsHuYKIwqLxFU1d506wb7VVABsyzJ6sQrAL9yuM9ZPd5y2v65cJixpvhkxSIAPppfYz5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. A rise in DC [Ca++]i follows interaction with CD8+ but not CD4+ T cells. / Enriched CD8+ (A) or CD4+ (B) alloreactive T cells loaded with FURA 2-am were added to FURA 2-am–loaded DCs and the oscillations in [Ca++]i were monitored at the single cell level. Pictures were taken at intervals of 10 minutes. Panels C and D show the mean of 15 Ca++ responses induced in DCs by CD8+ alloreactive T cells (C) and in CD4+alloreactive T cells by DCs (D). Results are expressed as ratio of the light emitted at 510 nm after excitation at 334 nm or 380 nm. One representative experiment of 3 is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2152/5/m_h81911583006.jpeg?Expires=1769094603&Signature=yivlMT~ExvXTVWVW6ZcgTaY87L8gf9LHcyrqrjGcPwLgzfu9jk4lQ6nPcobcKjDf67Zl40v30YZK4pjYoGzY4C9zFcs67R-C~eaq6ByUgIZItRePSVwmDsO8xqVaX75cDCwpfgEcvMR9SFoJdA0ds75yCqeLZzddPFrfSgpKENgQ3mOsicKJJjdkZ-ODHu4kjXVV4gA5Nkn0h4oRv4lUVIIdNNbQFxNbVolYSZxGC7Tv3DoUgax7FT6DwdY2QUoqfaIh1eOv2CkFO1qINir1h-0LAUePtJFqFOYrZsx2c8egujH4zrptpPyEQYdKQUSMkHxM5b7okVdro8Phd3UEig__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal