Sperm protein 17 (Sp17) is a highly conserved mammalian protein present on acrosome-reacted sperm that is thought to promote fertilization by binding sulfated carbohydrates of the oocyte zona pellucida. Although Sp17 was originally described as a testis-specific antigen, emerging evidence indicates that it may be more ubiquitously expressed than was previously thought. With the use of a specific antiserum, Sp17 was found to be present on the surface of malignant lymphoid cells, including B- and T-lymphoid cell lines, and on the surface of primary cells isolated from 2 patients having B-lymphoid tumors. Surprisingly, circulating B lymphocytes isolated from healthy volunteers also expressed Sp17, while circulating T lymphocytes exhibited only very weak expression. The role of Sp17 in promoting lymphoid cell adhesion was addressed with the use of recombinant Sp17 (rSp17). The rSp17 binds to the surface of myeloma cells but not to cells pretreated with heparitinase, an enzyme that removes heparan sulfate from the cell surface. Moreover, rSp17 promotes extensive aggregation of cells that express the syndecan-1 heparan sulfate proteoglycan, but in contrast, cells lacking syndecan-1 expression fail to aggregate in the presence of rSp17. These findings suggest that Sp17 promotes heparan sulfate–mediated cell aggregation and thereby plays a role in regulating adhesion and migration of normal and malignant lymphocytes.
Introduction
Sperm protein 17 (Sp17) is a 17.5-kd protein that is highly conserved with 61% homology among the protein sequences of mouse, rabbit, and human and with 97% homology between human and baboon.1-4 Analysis of the open reading frame sequence of human Sp17 reveals no transmembrane sequence and no signal peptide sequence.1 Sp17 is expressed at high levels in acrosome-reacted spermatozoa, where it is thought to promote fertilization by binding carbohydrates of the oocyte zona pellucida.5 In vitro analysis indicates Sp17 binds dextran sulfate and perhaps other sulfated carbohydrates, such as heparan sulfate, and despite its lack of a transmembrane domain, Sp17-transfected COS cells express the protein on the cell surface.2,6 Thus, Sp17 could play a role in adhesion of cells to extracellular matrix or to adjacent cells. In addition to its adhesive properties, Sp17, via its C terminus, binds to calmodulin and thus may regulate its activity.7 The N terminus of Sp17 has similarity to the dimer interaction site of the adenosine 3′,5′-cyclic monophosphate–dependent protein kinase IIα regulatory subunit and to A-kinase anchoring proteins.8 9
By Northern blotting, Sp17 messenger RNA (mRNA) is detected only within testis and thus was initially thought to be a testis-specific antigen. However, recent studies using reverse transcriptase–polymerase chain reaction (RT-PCR) have detected mRNA for Sp17 in murine metastatic squamous cell carcinoma cell lines and within Peyer patches of fetal lambs infected with bovine rotavirus.2,10 11 Although these studies did not assess Sp17 protein expression, they indicate that Sp17 may be more widely distributed than originally thought.
The present study was undertaken when we discovered during differential display experiments that Sp17 is expressed by human myeloma cells. Using a well-characterized antiserum specific for Sp17, we show that it is expressed on malignant lymphoid cells and, surprisingly, that it is also present on normal lymphocytes. In addition, we find that cell surface Sp17 promotes heparan sulfate–mediated adhesion between myeloma cells. These findings suggest that Sp17, via interactions with cell surface heparan sulfate, regulates the adhesion and migration of normal and malignant lymphocytes.
Materials and methods
Cell culture
Myeloma cell lines ARK and ARP-1 were established at the Arkansas Cancer Research Center from Ficoll-Hypaque–separated bone marrow aspirates obtained from myeloma patients.12,13ARH-77 cells, obtained from the American Type Culture Collection (Rockville, MD), were established from the peripheral blood of a patient with plasma cell leukemia.14 ARH-77 cells express little, if any, endogenous syndecan-1.15Syndecan-1–transfected ARH-77 cells were established by electroporation in the presence of the pMAMneo plasmid containing the complementary DNA (cDNA) for murine syndecan-1.16 Raji, Daudi, and Jurkat cell lines were obtained from the American Type Culture Collection. All cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 0.05 mM 2-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin sulfate, except for syndecan-1–transfected ARH-77 cells, which were maintained and selected in the above media containing 5% FBS and 0.3 mg/mL geneticin (Life Technologies, Gaithersburg, MD).
Normal peripheral blood leukocytes from healthy donors were isolated from blood samples collected in EDTA. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque separation, and peripheral blood total leukocytes were isolated following ammonium chloride lysis of red blood cells. Primary myeloma cells from patient samples were isolated with Ficoll-Hypaque separation.
Reverse transcriptase–polymerase chain reaction
Total RNA was isolated from cell lines or normal PBMCs with Trizol reagent (Life Technologies) according to the manufacturer's instructions. Reverse transcription was performed on 1.0 μg total RNA with an oligo(dT)15 primer (Promega, Madison, WI) by means of Moloney murine leukemia virus reverse transcriptase (Promega). From the resulting cDNAs, a 456–base pair (bp) cDNA fragment was amplified by means of primers specific for the human Sp17 open reading frame: 5′ oligonucleotide, 5′CGCGGATCCATGTCGATTCCATTCTCC-3′, and 3′ oligonucleotide, 5′-CGCCTCGAGACCAGTGTCCTCACTTGT-3′. PCR was performed with the following steps repeated for 28 cycles: melting at 94°C for 1 minute; annealing at 61°C for 1 minute, and elongation at 72°C for 1 minute. As an internal standard, a 983-bp fragment of glyceraldehyde-3–phosphate dehydrogenase (G3PDH) was amplified in the same PCR reaction as Sp17 by the addition of 40 nM G3PDH primers (Clontech Laboratories, Palo Alto, CA). A negative control, with water used instead of template, was run each time RT-PCR was performed. PCR products were visualized by means of a 1% agarose gel. For DNA sequencing, the 456-bp cDNA amplified from total RNA of ARH-77 cells was isolated from the PCR agarose gel, purified with the Qiaex II Gel Extraction System (Qiagen, Valencia, CA), and cloned by blunt end ligation into the pCR-TRAP cloning vector (Genhunter, Nashville, TN). Sequencing was performed with primers specific for the pCR-TRAP vector by means of the Dye Terminator Ready Reactionkit (Applied Biosystems, Foster City, CA) and by analysis on a 377 Automated DNA Sequencer (Applied Biosystems).
Expression and purification of recombinant Sp17
A fusion protein consisting of Sp17 attached to the C terminus of glutathione S-transferase–recombinant Sp17 (GST-rSp17) with a thrombin cleavage site separating the 2 proteins was expressed inEscherichia coli with the use of the GST Gene Fusion System (Amersham Pharmacia Biotech, Piscataway, NJ) according to manufacturer's instructions. Briefly, the Sp17 open reading frame was amplified by means of primers containing restriction enzyme sites BamHI (5′) or XhoI (3′). Sequences of primers were identical to those described above. The PCR-amplified Sp17 gene was ligated in-frame into the BamHI and XhoI sites of the pGEX-4T-2 vector 3′ to the GST gene. The resulting construct was used to transform E colistrain BL21. Restriction enzyme digestion was used to identify colonies containing plasmids with inserts, and the Sp17 cDNA insert was sequenced to confirm its accuracy. GST-rSp17 was purified from bacterial lysates by means of glutathione-Sepharose beads according to the manufacturer's instructions for bulk purification of fusion proteins (Amersham Pharmacia Biotech). GST-rSp17 bound to glutathione beads was digested with 5 NIH units of thrombin per milligram of fusion protein for 1 hour at room temperature to release rSp17. Thrombin was removed from the thrombin-released rSp17 with benzamidine-Sepharose (Amersham Pharmacia Biotech).
Immunostaining and flow cytometry
An extensively characterized rabbit antiserum specific for Sp17 was generously provided by Dr Michael O'Rand (University of North Carolina, Chapel Hill).3 For indirect immunostaining, 4 to 5 × 105 cells were incubated for 30 minutes on ice with 120 μg/mL Sp17 antiserum or control normal rabbit serum (Jackson ImmunoResearch Laboratories, West Grove, PA). After washing, cells were resuspended in 15 mg/mL biotinylated goat anti–rabbit immunoglobulin G (IgG) (Vector Laboratories, Burlingame, CA) for 30 minutes, washed with phosphate-buffered saline (PBS), and incubated with 1.5 μg/mL streptavidin phycoerythrin (Becton Dickinson, San Jose, CA). After washing in PBS, cells were fixed with 0.5 mL 1% paraformaldehyde and analyzed on a FACScan flow cytometer (Becton Dickinson). Gates were set to include only live cells in the analysis.
For dual staining of cells, fluorescein isothiocyanate (FITC)–labeled antibody to CD3 (Becton Dickinson), CD19 (Becton Dickinson), or CD138 (clone B-B4) (Serotec, Oxford, United Kingdom) was used in combination with antiserum to Sp17. The staining procedure described above was employed except that 10 μg/mL FITC-labeled monoclonal antibody (or 10 μg/mL of its isotype-matched control) was added for incubation concurrently with the biotinylated goat anti–rabbit immunoglobulin.
Western blotting
The rSp17 was analyzed on a 14% Tris glycine sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel (Novex, San Diego, CA) and transferred to nitrocellulose. The membrane was probed with Sp17 antiserum followed by incubation with a biotinylated goat anti–rabbit IgG (Vector Laboratories) and then with avidin–horseradish peroxidase (Vector Laboratories). Bound horseradish peroxidase was detected by chemiluminescence (Amersham Pharmacia Biotech).
Binding of rSp17 to cells via heparan sulfate chains
ARP-1 cells were treated twice (sequentially) with 1 mU/mL heparitinase (Seikagaku, Rockville, MD) or 50 mU/mL chrondroitinase ABC (Seikagaku) at 37°C for 30 minutes to remove heparan sulfate or chrondroitin sulfate chains, respectively. Cells (5 × 105) were then incubated with 50 μg/mL rSp17 for 1 hour at 37°C, washed 4 times, immunostained with antiserum to Sp17, and analyzed by flow cytometry.
Cell aggregation assays
Cell aggregation assays were performed as previously described.17 Briefly, cells growing in suspension were harvested by centrifugation, washed twice, and resuspended at a concentration of 4 × 106/mL in Hanks balanced salt solution containing 1 mM calcium chloride, 1 mM magnesium sulfate, 1% bovine serum albumin, and 10 mM Hepes. Cell aggregates were dispersed by pipetting, and 0.5 mL of the cell suspension was placed into wells of a 24-well culture plate (Corning, Corning, NY) along with 100 μg/mL rSp17 or rGST. The culture plate was rotated on a gyratory shaker (100 rpm) at 37°C for 2 hours. Cells were removed and gently resuspended so as not to disturb aggregates, and 10 μL cells were loaded onto each side of a Neubauer-type hemocytometer. Cells in a total area of 4 mm2 were counted to determine the percentage of cells in aggregates containing 3 or more cells.
Results
Sp17 is expressed on normal and malignant lymphocytes
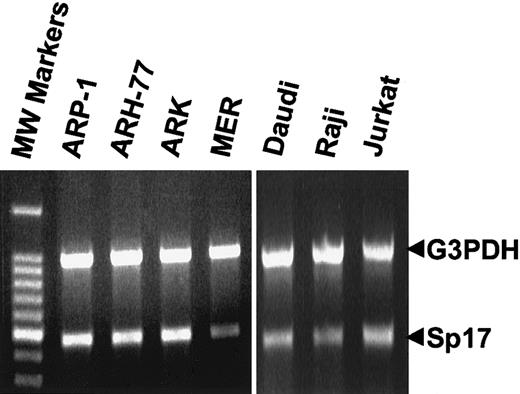
By means of RT-PCR, the 456-bp open reading frame of human Sp17 mRNA was detected at similar levels among tumor cell lines of B-lymphoid and T-lymphoid lineage (Figure1). To verify its identity, the amplified product from the ARH-77 cells was extracted from the PCR gel, cloned, sequenced, and found to be 100% identical to human Sp17 (not shown). Sp17 mRNA is present in lymphoid cells in relatively low abundance because Sp17 mRNA from sperm, but not lymphoid cells, could be detected by Northern analysis (not shown). This is consistent with Northern blot data in a previous report in which Sp17 was detected solely in sperm and not other human tissues.2
Sp17 mRNA in human lymphoid cell lines.
The 456-bp open reading frame of Sp17 was amplified by RT-PCR from the total RNA of lymphoid tumor cell lines (ARH-77, plasma cell leukemia; ARP-1, ARK, and MER, myeloma; Daudi and Raji, Burkitt lymphoma; Jurkat, T-cell lymphoma). As an internal standard, the 983-bp fragment of G3PDH was amplified along with the Sp17 in the same PCR reaction. A negative control (no template) was performed during each RT-PCR reaction (not shown).
Sp17 mRNA in human lymphoid cell lines.
The 456-bp open reading frame of Sp17 was amplified by RT-PCR from the total RNA of lymphoid tumor cell lines (ARH-77, plasma cell leukemia; ARP-1, ARK, and MER, myeloma; Daudi and Raji, Burkitt lymphoma; Jurkat, T-cell lymphoma). As an internal standard, the 983-bp fragment of G3PDH was amplified along with the Sp17 in the same PCR reaction. A negative control (no template) was performed during each RT-PCR reaction (not shown).
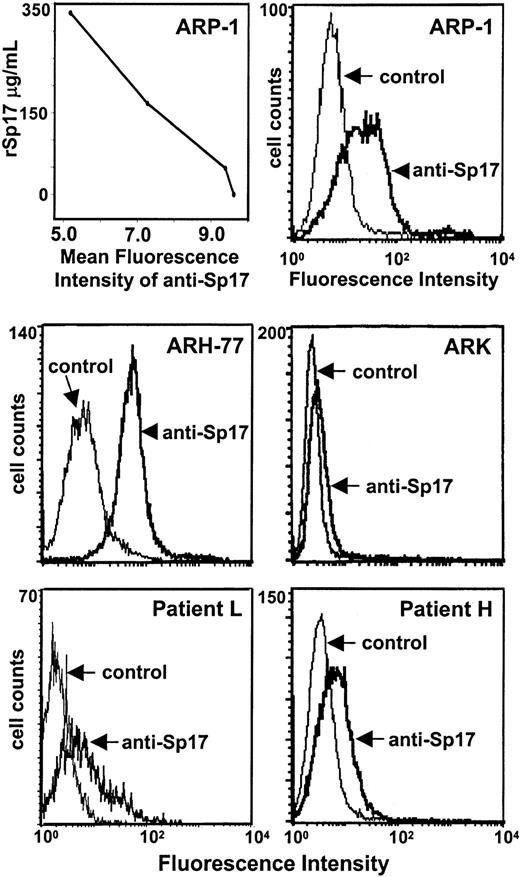
To determine if the Sp17 protein is expressed on the surface of lymphoid cells, a previously characterized antiserum to human Sp17 was employed.3 Analysis by flow cytometry demonstrates that ARH-77 cells express relatively high levels of Sp17 on their surface, while ARK cells express little, if any, Sp17 (Figure2). Staining of ARP-1 myeloma cells reveals a broad distribution in the level of Sp17 expression, with a small population of cells exhibiting high levels of expression. Thus, the level of Sp17 expression varied considerably among the cell lines tested. Specificity of the antiserum was confirmed by demonstrating a dose-dependent decrease in immunofluorescence staining of ARP-1 cells when antiserum is preincubated with rSp17 (Figure 2, upper left panel). To evaluate Sp17 expression on primary tumor cells, peripheral blood cells from a plasma cell leukemia patient and cells from a pleural effusion of a myeloma patient were harvested. Cells were dual stained with Sp17 antiserum (or control serum) and B-B4, a monoclonal antibody against CD138 (syndecan-1), a widely used marker that detects human plasma cells.18 Cells from both patients express detectable levels of Sp17 on their surface (Figure 2, patients L and H). In the sample from patient L, the shoulder in the peak indicates the presence of a subpopulation of tumor cells expressing higher levels of Sp17 than other cells within the sample.
Sp17 is expressed on the surface of B-lymphoid cell lines and primary tumor cells.
Sp17 is detected on the surface of ARP-1, ARH-77, and ARK cells by flow cytometry. The graph in the upper left panel demonstrates that the mean fluorescence intensity of staining with anti-Sp17 decreases in a dose-dependent manner as the concentration of rSp17 increases, thereby confirming antibody specificity. (Sp17 antiserum was incubated with 0, 47, 167, or 333 μg/mL rSp17 before it was used for immunostaining of ARP-1 cells as described in “Materials and methods.”) Data shown are representative of a minimum of 2 independent experiments. The lower panels show staining of freshly isolated tumor cells from the blood of a patient with plasma cell leukemia (patient L) and from a pleural effusion of a myeloma patient (patient H). Samples were dual stained with antiserum to Sp17 and antibody B-B4, a monoclonal antibody that recognizes the plasma cell marker CD138 (syndecan-1). The histograms shown are Sp17 staining of the CD138+tumor cells.
Sp17 is expressed on the surface of B-lymphoid cell lines and primary tumor cells.
Sp17 is detected on the surface of ARP-1, ARH-77, and ARK cells by flow cytometry. The graph in the upper left panel demonstrates that the mean fluorescence intensity of staining with anti-Sp17 decreases in a dose-dependent manner as the concentration of rSp17 increases, thereby confirming antibody specificity. (Sp17 antiserum was incubated with 0, 47, 167, or 333 μg/mL rSp17 before it was used for immunostaining of ARP-1 cells as described in “Materials and methods.”) Data shown are representative of a minimum of 2 independent experiments. The lower panels show staining of freshly isolated tumor cells from the blood of a patient with plasma cell leukemia (patient L) and from a pleural effusion of a myeloma patient (patient H). Samples were dual stained with antiserum to Sp17 and antibody B-B4, a monoclonal antibody that recognizes the plasma cell marker CD138 (syndecan-1). The histograms shown are Sp17 staining of the CD138+tumor cells.
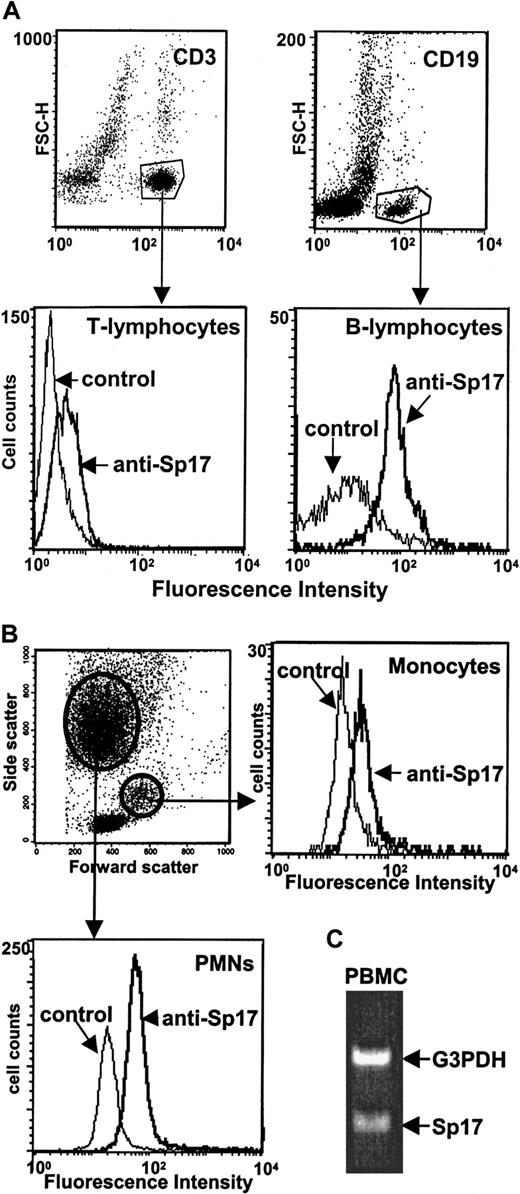
Next, PBMCs from healthy volunteers were assessed to determine if Sp17 is expressed on normal circulating lymphocytes. Lymphocytes were identified by dual staining with a monoclonal antibody specific for T lymphocytes (CD3) or B lymphocytes (CD19) and Sp17 antiserum (Figure3A). B-lymphocyte cell surfaces were clearly positive for Sp17 expression, while T lymphocytes exhibited only weak expression. Relative to controls, the B lymphocytes exhibited a much higher ratio of mean fluorescence intensity than did the T lymphocytes. Interestingly, Sp17 is also present at detectable levels on the surface of polymorphonuclear leukocytes (PMNs) and is weakly expressed on monocytes (Figure 3B). Also of interest was the observation that Sp17 is present in similar amounts on leukocytes harvested from both males and females (not shown). Analysis by RT-PCR of PBMCs reveals the presence of mRNA for Sp17 in these normal circulating cells (Figure 3C), indicating that the Sp17 detected on the cell surfaces is produced by the cells and is probably not derived from soluble Sp17 that could be present in the circulation. Together, these data indicate that expression of Sp17 is not restricted to testis and tumor cells; rather, it is also expressed on normal circulating PBMCs.
Sp17 is expressed on the surface of normal circulating lymphocytes.
(A) PBMCs from healthy donors were dual stained with antiserum to Sp17 and a monoclonal antibody specific for T lymphocytes (anti-CD3) or B lymphocytes (anti-CD19) and then analyzed by flow cytometry. Dot plots (upper panels) show the subpopulation of CD3+ and CD19+ cells, and histograms (lower panels) show the staining profile for Sp17 of those 2 subpopulations. (B) Sp17 is also present on polymorphonuclear leukocytes (PMNs) and monocytes. To distinguish PMN and monocyte populations, the light scatter pattern (forward versus side light scatter) was used. Data in panels A and B are representative of 2 to 4 independent experiments. (C) Amplification of Sp17 mRNA by RT-PCR confirms that normal PBMCs are producing the cell surface Sp17 that is detected by flow cytometry.
Sp17 is expressed on the surface of normal circulating lymphocytes.
(A) PBMCs from healthy donors were dual stained with antiserum to Sp17 and a monoclonal antibody specific for T lymphocytes (anti-CD3) or B lymphocytes (anti-CD19) and then analyzed by flow cytometry. Dot plots (upper panels) show the subpopulation of CD3+ and CD19+ cells, and histograms (lower panels) show the staining profile for Sp17 of those 2 subpopulations. (B) Sp17 is also present on polymorphonuclear leukocytes (PMNs) and monocytes. To distinguish PMN and monocyte populations, the light scatter pattern (forward versus side light scatter) was used. Data in panels A and B are representative of 2 to 4 independent experiments. (C) Amplification of Sp17 mRNA by RT-PCR confirms that normal PBMCs are producing the cell surface Sp17 that is detected by flow cytometry.
Sp17 promotes heparan sulfate–mediated adhesion of B-lymphoid cells
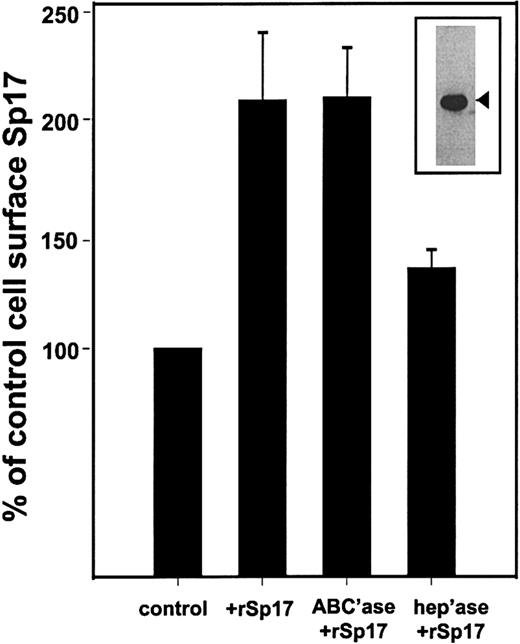
In previous studies, we demonstrated that syndecan-1 mediates B-lymphoid cell-cell adhesion via interactions between the heparan sulfate chains of syndecan-1 and an unidentified heparin-binding molecule. The amino acid sequence of Sp17 contains 2 linear consensus sequences for heparin binding (one at amino acid [aa] 49-52 and another at aa 135-138), and published binding assays demonstrate binding of recombinant Sp17 to immobilized dextran sulfate.2 This led us to examine the possibility that interactions between syndecan-1 and Sp17 promote lymphoid cell-cell adhesion. First, to determine if binding of Sp17 to cells is dependent on cell surface heparan sulfate, rSp17 was added to ARP-1 cells (which express endogenous syndecan-1) with and without pretreatment of the cells with heparitinase, an enzyme that strips heparan sulfate chains from the cell surface. Immunostaining of cells demonstrates that following addition of the protein, the level of detectable Sp17 at the cell surface increases more than 2-fold (Figure4). Removal of chondroitin sulfate chains from the cell surface has no effect on binding of Sp17, while removal of heparan sulfate chains dramatically reduces the amount of bound Sp17. This indicates that Sp17 binding to the cell surface is dependent on the presence of cell surface heparan sulfate.
Binding of rSp17 to cells is dependent on cell surface heparan sulfate.
The rSp17 (50 μg/mL) was added to ARP-1 cells and the level of Sp17 determined by flow cytometry following immunostaining. Data are expressed as a percentage of control, where the control was the value of staining for Sp17 in the absence of rSp17. Prior to addition of rSp17, some cells were treated with chondroitinase ABC (ABC'ase), to remove chondroitin sulfate chains, or heparitinase (hep'ase), to remove heparan sulfate chains. Removal of chondroitin sulfate chains has no effect on binding of rSp17 to cells. However, in comparison with these cells, removal of heparan sulfate significantly (P < .03) reduces the amount of rSp17 bound to cells. Values represent the mean of 3 experiments ± SE. (Insert) The rSp17 used in these experiments was purified as described in “Materials and methods” and analyzed for purity by Western blotting. The rSp17 is detected with antiserum as a 24.5-kd protein (arrowhead).
Binding of rSp17 to cells is dependent on cell surface heparan sulfate.
The rSp17 (50 μg/mL) was added to ARP-1 cells and the level of Sp17 determined by flow cytometry following immunostaining. Data are expressed as a percentage of control, where the control was the value of staining for Sp17 in the absence of rSp17. Prior to addition of rSp17, some cells were treated with chondroitinase ABC (ABC'ase), to remove chondroitin sulfate chains, or heparitinase (hep'ase), to remove heparan sulfate chains. Removal of chondroitin sulfate chains has no effect on binding of rSp17 to cells. However, in comparison with these cells, removal of heparan sulfate significantly (P < .03) reduces the amount of rSp17 bound to cells. Values represent the mean of 3 experiments ± SE. (Insert) The rSp17 used in these experiments was purified as described in “Materials and methods” and analyzed for purity by Western blotting. The rSp17 is detected with antiserum as a 24.5-kd protein (arrowhead).
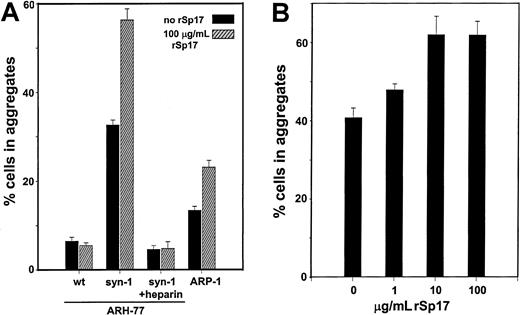
To determine if Sp17 can promote syndecan-1–mediated cell-cell adhesion, syndecan-1–positive (syndecan-1–transfected ARH-77 and ARP-1) and syndecan-1–negative (wild-type ARH-77) cell lines were evaluated for their ability to form aggregates following addition of rSp17. As previously shown,17 syndecan-1–transfected ARH-77 cells aggregate substantially, while the wild-type, syndecan-1–negative cells aggregate poorly (Figure5; compare wild-type and syndecan-1 ARH-77 cells in the absence of rSp17). Following addition of rSp17, aggregation of the syndecan-1–expressing ARH-77 cells almost doubles. In contrast, addition of rSp17 has no effect on the aggregation of the wild-type cells that lack expression of syndecan-1. Addition of heparin abolishes aggregation of syndecan-1–expressing ARH-77 cells, even in the presence of exogenous rSp17. For ARP-1 cells, rSp17 also promotes their aggregation, although these cells do not aggregate as extensively as syndecan-1–transfected ARH-77 cells. No increase in aggregation was seen in any of the cell lines in the presence of 100 μg/mL recombinant GST (data not shown). Dose-response experiments indicate a significant increase in aggregation at levels of rSp17 as low as 10 μg/mL (P < .005) (Figure 5B). Together, these data indicate that Sp17 promotes heparan sulfate–mediated adhesion of B lymphoid cells.
Sp17 promotes syndecan-1–mediated cell-cell adhesion.
(A) In the absence of rSp17, syndecan-1–transfected ARH-77 cells (syn-1) aggregate significantly more than wild-type (wt) cells that lack syndecan-1 expression (compare solid bars of syn-1 vs wt;P < .00000001). This illustrates the requirement of syndecan-1 for cell aggregation. Addition of rSp17 to syndecan-1–transfected cells significantly increases the extent of their aggregation (compare solid and hatched bars of syn-1;P < .0000001). In contrast, rSp17 has no effect on aggregation of the syndecan-1–negative wild-type cells (compare solid and hatched bars of wt). Addition of heparin blocks aggregation of the syndecan-1–transfected ARH-77 cells in either the absence or presence of rSP17 (syn-1 plus heparin). Addition of rSp17 also promotes aggregation of syndecan-1–expressing ARP-1 cells (compare solid vs hatched bars; P < .0001). Values represent the mean of a minimum of 3 experiments ± SE. In control experiments, addition of 100 μg/mL rGST had no effect on the extent of aggregation (not shown). (B) Dose-response experiments with rSp17 demonstrate a significant increase in aggregation of ARH-77 cells (syn-1) at 10 μg/mL (P < .005). An increase in aggregation is also noted at 1 μg/mL although at this level the change was not stastistically significant (P < .06).
Sp17 promotes syndecan-1–mediated cell-cell adhesion.
(A) In the absence of rSp17, syndecan-1–transfected ARH-77 cells (syn-1) aggregate significantly more than wild-type (wt) cells that lack syndecan-1 expression (compare solid bars of syn-1 vs wt;P < .00000001). This illustrates the requirement of syndecan-1 for cell aggregation. Addition of rSp17 to syndecan-1–transfected cells significantly increases the extent of their aggregation (compare solid and hatched bars of syn-1;P < .0000001). In contrast, rSp17 has no effect on aggregation of the syndecan-1–negative wild-type cells (compare solid and hatched bars of wt). Addition of heparin blocks aggregation of the syndecan-1–transfected ARH-77 cells in either the absence or presence of rSP17 (syn-1 plus heparin). Addition of rSp17 also promotes aggregation of syndecan-1–expressing ARP-1 cells (compare solid vs hatched bars; P < .0001). Values represent the mean of a minimum of 3 experiments ± SE. In control experiments, addition of 100 μg/mL rGST had no effect on the extent of aggregation (not shown). (B) Dose-response experiments with rSp17 demonstrate a significant increase in aggregation of ARH-77 cells (syn-1) at 10 μg/mL (P < .005). An increase in aggregation is also noted at 1 μg/mL although at this level the change was not stastistically significant (P < .06).
Discussion
This work demonstrates that Sp17 is present on the surface of normal and metastatic cells of hematopoietic lineage where it promotes heparan sulfate–mediated cell adhesion. By means of RT-PCR and specific antiserum, Sp17 was detected at various levels on cell lines of malignant B and T lymphocytes, freshly isolated cells from myeloma patients, and normal circulating B and T lymphocytes, PMNs, and monocytes. Its broad distribution on hematopoietic lineage cells suggests it may participate in important regulatory events related to adhesion and immune cell trafficking.
Sp17 was initially discovered on rabbit sperm, and Northern blot analysis of a range of tissues detects Sp17 mRNA only in testis.2 Our finding of Sp17 expression on hematopoietic lineage cells was aided by RT-PCR, which can detect mRNA at much lower levels than Northern analysis. PCR products for both Sp17 and G3PDH ran at their predicted cDNA size, indicating that the bands are not the result of genomic DNA contamination within our sample. If genomic DNA were present, the size of PCR products would be much larger because both the G3PDH and the Sp17 coding regions contain introns.1,19 Importantly, we were also able to confirm that the Sp17 protein is expressed on the surface of these cells using a highly specific and well-characterized antiserum. Although expression levels are not high on the surface of cells examined in this study, the Sp17 is easily detected by flow cytometry, particularly on B lymphocytes and PMNs. We do not yet know if cell surface levels of Sp17 are altered during the immune response, but a recent study suggests that this indeed may occur. Following bovine rotavirus stimulation of jejunal Peyer patches in fetal lambs, upregulation of 10 cDNAs was detected by differential display PCR.11 Interestingly, one of the ones upregulated was Sp17. Although not detected by Northern blotting on RNA from naive Peyer patch tissue, Sp17 mRNA was detected on Northern blots by means of poly(adenylic acid) RNA following immunostimulation. By RT-PCR, Sp17 was detected on mucosal-associated lymphoid tissue, including jejunal Peyer patches, non–Peyer patch intestine, and retropharyngeal lymph node, but was not detected on RNA from spleen, thymus, or prescapular lymph node.11 When these results are taken together with our discovery that Sp17 is present on the surface of hematopoietic lineage cells, it seems likely that this molecule plays an important role in the normal immune response.
Although the function of Sp17 is not clearly defined, we provide evidence that it can promote heparan sulfate–mediated cell-cell adhesion. By enzyme-linked immunosorbent assays, prior work demonstrated that Sp17 binds to dextran sulfate, which leads to speculation that Sp17 on sperm binds to heparan sulfate present on the zona pellucida.2 Analysis of the amino acid sequence of human Sp17 reveals 2 potential heparinbinding consensus regions, one at aa 49-52 and another at aa 135-138, while other heparin-binding sites may be present that result from alignment of basic residues upon folding of the protein. Interestingly, the potential heparin-binding site at aa 49-52 is conserved among all forms of Sp17 sequenced to date, including human, rabbit, baboon, rat, mouse, marmoset, sheep, opossum, and wallaby, and the site at aa 135-138 is conserved among all except the opossum and wallaby. When exogenous Sp17 is added to cells expressing syndecan-1, a substantial increase in cell-cell adhesion occurs (Figure 5). Importantly, this adhesion is blocked by addition of exogenous heparin. Moreover, cells lacking syndecan-1 expression fail to aggregate in the presence of Sp17. Together, these results demonstrate that Sp17 promotes heparan sulfate–mediated cell adhesion. This may be important in a number of immune cell interactions. For example, endothelial cells express heparan sulfate proteoglycans on their luminal surface, and these may interact with circulating white blood cells, particularly during the immune cell extravasation that accompanies lymphocyte recirculation or inflammatory responses. Indeed, the up-regulation of Sp17 mRNA during induction of an immune response led to the suggestion that this molecule may influence trafficking of immune cells.11 Our finding that Sp17 promotes heparan sulfate–mediated cell adhesion provides a potential mechanism to support this hypothesis.
By means of differential display PCR, Sp17 mRNA was also found to be up-regulated on metastatic vs nonmetastatic murine squamous cell carcinoma cell lines.10 This led to speculation that Sp17 may play a role in cell migration. This might also occur through Sp17 binding to heparan sulfate, which is present within squamous cell carcinoma. Such interactions may be relatively weak in affinity, allowing cells to maintain contact with adjacent cells to permit traction but not so tight as to inhibit migration. Interestingly, Sp17 on tumor cells may also be important in extravasation from blood vessels. This is supported by studies demonstrating that administration of heparin, or other sulfated glycosaminoglycans, can prevent extravasation of circulating tumor cells into tissue compartments.20 The mechanism for this is thought to be the blocking of heparan sulfate–ligand interactions at the endothelial cell surface.
Sp17 shares striking similarities to another well-characterized molecule known as heparin/heparan sulfate interacting protein (HIP).21 22 Sp17 and HIP are relatively small proteins of 152 and 159 amino acids, respectively; both lack transmembrane domains, yet are found on the surface of cells. Although they do not share strong protein sequence homology, they both contain multiple consensus sequences for heparin binding. Our finding that Sp17 can act to promote cell-cell adhesion through binding to heparan sulfate also parallels the well-defined role of HIP, which, via interaction with heparan sulfate, mediates binding of the embryo to uterine epithelium during implantation. The similarities between Sp17 and HIP suggest these may represent a new class of molecules present at the cell surface that perform regulatory roles by interacting with heparan sulfate present within the matrix or on adjacent cells.
Finally, the recent suggestion by Lim et al23 that Sp17 is a novel cancer-testis antigen in multiple myeloma is not supported by our results. Cancer-testis antigens, such as members of theMAGE family, BAGE and GAGE, are found in testis, absent in other normal tissues, but aberrantly expressed by tumor cells.24 The lack of expression of these antigens on normal tissues makes them important candidates as targets for cancer immunotherapy. Our finding that Sp17 is present on normal human hematopoietic cells, coupled with the observation that Sp17 mRNA is up-regulated substantially during a normal immune response in fetal lambs, strongly suggests that Sp17 is not a cancer-testis antigen. This is further supported by the recent detection of the Sp17 protein in numerous somatic tissues.25 Therefore, Sp17 may not be a safe and effective target for immunotherapy of multiple myeloma or other cancers.
The authors thank Dr Michael G. O'Rand, Department of Cell Biology and Anatomy, University of North Carolina at Chapel Hill, for generously supplying antiserum to Sp17 and for providing helpful advice during this study.
Supported by grants CA55819 and CA68494 from the National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ralph D. Sanderson, Department of Pathology, Slot 517, University of Arkansas for Medical Sciences, 4301 West Markham, Little Rock, AR 72205; e-mail: sandersonralphd@uams.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal