The chimeric monoclonal anti-CD20 antibody has been widely used for the treatment of relapsed or refractory low-grade B-cell non-Hodgkin lymphoma (NHL). However, in patients with a high number of tumor cells in blood, severe infusion-related toxicities have been reported.1 On the basis of elevated serum cytokine levels (tumor necrosis factor-α [TNF-α], interleukin-6 [IL-6]) the infusion-related complex consisting of fever, chills, hypotension, and dyspnea has been associated with an antibody-mediated cytokine-release syndrome.2 So far, the mechanism of cytokine release upon rituximab infusion remains unclear and histopathologic studies to enforce this hypothesis have not been performed. Here, we report the fatal course, and, for the first time, autopsy results of a patient with chronic lymphocytic leukemia treated with rituximab.
A 65-year-old man with heavily pretreated B-cell chronic lymphocytic leukemia (B-CLL) presented with rapidly increasing circulating lymphocyte counts (from 80.0 × 109/L to 260.0 × 109/L over a 2-week period), bulky lymphadenopathy, hepatosplenomegaly, and B symptoms (stage Rai IV). Previous treatment included chlorambucil, 4 cycles of COP (cyclophosphamide, vincristine, prednisone), and 2 cycles of fludarabine, which was discontinued because of development of an autoimmune thrombocytopenia. During the course of his disease the patient suffered from several infectious complications (bilateral pneumonia, listeria sepsis) and fully recovered from a right lobular pneumonia 4 weeks before rituximab treatment. After receiving local radiotherapy (10 Gy) for cervical bulky lymphadenopathy, the patient was admitted for the anti-CD20 monoclonal antibody therapy because he was refractory to standard chemotherapy. Pretreatment hematological parameters revealed the leukocyte count to be 271.0 × 109/L with 96% small-to-intermediate lymphocytes, hemoglobin level 8.6 g/dL, and platelet count 41.0 × 109/L. Flow cytometry of peripheral blood lymphocytes confirmed a typical B-CLL phenotype (CD19+, CD5+, CD23+, CD10−) with an unusually bright CD20 expression. After intravenous hydration and pretreatment with intravenous clemastine (2 mg), subcutaneous atropine (0.25 mg), oral acetaminophen (1000 mg), and allopurinol (300 mg), rituximab infusion was started. Initially, the antibody was given with a reduced infusion rate of 2.5 mg/h during the first 2 hours according to a fractionated dosing schedule recommended for patients with high tumor burden.2 The infusion rate was gradually increased to 10 mg/h, and 6.5 hours after start of infusion (42.5 mg total dose of rituximab given), the patient developed tachycardia, acetaminophen-resistant fever (39.7°C), and chills. A blood count revealed a decline of leukocytes to 190.0 × 109/L, hemoglobin level to 7.5 g/L, and platelets to 7.0 × 109/L. Rituximab therapy was discontinued and partial improvement of symptoms was noted following administration of metamizole (500 mg), diuretic therapy, O2-supplementation, and blood transfusion.
However, 3 hours after discontinuation of rituximab, the clinical condition of the patient again deteriorated and he developed progressive hypotension, dyspnea, tachypnea, and hypoxemia with basal crepitations in his lungs as well as heart and abdominal complaints. In addition, he developed progressive confusion and anuresis. Laboratory parameters showed an increase of serum lactate dehydrogenase from 1750 U/L to 3440 U/L, a hyperkalemia of 9.0 mM, and an elevated serum creatinine of 2.1 mg/dL, whereas calcium, phosphate, and uric acid levels remained in normal range. Plasma prothrombin time fell from 77% to 20%, fibrinogen level fell from 4.5 g/L to 3.7 g/L, and D-dimers increased moderately from > 0.1 mg/L to 0.4 mg/L, thus resembling a tumor lysis syndrome with disseminated intravascular coagulation. In spite of starting inotropic support, the patient died from cardiopulmonary failure 13 hours after initiation of rituximab infusion.
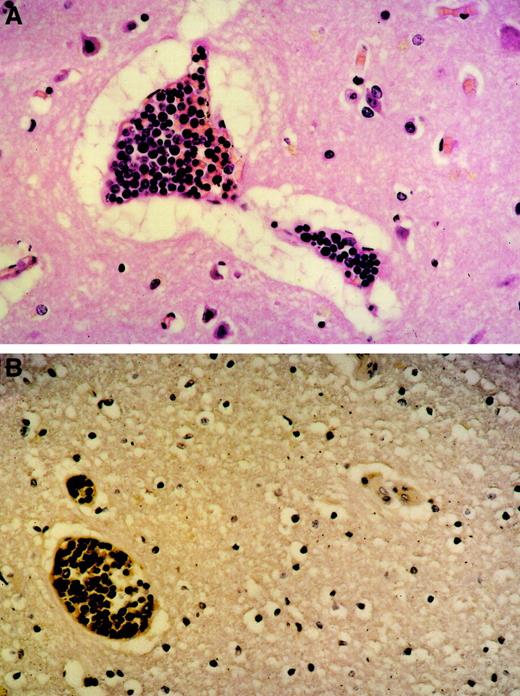
An autopsy was performed that revealed the transformation of the B-CLL into a diffuse, large-cell B-NHL (Richter syndrome) contributing to lymphoma infiltrates in lymph nodes, spleen, liver, and bone marrow. Interestingly, extensive intravascular leukostasis was observed in small vessels, most prominently in the heart, lung, and brain (Figure1), which was clearly different from postmortem findings in other patients with similarly high leukocyte counts. Importantly, neither significant apoptosis of tumor cells nor activation of the complement system could be demonstrated in this patient. These histopathologic findings are consistent with leukemic cell agglutination causing the clinical symptoms mentioned above and the fatal cardiopulmonary failure.
Tumor cell agglutination within blood vessels.
Original magnification was × 100. (A) Brain (H&E stain) agglutination of large lymphoid blasts with abundant mitoses within small capillaries. Marked perivascular edema. (B) Brain (immunhistology) CD20 expression of the tumor cells within the blood vessels proving them to be of B lineage.
Tumor cell agglutination within blood vessels.
Original magnification was × 100. (A) Brain (H&E stain) agglutination of large lymphoid blasts with abundant mitoses within small capillaries. Marked perivascular edema. (B) Brain (immunhistology) CD20 expression of the tumor cells within the blood vessels proving them to be of B lineage.
In contrast to myelocytic leukemias, leukostasis is a rare complication in patients with B-CLL or Richter syndrome.3 The leukostasis syndrome has been extensively investigated in a rat model of acute myelocytic leukemia. Physical characteristics of the tumor cells, such as cell size or hyperviscosity, as well as the activation of the complement system, contribute to the development of this syndrome.4 Patients with initially high or rapidly increasing tumor cell counts in the peripheral blood seem to be at higher risk for developing a leukostasis syndrome.5
Severe infusion-related adverse effects during first rituximab treatment in patients with very high numbers of circulating tumor cells (> 200.0 × 109/L) have been reported to be life-threatening or fatal, with a characteristic symptom complex that included pulmonary toxicity, hypotension, and a rapid reduction of circulating leukocytes and platelets, which might not be explained by a cytokine release syndrome alone.1 The development of these symptoms shortly after application of rituximab argues for a causative role of this anti-CD20 antibody in mediating tumor cell agglutination in blood vessels producing the fatal complications. Rituximab can contribute to leukostasis either because of direct cross-linking tumor cells or by more complex effects as opsonization of tumor cells. By increasing the antigen concentration (either high numbers of CD20 expressing circulating tumor cells or augmented density of the CD20 antigen), a zone of equivalence can be reached, where immune complexes consisting of tumor cells and anti-CD20 antibodies are formed, resulting in tumor cell agglutination followed by vessel obstruction.
This case report provides the first evidence based on histopathologic findings that tumor cell agglutination could be responsible for severe infusion-related adverse events during rituximab treatment. Patients with very high blood tumor cell numbers have an increased risk of developing this serious complication. Rituximab therapy in such patients should therefore be initiated with caution, and tumor cell reduction (< 50.0 × 109/L) prior to administration of rituximab is currently recommended because fractionated dosing schedules of rituximab, as in the case described here, obviously do not prevent this complication.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal