Abstract
Relapse is the major cause of treatment failure after allogeneic stem cell transplantation (SCT) in patients with acute lymphoblastic leukemia. Minimal residual disease (MRD) was analyzed before SCT in 30 patients with acute lymphoblastic leukemia. The aim was to determine whether the level of MRD before transplantation was correlated with outcome. Fifteen patients were found to have high-level MRD (10−2 to 10−3), 10 had low-level MRD (< 10−3), and 5 were MRD−. Among MRD− patients the probability of relapse was 0 in 5, which was less than in MRD+ patients (13 of 25) (P = .05). No major difference was found between the high- and low-level MRD+ groups. Among the MRD+ patients, only 2 of 11 with acute and chronic graft-versus-host disease had a relapse, versus 11 of 14 without (P = .005). In conclusion, for patients entering transplantation while they have residual disease, a combination of acute and chronic graft-versus-host disease may be needed to decrease the risk of relapse after SCT.
Introduction
Relapse still remains an obstacle to successful allogeneic stem cell transplantation (SCT) for patients with acute lymphoblastic leukemia (ALL).1,2 Graft-versus-host (GVH) disease, however, has been shown to protect against relapse.1,3 In particular, the combination of acute and chronic GVH disease seems to have the best antitumor effect.3-5
Analysis of antigen receptor (immunoglobulin [Ig] and T-cell receptor [TcR]) gene rearrangements to assess minimal residual disease (MRD) has started to become a part of routine laboratory work, and standardized protocols for monitoring MRD have therefore been developed.6 Using the polymerase chain reaction, MRD techniques are now sensitive enough to detect 1 leukemic cell among 104 to 106 normal cells.7 8
MRD studies after SCT have found a strong correlation between the presence of MRD and relapse.9,10 Increasing MRD levels have usually preceded a hematologic relapse. The importance of a pretransplant tumor burden to transplantation outcome has been shown by a better outcome in patients receiving transplants in complete remission (CR) than in those receiving transplants during relapse or with high MRD levels.11 12 However, many patients receiving transplants in remission still relapse after allogeneic SCT. The existence of residual disease not detected with morphologic analysis may therefore have an effect on outcome.
In this study of 30 patients with ALL, we quantified the leukemic cell burden before SCT to determine whether the level of MRD was correlated with outcome.
Study design
Patients
Between May 1989 and February 1999, 91 ALL patients received transplants at the Center for Allogeneic Stem Cell Transplantation, Huddinge University Hospital. Of these 91, 80 received transplants in remission. Nine patients were excluded from the study because of transplant-related mortality before day 100 after SCT, and another 37 were excluded for whom no samples were available at diagnosis or before SCT. Antigen receptor rearrangement was not found in 4 of the remaining 34 patients. Table 1 summarizes patient and donor characteristics of the remaining 30 patients in relation to the MRD results.
Patient characteristics, MRD results, and relapse incidence
| . | MRD, no. of relapse/no. of patients . | |||
|---|---|---|---|---|
| Total . | Negative . | Low* . | High† . | |
| All patients | 13/30 | 0/5 | 5/10 | 8/15 |
| Diagnosis | ||||
| T-cell ALL | 3/4 | 0/0 | 1/1 | 2/3 |
| Pre-B-cell ALL | 10/26 | 0/5 | 4/9 | 6/12 |
| Status at SCT | ||||
| CR1 | 4/16 | 0/3 | 3/7 | 1/6 |
| CR2 | 8/11 | 0/1 | 2/3 | 6/7 |
| CR3 | 1/3 | 0/1 | 0/0 | 1/2 |
| Cytogenetics | ||||
| Normal | 5/13 | 0/1 | 0/4 | 5/8 |
| t(9;22) | 1/7 | 0/2 | 1/2 | 0/3 |
| t(4;11) | 3/4 | 0/1 | 2/2 | 1/1 |
| Other abnormal | 3/5 | 0/1 | 2/2 | 1/2 |
| Failed | 1/1 | 0/0 | 0/0 | 1/1 |
| Donor | ||||
| HLA-identical sibling | 7/14 | 0/1 | 4/7 | 3/6 |
| MUD | 3/13 | 0/4 | 1/3 | 2/6 |
| MM sibling | 1/1 | 0/0 | 0/0 | 1/1 |
| MM unrelated | 2/2 | 0/0 | 0/0 | 2/2 |
| Conditioning | ||||
| Cy + TBI | 6/21 | 0/5 | 3/6 | 3/10 |
| Cy + fTBI | 4/5 | 0/0 | 1/2 | 3/3 |
| Bu + Cy | 3/4 | 0/0 | 1/2 | 2/2 |
| ATG/OKT-3 | 4/16 | 0/5 | 1/3 | 3/8 |
| GVHD prophylaxis | ||||
| MTX + CsA | 10/25 | 0/4 | 5/10 | 5/11 |
| MTX | 0/1 | 0/0 | 0/0 | 0/1 |
| CsA | 1/2 | 0/1 | 0/0 | 1/1 |
| MTX + CsA + TcD | 2/2 | 0/0 | 0/0 | 2/2 |
| GVHD | ||||
| No GVHD | 3/3 | 0/0 | 0/0 | 3/3 |
| Only cGVHD | 2/3 | 0/0 | 1/1 | 1/2 |
| Only aGVHD I/II | 6/9 | 0/1 | 3/3 | 3/5 |
| aGVHD + cGVHD | 2/15 | 0/4 | 1/6 | 1/5 |
| Recipient and donor | ||||
| Recipient age, y (median) | 13 (2-53) | 10 (7-17) | 19 (3-40) | 8 (2-53) |
| Donor age, y (median) | 28 (6-60) | 34 (15-37) | 22 (9-49) | 30 (6-60) |
| Recipient sex, M/F | 17/13 | 2/3 | 5/5 | 10/5 |
| Donor sex, M/F | 18/12 | 4/1 | 5/5 | 9/6 |
| Cell dose, 108/kg (median) | 3.0 (1.2-9.7) | 2.9 (2.4-3.6) | 3.6 (1.4-6.7) | 3.0 (1.2-9.7) |
| Days in remission before SCT (median) | 68 (19-355) | 66 (31-150) | 50 (11-355) | 75 (19-222) |
| . | MRD, no. of relapse/no. of patients . | |||
|---|---|---|---|---|
| Total . | Negative . | Low* . | High† . | |
| All patients | 13/30 | 0/5 | 5/10 | 8/15 |
| Diagnosis | ||||
| T-cell ALL | 3/4 | 0/0 | 1/1 | 2/3 |
| Pre-B-cell ALL | 10/26 | 0/5 | 4/9 | 6/12 |
| Status at SCT | ||||
| CR1 | 4/16 | 0/3 | 3/7 | 1/6 |
| CR2 | 8/11 | 0/1 | 2/3 | 6/7 |
| CR3 | 1/3 | 0/1 | 0/0 | 1/2 |
| Cytogenetics | ||||
| Normal | 5/13 | 0/1 | 0/4 | 5/8 |
| t(9;22) | 1/7 | 0/2 | 1/2 | 0/3 |
| t(4;11) | 3/4 | 0/1 | 2/2 | 1/1 |
| Other abnormal | 3/5 | 0/1 | 2/2 | 1/2 |
| Failed | 1/1 | 0/0 | 0/0 | 1/1 |
| Donor | ||||
| HLA-identical sibling | 7/14 | 0/1 | 4/7 | 3/6 |
| MUD | 3/13 | 0/4 | 1/3 | 2/6 |
| MM sibling | 1/1 | 0/0 | 0/0 | 1/1 |
| MM unrelated | 2/2 | 0/0 | 0/0 | 2/2 |
| Conditioning | ||||
| Cy + TBI | 6/21 | 0/5 | 3/6 | 3/10 |
| Cy + fTBI | 4/5 | 0/0 | 1/2 | 3/3 |
| Bu + Cy | 3/4 | 0/0 | 1/2 | 2/2 |
| ATG/OKT-3 | 4/16 | 0/5 | 1/3 | 3/8 |
| GVHD prophylaxis | ||||
| MTX + CsA | 10/25 | 0/4 | 5/10 | 5/11 |
| MTX | 0/1 | 0/0 | 0/0 | 0/1 |
| CsA | 1/2 | 0/1 | 0/0 | 1/1 |
| MTX + CsA + TcD | 2/2 | 0/0 | 0/0 | 2/2 |
| GVHD | ||||
| No GVHD | 3/3 | 0/0 | 0/0 | 3/3 |
| Only cGVHD | 2/3 | 0/0 | 1/1 | 1/2 |
| Only aGVHD I/II | 6/9 | 0/1 | 3/3 | 3/5 |
| aGVHD + cGVHD | 2/15 | 0/4 | 1/6 | 1/5 |
| Recipient and donor | ||||
| Recipient age, y (median) | 13 (2-53) | 10 (7-17) | 19 (3-40) | 8 (2-53) |
| Donor age, y (median) | 28 (6-60) | 34 (15-37) | 22 (9-49) | 30 (6-60) |
| Recipient sex, M/F | 17/13 | 2/3 | 5/5 | 10/5 |
| Donor sex, M/F | 18/12 | 4/1 | 5/5 | 9/6 |
| Cell dose, 108/kg (median) | 3.0 (1.2-9.7) | 2.9 (2.4-3.6) | 3.6 (1.4-6.7) | 3.0 (1.2-9.7) |
| Days in remission before SCT (median) | 68 (19-355) | 66 (31-150) | 50 (11-355) | 75 (19-222) |
MUD indicates matched unrelated donor; MM, mismatch; Cy, cyclophosphamide; fTBI, fractionated total body irradiation; Bu, busulfan; OKT, orthoclone; ATG, antithymocyte globulin; GVHD, GVH disease; MTX, methothrexate; CsA, cyclosporine A; TcD, T-cell depletion; a/cGVHD, acute/chronic GVHD.
MRD < 10−3.
MRD = 10−2-10−3.
Remission and relapse
Patients with regenerating peripheral blood values were considered in clinical remission when fewer than 5% blast cells among at least 200 nucleated cells were found in a bone marrow (BM) sample as defined by morphology. Clinical relapse was defined as when at least 30% blast cells were found in BM or when leukemic cells were detected extramedullary.
DNA samples and MRD analysis
All DNA material used in the MRD analysis was extracted from archival slides from BM aspirates. A salting-out procedure was performed as described by others.15
For MRD detection, the junctional regions of Ig and TcR gene rearrangements were amplified, cloned, and sequenced, and “patient-specific” primers were constructed for each patient. The methodology, polymerase chain reaction protocols, and primers for IgH, TcRδ, TcRγ, and Igκ (Kde) gene rearrangements are described in detail elsewhere.6,16 17
Quantification was performed by parallel amplification of 1 μg pre-SCT DNA with a 10-fold serial dilution of leukemic cell DNA in mononuclear cell DNA from 5 healthy donors. The percentage of leukemic cells in the diagnosis sample was known from morphology and immunophenotype analysis done on the same day as the preparation of the slides.
MRD levels were defined as high (10−2 to 10−3), low (10−4 to 10−5), or negative.
Samples
The diagnosis or relapse samples from which the patient-specific primers were generated were taken at a median of 4 (range, 2-13) months before SCT. The pre-SCT samples, analyzed for the presence of MRD, were taken at a median of 9 (range, 0-30) days before SCT.
Statistical analysis
The probability of relapse was calculated according to the Kaplan-Meier method. Differences in the incidence of GVH disease and relapse were compared with the Fisher exact test. The logistic regression model was used for multivariate analysis, which included risk factors such as sex, age, CR status, and acute and chronic GVH disease.
Results and discussion
Antigen receptor rearrangements and primer sensitivity
To avoid the problem of false negative results due to continuing rearrangements, usually observed in IgH rearrangements, several gene targets were used to identify clone-specific rearrangements.18 19
Twenty-seven patients were analyzed with primers reaching a sensitivity of 10−4 (n = 17) or 10−5 (n = 10). A target sensitivity of 10−3 was observed in 3 patients. All 3 patients, however, had an MRD level of more than 10−3 in the pre-SCT sample.
Patients
Thirteen patients died at a median of 10 (range, 2-22) months after SCT. Causes of death were BM relapse in 12 cases (median 8 [range, 2-22] months) and multiorgan failure in 1. Sixteen patients are alive and without relapse with a median follow-up of 39 (range, 13-119) months. One is alive with relapse.
MRD results and outcome
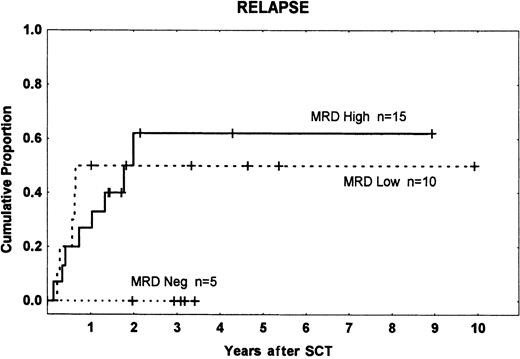
Fifteen patients had high-level MRD (10−2 to 10−3), 10 low-level MRD (10−4 to 10−5), and 5 were MRD−. There were 8, 5, and 0 relapses in the 3 groups, respectively, with a higher incidence among patients who were MRD+ (13 of 25) than in those who were MRD− (0 of 5) (P = .05). We found no significant difference in relapse rates between the high- and low-level MRD groups (Figure 1). This result does not accord with the findings of Knechtli et al,12 who had a relapse incidence of 100% in patients with high-level MRD, about 50% in those with low-level MRD, and about 20% in MRD−patients. This may be because most of their patients received a T-cell–depleted graft, which is associated with an increased risk of relapse.5,20 21
Time to and cumulative incidence of relapse among different MRD groups.
MRD high: 10−2 to 10−3; MRD low: less than 10−3; and MRD neg: MRD− before transplantation. Tick marks indicate patients without leukemic relapse.
Time to and cumulative incidence of relapse among different MRD groups.
MRD high: 10−2 to 10−3; MRD low: less than 10−3; and MRD neg: MRD− before transplantation. Tick marks indicate patients without leukemic relapse.
The importance of an alloreaction was seen when GVH disease was analyzed in the 3 MRD-level groups (Table 1). Among patients with both acute and chronic GVH disease, only 2 of 15 patients relapsed, compared with 11 of 15 in patients without or only acute or chronic GVH disease (P = .003).
In the 25 patients with detectable MRD before SCT, 2 of 11 patients with both acute and chronic GVH disease developed a hematologic relapse, as compared with 11 of 14 patients with no GVH disease or only acute or chronic GVH disease (P = .005).
In multivariate analysis, the combination of acute and chronic GVH disease was significantly associated with lower risk of relapse (odds ratio 0.07; 95% confidence interval, 0.01-0.52;P = .014). The incidence of relapse was also higher in patients receiving transplants in second or later remission than in those receiving transplants in first remission (P = .077). MRD could not be included in the multivariate analysis because there was no relapse in the MRD− group.
Although the present study is retrospective and includes a small number of patients, it indicates that patients with persistent disease are more likely to relapse than those in molecular remission. Patients at higher risk of relapse should therefore be followed more frequently after SCT, and those with persistent or increasing MRD levels may be given additional antitumor therapy, such as withdrawal of immunosuppression and/or donor lymphocyte infusions.22 It may be desirable to induce acute as well as chronic GVH disease to achieve the best antileukemic effect, as shown by several other studies.4,5,23 Our data may support this in patients with ALL who are MRD+ at the time of transplantation. Knowledge of the MRD status before SCT therefore may permit us to individually design the posttransplantation immunosuppressive strategy to decrease the risk of a threatening relapse.24
We are indebted to Inger Buskas and Anita Lindström for their help with the patient material. We thank the staff at the Center for Allogeneic Stem Cell Transplantation, Department of Hematology and Pediatrics, for compassionate and competent patient care.
Supported by grants from the Swedish Cancer Foundation (0070-B95-09XCC), the Children's Cancer Foundation (1995-035), the Swedish Medical Research Council (B96-16X-05971-16C), the FRF Foundation, the Tobias Foundation, and the Ellen Bachrach Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mehmet Uzunel, Dept of Clinical Immunology, Huddinge University Hospital, SE- 141 86 Stockholm, Sweden; e-mail:mehmet.uzunel@impi.ki.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal