Abstract
The adherence of sickle red blood cells (RBCs) to the vascular endothelium may contribute to painful vaso-occlusion in sickle cell disease. Sickle cell adherence involves several receptor-mediated processes and may be potentiated by the up-regulated expression of adhesion molecules on activated endothelial cells. Recent results showed that thrombin rapidly increases the adhesivity of endothelial cells for sickle erythrocytes. The current report presents the first evidence for the novel adhesion of normal and, to a greater extent, sickle RBCs to endothelial P-selectin. Studies of the possible interaction of erythrocytes with P-selectin revealed that either P-selectin blocking monoclonal antibodies or sialyl Lewis tetrasaccharide inhibits the enhanced adherence of normal and sickle cells to thrombin-treated endothelial cells. Both RBC types also adhere to immobilized recombinant P-selectin. Pretreating erythrocytes with sialidase reduces their adherence to activated endothelial cells and to immobilized recombinant P-selectin. Herein the first evidence is presented for the binding of normal or sickle erythrocytes to P-selectin. This novel finding suggests that P-selectin inhibition be considered as a potential approach to therapy for the treatment of painful vaso-occlusion in sickle cell disease.
Introduction
The interaction of P-selectin and its ligands contributes to the specificity of interactions among endothelial cells, platelets, and leukocytes during inflammation, coagulation, and atherosclerosis.1-8 The expression of P-selectin on endothelial cells and platelets requires activation of these cells by specific biologic response modifiers, such as thrombin, which has the capacity in both cell types to trigger rapid translocation of P-selectin from intracellular storage granules to the membrane surface and in endothelial cells to induce also slower transcriptional up-regulation of the P-selectingene.9-11 Another requisite for P-selectin–mediated adhesive interactions is a P-selectin ligand whose specificity is conferred by the particular transferases that generate its unique carbohydrate composition.1,4,6-8,12,13Erythrocytes are not considered to participate in these receptor-mediated processes,8 because normal red blood cells (RBCs) are not known to bear selectin ligands or to bind to P-selectin.14
The conventional view that erythrocyte sickling in small blood vessels causes the painful vaso-occlusion of sickle cell disease does not explain why only 5% of patients account for 30% of pain crises in this common debilitating genetic condition.15,16 An emerging hypothesis is that vaso-occlusion involves factors besides erythrocyte sickling. The adherence of sickle cells to vascular endothelium may be particularly important to vaso-occlusion.17 Hebbel and colleagues found that sickle erythrocytes are abnormally adherent to endothelial cells in vitro18 and that the adhesivity of sickle RBCs in vitro correlates with vaso-occlusive severity.19 Kaul and associates determined that endothelial adherence of human sickle cells infused into the rat microcirculation has the remarkable capacity to initiate vaso-occlusion ex vivo.20 Adhesion pathways implicated in sickle cell disease involve several specific interactions among the sickle cell molecules α4β1, CD36, band 3, sulfated glycolipid, and basal cell adhesion molecule/Lutheran protein, the endothelial molecule vascular cell adhesion molecule-1 (VCAM-1), and possibly glycoprotein Ib and CD36, the bridging molecules von Willebrand factor (vWF) and thrombospondin (TSP), and subendothelial matrix molecules TSP, vWF, laminin, and fibronectin.21-23
Endothelial adhesivity is enhanced by the additional adhesion molecules expressed when endothelial cells are activated.24 Several biologic modifiers of endothelial cell adhesivity are operative in sickle cell disease, including thrombin,25,26platelet-activating factor (PAF),27histamine,28 tumor necrosis factor-α,29interleukin-1β,29,30 interferon-γ,31erythropoietin,32 vascular endothelial growth factor,33 hypoxia,34,35reperfusion,36 reactive oxygen species,37viruses,38 and sickle cells themselves.39Consistent with these findings is the evidence that in sickle cell disease at least a fraction of endothelial cells are activated. Nadeau and associates used quantitative reverse transcriptase–polymerase chain reaction to determine that messenger RNA levels for intercellular adhesion molecule-1 (ICAM-1) and VCAM-1 are increased 2- to 7-fold in kidney biopsy samples from adult sickle cell patients compared to nonsickle controls.40 Duits and coworkers found plasma levels of VCAM-1 to be increased in sickle cell patients in steady state and to increase further during painful vaso-occlusion.41 Solovey and associates in the Hebbel laboratory reported that circulating endothelial cells are increased in number and have activated phenotypes (ie, express ICAM-1, VCAM-1, E-selectin, and P-selectin).42 43 These findings taken together indicate that at least a portion of the endothelial cells are activated in sickle cell disease.
The importance of endothelial cell activation to sickle cell vaso-occlusion is demonstrated by recent findings regarding the effects of PAF and thrombin, 2 endothelial cell agonists found at increased levels in sickle cell disease.25,27 The discovery that blocking αVβ3-integrin on PAF-activated rat microvascular endothelium reduces the postcapillary adherence and improves circulatory dynamics of human sickle cells infused into this ex vivo circulation44 led to the suggestion that a clinical trial of antiadhesion therapy aimed at blocking αVβ3 be considered for sickle cell disease.23,45 The increased generation of thrombin in sickle cell disease and its further enhancement during painful vaso-occlusion25 led to our recent determination that thrombin treatment of endothelial cells rapidly increases their adhesivity for sickle erythrocytes in vitro.46 The temporal consistency of our findings with P-selectin expression led us to explore whether the adherence of sickle and nonsickle RBCs to thrombin-stimulated endothelial cells is mediated by P-selectin.
Materials and methods
Blood samples
Heparinized blood samples were obtained from subjects with sickle cell disease and from healthy control subjects with approval of the Committee on Human Research of the University of California, San Francisco.
Thrombin treatment of endothelial monolayers, static gravity adherence with dip rinse, and adherence inhibition assays
Thrombin treatment of human umbilical vein endothelial cells (HUVECs) and the static gravity adherence assay with dip rinse were performed as previously described.46 When 90% confluent, HUVECs (Clonetics, San Diego, CA) were treated with 0.1 U/mL thrombin (Sigma Chemicals, St Louis, MO) or medium alone for 5 minutes before assaying erythrocyte adherence. Adherent RBCs were counted microscopically in 8 randomly selected 0.15-mm2 fields for each study condition. The adherence data are presented as percent adherence where 100% is the mean adherence of nonsickle RBCs to untreated HUVECs. The adherent RBCs observed in our microscopic gravity adherence assay are biconcave disks, which is consistent with the observation by several laboratories that the most adhesive sickle cells are the less dense fraction that is relatively devoid of irreversibly sickled cells.22
Because of potential modulatory effects of heparin on adherence, we used the static adherence assay to compare the adhesivity of sickle cells and autologous plasma according to whether they were prepared with citrate anticoagulant or with heparin. We detected no significant difference in these 2 anticoagulants.
The contribution of P-selectin to thrombin-enhanced adherence was determined by comparing the effects on adherence of exposing HUVECs to no antibody, a 1:200 dilution of nonblocking P-selectin monoclonal antibodies (mAbs) AC1.2 (BD Pharmingen, San Diego, CA), or a 1:200 dilution of blocking P-selectin mAb 9E1 (R & D Systems, Minneapolis, MN). The contribution of P-selectin to thrombin-enhanced adherence was confirmed by comparing the effects on adherence of adding to each well medium alone, 100 μM sialyl Lewis X (sLeX) tetrasaccharide (sLeX, Sigma), or 500 μM 3′-sialyl-lactose (sLac, Glycotech, Rockville, MD), an analogous sugar that does not bind P-selectin.
Flow cytometry
We used as primary antibodies for indirect immunofluorescence staining of erythrocytes in flow cytometry the mAbs AC1.2 and 9E1, which are specific for P-selectin. For each mAb we used an isotype-matched nonspecific mAb or the secondary antibody alone as negative controls. The secondary antibody was goat anti–mouse or goat anti–rat IgG conjugated to biotin (Sigma), which was reacted with fluorescein-conjugated Neutravidin (Molecular Probes, Eugene, OR). Fluorescence intensity of 20 000 cells/experiment was quantified on a FACS-scan flow cytometry system and analyzed using Cell-Quest software (Becton Dickinson, San Jose, CA).
Nonstatic adherence to immobilized sialic acid-binding lectin-Ig chimeras or P-selectin-Ig chimeras using a rotatory adherence assay
We immobilized on microtiter wells 400 ng bovine serum albumin (BSA; Sigma), a sialic acid-binding lectin (Siglec)-6-Ig chimera, mutated Siglec-7-Ig chimera, or P-selectin-Ig chimera.47-49 Siglec-6 is a sialic acid-binding lectin of the immunoglobulin superfamily that does not bind P-selectin ligands or erythrocytes, which we used as negative controls. Whereas Siglec-7 does bind RBCs, the mutated Siglec-7 we used as a negative control does not.49 These chimeras were applied to the wells in 10 mM carbonate buffer overnight and then blocked with 0.5% BSA in Hanks buffered salt solution (University of California, San Francisco, Cell Culture Facility) before incubation with RBCs using a published rotatory adhesion assay.50 Erythrocyte adherence to BSA, Siglec-6, mutant Siglec-7, or P-selectin was measured as the number of RBCs observed in 8 random 0.04-mm2 fields for each condition. The adherence data are presented as percent adherence where 100% is the mean number of untreated nonsickle erythrocytes/field in a well in which BSA or Siglec was immobilized.
Enzyme treatment of test RBCs
To determine whether sialic acid on erythrocytes is a recognition determinant for P-selectin, prior to testing RBC adherence we treated the erythrocytes with sialidase using a published method.51 Packed RBCs were mixed with an equal volume of buffer or 0.1 U/mL Vibrio cholerae sialidase (Calbiochem, San Diego). No hemolysis was detected with 0.1 U/mL sialidase as assayed by colorimetric spectrophotometry. Efficacy of sialidase on RBCs was confirmed by agglutination with peanut extract lectin (Arachis hypogea lectin, Sigma).
Statistical analyses
For each experiment mean adherence was set arbitrarily at 100% for the control data. The mean adherence of data derived from perturbations of control conditions were calculated as a percentage relative to the control. We then calculated the average of the means from replicate experiments. The uncertainty of the estimate of the means from the data distributed in each set is described as SEM. An SEM of 0% resulted when control data sets were normalized to 100%. The number of replications for each study is stated in the figure legends. We used the paired one-tailed Studentt test to compare changes in adhesion resulting from different perturbations of the system.52
Results
Effect of P-selectin mAb on erythrocyte adherence to endothelial cells
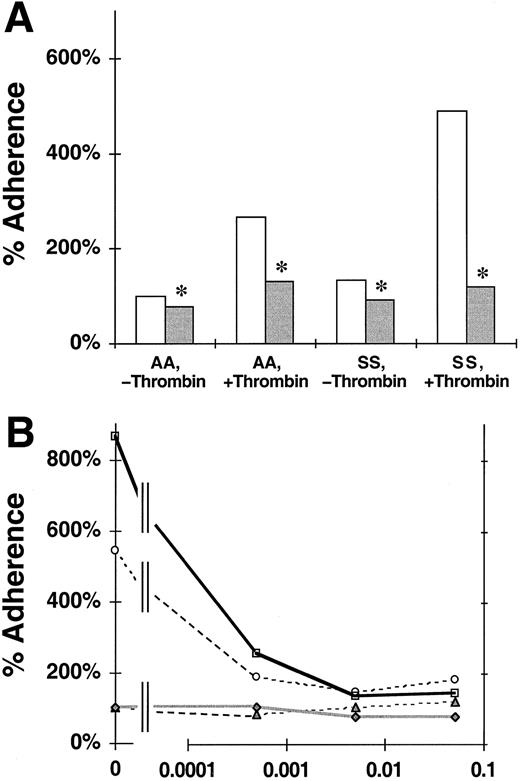
We assessed the effects of the P-selectin blocking mAb 9E1 on the static adherence of nonsickle or sickle RBCs to untreated or thrombin-treated HUVEC monolayers.46 The data shown in Figure 1A are consistent with previous reports that the adherence of sickle cells to untreated endothelial cells is greater than that of nonsickle RBCs18,46 and that the adherence of both RBC types to thrombin-treated endothelium is increased compared to untreated endothelium.46 Figure 1A also shows that blocking endothelial monolayers with mAb 9E1 reduced the adherence of nonsickle RBCs by 21% to untreated endothelium (100% ± 0% to 79% ± 11%; P = .046) and 51% to thrombin-treated endothelium (266% ± 72% to 131% ± 34%;P = .006). Blocking with mAb 9E1 reduced the adherence of sickle cells by 30% to untreated endothelium (132% ± 0% to 93% ± 11%; P = .002) and by 76% to thrombin-treated endothelium (490% ± 188% to 119% ± 18%;P = .038). These reductions in adherence were statistically significant but partial. The persistence of a portion of adhesivity after blocking P-selectin reveals that other adhesion mechanisms also are involved.
Effect of P-selectin antibodies on the adherence of nonsickle and sickle erythrocytes to HUVEC treated with or without thrombin.
The data shown are the static adherence of RBCs to HUVECs that were treated with thrombin or medium alone and then exposed to medium with or without blocking P-selectin antibody 9E1. The 100% adherence is the mean number of nonsickle (AA) RBC/field adherent to untreated HUVECs. SS, sickle erythorocytes. (A) The reduction of erythrocyte adherence to untreated or thrombin-treated HUVECs in the presence ( ) or absence (■) of mAb 9E1. The data are mean percent adherence from 12 replicate experiments. Significant inhibition of adherence due to mAb 9E1 with P < .05 is denoted by *. (B) Titration of adhesion inhibition by mAb 9E1. The data from this experiment are mean percent adherence plotted versus the dilution of mAb 9E1. ▴, AA, −thrombin; ○, AA, +thrombin; ♦, SS, −thrombin; ■, SS, +thrombin.
) or absence (■) of mAb 9E1. The data are mean percent adherence from 12 replicate experiments. Significant inhibition of adherence due to mAb 9E1 with P < .05 is denoted by *. (B) Titration of adhesion inhibition by mAb 9E1. The data from this experiment are mean percent adherence plotted versus the dilution of mAb 9E1. ▴, AA, −thrombin; ○, AA, +thrombin; ♦, SS, −thrombin; ■, SS, +thrombin.
Effect of P-selectin antibodies on the adherence of nonsickle and sickle erythrocytes to HUVEC treated with or without thrombin.
The data shown are the static adherence of RBCs to HUVECs that were treated with thrombin or medium alone and then exposed to medium with or without blocking P-selectin antibody 9E1. The 100% adherence is the mean number of nonsickle (AA) RBC/field adherent to untreated HUVECs. SS, sickle erythorocytes. (A) The reduction of erythrocyte adherence to untreated or thrombin-treated HUVECs in the presence ( ) or absence (■) of mAb 9E1. The data are mean percent adherence from 12 replicate experiments. Significant inhibition of adherence due to mAb 9E1 with P < .05 is denoted by *. (B) Titration of adhesion inhibition by mAb 9E1. The data from this experiment are mean percent adherence plotted versus the dilution of mAb 9E1. ▴, AA, −thrombin; ○, AA, +thrombin; ♦, SS, −thrombin; ■, SS, +thrombin.
) or absence (■) of mAb 9E1. The data are mean percent adherence from 12 replicate experiments. Significant inhibition of adherence due to mAb 9E1 with P < .05 is denoted by *. (B) Titration of adhesion inhibition by mAb 9E1. The data from this experiment are mean percent adherence plotted versus the dilution of mAb 9E1. ▴, AA, −thrombin; ○, AA, +thrombin; ♦, SS, −thrombin; ■, SS, +thrombin.
Further evidence for the involvement of other pathways was derived from a single titration experiment in which we tested the effect of 1:2000, 1:200, and 1:20 dilutions of mAb 9E1 on erythrocyte adherence to thrombin-activated HUVECs (Figure 1B). We determined that the adherence of nonsickle and sickle RBCs was reduced, respectively, 64% and 70% by a 1:2000 dilution of the mAb, 72% and 84% by a 1:200 dilution, and 66% and 83% by a 1:20 dilution. The persistence of similar levels of adherence at our standard 1:200 mAb dilution and at a 10-fold higher titer of 1:20 further supports the involvement of other adhesion pathways.
To verify the specificity of P-selectin blocking, in 3 replicate experiments we compared the effects of a pair of isotype-matched P-selectin mAbs, the blocking mAb 9E1 and the nonblocking mAb AC1.2, on RBC adhesion to thrombin-treated endothelial cell monolayers (data not shown). Treatment with AC1.2 resulted in no significant reduction in the adherence of either nonsickle (148% ± 25% to 160% ± 6%;P = .227) or sickle (318% ± 29% to 359% ± 25%;P = .242) RBCs from that observed without mAb. Compared to the adherence observed with AC1.2, treatment of endothelial cells with 9E1 reduced adherence by 48% for nonsickle cells (160% ± 6% to 84% ± 23%; P = .005) and 58% for sickle cells (359% ± 25% to 150% ± 38%; P = .039). Although the exact number of adherent erythrocytes and fractional inhibition varied among different experiments, different patients, patient status, and preparations of HUVECs, we consistently found statistically significant adhesion of both nonsickle and sickle RBCs to endothelial P-selectin.
These data taken together provide evidence for the novel adherence of normal and, to a greater degree, sickle erythrocytes to P-selectin on activated endothelial cells. They also support the contribution of P-selectin–independent pathways in thrombin-enhanced adherence.
Effect of sLeX tetrasaccharide on erythrocyte adherence to endothelial cells
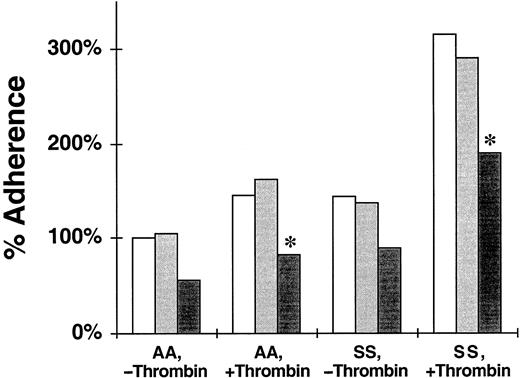
The sLeX antigen is a recognition determinant for selectins that selectively inhibits their adhesivity; sLac is a saccharide that is structurally related to sLeX but does not bind to P-selectin.1,4,6-8,13 In 4 replicate experiments we compared the static adherence of RBCs to endothelial cells in the absence of either saccharide, the presence of sLac, and the presence of sLeX. As shown in Figure 2, adherence to untreated endothelial cells with no added saccharide was not reduced significantly by the addition of sLac for either nonsickle (100% ± 0% to 105% ± 15%; P = .373) or sickle cells (143% ± 0% to 144% ± 44%; P = .493). Neither did sLac reduce significantly the adherence to thrombin-treated endothelial cells for either nonsickle (145% ± 31% to 162% ± 28%; P = .207) or sickle cells (325% ± 73% to 304% ± 74%; P = .240). The inhibitory effect of sLex on adherence was not significant with untreated endothelial cells but significant with thrombin-treated cells. Although not significant, compared with the adherence to untreated endothelial cells with sLac present, the addition of sLeX reduced adherence by an estimated 48% for nonsickle (105% ± 15% to 55% ± 12%; P = .063) and 37% for sickle cells (144% ± 44% to 91 ± 8%; P = .121). Compared with the adherence to thrombin-treated endothelial cells when sLac is present, adherence was reduced significantly by the addition of sLeX by 49% for nonsickle (162% ± 28% to 82% ± 41%;P = .047) and 36% for sickle cells (304% ± 74% 196% ± 38%; P = .037). These findings are consistent with the adhesion of nonsickle and sickle erythrocytes to P-selectin on activated endothelial cells. The incomplete inhibition observed with sLeX is not surprising because the inhibitory potency of this saccharide for P-selectin, while specific, is not strong.7The absence of an inhibitory effect by sLac confirms the specificity of sLeX for P-selectin in the static adhesion assay.
Effect of sLeX tetrasaccharide on the adherence of nonsickle and sickle erythrocytes to HUVECs treated with or without thrombin.
The data shown are the static adherence of RBCs to HUVEC that were treated with thrombin or medium alone. The 100% adherence is the mean number of nonsickle (AA) RBCs/field adherent to untreated HUVECs. SS, sickle erythrocytes. The reduction of erythrocyte adherence to untreated or thrombin-treated HUVECs in the presence of sLac ( ) or sLeX (▪) tetrasaccharide is shown. ■, control. The data are mean percent adherence from 4 replicate experiments. Significant inhibition of adherence due to sLeX with P < .05 is denoted by *.
) or sLeX (▪) tetrasaccharide is shown. ■, control. The data are mean percent adherence from 4 replicate experiments. Significant inhibition of adherence due to sLeX with P < .05 is denoted by *.
Effect of sLeX tetrasaccharide on the adherence of nonsickle and sickle erythrocytes to HUVECs treated with or without thrombin.
The data shown are the static adherence of RBCs to HUVEC that were treated with thrombin or medium alone. The 100% adherence is the mean number of nonsickle (AA) RBCs/field adherent to untreated HUVECs. SS, sickle erythrocytes. The reduction of erythrocyte adherence to untreated or thrombin-treated HUVECs in the presence of sLac ( ) or sLeX (▪) tetrasaccharide is shown. ■, control. The data are mean percent adherence from 4 replicate experiments. Significant inhibition of adherence due to sLeX with P < .05 is denoted by *.
) or sLeX (▪) tetrasaccharide is shown. ■, control. The data are mean percent adherence from 4 replicate experiments. Significant inhibition of adherence due to sLeX with P < .05 is denoted by *.
Although it is reasonable to conclude that the P-selectin mAb 9E1 and sLeX affect the same molecular interaction,7 we directly tested this precept in our system. We performed an experiment in which we compared the effects of these inhibitors singly and in combination. RBC adherence to thrombin-treated endothelial cells was inhibited nearly identically by mAb 9E1, sLeX, and their combination for nonsickle cells (17%-22%) and for sickle cells (43%-47%). The lack of additive effect is consistent with the knowledge that sLeX and the P-selectin participate in the same molecular interaction.7Because neither inhibitor nor their combination completely abrogated adherence, these results too are consistent with the existence of a P-selectin independent pathway for adhesion to thrombin-treated endothelial cells.
In our studies of mAb 9E1 only endothelial cells were exposed to the blocking agent, but in these studies of sLeX both endothelial and RBCs were exposed to the inhibitor. To assess whether the P-selectin blocked by sLeX may also have been on RBCs, we performed a flow cytometry study of nonsickle and sickle RBCs using P-selectin mAbs AC1.2 and 9E1. We detected no signal indicative of the presence of P-selectin on either type of erythrocyte (data not shown), which is consistent with prior reports of the absence of P-selectin from erythroid cells.53
Adherence of erythrocytes to immobilized recombinant P-selectin
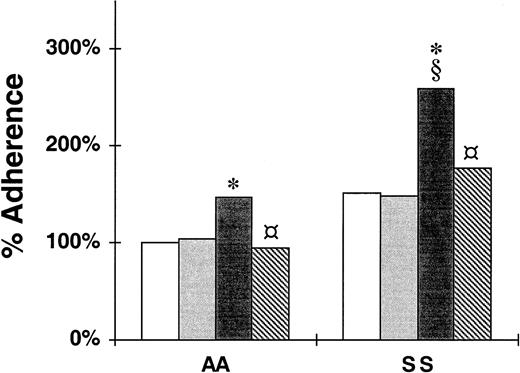
Multiple molecular mechanisms have been described for sickle cell adherence to unperturbed and activated endothelium. Our results from these studies on thrombin-activated HUVECs also may reflect the participation of adhesion molecules besides P-selectin, such as β1- and β3-integrins dissociated from their matrix binding sites (our unpublished data, 2001), and unmasked matrix proteins.54 As with leukocyte-endothelial interactions, the actual binding we observe may involve other molecules as well. Yet, selectin interactions typically initiate such adhesion cascades and are critically required. To directly confirm the role of P-selectin in sickle cell adhesion, we tested the nonstatic adhesion of RBCs to a recombinant P-selectin-Ig chimera47 immobilized on plastic microtiter wells using a rotatory adherence assay.50 The data shown in Figure3 demonstrate our comparison of the adherence of RBCs to P-selectin, to BSA, or to nonbinding Siglec-6 or mutated Siglec-7 chimeras, which share the Ig-Fc domain with the P-selectin construct.48,49 The adherence of nonsickle cells to P-selectin is a significant 46% greater than to BSA (146% ± 16% compared to 100% ± 0%; P = .031) and a nearly significant 41% greater than to Siglec-6 or mutated Siglec-7 (146% ± 16% compared to 104% ± 9%; P = .056). The adherence of sickle cells to P-selectin is 72% greater than to BSA (259% ± 44% compared to 151% ± 0%; P = .030) and 74% greater than to Siglec-6 or mutated Siglec-7 (259% ± 44% compared to 149% ± 7%; P = .017). The presence of 5 mM EDTA reduced the adherence to P-selectin by 35% for nonsickle cells (146% ± 16% to 95% ± 11%; P = .027) and 32% for sickle cells (259% ± 44% to 177% ± 18%;P = .016). These statistically significant and near significant differences provide further evidence that normal and, to a larger measure, sickle erythrocytes adhere to P-selectin. The statistically significant reduction of binding resulting from chelating calcium with EDTA confirms the specificity of P-selectin binding. Regarding the EDTA-resistant binding to P-selectin, we previously have established that P-selectin has 2 binding components, one EDTA sensitive and a second that is only sensitive to high (20 mM) concentrations of EDTA.47 The second component, which is not inhibited by the calcium chelating effect of EDTA but by its polycarboxylic acid nature, may represent the second anion binding site postulated for P- and L-selectin.
Adhesion of nonsickle and sickle erythrocytes to immobilized Siglec-6, P-selectin, or P-selectin in the presence of EDTA.
The data shown are the nonstatic adherence (in the rotatory adhesion assay) of untreated or sialidase-treated RBCs to immobilized BSA (■), Siglec-Ig chimera ( ), P-selectin-Ig chimera (▪), or P-selectin-Ig chimera in the presence of 5 mM EDTA (
), P-selectin-Ig chimera (▪), or P-selectin-Ig chimera in the presence of 5 mM EDTA ( ). The 100% adherence is the mean number of untreated nonsickcle (AA) RBCs/field in a well in which BSA was immobilized. SS, sickle erythrocytes. The data are mean percent adherence for 4 nonsickle and 6 sickle RBC samples. Differences in adherence with P < .05 were determined to be significant. Significant adhesion is compared to adhesion to BSA (*) and to Siglec (§), and significant inhibition of adhesion to P-selectin due to EDTA is denoted by
). The 100% adherence is the mean number of untreated nonsickcle (AA) RBCs/field in a well in which BSA was immobilized. SS, sickle erythrocytes. The data are mean percent adherence for 4 nonsickle and 6 sickle RBC samples. Differences in adherence with P < .05 were determined to be significant. Significant adhesion is compared to adhesion to BSA (*) and to Siglec (§), and significant inhibition of adhesion to P-selectin due to EDTA is denoted by .
.
Adhesion of nonsickle and sickle erythrocytes to immobilized Siglec-6, P-selectin, or P-selectin in the presence of EDTA.
The data shown are the nonstatic adherence (in the rotatory adhesion assay) of untreated or sialidase-treated RBCs to immobilized BSA (■), Siglec-Ig chimera ( ), P-selectin-Ig chimera (▪), or P-selectin-Ig chimera in the presence of 5 mM EDTA (
), P-selectin-Ig chimera (▪), or P-selectin-Ig chimera in the presence of 5 mM EDTA ( ). The 100% adherence is the mean number of untreated nonsickcle (AA) RBCs/field in a well in which BSA was immobilized. SS, sickle erythrocytes. The data are mean percent adherence for 4 nonsickle and 6 sickle RBC samples. Differences in adherence with P < .05 were determined to be significant. Significant adhesion is compared to adhesion to BSA (*) and to Siglec (§), and significant inhibition of adhesion to P-selectin due to EDTA is denoted by
). The 100% adherence is the mean number of untreated nonsickcle (AA) RBCs/field in a well in which BSA was immobilized. SS, sickle erythrocytes. The data are mean percent adherence for 4 nonsickle and 6 sickle RBC samples. Differences in adherence with P < .05 were determined to be significant. Significant adhesion is compared to adhesion to BSA (*) and to Siglec (§), and significant inhibition of adhesion to P-selectin due to EDTA is denoted by .
.
Effect of erythrocyte sialidase treatment on their adherence to endothelium and to immobilized P-selectin
The above findings indicate that normal erythrocytes have a ligand for P-selectin, which is enhanced markedly on sickle cells. The only published precedent for P-selectin binding activity on mature RBCs is on malarial parasitized cells, but the origin and nature of that ligand was incompletely characterized and appears to be malarial in origin.55,56 Ligand activity for P-selectin is typically mediated by sialylated, fucosylated, sulfated recognition determinants of membrane glycoproteins and glycolipids.6 8
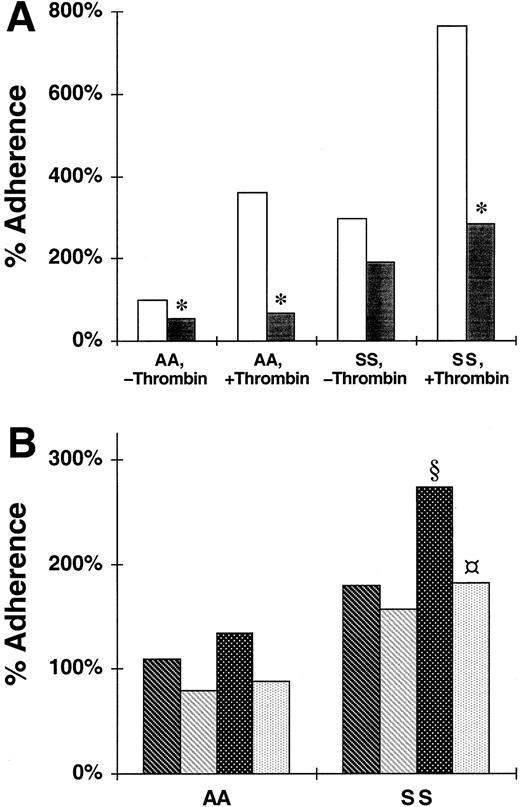
To assess the importance of erythrocyte membrane sialic acid as a binding determinant for P-selectin, we treated RBCs with sialidase before assaying their static adherence to endothelial monolayers, as has been described.50 Figure4A demonstrates that sialidase treatment of erythrocytes reduced adherence to untreated HUVECs by 47% for nonsickle cells (100% ± 0% to 53% ± 13%;P = .018) and by 36% for sickle cells (297% ± 0% to 191% ± 67%; P = .106), Sialidase treatment of RBCs significantly reduced adherence to thrombin-treated HUVECs by 81% for nonsickle (360% ± 125% to 68% ± 15%; P = .047) and 63% for sickle cells (766% ± 168% to 282% ± 133%;P = .044). These data provide a partial characterization of a novel erythrocyte P-selectin ligand that uses sialic acid as a recognition determinant.
Effect of sialidase treatment of nonsickle and sickle erythrocytes on their adherence to HUVECs treated with or without thrombin and to immobilized Siglec-6 or P-selectin.
The data shown are the adherence of RBCs that had or had not been treated with sialidase either to HUVECs, which had been treated with thrombin or medium alone, or to immobilized BSA, Ig-Ig chimera, or P-selectin–Ig chimera. The 100% adherence is the mean number of untreated nonsickle (AA) RBCs/field adherent to untreated HUVECs. SS, sickle erythrocytes. (A) The reduction of static erythrocyte adherence to untreated (■) or thrombin-treated HUVECs due to treatment of the RBCs with sialidase (▪). The data are mean percent adherence from 4 replicate experiments. Significant inhibition due to sialidase withP < .05 was denoted by *. (B) The reduction of nonstatic erythrocyte adherence to immobilized Siglec-6 or P-selectin due to treatment of the RBCs with sialidase. , Siglec, untreated;
, Siglec, untreated; , Siglec, sialidase;
, Siglec, sialidase; , P-seclectin, untreated;
, P-seclectin, untreated; , P-selectin, sialidase. The data are mean percent adherence for 3 nonsickle and 5 sickle RBC samples. Significant adhesion (P < .05) is compared to adhesion to Siglec-6 (§) and significant inhibition of adhesion to P-selectin due to sialidase is denoted by
, P-selectin, sialidase. The data are mean percent adherence for 3 nonsickle and 5 sickle RBC samples. Significant adhesion (P < .05) is compared to adhesion to Siglec-6 (§) and significant inhibition of adhesion to P-selectin due to sialidase is denoted by  .
.
Effect of sialidase treatment of nonsickle and sickle erythrocytes on their adherence to HUVECs treated with or without thrombin and to immobilized Siglec-6 or P-selectin.
The data shown are the adherence of RBCs that had or had not been treated with sialidase either to HUVECs, which had been treated with thrombin or medium alone, or to immobilized BSA, Ig-Ig chimera, or P-selectin–Ig chimera. The 100% adherence is the mean number of untreated nonsickle (AA) RBCs/field adherent to untreated HUVECs. SS, sickle erythrocytes. (A) The reduction of static erythrocyte adherence to untreated (■) or thrombin-treated HUVECs due to treatment of the RBCs with sialidase (▪). The data are mean percent adherence from 4 replicate experiments. Significant inhibition due to sialidase withP < .05 was denoted by *. (B) The reduction of nonstatic erythrocyte adherence to immobilized Siglec-6 or P-selectin due to treatment of the RBCs with sialidase. , Siglec, untreated;
, Siglec, untreated; , Siglec, sialidase;
, Siglec, sialidase; , P-seclectin, untreated;
, P-seclectin, untreated; , P-selectin, sialidase. The data are mean percent adherence for 3 nonsickle and 5 sickle RBC samples. Significant adhesion (P < .05) is compared to adhesion to Siglec-6 (§) and significant inhibition of adhesion to P-selectin due to sialidase is denoted by
, P-selectin, sialidase. The data are mean percent adherence for 3 nonsickle and 5 sickle RBC samples. Significant adhesion (P < .05) is compared to adhesion to Siglec-6 (§) and significant inhibition of adhesion to P-selectin due to sialidase is denoted by  .
.
To further explore the importance of sialic acid to the erythrocyte-binding determinant for P-selectin we treated RBCs with sialidase50 before assaying their nonstatic adherence to P-selectin and to control Siglec-6. The results shown in Figure 4B demonstrate that treatment of nonsickle cells with sialidase has no significant effect on their adherence to Siglec-6 (110% ± 11% to 79% ± 13%; P = .089) or to P-selectin (134% ± 14% to 88% ± 11%; P = .066). Treatment of sickle cells with sialidase also had no significant effect on their adherence to Siglec-6 (179% ± 7% to 157% ± 18%;P = .089) but a significant 33% reduction in their adherence to P-selectin (273% ± 28% to 182% ± 17%;P = .004). The finding that sialidase causes a statistically significant reduction in the adherence of sickle cells to P-selectin is consistent with the sialidase effects on sickle cell binding to thrombin-treated HUVECs described above. These results further support the partial characterization of a sickle cell P-selectin ligand, which uses sialic acid as a recognition determinant.
To confirm in our system the canonical requirement of sialic acid for P-selectin binding,7 50 we compared the effects of treating erythrocytes with sialidase and endothelial cells with mAb 9E1 singly and in combination. We observed that adherence to thrombin-treated endothelial cells was inhibited to a similar degree by sialidase, mAb 9E1, and their combination for nonsickle (17%-24%) and for sickle cells (29%-38%). The lack of additive effect is consistent with the participation of sialic acid and P-selectin in the same molecular interaction and with the adhesion of erythrocytes to P-selectin requiring a sialidase recognition determinant. Still, the existence of a sialidase-sensitive pathway not involving P-selectin cannot be excluded.
Discussion
Taken together, our data indicate a novel mechanism for sickle cell adherence to thrombin-treated endothelial cells via P-selectin. The partially characterized P-selectin ligand contains sialic acid and is the first reported selectin ligand activity on circulating erythrocytes that are not infected by malaria. The potential importance of P-selectin in sickle cell vaso-occlusion was implicit in the suggestion that sickle cell adhesion may resemble the process of leukocyte adherence.22 Erythrocyte adhesion to P-selectin also suggests possible molecular mechanisms for the adherence of activated platelets to sickle cells,57 cooperative heterocellular interactions in sickle cell vaso-occlusion,57-60 and the retention of erythrocytes in red thrombi.61,62 The modest adherence that we noted of nonsickle cells to P-selectin does not diminish the importance of sickle cell adherence. Indeed, our finding is consistent with previous reports of a lesser degree of nonsickle RBC adherence to endothelial cells.18,46 We believe the binding of sickle cells is much more robust than that of nonsickle cells because multiple adhesion systems are involved.22 The binding of nonsickle cells is weaker and therefore more susceptible to variation in relation to the background “noise” in adherence. When all of our data are taken together, particularly those shown in Figure 1A, there is evidence for lower level but significant P-selectin–mediated binding of nonsickle cells. The adherence of nonsickle erythrocytes may have little impact on blood flow in a physiologic setting, where normally deformable RBCs easily maneuver past a potential nidus of occlusion. However, 3 important differences distinguish the pathophysiologic setting of sickle cell disease from normal. First, the likely activated condition of endothelial cells in sickle cell disease40-43 results in the expression of additional adhesion molecules that presumably strengthen low affinity P-selectin–mediated adherence. Second, the delay in microvascular transit time imposed on circulating sickle cells by the adherent nidus of highly adhesive cells promotes deoxygenation and polymerization of hemoglobin S to generate poorly deformable, reversibly sickled cells.63 Third, these reversibly sickled cells and the inherently poorly deformable, irreversibly sickled cells reach an impasse behind the adherent nidus to complete the vaso-occlusive process.20 These conditions that contribute to vaso-occlusion in sickle cell disease are not extant in the normal circulation.
In our studies, P-selectin had significant effects both on static adhesion against the force of gravity orthogonal to the cell surface and on nonstatic adhesion against the tangential shear forces in a rotatory adhesion assay. Hebbel has elucidated the potential enunciated importance of both static and flow adherence studies to sickle cell vaso-occlusion.22 Based on the flow adhesion models of Springer and coworkers for leukocytes64-66 and of Ho and colleagues for Plasmodium falciparum–infected erythrocytes,55,56 in which tethering and rolling adhesion is mediated by P-selectin and firm adherence is effected by higher affinity adhesion molecules, it is tempting to predict a greater role for P-selectin in flow than in static adherence. Current experiments in our laboratory are comparing the effects of P-selectin in assays of the flow and static adherence in vitro and being tested in vivo using our sickle cell mouse model.67 68
Our finding of only partial inhibition of sickle and normal erythrocytes adherence to thrombin-activated HUVECs using blocking P-selectin mAbs with or without sLeX indicates the presence of P-selectin–independent mechanisms of activated adhesion. This is further supported by the partial inhibition of erythrocyte adherence to recombinant P-selectin in the presence of EDTA or with prior sialidase treatment of the RBCs. Possible alternative mechanisms of sickle cell adhesion to thrombin-activated endothelial cells include adherence to the redistributed endothelial integrins (our unpublished data, 2001) or exposed matrix proteins,54 the use of the putative second ligand binding site of endothelial P-selectin,47 and the adhesion of endothelial P-selectin to sulfatide in erythrocyte membranes.69-72 These sulfated glycolipids have ligand activity for P-selectin70 and bind the matrix proteins vWF, laminin, and TSP.73 The sulfated lipid purified by Hillery and colleagues from sickle cell membranes binds TSP and laminin, is resistant to sialidase treatment,74 and may comprise the sialidase-resistant component of erythrocyte P-selectin ligand activity that we identified. Identifying these additional possible pathways for P-selectin–mediated sickle cell adherence may provide important insights into sickle cell adherence and added guidance for the development of new therapy for sickle cell vaso-occlusion.
Detailed understandings of hemoglobin S polymerization notwithstanding, the factors that initiate painful vaso-occlusion in sickle cell disease have not been identified.17 In this regard, the novel adhesion mechanism we have discovered involves 2 temporal variations with the potential to influence adhesion and occlusion. The expression of P-selectin on endothelial cell surfaces in response to conditions active in sickle cell disease suggests a possible role for such variations in endothelial adhesivity as a determinant of painful vaso-occlusion. Another possible influence on the seemingly random vaso-occlusive events of sickle cell disease could stem from fluctuations in the presentation of P-selectin ligand on sickle RBCs. Our characterization of this ligand is incomplete. We have demonstrated that, as with other P-selectin ligands,7,50 sialic acid is an important recognition determinant. The primary ligand for P-selectin is P-selectin glycoprotein ligand-1 (PSGL-1),7 and the precise molecular interactions between P-selectin and this counterreceptor and with the recognition determinant sLeX have recently been solved.75 These interactions are germane to our preliminary attempts at defining the ligand. In pilot studies we found no evidence of PSGL-1 on sickle RBCs using mAb 2PH1, which is specific for PSGL-1, or KPL1, which is specific for tyrosine sulfated PSGL-1 (both from BD Pharmingen) in flow cytometry (data not shown). We did, however, detect a flow cytometry signal from a fraction of sickle cells using the sLeX mAb HECA-452 (BD Pharmingen; data not shown).6 The intensity of this sLeX signal varied among patients and over time. The nature of the sickle cell ligand for P-selectin is currently under further investigation in our laboratory. Our preliminary evidence for the presence of sLeX on sickle cell membranes suggests a second possible temporal determinant derived from P-selectin–dependent adhesion. For instance, Lewis RBC antigens are not synthesized by erythrocytes, but consist of glycolipids incorporated into erythrocyte membranes from the plasma into which they are secreted by intestinal epithelial cells.76 It is tempting to speculate that fluctuations in the synthesis, constitution, plasma concentrations, and membrane incorporation of sLeX and other P-selectin recognition determinants may influence the acquisition of P-selectin ligands by circulating erythrocytes. Such variations could contribute to the variability in sickle cell adhesivity and occurrence of pain. Alternatively, P-selectin ligand on sickle cells, as with certain other adhesion molecules on RBC membranes, may be residual from less mature stages of erythroid development,77 78 in which case fluctuations in rates of reticulocytosis may contribute to variations in the adhesivity of sickle cells and the occurrence of pain. We found in pilot flow cytometry experiments that recombinant P-selectin binds only to subpopulations of sickle and nonsickle erythrocytes (data not shown), a binding pattern consistent with either of these 2 mechanisms of ligand presentation.
The complexity of sickle cell adhesion mechanisms will most certainly deepen as the molecular nature of these interactions is defined. For instance, in our preliminary attempts to define the P-selectin ligand we have pretreated erythrocytes with 0.02% trypsin. This reduced substantially the adherence of sickle and nonsickle cells to untreated and thrombin-treated endothelial cells but did not reduce significantly their adhesion to immobilized P-selectin (data not shown). These results suggest that the erythroid P-selectin ligand is probably not a glycoprotein, but that proteolysis of erythrocyte membranes reduces RBC adherence to endothelial cell adhesion molecules other than P-selectin. These preliminary results and the full characterization of this P-selectin ligand are currently being pursued in our laboratory. It will be useful to correlate levels of adhesivity with independent markers of cell age, such as cell density or RNA content, as an indication of whether the ligand is acquired from the plasma by circulating sickle cells or residual from RBC precursors.
Our results suggest that P-selectin be considered as a candidate molecule for new therapeutic approaches for the painful vaso-occlusion of sickle cell disease. Potential new therapeutic strategies include the use of antagonists of endothelial cell activation79 and inhibitors of P-selectin–ligand interactions,80 the latter of which includes heparin.47,74,81,82 The published suggestion that heparin therapy may be efficacious in reducing the frequency of sickle cell pain crises83 could be verified presently, because there is much clinical experience using heparin for other vaso-occlusive diseases.
We wish to thank Dr Charles McCulloch for statistical advice; Drs Vishwanath Lingappa, Marion Reid, and Steven Spitalnik for helpful discussions; Dr Reid for the gift of peanut agglutinin; and especially our patients whose cooperation, support, and trust made this work possible.
Supported in part by a Sickle Cell Scholar's Award from the National Institutes of Health (to N.M.) and grants from the National Institutes of Health (to S.R., A.V., and S.E.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen H. Embury, Bldg 100, Rm 263, San Francisco General Hospital, 1001 Potrero Ave, San Francisco, CA 94110; e-mail:sembury@itsa.ucsf.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal