Abstract
Lymphohematopoietic progenitors derived from midgestation mouse embryos were established in long-term cultures with stromal cell monolayers and interleukin 7 (IL-7), giving rise to B-lineage cell lines. The initial emergence and in vitro establishment of these early embryo cell lines were highly sensitive to IL-7–mediated signals, in comparison to cell lines similarly obtained using precursors from late fetal liver (> 13 days postcoitum) and adult bone marrow. The early embryo-derived progenitors spontaneously differentiated in vitro to CD19+IgM+ immature B cells in the presence of optimal concentrations of IL-7, in contrast to those progenitors obtained from late gestation and adult mice, whose differentiation only occurred in the absence of IL-7. The newly in vitro–generated B cells of the early embryo cell lines repopulated adult immunodeficient severe combined immunodeficient mice on their adoptive transfer in vivo and generated specific humoral immune responses after immunization.
Introduction
The scenario of mouse embryo lymphohematopoiesis has been transformed during recent years. Novel intraembryonic sites (para-aortic splanchnopleura/aorta-gonad-mesonephros region [P-Sp/AGM], blood, omentum) and distinctive progenitors have been revealed in early, preliver midgestation periods (reviewed in Morales-Alcelay et al,1 Melchers and Rolink,2and Keller et al3). Stem cells giving rise to definitive lymphohematopoiesis are detected in para-aortic mesoderm,4,5 as well as in yolk sac (YS), liver, blood, spleen and, finally, in bone marrow (BM) microenvironments.6,7 Not only multipotential stem cells exist, but lineage-specific gene programs are also activated at the early ontogenic periods (days 9-12 postcoitum [dpc]); a limited process of B lymphopoiesis occurs, as it is revealed ex vivo by the detection of ckit+ AA4.1+ CD19+cells, IgH DJ rearrangements, and the transcription of pre-B–specific genes (RAGs, VpreB, Igβ, etc) in P-Sp/AGM, YS, and blood, and in liver at 11 to 12 dpc,8-10 (De Andrés B et al, manuscript in preparation). These B-primed early progenitors maintain low cell numbers until days 12 to 13 of mouse gestation, and thereafter they grow exponentially.9,11-15 The early mouse embryo progenitors fully differentiate into B cells on embryo tissue grafting into severe combined immunodeficient (SCID) mice,4 or after adoptive transfer experiments into conditioned newborn mice,16 and they also mature in vitro on stromal cell/interleukin 7 (IL-7) cultures.6
An efficient B lymphopoiesis relies on sequentially acting transcription factors (eg, Ikaros, Pax5, Id) that commit multipotential progenitors to the B-cell lineage, while restricting other cellular fates.17-19 Supportive cytokines (especially IL-7) and interactions with stromal cells and components of the extracellular matrix, maintain viability and promote growth of early B-cell precursors (pro-B I cells) in inductive microenvironments.20,21 The expression of pre-B–specific genes encoding for the recombinase enzymatic complex (RAGs, DNA-PK, Ku, others) and for the components of the surrogate light chain (SLC) (λ5, VpreB), is needed to proceed into differentiation.22,23 The IgH chains that emerge from productive VHDJH rearrangements and that are able to pair with the SLCs, are expressed in the membrane as pre-B-cell receptors (PreBCRs).24-26 A second round of rearrangements takes place on the κ/λ loci of resting IgH+ pre-B cells to generate the light chains that are required to form a complete clonotypic H/L Ig receptor, which defines a B lymphocyte. The study of B-lineage cells in specific culture systems (stromal cells plus IL-7 and additional factors) has been highly instructive in elucidating the molecular steps of B-cell differentiation.27 A drawback of the later protocols was the difficulty obtaining mature B cells in these cultures, leading to the suggestion of an IL-7–induced inhibition of late pre-B-cell differentiation.27-29
The work reported here focuses on the B lymphopoiesis emerging from midgestation mouse embryo progenitors, based on the establishment of long-term growing, untransformed CD19+ B-lineage cell lines. The in vitro cell lines obtained from 11-dpc embryos showed a much lower threshold of response to limiting amounts of IL-7 than the one of cell lines derived from late fetal liver (LFL; after 13 dpc) and BM progenitors (LFL/BM B-cell lines). Surprisingly, and in contrast with those, the cell lines generated from 11-dpc mouse embryo progenitors spontaneously differentiated in vitro to functional IgM+ B cells, even in the presence of high concentrations of IL-7. These cell lines reconstituted the B-cell compartment of adult SCID mice, giving rise to CD19+IgM+ cells in them. On T-cell–independent immunization, the B-cell–repopulated mice generated humoral immune responses. Midgestation mouse embryo progenitors may consequently represent an optimal source to establish long-term, in vitro growing, polyclonal B-cell lines.
Materials and methods
Mice, microsurgery, and cell purification from ex vivo samples
BALB/c, BALB/cnu/nu, and CB17.SCID mice were bred under specific pathogen-free conditions in the animal facilities of Instituto de Salud Carlos III (ISCIII) and Centro de Biologı́a Molecular S. O. (CBMSO). Mouse embryo age was calculated by detection of vaginal plug after overnight mating (day 0). Mouse embryos (11 dpc) were microdissected under a stereomicroscope (Nikon SMZ-1, Tokyo, Japan). Blood, YS, P-Sp/AGM, and liver were isolated and cell suspensions were obtained by mechanical dissociation. BM was flushed out by injection of phosphate-buffered saline (PBS)/2% fetal calf serum (FCS) with a 25-gauge needle into the femur of 2-month-old BALB/c mice. Viable cells were counted by trypan blue exclusion. Recipient CB17.SCID mice were maintained in air-filtered, positive-pressure cages, and received a single dose of 150 cGy total body x-irradiation from a 137Cs source (IBL 437C, CIS Biointernational, Gif-sur-Yvette Cedex, France), 24 hours before cell transfers. Adoptive cell transfer and bleeding of the mice were done by injections into the retro-orbitary plexus.
Cell cultures and limiting dilution analyses
The stromal cell line ST230 was grown to confluence in Iscoves modified Dulbecco medium (IMDM, Biowhittaker Europe, Verviers, Belgium) supplemented with 10% heat-inactivated FCS (Labtech, Uckfield, United Kingdom), 100 U/mL penicillin-streptomycin (Biowhittaker), 2 mM l-glutamine (Gibco BRL, Paisley, Scotland), 1 mM piruvate (Sigma Chemical, St Louis, MO), 5 × 10−5 M 2-mercaptoethanol (Sigma) and 1 × nonessential amino acids (Biowhittaker). Preconfluent ST2 cells were recovered after trypsinization, treated with 50 μg/mL mitomycin C (Sigma), seeded in 96-well plates (0.5-1 × 106cells/well) and cultured overnight at 37°C, 5% CO2. Cells obtained from P-Sp/AGM, YS, blood, liver, and BM were plated in limiting dilution conditions31 on the layer of ST2 cells. Mouse recombinant IL-7 (rIL-7; Sigma) was added to the cultures at doses ranging from 0 to 30 ng/mL. At least 48 wells were seeded for each cell dilution and rIL-7 dose in 10% FCS/IMDM medium. Fresh medium containing rIL-7 was added every 96 hours. The supernatant of the 3T3 fibroblast cell line, stably transfected with murine IL-7 complementary DNA (cDNA),12 was used as a source of IL-7 for cell line maintenance at a final concentration of 3 ng/mL. IL-7–3T3 cells were grown in 10% FCS/RPMI 1640 medium (Gibco BRL). The amount of IL-7 present on supernatants of confluence-grown cultures was determined on cells from an IL-7–dependent cell line32 against rIL-7 as standard and was adjusted to 30 ng/mL. The 70Z/3 pre-B-cell line33 was used as control for reverse transcription-polymerase chain reaction (RT-PCR) analyses.
Flow cytometry and immunofluorescence microscopy
The following monoclonal antibodies (mAbs) were purified from hybridoma supernatants by affinity chromatography on protein G columns (Pharmacia, Uppsala, Sweden), and fluoresceinated or biotinylated by standard methods: anti-IgMa (RS3.1),34anti-CD191D3,35 anti-B220 (RA3.6B2),36anti-I-Ek, d, r, p (14-4-4S),37 anti-CD43 (S7),38 anti-CD553-7.313,39anti-BP-1,40 and anti-PB76 (G-5-2).41Fluoresceinated anti-IgD and biotinylated anti–IL-7 receptor α chain (IL-7Rα) were purchased from Pharmingen (San Diego, CA). Fluoresceinated sheep F(ab′)2 antimouse Ig(H and L) was obtained from Silenus Lab (Hawthorn, Australia). Biotinylated antibodies were revealed with phycoerythrin (PE)-conjugated streptavidin (Southern Biotechnology, Birmingham, AL). Two-color stainings were performed as described.9 Cell debris and dead cells were excluded on the basis of forward- and side-light scatter parameters and propidium iodide-stained cells. Specific mAb signals were defined against the background fluorescence of isotype-matched irrelevant mAbs and after Fc block with anti-CD16/CD32 purified mAb (Pharmingen). The flow cytometry analysis of cytoplasmic μH was done as described.24 Flow cytometry was performed on a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA) and analyzed with the CellQuest Immunocytometry analysis system (Becton Dickinson).
Cytospin preparations were stained as described42 with either rhodamine-labeled goat antimouse IgM or rhodamine-labeled goat antimouse IgG (Southern Biotechnology), and countersained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI; Molecular Probes, Eugene, OR). The slides were analyzed in a Leitz DMRD microscope (Leica, Wetzlar, Germany).
Immunomagnetic cell subset purification
B-cell precursor purification from embryo cell lines and from spleens of reconstituted SCID mice was performed by immunomagnetic cell sorting with the VarioMACS system (Miltenyi Biotec, Bergisch, Germany). IgM+ B cells were labeled with biotinylated anti-IgMa mAb and incubated later on with streptavidin-conjugated beads (Miltenyi Biotec). The degree of contamination with IgM+ cell populations was verified by reanalysis in the FACScalibur (Becton Dickinson), and was below 1%.
Cell proliferation and enzyme-linked immunosorbent assays
Cell proliferation was measured after a 12-hour pulse of 1 μCi3[H]-thymidine (Amersham-Pharmacia Biotech, Buckinghamshire, United Kingdom), and incorporated radioactivity was quantified by scintillation counting (Wallac, Turku, Finland). The enzyme-linked immunosorbent assays (ELISAs) were performed as described.43 Serum IgMa, IgG2aa, and IgG1 Abs were detected by using plates coated with 3 μg/mL purified mAb specific for mouse IgMa (RS3.1), mouse IgG2aa/2ba (clone 21-48.31, Pharmingen), and mouse IgG1 (clone A85-3, Pharmingen), respectively. The assays were revealed with biotinylated goat antimouse IgM (Southern Biotechnology), biotinylated mouse antimouse IgG2aa (clone 8.3, Pharmingen), and biotinylated rat antimouse κ chain (clone 187.1), respectively. Plates were then incubated with streptavidin-conjugated peroxidase (Southern Biotechnology) and developed with 0.5 M o-phenylenediamine (Sigma). The absorption values were read at 405 nm. A normalized IgM titer was calculated for each serum using the GraphPad Prism 2.0 software (Graphpad Software, San Diego, CA), with the dilution value (D50) representing the 50% of the absorbance obtained by a given dilution of BALB/c serum. The plates used for the anti-DNP (2,4-dinitrophenyl) IgMa ELISA were coated with DNP-OVA (10 μg/mL), and antimouse biotinylated anti-IgMa mAb (RS3.1) was used as developing Ab. The DNP-specific Ab titers (D50) were calculated as above.
RT-PCR
Total RNA was isolated from cell pellets and the corresponding cDNAs were prepared on heat-denatured RNA (5 μg), by using 1 μg oligo-(dT) as primer and avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI), as described.44Equal amounts of cDNA were used to amplify λ5, VpreB, RAG-2, TdT, BSAP (Pax5), and β-actin transcripts. Enzymatic amplifications were performed as described,9 with 2 U Taq DNA polymerase (Sigma), for 30, 35, and 40 cycles (RAG-2, TdT and BSAP) or for 20, 25, and 30 cycles (λ5, VpreB and β-actin). The annealing temperature was 63°C for λ5, VpreB, and RAG-2 amplifications; 60°C for TdT and BSAP amplifications; and 52°C for β-actin amplifications. The oligonucleotides used as primers for VpreB were: sense 5′-ATGTTCTCCAGAGCCTAAGATC-3′ and reverse 5′-CTGGCCTATCTCACAGGTT-3′, and for β-actin sense: 5′-TTCTTGGCTATGGAATCCTGT-3′. The primers for reverse β-actin, and for λ5, RAG-2, TdT, and BSAP have been described.9,45,46 The amplification products were separated electrophoretically on 2% agarose gels and then transferred to Zeta-probe membranes (Bio-Rad, Hercules, CA) after treatment with 0.4 M NaOH. Hybridization was performed with probes32P-labeled by random priming.47 The probes used for VpreB, RAG-2, and TdT hybridizations were obtained after cloning the PCR-amplified fragments into a modified Bluescript vector (Stratagene, La Jolla, CA; pBS-T).48 The β-actin probe was the PstI insert of the plasmid p41AL49 and the λ5 probe was the HincII/BamHI insert from the λ5 cDNA clone pZ183-1™.33 An internal oligonucleotide was used as a32P-labeled BSAP probe.46 PCR signal intensities were quantified by densitometry (Fujibas-1000 detector; Fuji, Tokyo, Japan) with the TINA software (Raytest, Strauhenhardt, Germany).
Cloning and sequencing of genomic DNA
Genomic DNA was prepared from cell pellets.50VHDJH-rearranged alleles were amplified by PCR, with VH7183-, VHJ558-, and JH4-specific primers as previously described.51 The reaction products were separated electrophoretically on 2% agarose gels and visualized by ethidium bromide staining. Reaction aliquots containing the correct size fragments were cloned into pBS-T. White colonies obtained from the transformation of electrocompetent DH5α bacteries were selected with X-Gal (Sigma) and checked for the size of the inserts by specific PCR as above. Plasmid DNA was purified using the FlexiPrep Kit (Amersham Pharmacia Biotech), and sequenced with a DNA sequencing kit (PE Applied Biosystems, Warrington, United Kingdom), according to the manufacturer's instructions. The final reactions were analyzed in the ABI PRISM 377 (PE Applied Biosystems) DNA sequencer.
In vivo reconstitution and immune response studies
Established BALB/c embryo-derived cell lines (2-3 months in culture) were collected from cultures and injected into low-dose irradiated CB17.SCID mice (106 cells/mouse, 2-4 mice/cell line). Control mice were injected with LFL/BM-derived cell lines or with PBS. IgMa-specific serum concentrations were sequentially analyzed by ELISA. Mice were killed at different times after adoptive cell transfers and cell suspensions were recovered from BM, peritoneum, and spleen for flow cytometry analyses. A group of mice was immunized intraperitoneally with 100 μg 2,4-dinitrophenyl-lipopolysaccharide (DNP-LPS) in complete Freund adjuvant (CFA). Serum samples were obtained weekly and tested for specific Ab titers.
Results
Low IL-7 concentrations rescued progenitors from midgestation mouse embryos, leading to the establishment of high numbers of B-lineage cell lines
Cell suspensions from 11-dpc BALB/c mouse embryo lymphohematopoietic sites, after 13 dpc LFL and adult BM were seeded in limiting dilution conditions on 96-well plates, which were previously covered with a confluent layer of ST2 cells. Titrated dilutions of rIL-7 were added at the start point of the cell cultures. Cultures harboring well-defined cell clones (> 100 cells/clone) after 7 to 10 days of culture were considered positive. As shown in Figure1A for 11-dpc and BM progenitors, the former gave rise to higher numbers of clonable B-lineage cell lines than the latter ones, under limiting doses of rIL-7 (0.3 ng/mL, 1 of 2900 versus 1 of 10 000, respectively). Even in the absence of any rIL-7 addition a few short-lived embryo-derived clones appeared in the cultures, whereas this was never the case in cell cultures of BM progenitors (1 of 6000 versus < 1 of 105). In contrast, the clonable BM-derived cell lines that appeared in the cultures established under optimal rIL-7 supplementation (3 ng/mL) were similar to those derived from embryonic sites (1 of 2000 versus 1 of 2500, respectively). After their initial emergence, the viability of B-lineage clones underwent a fluctuant period in culture, being stabilized at 20 to 25 days and remaining so for long periods of time (1-5 months). The generated B-lineage clones could be frequently frozen, thawed, and recultured. LFL cells were established and progressed moderately better in vitro than adult BM cells, although never reaching the high recoveries of midgestation embryo-derived cells. Because the in vitro behavior of the 11-dpc embryo cell lines was markedly different from those cell lines originated both from LFL and BM, these latter 2 were considered equivalent and their individual data were pooled. Figure 1B shows the kinetics of appearance and early evolution of cell lines obtained from 11-dpc progenitors and from 15- to 18-dpc LFL and BM progenitors under 3 ng/mL rIL-7. Most of the LFL/BM-derived clones could not be maintained in long-term cultures (80%). Eleven-dpc embryo-derived clones, however, emerged 2 to 3 days later (probably due to a higher ratio of immature stem versus intermediate cells), and they were efficiently established in long-term cultures, at a frequency that was 3 times higher than the one of LFL/BM-derived progenitors. These analyses suggest that the embryo-clonable B-lineage cell progenitors were highly sensitive to IL-7 signals leading to in vitro establishment and maintenance of long-term growing cell lines. No major differences were revealed by using precursor cells from various hematopoietic 11-dpc mouse embryo locations, despite some quantitative variations in B-cell precursor frequencies: 1 of 2500 plated cells for liver, 1 of 2800 for YS, 1 of 5000 for blood, and 1 of 9000 for P-Sp/AGM (data not shown). Embryo- and LFL/BM-derived cells did not show other significant differences in proliferation or Ig secretion when exposed to non–IL-7 stimuli, such as anti-IgM, LPS, IL-4, or anti-CD40 (data not shown).
Establishment of B-lineage cell lines in ST2/IL-7 cell cultures of lymphohematopoietic progenitors obtained from 11-dpc BALB/c mouse embryos.
(A) rIL-7 dose-dependent emergence of cell clones under limiting dilution conditions at 8 days of culture; · · · · · indicates 0 ng/mL, – – –, 0.3 ng/mL, and _____, 3 ng/mL. One representative experiment of 3 is shown for 11-dpc liver (embryo cells) and for BM cells. (B) Kinetics of establishment of long-term growing embryo and LFL/BM cell lines at 3 ng/mL rIL-7. The cell lines were obtained by seeding 105 embryo or LFL/BM cells in 96-well plates at 1000 cells/well. Data are presented as mean ± SD of 3 independent experiments for embryo (circles) and LFL/BM (triangles) cell lines, and represent the number of B-lineage cell lines growing at each time point.
Establishment of B-lineage cell lines in ST2/IL-7 cell cultures of lymphohematopoietic progenitors obtained from 11-dpc BALB/c mouse embryos.
(A) rIL-7 dose-dependent emergence of cell clones under limiting dilution conditions at 8 days of culture; · · · · · indicates 0 ng/mL, – – –, 0.3 ng/mL, and _____, 3 ng/mL. One representative experiment of 3 is shown for 11-dpc liver (embryo cells) and for BM cells. (B) Kinetics of establishment of long-term growing embryo and LFL/BM cell lines at 3 ng/mL rIL-7. The cell lines were obtained by seeding 105 embryo or LFL/BM cells in 96-well plates at 1000 cells/well. Data are presented as mean ± SD of 3 independent experiments for embryo (circles) and LFL/BM (triangles) cell lines, and represent the number of B-lineage cell lines growing at each time point.
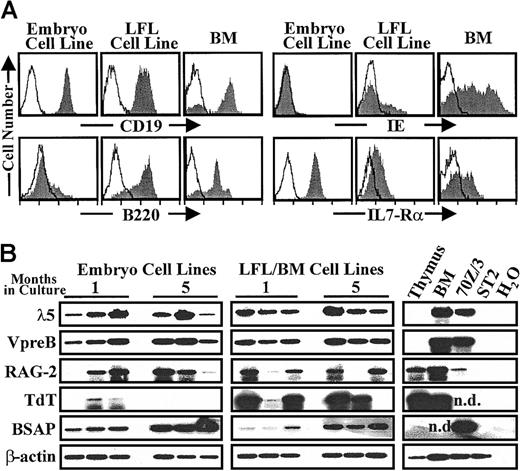
The in vitro generated cells were characterized as B-lineage cells by the surface expression of CD19 and IL-7Rα (Figure2A). Most of the mouse embryo-derived cell lines lacked major histocompatibility complex (MHC) class II IE and B220/6B2 molecules. These findings agree with previous reports about IE− B-lineage cells in early ontogeny52and lower B220 antigen (Ag) levels in fetal pre-B cells.53Other authors have communicated the B220 expression in short-term B-cell lines from 12-dpc fetal liver progenitors.54 The use of different mAbs (RA3.6B2 versus 14.81),55 and other experimental variables (stromal cell lines, time in culture, gestational age, genetic backgrounds of the mice) may explain these divergences. A significant feature was the great expression of IL-7Rα in embryo B-cell lines. To further elucidate the cell lineage and developmental stage of the midgestation embryo cell lines, we studied the expression of pre-B cell–specific genes on cDNA samples obtained from them after 1 and 5 months in culture. As shown in Figure 2B, both groups expressed variable transcript levels of λ5, VpreB, RAG-2, TdT, and BSAP/Pax-5 genes, which revealed the presence of B-cell precursors in the lines. It is interesting to note the minor or absent TdT expression and the augmented transcript levels of BSAP/Pax-5 (10-fold of those expressed by LFL/BM cell lines) found in the 11-dpc embryo-derived cell lines.
Surface phenotype and pre-B specific gene expression pattern of in vitro embryo-derived B-cell lines.
(A) Expression of cell surface Ags in representative embryo and LFL cell line (1 of 10-20 cells lines tested in each group; 2 months of culture), and in BALB/c BM lymphoid cell populations was detected with specific biotinylated mAbs. The mAbs were revealed with PE-conjugated streptavidin. Shaded and empty histograms correspond to positive signals and to background fluorescence of irrelevant mAbs, respectively. Log10 of fluorescence intensity is represented. (B) Transcript levels of λ5, VpreB, RAG-2, TdT, BSAP and β-actin, detected by semiquantitative RT-PCR in 3 representative cell lines of each group (embryo and LFL/BM) after 1 and 5 months of continuous cell culture. Controls for gene transcription are shown in the right columns; nd indicates not done.
Surface phenotype and pre-B specific gene expression pattern of in vitro embryo-derived B-cell lines.
(A) Expression of cell surface Ags in representative embryo and LFL cell line (1 of 10-20 cells lines tested in each group; 2 months of culture), and in BALB/c BM lymphoid cell populations was detected with specific biotinylated mAbs. The mAbs were revealed with PE-conjugated streptavidin. Shaded and empty histograms correspond to positive signals and to background fluorescence of irrelevant mAbs, respectively. Log10 of fluorescence intensity is represented. (B) Transcript levels of λ5, VpreB, RAG-2, TdT, BSAP and β-actin, detected by semiquantitative RT-PCR in 3 representative cell lines of each group (embryo and LFL/BM) after 1 and 5 months of continuous cell culture. Controls for gene transcription are shown in the right columns; nd indicates not done.
Polyclonal VHDJH repertoires in long-term, in vitro growing B-lineage cell lines derived from mouse embryo progenitors
To know whether the embryo cell lines represented an in vitro model of normal B-cell lymphopoiesis that gives rise to diverse Ig repertoires, we sequenced a pool of VHDJHrearrangements in 2 11-dpc mouse embryo cell lines (Figure3). One half of the VHDJH-joint sequences lacked nontemplated N/P nucleotides, whereas the rest had a limited number of these nucleotides at the junctions. Seven of the 16 IgH rearrangements sequenced were productive. No significant IgH gene biases were observed, except for a high usage of JH4 (14 of 16 VHDJH rearrangements). This JH4 bias of the embryo-derived VHDJH rearrangements was also noticed when DJH rearrangements were analyzed in a larger sample of embryo- and LFL/BM-derived cell lines by specific PCR.9 56 JH4 was found in 66.6% and 36.3%, respectively, of the 48 embryo- and 55 LFL/BM-derived DJHrearrangements that were detected in a total number of 10 to 12 cell lines analyzed per group (data not shown). Novel IgH rearrangements, which were previously undetected at 2 months of evolution, appeared after 5 months in culture (Figure 3, cell line no. 2.11). Yet, the number of sequences found repeated at the later time-point cultures significantly increased (none of 13 sequences at 2 months versus 17 of 20 sequences at 5 months of continuous culture), showing a tendency to restriction of IgH repertoires and oligoclonality.
Ig VHDJH rearrangements from long-term in vitro growing embryo cell lines.
VH7183-D-JH and VHJ558-D-JH rearrangements were amplified, cloned, and sequenced as described in “Materials and methods.” The VHDJH joint sequences of clones obtained from the progeny of 2 independent embryo-derived precursors are shown. The sequences are aligned under the germline sequences corresponding to the 3′ region of VH 7183 and J558 families and the 5′ region of germline JH4 (top line). Dashed lines denote identities. N nucleotides are underlined. Parentheses indicate probable D-D joints. Nucleotides in italics may be assigned to germline sequences of V, D, or J segments and are putative sites of homology-directed recombination. RF indicates reading frame.
Ig VHDJH rearrangements from long-term in vitro growing embryo cell lines.
VH7183-D-JH and VHJ558-D-JH rearrangements were amplified, cloned, and sequenced as described in “Materials and methods.” The VHDJH joint sequences of clones obtained from the progeny of 2 independent embryo-derived precursors are shown. The sequences are aligned under the germline sequences corresponding to the 3′ region of VH 7183 and J558 families and the 5′ region of germline JH4 (top line). Dashed lines denote identities. N nucleotides are underlined. Parentheses indicate probable D-D joints. Nucleotides in italics may be assigned to germline sequences of V, D, or J segments and are putative sites of homology-directed recombination. RF indicates reading frame.
Clonable B-lineage cell lines derived from midgestation mouse embryo progenitors differentiated in vitro to surface IgM+ B cells in the presence of rIL-7
The differentiation stage reached by both midgestation mouse embryo- and LFL/BM-derived B-lineage cell lines was analyzed studying the membrane IgM expression by flow cytometry (Figure4A). The vast majority of LFL/BM-derived B-lineage cell lines were unable to generate IgM+ B cells in cultures supplemented with 3 ng/mL rIL-7, as reported.27 29 In contrast, most of early mouse embryo-derived cell lines (> 60%) contained small but clear subsets of surface IgM+ B cells (1%-10% IgM+cells/line) at 1 month in culture. After longer periods of time (2-5 months), one half of the embryo cell lines harbored significant IgM+ B cell populations, whereas only rare “escape” IgM+ clones were detected in the long-term cultures of adult cell lines. The relative levels of B cells increased up to a mean value of 40% IgM+ B cells/IgM+ embryo cell line at 5 months.
Differentiation of embryo cell lines to CD19+IgM+ immature B cells in ST2/IL-7 cell cultures.
(A) Relative number of sIgM+ B cells in both embryo and LFL/BM cell lines after 1, 2, and 5 months of continuous ST2/IL-7 (3 ng/mL) cell cultures, as obtained by flow cytometry analyses. Each point corresponds to one independent cell line. The cell lines were considered IgM+ when containing 1% sIgM+ cells or more (horizontal dotted bar). The mean values of IgM+ B cells present in each of the IgM+ embryo cell lines are shown as thick horizontal lines. The average percentage of IgM+ cell lines found at each time point is shown in the bottom line. (B) Cell surface phenotype of IgM+ B cells from a representative embryo cell line cultured for 2 months. This particular cell line contained 12% B cells. The mAbs and protocols used for double stainings are described in “Materials and methods.” The forward side scatter (FSC) is displayed in a linear scale. The fluorescein isothiocyanate (FITC) and PE fluorescence intensity of the different mAbs are represented in a log10 scale.
Differentiation of embryo cell lines to CD19+IgM+ immature B cells in ST2/IL-7 cell cultures.
(A) Relative number of sIgM+ B cells in both embryo and LFL/BM cell lines after 1, 2, and 5 months of continuous ST2/IL-7 (3 ng/mL) cell cultures, as obtained by flow cytometry analyses. Each point corresponds to one independent cell line. The cell lines were considered IgM+ when containing 1% sIgM+ cells or more (horizontal dotted bar). The mean values of IgM+ B cells present in each of the IgM+ embryo cell lines are shown as thick horizontal lines. The average percentage of IgM+ cell lines found at each time point is shown in the bottom line. (B) Cell surface phenotype of IgM+ B cells from a representative embryo cell line cultured for 2 months. This particular cell line contained 12% B cells. The mAbs and protocols used for double stainings are described in “Materials and methods.” The forward side scatter (FSC) is displayed in a linear scale. The fluorescein isothiocyanate (FITC) and PE fluorescence intensity of the different mAbs are represented in a log10 scale.
The IgM+ B cells in vitro generated by the embryo cell lines were small resting cells (as defined by forward side scatter), which expressed CD19, CD43, BP-1, and PB76, but were negative for B220/6B2, MHC class II IE, CD5, and IgD Ags (Figure 4B). These B cells therefore can be ascribed to the stage of BP-1+IgM+IgD− immature B cells. They also share phenotypical traits with ontogenically early B cells, as it is the expression of CD43 and PB76, and the absence of IE Ags.52 Surprisingly, IL-7Rα, which is usually restricted to early B-cell progenitors, persisted in the in vitro–generated IgM+ B cells of the embryo cell lines. These findings suggest that, in contrast to those progenitors present in LFL/BM, midgestation mouse embryo progenitors have the potential to differentiate into the stage of IgM+IgD−immature B cells in stromal cell plus IL-7 culture conditions.
IL-7 selectively regulated pre-B cell numbers, but did not block the last differentiation step to sIgM+ B cells, in midgestation mouse embryo cell lines
We decided then to analyze the effect of IL-7 on the populations of pre-B and B cells of embryo cell lines. When 6 representative embryo cell lines and 2 LFL/BM cell lines (Figure5, black and dashed bars, respectively) were plated on ST2 monolayers without rIL-7, their proportions of IgM+ B cells selectively increased at 48 to 72 hours, in comparison with the same cell lines cultured with 3 ng/mL rIL-7 (Figure5A). However, the absolute numbers of B cells recovered did not significantly change in the 11-dpc embryo-derived cell lines cultured with or without rIL-7 (3 ng/mL), whereas minor increases in B-cell numbers were observed in the LFL/BM cell lines, on rIL-7 depletion (Figure 5B). IL-7 also stimulates the proliferation of B-cell precursors, and this activity was responsible for the apparent discrepancy between relative and absolute B-cell recoveries obtained with the embryo cell lines. Both cell proliferation and total recovered cells increased in a rIL-7 dose-dependent manner to reach a plateau at the 3 ng/mL rIL-7 dose both in 11-dpc embryo- and LFL/BM-derived cell lines (Figure 5C). The total numbers of the B-cell precursor population were selectively expanded in response to rIL-7 (Figure 5D). Subsequently, the B-cell population was diluted by this expansion of pre-B cells at increasing doses of rIL-7 and was concentrated in the absence of the cytokine (Figure 5A).
Selective proliferation and expansion of B-cell precursors from the embryo cell lines in response to IL-7 signals.
Six embryo cell lines and 2 LFL-derived cell lines harboring different percentages of B cells were used in these studies. The findings shown were obtained at 72 hours after culturing the cell lines in new ST2-coated plates with fresh medium and rIL-7. (A,B) Relative and absolute number of IgM+ B cells in embryo (black bars) and LFL (dashed bars) cell lines growing in cultures supplemented with or without rIL-7 at 3 ng/mL. (C) Dose-dependent rIL-7 cell proliferation (mean ± SD of triplicate cultures in each point) and total recovered cells in 4 independent embryo-derived cell lines (circles, continuous lines) and in LFL cell lines (triangles, dotted lines). (D) Total number of CD19+IgM− cells recovered from rIL-7–enriched (3 ng/mL)-cultures or from cultures without rIL-7 addition. The total number of CD19+IgM+ B cells and CD19+IgM− cells in both embryo and LFL cell lines was calculated by multiplying the total cells recovered by the frequency of IgM+ and IgM− cells obtained in the flow cytometry analysis.
Selective proliferation and expansion of B-cell precursors from the embryo cell lines in response to IL-7 signals.
Six embryo cell lines and 2 LFL-derived cell lines harboring different percentages of B cells were used in these studies. The findings shown were obtained at 72 hours after culturing the cell lines in new ST2-coated plates with fresh medium and rIL-7. (A,B) Relative and absolute number of IgM+ B cells in embryo (black bars) and LFL (dashed bars) cell lines growing in cultures supplemented with or without rIL-7 at 3 ng/mL. (C) Dose-dependent rIL-7 cell proliferation (mean ± SD of triplicate cultures in each point) and total recovered cells in 4 independent embryo-derived cell lines (circles, continuous lines) and in LFL cell lines (triangles, dotted lines). (D) Total number of CD19+IgM− cells recovered from rIL-7–enriched (3 ng/mL)-cultures or from cultures without rIL-7 addition. The total number of CD19+IgM+ B cells and CD19+IgM− cells in both embryo and LFL cell lines was calculated by multiplying the total cells recovered by the frequency of IgM+ and IgM− cells obtained in the flow cytometry analysis.
To show whether there was a component of IL-7–mediated differentiation arrest between pre-B and B cells in the embryo cell lines, CD19+IgM− pre-B cells were purified by immunomagnetic sorting and re-established in vitro with rIL-7 (3 ng/mL). Newly differentiated IgM+ B cells emerged in these cultures at 72 to 96 hours (Figure 6A). Purified pre-B cells from 11-dpc embryo cell lines and from LFL/BM cell lines were cultured on ST2 monolayers with sequential concentrations of rIL-7 (Figure 6B). Absolute pre-B cell numbers increased in direct correlation with rIL-7 until the dose of 30 ng/mL, regardless the origin of the cell line. The differentiation of purified pre-B cells to novel IgM+ B cells in embryo cell lines was also stimulated by rIL-7 at 0.3 and 3 ng/mL, and only moderately lower numbers of IgM+ B cells were recovered with 30 ng/mL rIL-7. IgM+ cell numbers were, however, always higher in the IL-7–enriched cultures than in the absence of added rIL-7. By contrast, IgM+ B cells were undetectable already at 0.3 ng/mL rIL-7 in cultures of purified pre-B cells from LFL/BM cell lines. These findings show that midgestation embryo-derived cell lines differentiated in vitro to the stage of immature IgM+ B cells in the presence of IL-7 concentrations that were optimal for growth/survival (3 ng/mL; Figure 5C). However, a partial IL-7–dependent blockade of late pre-B/B cell differentiation was also produced at high IL-7 concentrations (30 ng/mL).
IgM+ B cells generated in cultures from pre-B cell precursors obtained from embryo cell lines in the presence of rIL-7.
(A) CD19+IgM− B-cell precursors were purified by immunomagnetic depletion of IgM+ B cells from an embryo cell line, and they were replated in ST2/rIL-7 culture conditions. The numbers displayed in the contour plots correspond to the percentages of B cells inside the window, at the indicated time points. (B) CD19+IgM− B-cell precursors were purified as above from 2 embryo cell lines (circles) and one LFL/BM cell line (triangles) as control. The cells were cultured for 96 hours in the presence of ST2 cells and the indicated doses of rIL-7. At the end of the culture, the recovered cells were counted and analyzed as in Figure 5.
IgM+ B cells generated in cultures from pre-B cell precursors obtained from embryo cell lines in the presence of rIL-7.
(A) CD19+IgM− B-cell precursors were purified by immunomagnetic depletion of IgM+ B cells from an embryo cell line, and they were replated in ST2/rIL-7 culture conditions. The numbers displayed in the contour plots correspond to the percentages of B cells inside the window, at the indicated time points. (B) CD19+IgM− B-cell precursors were purified as above from 2 embryo cell lines (circles) and one LFL/BM cell line (triangles) as control. The cells were cultured for 96 hours in the presence of ST2 cells and the indicated doses of rIL-7. At the end of the culture, the recovered cells were counted and analyzed as in Figure 5.
Mouse embryo-derived cell lines reconstituted the B-cell compartment of adult SCID mice and mounted humoral immune responses
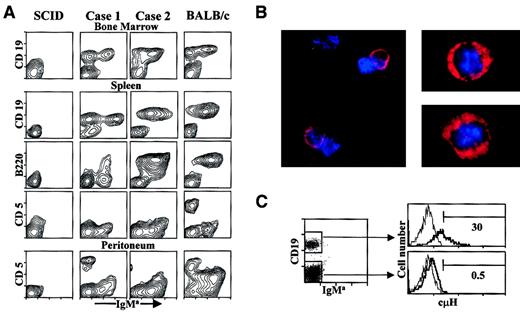
To elucidate the in vivo reconstitution capacity and functionality of the embryo cell lines, we transferred 8 representative cell lines from 11-dpc BALB/c (IgHa) mouse embryos to adult, low-dose irradiated CB17.SCID mice (IgHb). Transferred cells expanded in vivo, and donor-derived IgMa+ B-cell subsets were detected in BM, spleen, and peritoneal exudate of the recipient SCID mice. Two typical examples of the cell surface features of CD19+IgMa+ B cells present in the recipient mice are displayed in Figure 7A. The BM and the spleen of the recipient mice harbored significant populations of donor-derived IgMa+ B cells, and CD19+IgMa− cells whose genetic origin cannot be firmly established. We tested for the presence of cytoplasmic IgH in the splenic CD19+IgM− cell population purified by immunomagnetic sorting from reconstituted SCID mice, and found that one third of them were positive by both immunofluorescence microscopy (Figure 7B) and intracellular flow cytometry (Figure 7C). The embryo-derived B-lineage cells partially or completely up-regulated the B220/6B2 Ag in vivo. Donor-derived IgMa+ B cells also up-regulated MHC class II IE molecules and lost the in vitro expression of IL-7Rα (data not shown). Small subsets of CD5+IgMa− T cells, most likely of endogenous origin, due to the “leakiness” of the SCID deficiency further stimulated by the irradiation,57 were also present in the peritoneal exudate of recipient SCID mice. B-cell reconstitutions were detected up to 4 months in the recipient mice. Unfortunately, the transfer of embryo-derived cell lines into H-2b+RAG-2–deficient mice, performed to definitively ascertain the donor origin of the CD19+IgMa-cμH− and the CD5+IgMa− cell populations, repeatedly failed to produce reconstitutions.
Phenotypes of B-lineage cells present in SCID mice repopulated with embryo cell lines.
(A) Stainings of BM, spleen, and peritoneum cell populations are shown for SCID mice injected with 2 representative embryo cell lines. The organs of SCID mice inoculated with PBS and of BALB/c mice are shown as negative and positive controls, respectively. The contour plots show the CD19, B220/6B2, and CD5 Ag expressions (vertical axis) and the IgMa+ surface levels (horizontal axis). (B) Intracytoplasmic μH stainings were performed after immunomagnetic depletion of sIgMa+ splenic cells from reconstituted SCID mice, as described in “Materials and methods.” Photomicrographs of IgMa-depleted populations are stained with rhodamine-labeled goat antimouse IgM (red). Nuclei are counterstained with DAPI (blue). The magnification scales are × 63 and × 100 for left and right photomicrographs, respectively. The samples were analyzed in a Leitz DMRD system. (C) Flow cytometry analyses for intracytoplasmic H chains in the CD19+IgMa−-splenic cells of reconstituted SCID mice. The dot plot at the left shows the reanalysis performed after depletion of IgMa+ cells by immunomagnetic cell sorting. The boxes define the CD19+IgMa− and CD19−IgMa− populations present in the sample. The right overlaid histograms represent the findings obtained by labeling with a control FITC-sheep antiserum (dotted lines) and a FITC-sheep F(ab)′2 antimouse Ig(H+L) (continuous lines), in the CD19+IgMa− (upper histogram) and the CD19−IgMa− (lower histogram) cell populations. The numbers represent the percentage of cH+cells.
Phenotypes of B-lineage cells present in SCID mice repopulated with embryo cell lines.
(A) Stainings of BM, spleen, and peritoneum cell populations are shown for SCID mice injected with 2 representative embryo cell lines. The organs of SCID mice inoculated with PBS and of BALB/c mice are shown as negative and positive controls, respectively. The contour plots show the CD19, B220/6B2, and CD5 Ag expressions (vertical axis) and the IgMa+ surface levels (horizontal axis). (B) Intracytoplasmic μH stainings were performed after immunomagnetic depletion of sIgMa+ splenic cells from reconstituted SCID mice, as described in “Materials and methods.” Photomicrographs of IgMa-depleted populations are stained with rhodamine-labeled goat antimouse IgM (red). Nuclei are counterstained with DAPI (blue). The magnification scales are × 63 and × 100 for left and right photomicrographs, respectively. The samples were analyzed in a Leitz DMRD system. (C) Flow cytometry analyses for intracytoplasmic H chains in the CD19+IgMa−-splenic cells of reconstituted SCID mice. The dot plot at the left shows the reanalysis performed after depletion of IgMa+ cells by immunomagnetic cell sorting. The boxes define the CD19+IgMa− and CD19−IgMa− populations present in the sample. The right overlaid histograms represent the findings obtained by labeling with a control FITC-sheep antiserum (dotted lines) and a FITC-sheep F(ab)′2 antimouse Ig(H+L) (continuous lines), in the CD19+IgMa− (upper histogram) and the CD19−IgMa− (lower histogram) cell populations. The numbers represent the percentage of cH+cells.
A total of 70% to 80% of the recipient mice were restored with different levels of donor-derived, serum IgMa at 1 month after transfer, the titers increasing later on to reach values that were close to those of normal BALB/c mice (Figure8A). IgG2aa and IgG1 serum Abs were detected in 4 and 2 recipient mice of 20, respectively, at levels 1 log below those of BALB/c mice (data not shown). A group of 2-month reconstituted SCID mice with 2 independent embryo B-cell lines was immunized with DNP-LPS, using BALB/c and BALB/cnu/numice as immunization controls. The serum levels of anti-DNP–specific IgMa antibodies were studied by means of IgM allotype-specific ELISAs. As shown in Figure 8B (upper histogram), the IgMa+ B cells established in vivo mounted a primary immune response to the DNP-LPS Ag during the 2 months after immunization. The increases of serum anti-DNP Ab levels found in the reconstituted SCID mice were roughly similar to those of athymic BALB/cnu/nucontrols. BALB/c mice, however, displayed a more robust and faster anti-DNP response, perhaps due to a minor component of T-cell help in this B-cell immune response (Figure 8B, bottom histograms). These findings suggest that the B-cell lines generated in vitro from progenitors recovered from midgestation mouse embryos were not only able to reconstitute the B-cell compartment of adult SCID mice, but also were able to give rise to humoral immune responses after specific immunization.
B-cell repopulation of adult SCID mice with embryo cell lines and induction of specific humoral immune responses.
(A) Serum IgMa levels of reconstituted mice at 1 and 2 months after the adoptive transfer of embryo cell lines. They were defined by comparison to normal BALB/c serum levels (100titer). The data were obtained in IgM allotype-specific ELISAs as described in “Materials and methods.” Each point corresponds to one independent mouse. The mean values are indicated as in Figure 4. (B) Sequential anti-DNP IgMa serum titers were calculated with DNP-specific ELISAs after immunization with DNP-LPS. Black circles and triangles correspond to the mean values of 3 recipient SCID mice, each one injected with a different embryo cell line. Empty circles show the serum anti-DNP IgMa+ titers of reconstituted, but unimmunized, SCID mice (upper histogram). Empty squares and triangles are the mean values of 3 BALB/c and BALB/cnu/nu mice, respectively (lower histograms).
B-cell repopulation of adult SCID mice with embryo cell lines and induction of specific humoral immune responses.
(A) Serum IgMa levels of reconstituted mice at 1 and 2 months after the adoptive transfer of embryo cell lines. They were defined by comparison to normal BALB/c serum levels (100titer). The data were obtained in IgM allotype-specific ELISAs as described in “Materials and methods.” Each point corresponds to one independent mouse. The mean values are indicated as in Figure 4. (B) Sequential anti-DNP IgMa serum titers were calculated with DNP-specific ELISAs after immunization with DNP-LPS. Black circles and triangles correspond to the mean values of 3 recipient SCID mice, each one injected with a different embryo cell line. Empty circles show the serum anti-DNP IgMa+ titers of reconstituted, but unimmunized, SCID mice (upper histogram). Empty squares and triangles are the mean values of 3 BALB/c and BALB/cnu/nu mice, respectively (lower histograms).
Discussion
B lymphopoiesis proceeds during the whole life span of the individual, starting on early midgestational periods of the mouse embryo. The process varies throughout the mouse ontogeny related to organ location, developmentally controlled waves, microenvironment-bound signals, and peripheral cell turnover rates. Recent evidence indicates that genetic elements of B-cell development appear very close in time to the detection of the first lymphohematopoietic progenitors in the P-Sp/AGM (8.5 dpc).8-10 Whether these processes are part of bona fide B-cell differentiation or represent low-level, random gene transcription events in multipotent cell progenitors58,59is a question open to debate. As it happens for other somatic cell lineages, the in vitro establishment of cell progenitors in conditions promoting their full maturation and functionality is an important goal, both to approach the molecular bases of cell differentiation and to develop putative therapies of substitution in selected diseases.60 The cultures of stromal cells plus IL-7 have been a powerful tool to study mouse B-cell differentiation.27,54 These cultures, however, showed an unexpected IL-7–mediated blockade at the stage of late pre-B cells, with inhibition of the emergence of functional B cells in the cultures of LFL and BM progenitors. To differentiate into IgM+cells, IL-7 had to be removed from the cultures,27-29which then became short-lived and self-limited. We have now established untransformed B-lineage cell lines from 11-dpc mouse lymphohematopoietic sites (liver, P-Sp/AGM, YS) in cell cultures with stroma plus IL-7. In contrast to LFL/BM cell lines, these early embryo cell lines were able to spontaneously differentiate up to the stage of CD19+IgM+ B cells in the presence of IL-7 and to efficiently reconstitute functional B-cell compartments on their adoptive transfer into adult SCID mice.
The IL-7/IL-7R pair is critical for lymphoid development.61 Although factors alternative to IL-7 may also be involved in IL-7R signaling,62-64 the deletion of the IL-7Rα chain cannot be substituted.65,66 Signals starting in specific IL-7Rα intracytoplasmic domains are transmitted to the antiapoptotic and cell cycling machineries through tyrosine phosphorylation of signaling proteins, including PI3K.67Other IL-7Rα residues contribute to differentiation related with the genomic rearrangements of IgH and TCRγloci.68-71 The midgestation embryo cell progenitors showed a greater sensitivity to limiting IL-7 amounts, when compared with those of LFL/BM-derived cells. This difference was noteworthy in the early time points of in vitro cell line establishment and in the populations of CD19+IgM− pro-B/early pre-B cells. The IgM+ B cells newly emerging on the embryo cell lines did not respond to IL-7, although they still expressed the IL-7Rα, probably under the stimulus of the complementary cytokine.72 The increased IL-7 sensitivity of embryo cell lines may be partly due to their high IL-7Rα expression (10-fold over LFL/BM cell lines), but also to developmental changes in intracellular signaling pathways of B-cell precursors of the early mouse ontogeny, as compared with the lower IL-7 responsiveness of BM progenitors from aged individuals.12,73-75 At the early ontogeny, the hematopoietic progenitors strongly expand7,9 and are highly mobile between various microenvironments while the lineage differentiation programs start to be fixed in minor cell populations. Later on, a synchronous wave of B-committed precursors begins at day 13 in liver to peak at day 16.11 It is tempting to speculate that this LFL/BM B-cell development might depend more on the interplay of both microenvironment-specific cell interactions (including between pre-B cells themselves)21 and soluble factor signals. In contrast, early embryo B lymphopoiesis (pre-13 dpc) could rely more on responses to soluble factors (eg, IL-7) than on specific cell interactions with particular microenvironmental niches.
A synergistic cross-talk between IL7R and PreBCR-encodingλ5 genes has been recently described in adult progenitors at low doses of IL-7.21,76 We have observed that λ5 transcripts are barely detectable up to 12 dpc in the mouse embryo.9,59 It might happen that λ5-nonexpressing embryo B-cell progenitors are compelled to exclusively or preferentially use IL-7R pathways, subsequently lowering the threshold of response to available IL-7. Once established in vitro, the embryo cell lines expressed λ5 either by selection of λ5+ clones and/or by an IL-7–mediated up-regulation of the λ5gene.72 We realize that this explanation differs from the one proposed on the basis of findings obtained in λ5-deficient mice, that originated B-lineage cell lines more resistant to IL-7–mediated signals.21 76 Although we presently ignore the reason for such divergence between both models, it might depend on the different ontogenic origin of the B-cell progenitors, or on the fact that theλ5 gene is rapidly expressed in vitro in our embryo cell lines, whereas this cannot happen in λ5-deficient cell lines. These possibilities can be elucidated by analyzing the embryo progenitors of λ5-deficient mice.
The embryo cell lines were able to overcome the IL-7 differentiation arrest and efficiently mature to functional IgM+ B cells in the presence of saturating IL-7 concentrations. Only when cultures of purified B-cell precursors were submitted to superoptimal doses of rIL-7 (30 ng/mL), was the number of newly emerging B cells reduced. This implies that a partial IL-7–dependent differentiation arrest also existed, therefore, in embryo cell lines at the stage of late pre-B cell. A selective driving force of midgestation mouse embryo progenitors to complete differentiation along the B-cell lineage, as has been suggested with other postgastrulation hematopoietic cells,77 78 could represent an intrinsic feature of these early cells, contributing to overcome the IL-7–mediated arrest. Both the low threshold of proliferative responses to IL-7 at the immature cell stages and the strong bias to rapid differentiation may account for the distinctive behavior observed in the embryonic cell lines.
The in vitro establishment of normal, untransformed B cells represents a long-searched goal for studies of humoral immunity. We consider that the approach shown in this paper can represent a substantial contribution in that sense. This work shows that polyclonal CD19+IgM+ B lymphocytes were continuously generated in vitro when progenitors from midgestation mouse embryos were established in stromal monolayer plus IL-7 cell cultures. More importantly, these B cells efficiently reconstituted adult immunodeficient mice, showed a conventional cell surface phenotype, and became able to respond to Ag immunization. It seems likely that the in vivo expansion potential of the embryo cell lines depends on minor subsets of pro-B cells remaining in the in vitro growing cell lines. Similar repopulations of BM and spleen B-cell pools are absent in the adoptive transfers of LFL- and BM-derived cell lines (data not shown).79 80 Finally, the experimental approach shown here may be useful to analyze the progenitor's potentials from different genetic backgrounds, and in particular, those of gene-manipulated mice suffering gestational death. From a biomedical viewpoint, it would be interesting to know whether developmental characteristics similar to those observed here also apply to human progenitors from sources such as the cord blood, to consider them for putative substitution therapies in certain immunodeficiencies.
We acknowledge the suggestions and critical review of the manuscript of J. Andersson and his colleagues. We also thank A. Grandien for the ST2 cells and A. G. Rolink for the IL-7–transfected 3T3 cells. The technical support of P. Ferrero and the editorial assistance of M. Messman are also recognized.
Supported by grants from the Comisión Interministerial de Ciencia y Tecnologı́a (PM96-0072 and PM99-0104) and the CAM (08.3/0009). The CBMSO is partially founded by Fundación Ramón Areces.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marı́a-Luisa Gaspar, Centro Nacional de Biologı́a Fundamental, Instituto de Salud Carlos III, Ctra Majadahonda-Pozuelo Km 2, 28220 Majadahonda, Spain; e-mail:mlgaspar@isciii.es.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal