Abstract
Verotoxin-1 (VT-1)–producing Escherichia coli is the causative agent of postdiarrheal hemolytic uremic syndrome (D+HUS) of children, which leads to renal and other organ microvascular thrombosis. Why thrombi form only on arterioles and capillaries is not known. This study investigated whether VT-1 directly affected endothelial antithrombogenic properties promoting platelet deposition and thrombus formation on human microvascular endothelial cell line (HMEC-1) under high shear stress. Human umbilical vein endothelial cells (HUVECs) were used for comparison as a large-vessel endothelium. HMEC-1 and HUVECs were pre-exposed for 24 hours to increasing concentrations of VT-1 (2-50 pM) and then perfused at 60 dynes/cm2 with heparinized human blood prelabeled with mepacrine. Results showed that VT-1 significantly increased platelet adhesion and thrombus formation on HMEC-1 in comparison with unstimulated control cells. An increase in thrombus formation was also observed on HUVECs exposed to VT-1, but to a remarkably lower extent. The greater sensitivity of HMEC-1 to the toxin in comparison with HUVECs was at least in part due to a higher expression of VT-1 receptor (20-fold more) as documented by FACS analysis. The HMEC-1 line had a comparable susceptibility to the thrombogenic effect of VT-1 as primary human microvascular cells of the same dermal origin (HDMECs). The adhesive molecules involved in VT-induced thrombus formation were also studied. Blocking the binding of von Willebrand factor to platelet glycoprotein Ib by aurintricarboxylic acid (ATA) or inhibition of platelet αIIbβ3-integrin by chimeric 7E3 Fab resulted in a significant reduction of VT-1–induced thrombus formation, suggesting the involvement of von Willebrand factor–platelet interaction at high shear stress in this phenomenon. Functional blockade of endothelial β3-integrin subunit, vitronectin receptor, P-selectin, and PECAM-1 with specific antibodies was associated with a significant decrease of the endothelial area covered by thrombi. Confocal microscopy studies revealed that VT-1 increased the expression of vitronectin receptor and P-selectin and redistributed PECAM-1 away from the cell-cell border of HMEC-1, as well as of HDMECs, thus indicating that the above endothelial adhesion molecules are directly involved and possibly determine the effect of VT-1 on enhancing platelet adhesion and thrombus formation in microvascular endothelium. These results might help to explain why thrombi in HUS localize in microvessels rather than in larger ones and provide insights on the molecular events involved in the process of microvascular thrombosis associated with D+HUS.
Introduction
Verotoxin (VT)–producing Escherichia coli infection has been strongly implicated as the causative agent for most cases of postdiarrheal hemolytic uremic syndrome (D+HUS), a disorder of microangiopathic hemolytic anemia, thrombocytopenia, and acute renal failure that mainly affects infants and small children.1-3 The characteristic lesion, thrombotic microangiopathy, consists of swelling and detachment of endothelial cells from the basement membrane and deposition of platelet thrombi that occlude the microcirculation of the kidneys and other organs.4 Why thrombi form only in arterioles and capillaries is not known.
It is now clear that endothelial dysfunction plays a crucial role in the sequence of events leading to the microangiopathic processes, and evidence points to VT-1 and VT-2 as critical determinants for the development of vascular lesions. Verotoxins (also called Shiga toxins) are formed by a biologically active A subunit and a number of B subunits by which the toxin binds to a specific glycosphingolipid globotriaosyl ceramide (Gb3) receptor on the endothelial surface.2,5 After binding, the toxin penetrates the cytosol by endocytosis and exerts its cytotoxic effect by inhibiting protein synthesis and causing cell death.6-8Monocytes/macrophages in response to VT release cytokines such as interleukin-1 (IL-1) and tumor necrosis factor (TNF) that remarkably potentiate sensitivity of vascular endothelial cells to VT by up-regulating endothelial Gb3 receptor.9 Evidence also shows that renal microvascular endothelial cells have a higher sensitivity to the cytotoxic effect of VT as compared to endothelial cells derived from large vessels.7 8
The interaction between leukocytes and endothelial cells is instrumental in the development of microvascular injury in VT-associated HUS. Thus, evidence suggests that neutrophils isolated from children in the acute phase of D+HUS adhered to endothelial cells in culture more than normal neutrophils and induced endothelial injury by local release of proteases.10 We have demonstrated in vitro that VT-1 directly induced a massive leukocyte adhesion to cultured endothelial cells under flow conditions, by up-regulating the adhesive proteins E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1).11 Furthermore, a preliminary report has shown that glomerular endothelial cells exposed to VT became more susceptible to neutrophil-mediated oxidant injury.12 Taken together these studies indicate that VT causes cell injury by altering cell adhesive properties and by increasing endothelial susceptibility to leukocyte-mediated injury. The resulting injured endothelium changes its normal thromboresistant phenotype and becomes thrombogenic, initiating microvascular thrombus formation.
In HUS, structural damage of microvessels associated with narrowing of the lumina determines major changes in fluid shear stress, which would favor persistent endothelial damage, platelet activation, and progression of microvascular thrombosis.13 Changes in shear stress, the tractive force produced by blood flowing over the endothelial surface, have a profound influence on von Willebrand factor (vWF) handling by enhancing its susceptibility to proteolytic cleavage.14 Under conditions of high shear stress, vWF undergoes conformational changes and serves to bridge the subendothelial matrix to glycoprotein (GP) Ib expressed on platelet membranes.15 The engagement of this receptor promotes activation of the platelet αIIbβ3(GPIIb/IIIa) complex that binds to the RGD (Arg-Gly-Asp) sequence of vWF leading to thrombus formation.15 Via the RGD sequence, vWF may also bind vitronectin receptor (αvβ3), the major integrin expressed on endothelial cells16 that promotes endothelial cell adhesion to the vascular matrix.17 GPIb is also expressed on endothelial cells; however, controversial results about its function have been reported so far.18 19
Several distinct endothelial cell molecules have been reported to be involved in the binding of platelets to endothelial cells. P-selectin, which is stored in intracellular granules of platelets and endothelial cells together with vWF and which rapidly mobilizes to the cell surface on stimulation,20 is required for platelet rolling and adhesion on activated endothelium.21 Increased plasma levels of P-selectin have been measured in patients with HUS, possibly reflecting activation/damage of platelets and endothelial cells.22 Evidence also indicates that platelet-endothelial cell adhesion molecule-1 (PECAM-1) expressed on endothelial cells23 contributes to platelet adhesion and aggregation at sites of injured endothelium, as documented by the finding that anti-PECAM-1 antibody injection delayed thrombus formation in laser-induced microvessel injury of mouse brain.24
In the present study, we sought to (1) assess whether VT-1 directly affected the antithrombogenic properties of the endothelium under high shear stress; (2) evaluate whether microvascular endothelium had a higher sensitivity to VT-1–induced thrombus formation, as compared to endothelium derived from large vessels; and (3) identify platelet and endothelial cell adhesive proteins involved in the thrombotic process promoted by VT-1.
Materials and methods
Endothelial cell culture and incubation
The human microvascular endothelial cell line of dermal origin (HMEC-1)25 was a kind gift from Dr Francisco J. Candal (Centers for Disease Control and Prevention, Atlanta, GA). The growth medium consisted of MCDB 131 (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (Gibco), 1 μg/mL hydrocortisone, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM glutamine (Gibco), and 50 μg/mL endothelial cell growth factor prepared as described.26
Human umbilical vein endothelial cells (HUVECs) were obtained by collagenase digestion according to the method of Jaffé and coworkers.27 The cells were grown in Medium 199 (Gibco) supplemented with 10% newborn calf serum (Gibco) and 10% human serum, 5 mMN-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES; Sigma Chemical, St Louis, MO), 100 U/mL penicillin, 100 μg/mL streptomycin, 2.5 μg/mL fungizone, 2 mM glutamine (Gibco), 15 U/mL heparin (Parke-Davis, Milan, Italy), and 50 μg/mL endothelial cell growth factor. Cultured cells were identified as endothelial by their cobblestone morphology and the presence of vWF, using indirect immunofluorescence microscopy. Confluent HUVECs were used for experiments between the first and fifth passage.
Primary human microvascular endothelial cells of dermal origin (HDMECs) (Promocell, Heidelberg, Germany) were cultured in endothelial cell growth medium MV plus SupplementMix (Promocell). HDMECs were used between the second and sixth passage.
For the experiments, endothelial cells were plated on 60 × 20-mm plastic coverslips (Thermanox; Nunc, Naperville, IL) and used 1 day after reaching confluence.
To study the effect of VT-1 in inducing platelet adhesion and thrombus formation, HMEC-1 and HUVECs were pre-exposed for 24 hours in static condition to 2, 10, and 50 pM VT-1 (kindly provided by Dr M. A. Karmali, Hospital for Sick Children, Toronto, ON, Canada; endotoxin content <0.05 EU/mL using Limulus amoebocyte lysate assay) in medium plus 2% fetal calf serum (Hyclone Laboratories, Logan, UT); then cells were perfused at 60 dynes/cm2 in a parallel plate flow chamber with human blood. Blood was drawn from an antecubital vein through a 19-gauge needle (infusion set) directly into a polypropylene tube and prelabeled for 5 minutes with fluorescent dye mepacrine (10 μM, quinacrine dihydrochloride BP; Sigma Chemical). Blood was then transferred (5-mL aliquots) to test tubes and not disturbed until assay. The percentage of the surface occupied by thrombi was calculated by analysis of fluorescent thrombus images acquired by confocal microscopy.
The concentrations of VT-1 used for the adhesion experiments did not affect cell count after 24 hours of incubation either in HMEC-1 (10 pM: 75 ± 0.5 × 104, 50 pM: 75 ± 5 × 104versus control: 70 ± 5 × 104 cells) or in HUVECs (10 pM: 38.2 ± 1.1 × 104, 50 pM: 37.4 ± 0.7 × 104 versus control: 36.5 ± 0.5 × 104 cells).
To evaluate endothelial integrity, HMEC-1 pre-exposed for 24 hours to VT-1 (10 pM) were perfused with blood without mepacrine and then fixed with 0.5% glutaraldehyde (Fluka, Milan, Italy), dehydrated with methyl alcohol, and stained with May-Grunwald Giemsa technique (Carlo Erba Reagents, Milan, Italy).
By selected experiments we verified whether the HMEC-1 cell line exhibited a similar sensitivity to VT-1—as for the VT effect to induce thrombus formation—compared with primary HDMECs. For this purpose HMEC-1 and HDMECs were exposed to VT-1 (10 pM) for 24 hours. HUVECs were studied in parallel.
To compare the effect of VT-1 with respect to other thrombogenic stimuli, HMEC-1 and HUVECs were exposed to thrombin (2 U/mL, 10 minutes; Biosciences, La Jolla, CA), TNF-α (100 U/mL, 4 hours; BASF KNOLL, Ludwigshafen, Germany), IL-1β (100 U/mL, 4 hours; Becton Dickinson, Milan, Italy), or VT-1 (10 pM, 24 hours) and perfused with blood at 60 dynes/cm2.
To identify platelet receptors involved in VT-induced thrombus formation, HMEC-1 treated for 24 hours with VT-1 (10 pM) were perfused with human blood preincubated with inhibitors of GPIb and αIIbβ3 receptor binding. We used polymeric aurin tricarboxylic acid (ATA; ATA trisodium salt, Aldrich Chemical, Milwaukee, WI) that interacts with vWF and inhibits its binding to platelet receptor GPIb.28 Because the most effective inhibitors of vWF are ATA polymers of molecular weight greater than 2500 d,29 the higher molecular weight polymers of ATA were separated from the lower molecular weight ones by a 30-kd cutoff dialysis membrane concentrators (Centricon 30, Amicon, Beverly, MA). For experiments blood samples were incubated with ATA (100 μg/mL) for 5 minutes at 37°C before perfusion on VT-treated HMEC-1. The platelet receptor αIIbβ3 was blocked by incubating blood samples with chimeric 7E3 Fab anti-αIIbβ3 (20 μg/mL; Abciximab, Reopro; Eli Lilly, Indianapolis, IN)30 for 5 minutes at 37°C before blood perfusion.
To determine the endothelial adhesive proteins involved in VT-induced thrombus formation, HMEC-1 pretreated with VT-1 (10 pM) for 24 hours were incubated with chimeric 7E3 Fab (20 μg/mL) for 20 minutes, mouse monoclonal antibody (mAb) antihuman vitronectin receptor LM609 (10 μg/mL; Chemicon International, Temecula, CA) for 10 minutes, mouse mAb anti-GPIbα LJ-Ib1 (100 μg/mL, a kind gift from Dr Zaverio M. Ruggeri, The Scripps Research Institute, La Jolla, CA)—a competitive inhibitor of vWF binding—for 20 minutes,31 mouse mAb antihuman P-selectin (50 μg/mL; Endogen, Woburn, MA) for 20 minutes, or mouse mAb antihuman PECAM-1 (25 μg/mL, Endogen) for 10 minutes; then the adhesion assay was performed. Appropriate concentration and incubation time for each antibody were identified by preliminary experiments. Irrelevant antibody of mouse IgG1 (CAMfolio, Becton Dickinson) at concentrations of 10, 25, and 50 μg/mL were used.
The involvement of P-selectin and PECAM-1 in VT-1–induced thrombus formation on HDMECs was also assessed, using the same experimental condition described above for HMEC-1.
Adhesion assay under flow conditions and fluorescence confocal microscopy
Platelet adhesion assay was performed with whole blood perfused in a chamber using a syringe pump. A flow chamber regulated at 37°C was used, in which one surface of the perfusion channel was a coverslip (Thermanox; Nunc, Naperville, IL) seeded with a monolayer of endothelial cells. The chamber dimensions (30 × 1 mm and 150 μm in thickness) allow us to obtain a wide range of shear rates using low flow rates of blood. The flow conditions are well characterized for this geometry and are predicted to be laminar with very low Reynolds number (< 10); precise estimation of shear stress conditions on the adhesion surface was also performed using the computational fluid dynamic analysis (CFD package FIDAP, Fluid Dynamic International, Evanston, IL) to verify the inlet and outlet effect on flow velocity profiles. The system was filled with 10 mM phosphate-buffered saline (PBS) at pH 7.3; then the slide seeded with the endothelial monolayer was mounted in the flow chamber. Heparinized whole blood was incubated with the fluorescent dye mepacrine (10 μM). Mepacrine concentrates in the dense granules of platelets and in the granules of leukocytes. At this concentration it has no effect on normal platelet function.32 Any fluorescence within the erythrocytes is quenched by hemoglobin. Blood preincubated for 5 minutes at 37°C was then perfused into the chamber at constant flow rate of 1500 1/sec (60 dynes/cm2). After 3 minutes, perfusion was stopped and the slide with the endothelial cell monolayer was dehydrated and fixed in acetone for 20 minutes.
Images of platelet thrombi on endothelial cell surface were acquired by a confocal inverted laser microscope (InSight plus; Meridian Instruments, Okemos, MI). An argon laser emission filter at 488 nm was used to excite specimens. Fifteen fields, systematically digitized along the adhesion surface, were acquired using a computer-based image analysis system. The area occupied by thrombi was evaluated by automatic edge detection using built-in specific functions of the software Image 1.61 (National Institutes of Health, Bethesda, MD), and expressed as μm2/field analyzed (total area: 474 473 μm2/field).
Scanning electron microscopy
For scanning electron microscopy analysis HMEC-1 grown on coverslips were treated with VT-1 (10 pM, 24 hours), perfused with blood, and then fixed overnight at 4°C with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4. The slides were rinsed in 0.1 M sodium cacodylate buffer, osmicated for 1 hour, and then dehydrated in an ascending series of ethanol. The dehydration series concluded with 2 × 15-minute exchanges in 100% ethanol. After drying, the slides were coated with gold and examined in a scanning electron microscope (Stereoscan 200, Cambridge Instruments, Cambridge, MA).
Flow cytometry analysis
The surface expression of VT-1 receptor on HMEC-1 and HUVECs was evaluated by flow cytometry analysis (FACS; FACSort, Becton Dickinson). Endothelial cells in suspension were incubated for 45 minutes at room temperature with 1 μg/100 μL fluorescein isothiocyanate (FITC)–labeled albumin (Sigma Chemical) as control or 1 μg/100 μL FITC-labeled VT-1B subunit (a kind gift from Dr C. A. Lingwood, The Hospital for Sick Children, Toronto, ONT),33 washed 3 times, fixed with 2% paraformaldehyde, and assayed within 1 hour.
Fluorescence confocal microscopy
The HMEC-1 line and HDMECs grown on coverslips were incubated with medium alone or VT-1 (10 pM) for 24 hours and then fixed in 3% paraformaldehyde plus 2% sucrose in PBS, pH 7.4, for 15 minutes at room temperature. After 3 washings (5 minutes) with PBS plus 3% bovine serum to prevent nonspecific antibody binding, cells were treated with anti-P-selectin antibody (50 μg/mL) or antivitronectin receptor antibody LM609 (10 μg/mL). For PECAM-1 assessment, cells were permeabilized with Triton X-100 (0.1% in PBS; Sigma Chemical) for 4 minutes before incubation with anti-PECAM-1 antibody (10 μg/mL). Then cells were incubated with FITC-conjugated F(ab′)2 goat antimouse IgG + IgM (Jackson Immunoresearch Laboratories, West Grove, PA).
Negative control experiments with FITC-conjugated antibody alone were performed. Coverslips were washed, mounted in 1%N-propyl-gallate in 50% glycerol, 0.1 M Tris-HCl, pH 8, and examined under confocal inverted laser microscopy. Representative fields were digitized with millions of colors and printed.
Statistical analysis
Results are expressed as mean ± SE. Statistical analysis was performed using ANOVA followed by the Tukey test for multiple comparisons, as appropriate.34 Statistical significance was defined as P less than .05.
Results
VT-1 promotes platelet adhesion and thrombus formation on endothelial cells
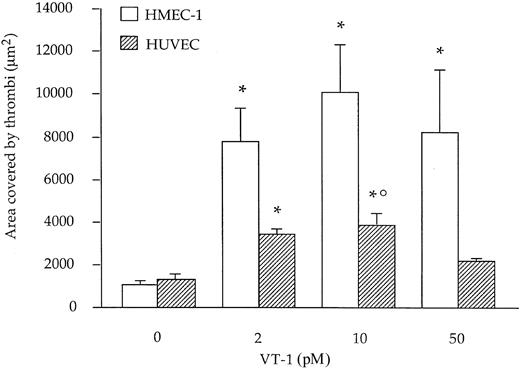
We studied the effect of VT-1 on platelet adhesion and thrombus formation under laminar flow at high shear rate on HMEC-1. HUVECs were used for comparison as large-vessel endothelium. Heparinized blood was prelabeled with mepacrine and perfused at 60 dynes/cm2 over resting or VT-1–treated endothelial cells; then thrombus formation was quantified by analyzing images acquired by confocal microscopy. On resting HMEC-1 only limited platelet deposits were observed, usually less than 0.3% of the total perfused area, corresponding to about 1200 μm2/field analyzed. Exposure of HMEC-1 to VT-1 for 24 hours led to a significant (P < .01) increase in platelet adhesion and thrombus formation in comparison to control cells, with a maximum effect at 10 pM (2 pM: 7754 ± 1592; 10 pM: 10 090 ± 2246; 50 pM: 8244 ± 2874 versus control: 1077 ± 140 μm2 area covered by thrombi) (Figure1).
Effect of VT-1 on platelet adhesion and thrombus formation on HMEC-1 and HUVECs.
HMEC-1 and HUVECs were treated for 24 hours with VT-1 (0, 2, 10, 50 pM) and then perfused with mepacrine-labeled blood at high shear stress (60 dynes/cm2). The area occupied by thrombi was measured on images acquired by confocal fluorescence microscopy, as described in “Materials and methods.” Data are expressed as mean ± SE of area covered by thrombi (n = 8 experiments). *P < .01 versus untreated cells; °P < .05 HUVEC + VT-1(10 pM) versus HMEC-1 + VT-1 (10 pM).
Effect of VT-1 on platelet adhesion and thrombus formation on HMEC-1 and HUVECs.
HMEC-1 and HUVECs were treated for 24 hours with VT-1 (0, 2, 10, 50 pM) and then perfused with mepacrine-labeled blood at high shear stress (60 dynes/cm2). The area occupied by thrombi was measured on images acquired by confocal fluorescence microscopy, as described in “Materials and methods.” Data are expressed as mean ± SE of area covered by thrombi (n = 8 experiments). *P < .01 versus untreated cells; °P < .05 HUVEC + VT-1(10 pM) versus HMEC-1 + VT-1 (10 pM).
In these experimental conditions the endothelial cell integrity was preserved as indicated by staining with May-Grunwald Giemsa of HMEC-1 treated with VT-1 (10 pM) and then perfused with blood.
As shown in Figure 1, VT-1 also promoted platelet adhesion and thrombus formation on HUVECs but to a remarkably lower extent than on HMEC-1 (2 pM: 3406 ± 297; 10 pM: 3849 ± 540; 50 pM: 2497 ± 99 versus control: 1311 ± 263 μm2).
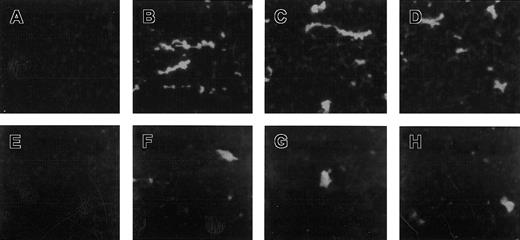
Figure 2 depicts digitized images from a representative experiment acquired by confocal fluorescent microscopy showing an increase in the number of mepacrine-labeled thrombi on HMEC-1 exposed to VT-1 in comparison to HUVECs.
Thrombi formed after treatment with VT-1.
Micrographs show thrombi formed on HMEC-1 (A-D) and HUVEC (E-H) treated with VT-1. Cells were incubated with control medium (A,E) or with VT-1 at 2 pM (B,F), 10 pM (C,G), 50 pM (D,H) for 24 hours, perfused with blood at 60 dynes/cm2, and examined by confocal microscopy. Digitized representative fields are shown.
Thrombi formed after treatment with VT-1.
Micrographs show thrombi formed on HMEC-1 (A-D) and HUVEC (E-H) treated with VT-1. Cells were incubated with control medium (A,E) or with VT-1 at 2 pM (B,F), 10 pM (C,G), 50 pM (D,H) for 24 hours, perfused with blood at 60 dynes/cm2, and examined by confocal microscopy. Digitized representative fields are shown.
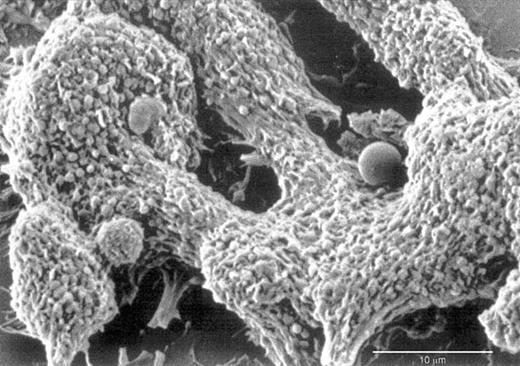
Scanning electron microscopy evaluation of VT-treated HMEC-1 illustrates the attachment of platelets to the endothelial cell monolayer to form organized thrombi in which leukocytes at different stages of activation are entrapped (Figure3).
Scanning electron micrograph of thrombi on HMEC-1.
HMEC-1 treated with VT-1 (10 pM) reveal organized thrombi with entrapped leukocytes at different stages of activation.
Scanning electron micrograph of thrombi on HMEC-1.
HMEC-1 treated with VT-1 (10 pM) reveal organized thrombi with entrapped leukocytes at different stages of activation.
In selected experiments, the behavior of the HMEC-1 cell line in response to the thrombogenic effect of VT-1 was compared with that of primary microvascular endothelial cells of similar dermal origin. VT-1 (10 pM) induced thrombus formation on the HMEC-1 line and primary HDMEC to a similar extent (HMEC-1: 11 831 ± 1303; HDMEC: 9226 ± 1979 μm2 area covered by thrombi). HUVECs used in these settings for comparison were significantly less susceptible to the effects of VT-1 (4061 ± 553 μm2, P < .01 versus HMEC-1 and P < .05 versus HDMECs).
VT-1 is more thrombogenic than thrombin and cytokines
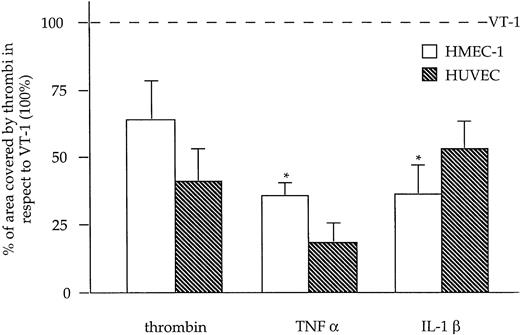
We compared the capability of VT-1 to induce thrombus formation on HMEC-1 and HUVECs with other thrombogenic agonists like thrombin, TNF-α, and IL-1β. As shown in Figure4, thrombin and cytokines were less effective in promoting platelet deposition than VT-1 in both endothelial cell types. The superior thrombogenic effect of VT-1 translated in larger thrombus size with respect to thrombin, TNF-α, and IL-1β, indicating a selective pattern of endothelial activation implemented by VT-1 (mean area of thrombi, HMEC-1 + VT-1: 2584 ± 511 μm2; HMEC-1 + thrombin: 1140 ± 132 μm2; HMEC-1 + TNF-α: 1204 ± 588 μm2; HMEC-1 + IL-1β: 950 ± 362 μm2; and HUVEC + VT-1: 1748 ± 399 μm2; HUVEC + thrombin: 637 ± 128 μm2; HUVEC + TNF-α: 427 ± 149 μm2; HUVEC + IL-1β: 1406 ± 618 μm2).
Effect of thrombin, TNF-α, and IL-1β on thrombus formation on HMEC-1 and HUVEC compared with VT-1.
Endothelial cells were incubated with thrombin (2 U/mL, 10 minutes), TNF-α (100 U/mL, 4 hours), IL-1β (100 U/mL, 4 hours), or VT-1 (10 pM, 24 hours), perfused with blood at 60 dynes/cm2, and examined under confocal microscopy. Data are expressed as mean ± SE of percent of area covered by thrombi in respect to VT-1 (100%) (n = 3 experiments). *P < .01 versus VT-1.
Effect of thrombin, TNF-α, and IL-1β on thrombus formation on HMEC-1 and HUVEC compared with VT-1.
Endothelial cells were incubated with thrombin (2 U/mL, 10 minutes), TNF-α (100 U/mL, 4 hours), IL-1β (100 U/mL, 4 hours), or VT-1 (10 pM, 24 hours), perfused with blood at 60 dynes/cm2, and examined under confocal microscopy. Data are expressed as mean ± SE of percent of area covered by thrombi in respect to VT-1 (100%) (n = 3 experiments). *P < .01 versus VT-1.
VT-1 receptor expression on endothelial cells
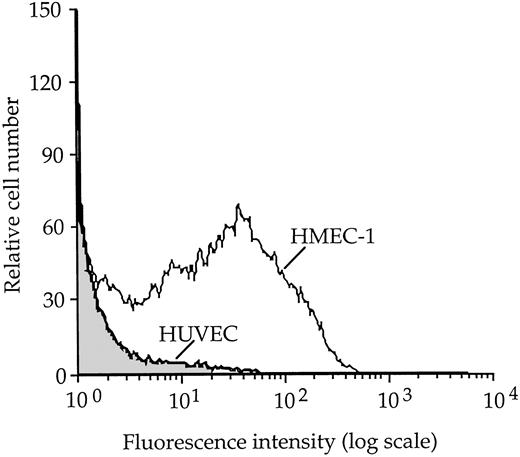
To investigate whether the higher sensitivity of HMEC-1 to VT-1 with respect to HUVECs was due to a different expression of VT-1 receptors, FACS studies were performed using fluorescent VT-1B subunit. Surface expression of VT-1 receptor, as a percentage of fluorescent cells, is depicted in Figure 5. The percentage of resting HMEC-1 stained with fluorescent VT-1B subunit was approximately 20-fold higher than that observed for HUVECs. FITC-albumin, used as control, bound to HMEC-1 and HUVECs at comparable extents (1% ± 0.08% and 0.9% ± 0.1% of fluorescent cells, respectively).
Flow cytometric analysis of VT-1 receptor expression on unstimulated HMEC-1 and HUVECs using FITC-labeled VT-1B subunit.
Percentage of fluorescent HMEC-1 cells was 34% and of fluorescent HUVEC cells, 2%.
Flow cytometric analysis of VT-1 receptor expression on unstimulated HMEC-1 and HUVECs using FITC-labeled VT-1B subunit.
Percentage of fluorescent HMEC-1 cells was 34% and of fluorescent HUVEC cells, 2%.
Effect of functional blockade of platelet and endothelial adhesive molecules on VT-1–induced thrombus formation in HMEC-1
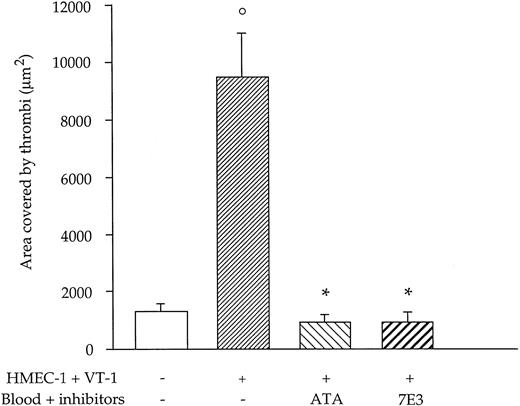
To identify adhesive proteins involved in thrombus formation induced by VT-1 at high shear rate, we first evaluated the effect of blocking the interaction between vWF and platelet receptors GPIb and αIIbβ3. As shown in Figure6, ATA, which inhibits vWF-GPIb interaction, completely prevented platelet deposition and thrombus formation induced by VT-1 (10 pM) on HMEC-1 surface (VT-1 + ATA: 919 ± 256 μm2 versus VT-1: 9502 ± 1475 μm,2P < .01). A similar significant (P < .01) reduction in the area occupied by thrombi was observed by blocking the platelet receptor αIIbβ3 with the chimeric 7E3 Fab (VT-1 + 7E3: 952 ± 278 μm2).
Effect of blockade of vWF binding to platelet GPIb or αIIbβ3 with specific inhibitors on VT-1–induced thrombus formation on HMEC-1.
ATA (100 μg/mL), which inhibits vWF binding to GPIb, or 7E3 Fab (20 μg/mL), an anti-αIIbβ3, was added to blood 5 minutes before perfusion over HMEC-1. Data are expressed as mean ± SE (n = 6 experiments). °P < .01 versus control; *P < .01 versus VT-1.
Effect of blockade of vWF binding to platelet GPIb or αIIbβ3 with specific inhibitors on VT-1–induced thrombus formation on HMEC-1.
ATA (100 μg/mL), which inhibits vWF binding to GPIb, or 7E3 Fab (20 μg/mL), an anti-αIIbβ3, was added to blood 5 minutes before perfusion over HMEC-1. Data are expressed as mean ± SE (n = 6 experiments). °P < .01 versus control; *P < .01 versus VT-1.
Considering that GPIb is also expressed on endothelial cells, we investigated the role of this receptor on VT-1–induced thrombus formation in HMEC-1. Functional blocking of GPIb with LJ-Ib1 mAb did not affect thrombus formation in response to VT-1 (VT-1 + anti-GPIb: 12 096 ± 2716 μm2 versus VT-1: 12 696 ± 1677 μm2), suggesting that in this experimental setting endothelial GPIb was not involved in vWF-induced thrombi at high shear stress.
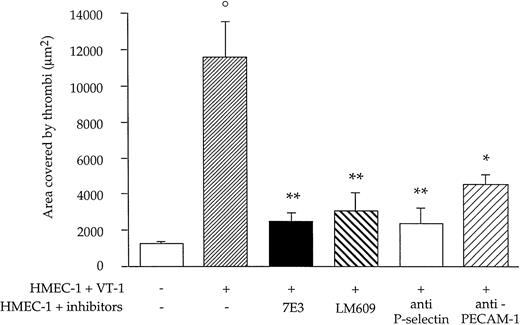
Because it is known that vWF, besides binding extracellular matrix proteins, can interact with endothelial β3-integrin subunit via RGD sequence, we assessed the role of β3-integrin and more specifically of αvβ3 in the thrombotic process induced by VT-1 in HMEC-1. As shown in Figure 7, blocking of β3-integrin subunit by 7E3 or inhibition of αvβ3-integrin complex by LM609 both resulted in a significant (P < .01) reduction of the area covered by thrombi (VT-1 + 7E3: 2477 ± 515 μm2; VT-1 + LM609: 3096 ± 972 μm2 versus VT-1: 11 609 ± 1961 μm2).
Effect of functional blockade of endothelial adhesion molecules on VT-1–induced thrombus formation on HMEC-1.
Cells pretreated with VT-1 (10 pM, 24 hours) were incubated with the following adhesion blocking antibodies: anti-β3-integrin subunit (7E3 Fab 20 μg/mL for 20 minutes), anti-αvβ3 (LM609 10 μg/mL for 10 minutes), anti-P-selectin (50 μg/mL for 20 minutes), and anti-PECAM-1 (25 μg/mL for 10 minutes) before blood perfusion at 60 dynes/cm2. Data are expressed as mean ± SE (n = 6 experiments). °P < .01 versus untreated cells. *P < .05, **P < .01 versus VT-1.
Effect of functional blockade of endothelial adhesion molecules on VT-1–induced thrombus formation on HMEC-1.
Cells pretreated with VT-1 (10 pM, 24 hours) were incubated with the following adhesion blocking antibodies: anti-β3-integrin subunit (7E3 Fab 20 μg/mL for 20 minutes), anti-αvβ3 (LM609 10 μg/mL for 10 minutes), anti-P-selectin (50 μg/mL for 20 minutes), and anti-PECAM-1 (25 μg/mL for 10 minutes) before blood perfusion at 60 dynes/cm2. Data are expressed as mean ± SE (n = 6 experiments). °P < .01 versus untreated cells. *P < .05, **P < .01 versus VT-1.
To investigate endothelial adhesive proteins that can directly interact with platelet receptors, HMEC-1 preincubated for 24 hours with VT-1 were exposed to anti-P-selectin and anti-PECAM-1 antibodies. Anti-P-selectin antibody almost completely (P < .01) prevented platelet adhesion (VT-1 + anti-P selectin: 2386 ± 826 μm2). Blocking of PECAM-1 had a less pronounced but still significant (P < .05) inhibitory effect on VT-1–induced thrombus formation with the area covered by thrombi averaging 4553 ± 532 μm2 (Figure 7). Irrelevant antibody did not significantly modify VT-1–induced platelet deposition (percent of reduction in area covered by thrombi compared with VT-1 alone: 10 μg/mL, 2%; 25 μg/mL, 9%; and 50 μg/mL, 15%).
The involvement of P-selectin and PECAM-1 in platelet deposition elicited by VT-1 was also confirmed on primary microvascular endothelial cells by using functional blocking antibodies that reduced by 79% ± 7% and 86% ± 4%, respectively, the area covered by thrombi (P < .01 versus VT-1).
Endothelial adhesive proteins involved in VT-1–induced thrombus formation
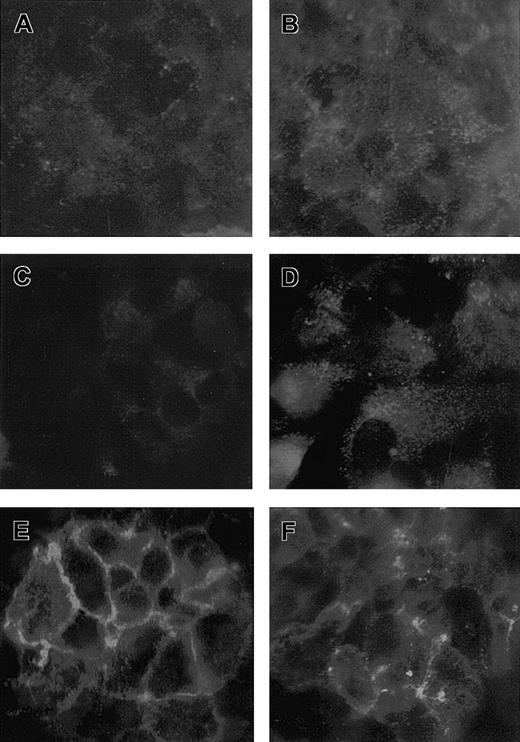
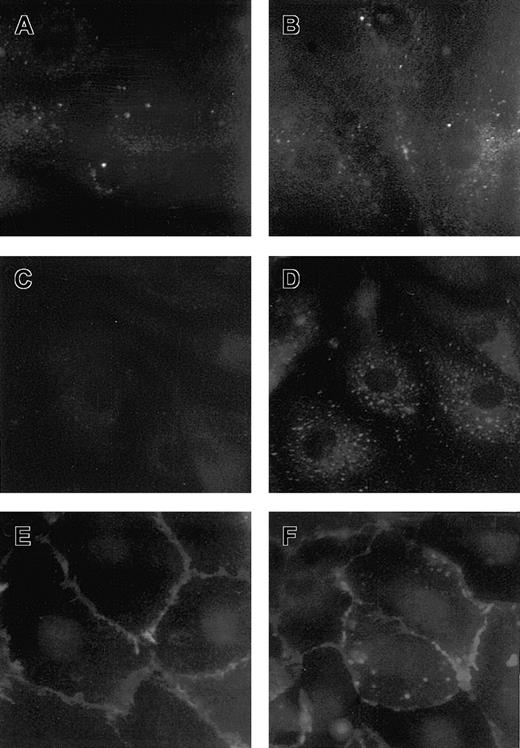
We characterized by confocal fluorescence microscopy the distribution on the endothelial surface of the adhesive molecules found in the above experiments to be implicated in the thrombotic process induced by VT-1. As shown in Figure 8A,B, HMEC-1 treated with VT-1 (10 pM, 24 hours) exhibited an increased expression of vitronectin receptor, as small diffuse granules on the luminal surface, in comparison to unstimulated cells.
Expression of adhesive molecules in HMEC-1 treated with VT-1.
Micrographs of HMEC-1 treated with control medium (A,C,E) or VT-1 (10 pM, 24 hours; B,D,F) and stained for vitronectin receptor αvβ3 (A,B), P-selectin (C,D), and PECAM-1 (E,F). HMEC-1 exposed to VT-1 showed an increased surface expression of vitronectin receptor and P-selectin as a diffuse granular pattern. PECAM-1 appeared redistributed away from the cell junctions after VT-1 treatment (n = 3 experiments).
Expression of adhesive molecules in HMEC-1 treated with VT-1.
Micrographs of HMEC-1 treated with control medium (A,C,E) or VT-1 (10 pM, 24 hours; B,D,F) and stained for vitronectin receptor αvβ3 (A,B), P-selectin (C,D), and PECAM-1 (E,F). HMEC-1 exposed to VT-1 showed an increased surface expression of vitronectin receptor and P-selectin as a diffuse granular pattern. PECAM-1 appeared redistributed away from the cell junctions after VT-1 treatment (n = 3 experiments).
The HMEC-1 cell line in a resting condition did not stain for P-selectin on the apical surface (Figure 8C). In contrast, on VT-1 challenge a strong fluorescence was observed with the P-selectin staining pattern of granules distributed on the apical side (Figure 8D).
PECAM-1 localized to the cell-cell border of adjacent unstimulated HMEC-1 as a linear staining (Figure 8E). After treatment with VT-1 PECAM-1 redistributed away from intercellular junctions and formed irregular patches of staining along the periphery of the cell or diffuse granules on the luminal surface and/or at the intracellular level (Figure 8F).
By studying the effect of VT-1 on the expression of these adhesive proteins in primary HDMECs we observed a distribution similar to that of the HMEC-1 line (Figure 9A-F).
Expression of adhesive molecules in HDMECs treated with VT-1.
Micrographs of HDMEC treated with control medium (A,C,E) or VT-1 (10 pM, 24 hours; B,D,F) and stained for vitronectin receptor αvβ3 (A,B), P-selectin (C,D), and PECAM-1 (E,F). HDMEC exposed to VT-1 showed an increased surface expression of vitronectin receptor and P-selectin as a diffuse granular pattern. PECAM-1 appeared redistributed away from the cell-cell border and irregular patches of staining were evident along the periphery of the cells after VT-1 treatment (n = 3 experiments).
Expression of adhesive molecules in HDMECs treated with VT-1.
Micrographs of HDMEC treated with control medium (A,C,E) or VT-1 (10 pM, 24 hours; B,D,F) and stained for vitronectin receptor αvβ3 (A,B), P-selectin (C,D), and PECAM-1 (E,F). HDMEC exposed to VT-1 showed an increased surface expression of vitronectin receptor and P-selectin as a diffuse granular pattern. PECAM-1 appeared redistributed away from the cell-cell border and irregular patches of staining were evident along the periphery of the cells after VT-1 treatment (n = 3 experiments).
Discussion
Verotoxin-producing E coli, the causative agent of D+HUS, activates endothelial cells to acquire a prothrombotic phenotype with corresponding lesions confined to microvessels mostly of renal glomeruli.1-4
In this report we show for the first time that VT-1 directly induces platelet adhesion and thrombus formation on cultured endothelial cells perfused with whole blood in a flow chamber system under shear stress levels high enough to mimic the ones encountered in the microcirculation. The effect of VT-1 was superior to that of other known thrombogenic agonists such as thrombin and cytokines.
The area occupied by thrombi was more pronounced on VT-1–treated endothelial cells of microvascular (HMEC-1) in comparison with large-vessel (HUVEC) origin. The HMEC-1 line had a similar sensitivity to the thrombogenic effect of VT-1 as HDMECs.
That microvascular endothelium is indeed more susceptible to the prothrombotic activity of VT-1 is consistent with previous findings. Thus, renal microvascular endothelial cell viability, as well as their protein synthesis capacity, were reduced by VT concentrations that were instead not cytotoxic for HUVECs.7 Basal Gb3 levels in renal microvascular endothelial cells were 50-fold higher than in HUVECs, which suggested a relationship between the degree of VT sensitivity and the amount of Gb3 receptor expressed by these cells.7 Similar to renal microvascular endothelial cells, we found that HMEC-1 expressed about 20-fold more VT-1 receptors than HUVECs, which might account for the different sensitivity of different vascular beds to VT-mediated disease.
In the attempt to identify the adhesive proteins involved in platelet-endothelial cell interactions elicited by VT-1, which eventually resulted in thrombus formation on HMEC-1, we first focused on vWF, which is the indispensable adhesive substrate to promote platelet thrombus formation in high shear stress environments.15 We found that ATA, an inhibitor of vWF-platelet GPIb interaction, completely prevented the deposition of thrombi. Furthermore, blockade of αIIbβ3 on activated platelets by chimeric 7E3 Fab also abrogated platelet adhesion and thrombus formation. These observations extend to our experimental condition what has been documented in other settings,15 35 that is, at high shear stress the mechanism supporting platelet adhesion and thrombus formation requires binding of platelet GPIb to vWF. The engagement of this receptor then promotes activation of platelet receptor αIIbβ3 that mediates irreversible adhesion by interacting with the RGD sequence of vWF. Therefore, the inhibition of one of these steps with ATA or 7E3 would result in a complete blocking of thrombus formation.
It has been widely described that interaction of vWF with platelet GPIb/αIIbβ3 is instrumental in mediating platelet adhesion to subendothelial matrix at high shear rates.15 Here, we provide a series of observations indicating that vWF-mediated platelet adhesion on VT-1 challenge occurred mainly on the endothelial surface rather than in the subendothelium. First, we have verified by light microscopy that the integrity of the endothelial layer was still preserved after blood perfusion at high shear stress. Moreover, based on the evidence that vWF via the RGD sequence17 binds vitronectin receptor (αvβ3), the major integrin expressed on endothelial cells,16 we have documented by confocal fluorescence microscopy that vitronectin receptors were up-regulated and/or redistributed on the apical aspect of HMEC-1 after VT-1 exposure. Finally, functional blocking of endothelial vitronectin receptor αvβ3 with LM609 almost completely abrogated thrombus formation. Altogether these data indicate that VT-1 alters endothelial thromboresistance by inducing changes in the surface expression of vitronectin receptor that leads to platelet deposition via a vWF-dependent bridging mechanism. These findings are in line with recent studies showing that prothrombotic mediators such as α-thrombin and IL-1β induced on the luminal surface of endothelial cells up-regulation of vitronectin receptors that in turn promoted platelet adhesion through an RGD-dependent pathway.36
Our finding that treatment of endothelial cells with anti-GPIb antibody did not inhibit thrombus formation induced by VT-1 suggested that GPIb expressed on endothelium is not engaged in the interaction with soluble vWF in a high shear stress environment.
The endothelial adhesive molecules P-selectin20 and PECAM-123 have been involved in the process of platelet deposition on activated or damaged endothelium by their direct binding to platelets.21,24,37 Thus, it has been shown by intravital fluorescence microscopy that ischemia/reperfusion injury caused overexpression of P-selectin on intestinal microvascular endothelial cells, in association with platelet rolling and adhesion.37 Antibodies against P-selectin significantly reduced microvascular thrombosis, which implied a direct role of this endothelial adhesive molecule in platelet deposition under flow conditions.37 We have found that inhibition of P-selectin with a specific antibody caused a significant decrease in VT-induced thrombus formation on HMEC-1. These data, along with our observation of a strong expression of P-selectin on the apical surface of HMEC-1 after VT-1 challenge, provide evidence for the involvement of P-selectin in the thrombotic process elicited by VT-1 at high shear stress.
As for endothelial PECAM-1, its contribution to platelet deposition was proved in a model of laser-induced endothelial injury in mouse brain arterioles by the observation that anti–PECAM-1 antibody reduced microvascular thrombosis over damaged but not denuded endothelium.24 Our present study showed that functional blocking of PECAM-1 resulted in a significant reduction of the area covered by thrombi in HMEC-1 exposed to VT-1. In addition, confocal microscopy experiments revealed that VT-1 induced a redistribution of this protein away from cell junctions, a pattern similar to that described in human endothelial cells after cytokine stimulation.38 We speculate that PECAM-1 once redistributed on the endothelial surface may undergo phosphorylation,23 which would render this adhesive receptor available for platelet interaction.
In primary microvascular endothelial cells treated with VT-1, expression of vitronectin receptor, P-selectin, and PECAM-1 was similar to that observed in the HMEC-1 line. Moreover, as in HMEC-1, blockade of P-selectin and PECAM-1 by specific antibodies markedly limited VT-1–induced thrombus formation, thus suggesting that thrombotic response elicited by VT-1 involved activation of the same endothelial adhesive proteins in both line and primary microvascular endothelial cells.
In conclusion, our results indicate for the first time that (1) VT-1 is a potent promoter of platelet adhesion and thrombus formation on endothelial cells under high shear stress; (2) microvascular endothelial cells demonstrate a remarkably greater sensitivity to the thrombogenic effect of VT-1 than endothelium derived from large vessels, possibly due to the higher expression of VT-1 receptor; (3) at high shear stress interaction of vWF with platelet GPIb/αIIbβ3 supports VT-1–induced platelet deposition through the binding to vitronectin receptor on the endothelial luminal surface; and (4) up-regulation and/or redistribution of endothelial vitronectin receptors, P-selectin, and PECAM-1 appear instrumental in the process of thrombus formation induced by VT-1.
These findings might help to clarify why thrombi in HUS preferentially localize in microvessels and provide insights on the determinants possibly involved in the process of microvascular thrombosis associated with D+HUS.
We are indebted to Prof Giuseppe Silva and Piero Pellini for helpful cooperation during scanning electron microscopy evaluations (Politecnico di Milano, Italy). We thank Dr Anna Falanga (Unit of Hematology, Azienda Ospedaliera, Ospedali Riuniti di Bergamo, Italy) for kind cooperation, and Stefania Angioletti, Chiara Rossi, and Federica Casiraghi for technical assistance.
E.B. is a recipient of a fellowship from “Foppolo aiuta i bambini.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marina Morigi, Mario Negri Institute for Pharmacological Research, Via Gavazzeni 11, 24125 Bergamo, Italy; e-mail: morigi@marionegri.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal