To prevent the development of acute graft-versus-host disease (GVHD) in lethally irradiated C57BL/6 (H-2b) recipient mice transplanted with bone marrow–splenocyte grafts from major histocompatibility complex (MHC) disparate BALB/c mice (H-2d), recipient mice were treated with the rationally designed JAK3 inhibitor WHI-P131 [4-(4′-hydroxyphenyl)-amino-6,7-dimethoxyquinazoline] (20 mg/kg, 3 times a day [tid]) daily from the day of bone marrow transplantation (BMT) until the end of the 85-day observation period. Total body irradiation (TBI)-conditioned, vehicle-treated control C57BL/6 mice (n = 38) receiving bone marrow–splenocyte grafts from BALB/c mice survived acute TBI toxicity, but they all developed histologically confirmed severe multi-organ GVHD and died after a median survival time of 37 days. WHI-P131 treatment (20 mg/kg intraperitoneally, tid) prolonged the median survival time of the BMT recipients to 56 days. The probability of survival at 2 months after BMT was 11% ± 5% for vehicle-treated control mice (n = 38) and 41% ± 9% for mice treated with WHI-P131 (n = 32) (P < .0001). Notably, the combination regimen WHI-P131 plus the standard anti-GVHD drug methotrexate (MTX) (10 mg/m2 per day) was more effective than WHI-P131 or MTX alone. More than half the C57BL/6 recipients receiving this most effective GVHD prophylaxis remained alive and healthy throughout the 85-day observation period, with a cumulative survival probability of 70% ± 10%. Taken together, these results indicate that targeting JAK3 in alloreactive donor lymphocytes with a chemical inhibitor such as WHI-P131 may attenuate the severity of GVHD after BMT.
Introduction
Graft-versus-host disease (GVHD), a donor T-cell–initiated highly complex pathologic condition that frequently follows allogeneic bone marrow transplantation (BMT), especially in the context of a major human leukocyte antigen (HLA) disparity, is associated with significant morbidity and mortality.1Severe GVHD remains a major obstacle to a more successful outcome of allogeneic BMT using HLA-matched unrelated donors and partially HLA-mismatched related donors.2-4 Contemporary methods for GVHD prophylaxis, including ex vivo T-cell depletion of marrow grafts,5-7 use of positively selected CD34+hematopoietic precursor cells,8 and systemic immunosuppression,4 are associated with an increased risk for relapse in patients with leukemia undergoing BMT.9 Therefore, dual-function anti-GVHD agents with potent antileukemic activity are urgently needed for effective prevention of GVHD after BMT without facilitating the recurrence of leukemia.
Signal transducers and activators of transcription (STAT) are pleiotropic transcription factors that mediate cytokine-stimulated gene expression in multiple cell populations.10,11 All STAT proteins contain a DNA-binding domain, a Src homology 2 (SH2) domain, and a transactivation domain necessary for transcriptional activation of target gene expression. Janus kinases (JAK)—including JAK1, JAK2, Tyk, and JAK3—are cytoplasmic protein tyrosine kinases (PTK) that play pivotal roles in the initiation of cytokine-triggered signaling events by activating the cytoplasmic latent forms of STAT proteins through tyrosine phosphorylation on a specific tyrosine residue near the SH2 domain.12 Tyrosine-phosphorylated STAT proteins dimerize through specific reciprocal SH2-phosphotyrosine interactions and translocate from the cytoplasm to the nucleus, where they stimulate the transcription of specific target genes by binding to response elements in their promoters.13 Among the 4 members of the JAK family, JAK3 is abundantly expressed in lymphoid cells and plays an important role in normal lymphocyte development and function, as evidenced by qualitative and quantitative deficiencies in the B-cell and T-cell compartments of the immune system of JAK3-deficient mice14,15 and the development of severe combined immunodeficiency in JAK3-deficient patients.16,17 JAK3 is expressed at very high levels in human lymphoblastic and myeloblastic leukemia cells, and several studies have correlated constitutive STAT activation in human leukemia cells with resistance to apoptosis-inducing chemotherapeutic agents.18 19
We recently reported the structure-based design of specific inhibitors of JAK3 as apoptosis-inducing antileukemic agents.20 The lead compound, 4-(4′-hydroxyphenyl)-amino-6,7-dimethoxyquinazoline (WHI-P131), inhibited JAK3 but not JAK1 or JAK2.20Similarly, the ZAP/SYK family tyrosine kinase SYK, TEC family tyrosine kinase BTK, SRC family tyrosine kinase LYN, and receptor family tyrosine kinase IRK were not inhibited by WHI-P131. The use of this compound in biologic assays confirmed that JAK3 is a vital target in human leukemia cells and demonstrated that WHI-P131 triggers apoptosis in lymphoblastic and myeloblastic leukemia cells.20WHI-P131 was well tolerated by mice and monkeys, and plasma concentrations of WHI-P131 that are cytotoxic to human leukemia cells in vitro could be achieved at nontoxic dose levels.21 The antileukemic activity and lack of significant systemic toxicity of WHI-P131 suggest that this JAK3 inhibitor may be useful in the treatment of patients with relapsed or therapy-refractive leukemia.
The pivotal role of JAK3 in normal lymphocyte development and function, along with the cytotoxic effects of WHI-P131 on leukemia cells, prompted the hypothesis that this JAK3 inhibitor could be useful as a dual-function anti-GVHD agent with potent antileukemic activity. The purpose of the present study was to examine the effectiveness of targeting JAK3 with WHI-P131 for the prevention and treatment of lethal GVHD across the major histocompatibility (MHC) barrier in mice. Here, we show that WHI-P131 attenuates the severity of GVHD in an acute GVHD model using BALB/c (H-2d) donor bone marrow–spleen cells and H-2 disparate C57BL/6 (H-2b) recipient mice.
Materials and methods
C57BL/6 and BALB/c mice
Eight- to 10-week-old C57BL/6 (H-2b) male mice and 6- to 8-week-old BALB/c (H-2d) male mice were purchased from Taconic (Germantown, NY). Mice were housed in a controlled environment (12-hour light/12-hour dark photoperiod, 22 ± 1°C, 60% ± 10% relative humidity) fully accredited by the United States Department of Agriculture. All husbandry and experimental contact made with the mice maintained specific pathogen-free conditions. All mice were kept in Micro-Isolator cages (Lab Products, Maywood, NY) containing autoclaved food (Harlan Teklad LM-485, Madison, WI), water, and bedding. Animal studies were approved by the Parker Hughes Institute Animal Care and Use Committee, and all animal care procedures conformed to the Principles of Laboratory Animal Care (National Institutes of Health publication #85-23, revised 1985).
Mitogen stimulation assays
Splenocytes (4 × 106/mL RPMI 1640 medium, supplemented by 10% fetal calf serum) from 9-week-old C57BL/6 males were used as responders in phytohemagglutinin (PHA)-or concanavalin A (ConA)-induced T-cell stimulation–proliferation assays.22Cells (2 × 105/200 μL final volume/sample) in triplicate wells of 96-well microplates were stimulated with PHA in the presence or absence of WHI-P131. PHA (Sigma, St Louis, MO) was used at a final concentration of 5 μg/mL. ConA (Sigma, St Louis, MO) was used at a final concentration of 2 μg/mL. WHI-P131 was used at the final concentrations of 0.1, 1, 10, and 50 μg/mL. Controls were treated with vehicle alone without any added WHI-P131. Cells were cultured at 37°C for 3 days in a humidified 5% CO2 atmosphere, and their mitogenic response was examined using a colorimetric assay for the quantification of cell proliferation, based on the cleavage of the tetrazolium salt WST-1 (Boehringer Mannheim, Indianapolis, IN) by mitochondrial dehydrogenases in viable cells. Absorbance at 450/690 nm was measured using a Multiskan MS microplate scanner. Optical density (OD) values of the nonstimulated samples were used for comparison as baseline controls.
Mixed lymphocyte reaction
We used a one-way mixed lymphocyte reaction (MLR) assay to measure the in vitro responses of mouse splenocytes to alloantigen. Single-cell suspensions of splenocytes obtained from 9-week-old C57BL/6 males were used as responder cells, and mitomycin-treated splenocytes obtained from 10- to 12-week-old BALB/c males were used as stimulators. Responders were plated in triplicate in 96-well plates (4 × 105/100 μL/sample). Stimulators (8 × 104 in 50 μL) were added to each well. WHI-P131 (50 μL) was added to the wells to yield final concentrations of 0.1, 1, 10, and 50 μg/mL. Controls were treated with vehicle alone without any added WHI-P131. Cells were cultured for 5 days, and a colorimetric WST-1 assay was performed as described above for the mitogen response studies.
Pretransplantation total body irradiation
For pretransplantation conditioning, recipient C57BL/6 and BALB/c mice, positioned in a pie-shaped Lucite holder (Braintree Scientific, Boston, MA), underwent total body irradiation (TBI) (7.5 Gy and 6.0 Gy, respectively) 1 day before bone marrow transplantation, which was delivered by a Cesium Instrument (JL Sheppard Labs, San Fernando, CA; 47.08 cGy/min). Recipients were given antibiotic-supplemented water (sulfamethoxazole–trimethoprim; Hi-Tech Pharmacal, Amityville, NY) starting the day before transplantation.
Bone marrow transplantation
Donor BM was collected into RPMI 1640 medium supplemented with L-glutamine (Cellgro; Mediatech, Herndon, VA) by flushing the shafts of femurs and tibias, and cell suspensions were prepared as previously described.23 In parallel, single-cell suspensions of donor splenocytes (S) were prepared from minced spleens as a source of GVHD-causing T cells. Cells were washed and resuspended for intravenous injection through the caudal vein. The standard BM/S inoculum consisted of 25 × 106 BM cells and 25 × 106splenocytes in 0.5 mL RPMI 1640.
Graft-versus-host disease monitoring
BMT recipients were monitored daily for any clinical evidence of GVHD (weight loss, manifestations of skin erythema, allopecia, hunching, diarrhea) and survival during the 85-day observation period. Survival times were measured from the day of BMT (day 0).
Evaluation of engraftment status after BMT
Allo-engraftment was documented by flow cytometric (FACScan; Becton Dickinson, Mountain View, CA) H-2Dd typing of peripheral blood nucleated cells using fluorescein isothiocyanate–labeled anti-H-2Dd antibody (clone 34-2-12; Pharmingen, San Diego, CA), which marks BALB/c cells. Immunofluorescent staining of cells and flow cytometry were performed using standard procedures.24 25
Drug treatments
For GVHD prophylaxis, injections of WHI-P131, WHI-P132, MTX (Immunex), or vehicle control were administered to recipient mice. The JAK3-inhibitory dimethoxyquinazoline compound WHI-P13120(60 mg/kg per day in 3 divided doses) and the structurally related control dimethoxyquinazoline compound WHI-P132 [4-(2′-hydroxyphenyl)-amino-6,7-dimethoxyquinazoline]20(50 mg/kg per day in 3 divided doses), which does not inhibit JAK3, were administered daily starting on day 0. These dimethoxyquinazoline compounds were synthesized and characterized as previously described in detail.20 WHI-P131 and WHI-P132 were administered intraperitoneally in 200 μL PBS supplemented with 10% dimethyl sulfoxide as the vehicle, as previously reported.21 In some experiments, treatment with WHI-P131 and WHI-P132 was delayed until day 18. The standard anti-GVHD drug MTX (10 mg/m2 per day once daily) was used for comparison. MTX was administered intraperitoneally on days 1, 3, 6, and 11 after BMT.
Statistical analysis
Group comparisons of continuous variables were made using Student t tests. Survival data were analyzed by life-table methods. P < .05 (log-rank test) was considered significant.
Histopathologic examination of tissues
Recipient mice were electively killed at specified time points; they underwent necropsy, and tissues were removed and fixed in 10% neutral-buffered formalin. Fixed tissues were embedded in paraffin, cut into 4-μm sections, and stained with hematoxylin and eosin. All histopathology slides were blindly coded and graded by a certified veterinary pathologist (B.W.). Livers were scored positive for GVHD when there was a periportal infiltrate, lungs were scored positive when there was evidence of vasculitis with a lymphocytic infiltrate, skin was scored positive when there was single-cell necrosis, and colon was scored positive when there was single-cell necrosis or crypt dropout. After initial scoring, all slides were reviewed for GVHD grading, as described.26 Our GVHD grading system was as follows:
Liver.
Grade 0.5, focal portal lymphoid infiltrate; grade 1, widespread portal lymphoid infiltrate; grade 2, focal bile duct invasion or cellular injury; grade 3, multiple foci of bile duct injury and regeneration; grade 4, widespread injury and destruction of bile ducts.
Small and large intestines.
Grade 0.5, occasional or rare necrotic cells in glands or crypts; grade 1, multiple foci of necrotic cells in glands or crypts; grade 2, necrosis involving several crypts or glands with focal abscess formation in crypts; grade 3, widespread crypt abscesses with focal glandular destruction; grade 4, loss of mucosa with granulation tissue response.
Skin-ear.
Grade 0.5, occasional or rare single basal vacuolar necrosis; grade 1, several foci of single basal vacuolar necrosis; grade 2, contiguous single-cell necrosis or multiple necrotic cells in proximity to lymphoid infiltrates; grade 3, confluent loss of cells with cleft formation or loss of skin appendages with extensive lymphoid infiltrates; grade 4, loss of epidermis or epithelium with or without granulation tissue response.
Results
Targeting JAK3 with the chemical inhibitor WHI-P131 inhibits mitogenic and MLR responses of JAK3+/+ splenocytes from wild-type C57BL/6 mice
We hypothesized that targeting JAK3 in donor lymphocytes with a chemical inhibitor may provide an effective means to prevent severe GVHD after BMT across the MHC barrier. We first used a one-way MLR assay to measure the in vitro alloresponses ofJak3+/+ splenocytes from wild-type C57BL/6 mice (H-2b) to mitomycin-treated splenocytes from BALB/c mice (H-2d) that were used as stimulators in the presence and absence of the rationally designed JAK3 inhibitor WHI-P131.20 As shown in Figure1A, WHI-P131 inhibited the MLR responses of C57BL/6 splenocytes in a concentration-dependent fashion with a near-complete inhibition at 1 μg/mL or more. WHI-P131 also inhibited the mitogenic responses of C57BL/6 splenocytes to PHA (Figure 1B) and ConA (Figure 1C). Notably, our previous studies have demonstrated that 10- to 100-μg/mL concentrations of WHI-P131 can be achieved in mice and monkeys without significant side effects.21 Thus, these in vitro findings show that targeting JAK3 with therapeutically achievable concentrations of WHI-P131 is an effective means to inhibit the antigen and mitogen responses of T cells.
Abrogation of proliferative allo-antigen and mitogen responses of murine splenocytes by the JAK3 inhibitor WHI-P131.
The effects of WHI-P131 on allo-antigen and mitogen responses of JAK3+/+ splenocytes from WT C57BL/6 mice were examined in MLR (A), PHA (B), and ConA (C) assays, as described in “Materials and methods.” WHI-P131 was applied at concentrations of 0 (vehicle alone), 0.1, 1, 10, and 50 μg/mL during the 5-day (MLR) or 3-day culture period (PHA and ConA assays). Proliferation was measured using the colorimetric WST-1 assay. Results are presented as mean OD (± SEM) values from 3 to 6 independent experiments. Statistical differences between the WHI-P131 treatment groups and vehicle-treated control groups were analyzed by Student ttests: *P < .05; **P < .0005; and ***P < .0001.
Abrogation of proliferative allo-antigen and mitogen responses of murine splenocytes by the JAK3 inhibitor WHI-P131.
The effects of WHI-P131 on allo-antigen and mitogen responses of JAK3+/+ splenocytes from WT C57BL/6 mice were examined in MLR (A), PHA (B), and ConA (C) assays, as described in “Materials and methods.” WHI-P131 was applied at concentrations of 0 (vehicle alone), 0.1, 1, 10, and 50 μg/mL during the 5-day (MLR) or 3-day culture period (PHA and ConA assays). Proliferation was measured using the colorimetric WST-1 assay. Results are presented as mean OD (± SEM) values from 3 to 6 independent experiments. Statistical differences between the WHI-P131 treatment groups and vehicle-treated control groups were analyzed by Student ttests: *P < .05; **P < .0005; and ***P < .0001.
Targeting JAK3 with the chemical inhibitor WHI-P131 prevents fatal acute graft-versus-host disease across the major histocompatibility barrier in mice
Severe GVHD was induced in lethally irradiated (7.5 Gy TBI) C57BL/6 mice (H-2b) across the MHC barrier by injection of BM/S grafts from BALB/c mice (H-2d). Within 3 weeks in the pilot experiment, 8 of 8 control mice developed severe multiorgan GVHD associated with overt diarrhea, hunching, weight loss, and ruffled fur. Histopathologic examination of multiple organs from 5 control mice that either died or were terminated in moribund condition on or before day 30 confirmed the diagnosis of GVHD with significant liver and skin involvement (Figure 2A.1-.3). Average GVHD scores in these mice were 3.1 ± 0.2 for the liver, 2.0 ± 0.2 for the skin, 0.9 ± 0.2 for the small intestine, and 1.1 ± 0.2 for the large intestine (Table 1). By comparison, none of the 8 mice treated with the JAK3 inhibitor WHI-P131 (20 mg/kg, tid) showed signs of severe GVHD. These mice were electively killed on day 30, and histopathologic examination of their organs showed evidence of mild to moderate GVHD (Figure 2B.1-.3). Average liver and skin GVHD scores in these mice were significantly lower than those of control mice (Table 1).
Anti-GVHD activity of the JAK3 inhibitor WHI-P131.
(A.1, A.2) Histopathologic examination of the liver from the representative vehicle-treated control C57BL/6 (H-2b) mouse transplanted with a BALB/c (H-2d) BM/S graft, killed on day 30 after BMT, revealed a severe, predominantly lymphocytic, inflammatory reaction around the portal area with destruction of bile ducts. (A.3) Skin GVHD with focal pyknotic cells and vacuolation in the basal cell layer of the epidermis in skin of the same mouse. (B.1, B.2) Histopathologic examination of the liver from the representative WHI-P131–treated C57BL/6 (H-2b) mouse transplanted with a BALB/c (H-2d) BM/S graft, killed on day 30 after BMT, revealed a mild lymphocytic infiltration of the portal area without a bile duct infiltration and destruction. (B.3) Normal-appearing skin in the same mouse.
Anti-GVHD activity of the JAK3 inhibitor WHI-P131.
(A.1, A.2) Histopathologic examination of the liver from the representative vehicle-treated control C57BL/6 (H-2b) mouse transplanted with a BALB/c (H-2d) BM/S graft, killed on day 30 after BMT, revealed a severe, predominantly lymphocytic, inflammatory reaction around the portal area with destruction of bile ducts. (A.3) Skin GVHD with focal pyknotic cells and vacuolation in the basal cell layer of the epidermis in skin of the same mouse. (B.1, B.2) Histopathologic examination of the liver from the representative WHI-P131–treated C57BL/6 (H-2b) mouse transplanted with a BALB/c (H-2d) BM/S graft, killed on day 30 after BMT, revealed a mild lymphocytic infiltration of the portal area without a bile duct infiltration and destruction. (B.3) Normal-appearing skin in the same mouse.
Attenuation of liver and skin GVHD by WHI-P131 and WHI-P131 + MTX
| Group . | Time of analysis (wk) . | N . | Organ scoring (mean ± SEM) . | |||
|---|---|---|---|---|---|---|
| Liver . | Skin . | Small intestine . | Large intestine . | |||
| A. Syngeneic | 12 | 8 | 0.2 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| B. No treatment | 4-6 | 5 | 3.1 ± 0.2 | 2.0 ± 0.2 | 0.9 ± 0.2 | 1.1 ± 0.2 |
| C. Vehicle | 4-6 | 10 | 2.7 ± 0.2 | 1.7 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.2 |
| D. WHI-P131 | 4-6 | 8 | 1.6 ± 0.1*(*) | 0.9 ± 0.3*(*) | 0.8 ± 0.1 | 0.7 ± 0.1 |
| E. WHI-P131 | 12 | 3 | 0.7 ± 0.2*(*) | 0.5 ± 0.3*(*) | 0.5 ± 0.0*(*) | 0.6 ± 0.0 |
| F. WHI-P131 + MTX | 12 | 7 | 1.5 ± 0.0*(*) | 0.2 ± 0.1*(*) | 1.1 ± 0.1 | 0.8 ± 0.1 |
| Group . | Time of analysis (wk) . | N . | Organ scoring (mean ± SEM) . | |||
|---|---|---|---|---|---|---|
| Liver . | Skin . | Small intestine . | Large intestine . | |||
| A. Syngeneic | 12 | 8 | 0.2 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| B. No treatment | 4-6 | 5 | 3.1 ± 0.2 | 2.0 ± 0.2 | 0.9 ± 0.2 | 1.1 ± 0.2 |
| C. Vehicle | 4-6 | 10 | 2.7 ± 0.2 | 1.7 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.2 |
| D. WHI-P131 | 4-6 | 8 | 1.6 ± 0.1*(*) | 0.9 ± 0.3*(*) | 0.8 ± 0.1 | 0.7 ± 0.1 |
| E. WHI-P131 | 12 | 3 | 0.7 ± 0.2*(*) | 0.5 ± 0.3*(*) | 0.5 ± 0.0*(*) | 0.6 ± 0.0 |
| F. WHI-P131 + MTX | 12 | 7 | 1.5 ± 0.0*(*) | 0.2 ± 0.1*(*) | 1.1 ± 0.1 | 0.8 ± 0.1 |
Scoring was performed as described in “Materials and methods.” Statistical analysis of the differences between the WHI-P131–treated and the untreated (vehicle-treated) groups was performed by Student t test;
(*)P < .01.
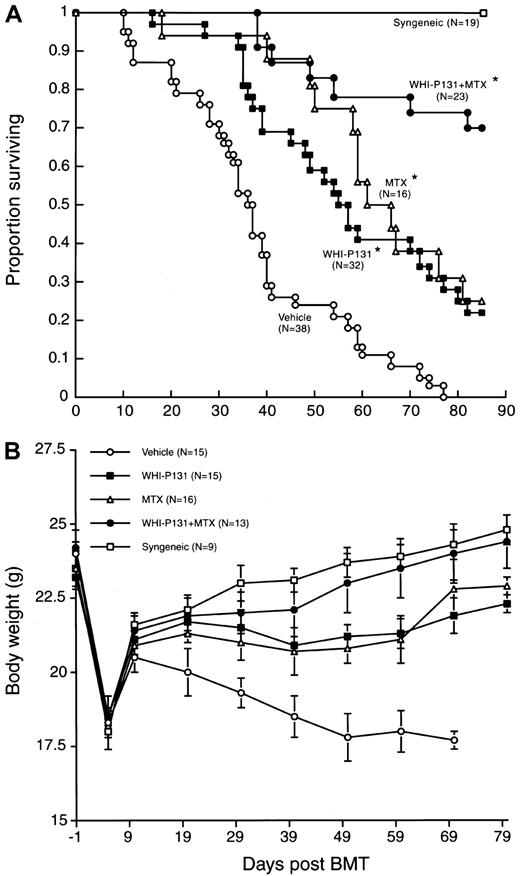
We next set out to determine the effect of WHI-P131 on post-BMT survival outcomes of mice in this murine model of GVHD. To prevent the development of fatal GVHD, recipient mice were treated with WHI-P131 (20 mg/kg, tid) every day from the day of BMT until the end of the 85-day observation period. Control mice were treated with vehicle alone. As shown in Figure 3 and Table2, all 19 lethally irradiated C57BL/6 mice injected with syngeneic BM/S grafts remained alive throughout the 85-day observation period. By comparison, the TBI-conditioned, vehicle-treated control C57BL/6 mice (n = 38) receiving BM/S grafts from BALB/c mice survived acute TBI toxicity, but within 2 to 3 weeks they all developed severe multi-organ GVHD, as clinically signaled by the development of overt diarrhea, hunching, weight loss, and ruffled fur, and they died after a median survival of 37 days (Figure 3, Table2). Histopathologic examination of multiple organs from moribund mice that were electively killed between 4 and 6 weeks after BMT (n = 10) confirmed the diagnosis of GVHD. Average GVHD scores were 2.7 ± 0.2 for the liver, 1.7 ± 0.1 for the skin, 1.1 ± 0.1 for the small intestine, and 1.2 ± 0.2 for the large intestine (Table 1). By comparison, the average GVHD scores for blindly evaluated syngeneic controls were 0.2 ± 0.1 for the liver, 0.0 ± 0.0 for the skin, 0.1 ± 0.1 for the small intestine, and 0.1 ± 0.1 for the large intestine (Table 1).
Effects of the JAK3 inhibitor WHI-P131 in combination with methotrexate on the post-BMT survival outcome in a murine model of acute GVHD.
In 2 independent experiments, irradiated (7.5 Gy) C57BL6 (H-2b) recipients were given BM and splenocytes (25 × 106 of each) from BALB/c (H-2d) mice. Some recipients were transplanted with syngeneic BM/S grafts (Syngeneic). WHI-P131 was administered intraperitoneally at a dose level of 60 mg/kg per day in 3 divided doses from day 0 to day 85. MTX was used at a dose level of 10 mg/m2 per day once daily and administered intraperitoneally on days 1, 3, 6, and 11 after BMT. *P < .0001 (vehicle controls vs WHI-P131, MTX, or WHI-P131 + MTX treatment groups, log-rank test). (A) Survival curves. See Table 2 for details of the life-table analysis. *MST of the control group B was 40 days in the first experiment involving 15 control mice and 33 days in the second experiment involving 23 control mice. The cumulative proportion of control mice surviving at 60 days was 13% ± 9% in the first experiment and 9% ± 6% in the second experiment. No control mouse was alive at 85 days in either of these 2 experiments. (B) Weight curves were obtained in the first, but not the second, experiment to minimize the handling of mice and the associated stress.
Effects of the JAK3 inhibitor WHI-P131 in combination with methotrexate on the post-BMT survival outcome in a murine model of acute GVHD.
In 2 independent experiments, irradiated (7.5 Gy) C57BL6 (H-2b) recipients were given BM and splenocytes (25 × 106 of each) from BALB/c (H-2d) mice. Some recipients were transplanted with syngeneic BM/S grafts (Syngeneic). WHI-P131 was administered intraperitoneally at a dose level of 60 mg/kg per day in 3 divided doses from day 0 to day 85. MTX was used at a dose level of 10 mg/m2 per day once daily and administered intraperitoneally on days 1, 3, 6, and 11 after BMT. *P < .0001 (vehicle controls vs WHI-P131, MTX, or WHI-P131 + MTX treatment groups, log-rank test). (A) Survival curves. See Table 2 for details of the life-table analysis. *MST of the control group B was 40 days in the first experiment involving 15 control mice and 33 days in the second experiment involving 23 control mice. The cumulative proportion of control mice surviving at 60 days was 13% ± 9% in the first experiment and 9% ± 6% in the second experiment. No control mouse was alive at 85 days in either of these 2 experiments. (B) Weight curves were obtained in the first, but not the second, experiment to minimize the handling of mice and the associated stress.
Attenuation of lethal GVHD in murine allogeneic BMT recipients by targeting JAK3 with WHI-P131
| Treatment protocol . | n . | MST (d) . | Cumulative proportion surviving (% ± SEM) . | P(Log-rank) . | |||
|---|---|---|---|---|---|---|---|
| 30 d . | 60 d . | 85 d . | vs B . | vs C . | |||
| A. TBI + syngeneic BMT | 19 | > 85 | 100 ± 0 | 100 ± 0 | 100 ± 0 | — | — |
| B. TBI + Allo-BMT (H-2d to H-2b) + vehicle* | 38 | 37 | 68 ± 8 | 11 ± 5 | 0 ± 0 | — | < .0001 |
| C. TBI + Allo-BMT (H-2d to H-2b) + WHI-P131 | 32 | 56 | 94 ± 4 | 41 ± 9 | 19 ± 7 | < .0001 | — |
| D. TBI + Allo-BMT (H-2d to H-2b) + WHI-P131 delayed treatment | 14 | 54 | 93 ± 7 | 43 ± 13 | 14 ± 9 | .007 | NS |
| E. TBI + Allo-BMT (H-2d to H-2b) + WHI-P132 | 9 | 44 | 89 ± 11 | 11 ± 11 | 0 ± 0 | NS | .02 |
| F. TBI + Allo-BMT (H-2d to H-2b) + WHI-P132 delayed treatment | 9 | 35 | 89 ± 11 | 0 ± 0 | 0 ± 0 | NS | .0002 |
| G. TBI + Allo-BMT (H-2d to H-2b) + MTX | 16 | 63 | 94 ± 6 | 56 ± 12 | 25 ± 11 | < .0001 | NS |
| H. TBI + Allo-BMT (H-2d to H-2b) + WHI-P131 + MTX2-160 | 23 | > 85 | 100 ± 0 | 78 ± 9 | 70 ± 10 | < .0001 | .0001 |
| Treatment protocol . | n . | MST (d) . | Cumulative proportion surviving (% ± SEM) . | P(Log-rank) . | |||
|---|---|---|---|---|---|---|---|
| 30 d . | 60 d . | 85 d . | vs B . | vs C . | |||
| A. TBI + syngeneic BMT | 19 | > 85 | 100 ± 0 | 100 ± 0 | 100 ± 0 | — | — |
| B. TBI + Allo-BMT (H-2d to H-2b) + vehicle* | 38 | 37 | 68 ± 8 | 11 ± 5 | 0 ± 0 | — | < .0001 |
| C. TBI + Allo-BMT (H-2d to H-2b) + WHI-P131 | 32 | 56 | 94 ± 4 | 41 ± 9 | 19 ± 7 | < .0001 | — |
| D. TBI + Allo-BMT (H-2d to H-2b) + WHI-P131 delayed treatment | 14 | 54 | 93 ± 7 | 43 ± 13 | 14 ± 9 | .007 | NS |
| E. TBI + Allo-BMT (H-2d to H-2b) + WHI-P132 | 9 | 44 | 89 ± 11 | 11 ± 11 | 0 ± 0 | NS | .02 |
| F. TBI + Allo-BMT (H-2d to H-2b) + WHI-P132 delayed treatment | 9 | 35 | 89 ± 11 | 0 ± 0 | 0 ± 0 | NS | .0002 |
| G. TBI + Allo-BMT (H-2d to H-2b) + MTX | 16 | 63 | 94 ± 6 | 56 ± 12 | 25 ± 11 | < .0001 | NS |
| H. TBI + Allo-BMT (H-2d to H-2b) + WHI-P131 + MTX2-160 | 23 | > 85 | 100 ± 0 | 78 ± 9 | 70 ± 10 | < .0001 | .0001 |
In 2 independent experiments, summarized in this table, C57BL/6 (H-2b) recipients were lethally irradiated (TBI, 7.5 Gy) and transplanted with BM/S grafts from MHC-disparate BALB/c (H-2d) mice and subjected to the treatment regimens presented above (the details of these treatment regimens are given in “Materials and methods)”;
P = .009 compared to group G; statistically significant differences obtained by life table analysis (log-rank test).
MST of control group B was 40 days in the first experiment involving 15 control mice and 33 days in the second experiment involving 23 control mice. The cumulative proportion of control mice surviving at 60 days was 13% ± 9% in the first experiment and 9% ± 6% in the second experiment. No control mouse was alive at 85 days in either of these two experiments. The weight curves were obtained in the first but not the second experiment to minimize the handling of mice and the associated stress.
Notably, WHI-P131 treatment significantly improved the survival of BMT recipients (P < .0001, log-rank test) and prolonged the median survival time (MST) to 56 days (Table 2). The probability of survival at 2 months after BMT was 11% ± 5% for vehicle-treated control mice and 41% ± 9% for mice treated with WHI-P131 (Figure 3, Table 2). Unlike control mice that showed progressive weight loss and clinical signs of severe GVHD only until the termination of the experiment, WHI-P131–treated mice had progressive weight loss after recovery from the acute TBI toxicity (Figure 3B).
The standard anti-GVHD drug MTX (10 mg/m2 per day once daily) was used for comparison. WHI-P131 was comparable to MTX in its efficacy to improve the survival outcome after BMT (Table 2). Weight curves of WHI-P131–treated and MTX-treated mice were superimposable and better than the weight curves of vehicle-treated allograft recipients but worse than the weight curves of mice undergoing syngeneic BMT (Figure 3B).
Histopathologic examination of the organs from the WHI-P131–treated long-term survivors (n = 3) electively killed on day 85 after BMT revealed evidence for subclinical mild GVHD involving the liver, small and large intestines, and skin. According to the scoring system, GVHD grades were 0.7 ± 0.2 for the liver, 0.5 ± 0.0 for the small intestine, 0.6 ± 0.0 for the large intestine, and 0.5 ± 0.3 for the skin (Table 1).
We next asked whether WHI-P131 could still improve the survival outcome if the therapy was delayed until the third week (day 18) after BMT. As shown in Table 2, this treatment also resulted in significant improvement of survival, with a 43% ± 13% probability of survival at 2 months and a median survival time of 54 days. In contrast to WHI-P131, the structurally related control dimethoxyquinazoline compound WHI-P132, which does not inhibit JAK3, did not improve survival after BMT even when its administration began on the day of BMT (Table 2). Taken together, these results indicate that targeting JAK3 in alloreactive donor lymphocytes with a chemical inhibitor such as WHI-P131 may improve survival after BMT across the MHC barriers by decreasing the probability of fatal GVHD.
Efficacy of WHI-P131 plus methotrexate combination in prevention of fatal acute graft-versus-host disease across the major histocompatibility barrier in mice
We next sought to identify an effective GVHD prevention regimen that uses the JAK3 inhibitor WHI-P131 in combination with a standard immunosuppressive agent. As shown in Table 2 and Figure 3A, the combination regimen WHI-P131 plus MTX was more effective than WHI-P131 or MTX alone. The weight curve for this group of mice was similar to the weight curve of syngeneic controls and better than the weight curves of WHI-P131–treated or MTX-treated mice (Figure 3B). More than half the C57BL/6 recipients receiving this most effective GVHD prophylaxis remained alive and healthy throughout the 85-day observation period, with a cumulative survival probability of 70% ± 10% (Table 2, Figure 3A).
The long-term survival of WHI-P131–, MTX-, or WHI-P131 + MTX–treated mice was not attributed to poor engraftment of donor cells (Table 3). Notably, 94.5% ± 2.3% H-2Dd-positive donor cell engraftment was observed in WHI-P131–treated mice (Table 3), indicating that WHI-P131 does not prevent donor cell engraftment under these experimental conditions, and the attenuation of GVHD in WHI-P131– or WHI-P131 + MTX–treated recipient mice was not attributed to lack of donor cell engraftment with concomitant autologous recovery. At the end of the observation period, flow cytometric H-2Dd-typing of nucleated peripheral blood cells obtained from 7 representative mice in the WHI-P131 + MTX treatment group showed 97% ± 2% H-2Dd–positive donor cell engraftment (Table 3). Although these long-term surviving allograft recipient C57BL/6 mice showed no clinical evidence of GVHD, the histopathologic examination of their organs revealed evidence for subclinical mild GVHD involving the liver, small and large intestines, and skin. According to the scoring system, the histologic GVHD grades were 1.5 ± 0.0 for the liver, 1.1 ± 0.1 for the small intestine, 0.8 ± 0.1 for the large intestine, and 0.2 ± 0.1 for the skin (Table 1).
Donor cell engraftment
| Group . | n . | H-2Dd (%) . |
|---|---|---|
| Vehicle | 5 | 94.7 ± 1.4 |
| WHI-P131 | 3 | 94.5 ± 2.3 |
| MTX | 3 | 97.6 ± 1.0 |
| WHI-P131 + MTX | 7 | 97.0 ± 2.0 |
| Syngeneic controls | 3 | 0.0 ± 0.0 |
| Group . | n . | H-2Dd (%) . |
|---|---|---|
| Vehicle | 5 | 94.7 ± 1.4 |
| WHI-P131 | 3 | 94.5 ± 2.3 |
| MTX | 3 | 97.6 ± 1.0 |
| WHI-P131 + MTX | 7 | 97.0 ± 2.0 |
| Syngeneic controls | 3 | 0.0 ± 0.0 |
Vehicle-treated mice were analyzed at the time of death between 50 and 77 days. All other mice were electively killed and analyzed at the end of the 85-day observation period. Data are presented as mean ± SEM values for the percentage of H-2Dd-positive donor cells in the nucleated peripheral blood cell population.
Discussion
GVHD and its complications significantly limit the success of allogeneic and unrelated BMT for patients with acute lymphoblastic leukemia (ALL), acute myeloblastic leukemia (AML), and chronic myelocytic leukemia (CML).9,27-30 Although the prevention and treatment of GVHD are essential for a successful outcome of BMT, the absence of GVHD has been associated with increased risk for relapse in patients with leukemia (especially with CML) undergoing BMT because of the lack of graft-versus-leukemia effects against residual host leukemia cells.9 Furthermore, for certain leukemia patients undergoing BMT, especially those with high-risk ALL, recurrence of leukemia remains the major cause of failure.27,31 The most commonly used drugs for GVHD prevention are cyclosporin A (CSA) and MTX.3,32-34 Prednisone (PDN) has been widely used for the treatment of GVHD, and its use for the prevention of GVHD is still under investigation.3,32-35 Of these 3 drugs, only PDN and MTX have antileukemic activity against ALL cells, and none exhibits activity against AML or CML cells. Furthermore, front-line use of steroids and MTX in patients with ALL is associated with steroid resistance and MTX resistance at relapse.36 Therefore, the currently available drugs for GVHD prevention or treatment are not expected to have a substantial positive impact on leukemic relapse rates after BMT. Therefore, dual-function immunosuppressive agents with potent antileukemic activity are urgently needed. Such agents could reduce the risk for severe GVHD and leukemic relapse after BMT.
Our earlier studies have shown that JAK3 is a vital target in human leukemia cells, and they demonstrated that the rationally designed JAK3 inhibitor WHI-P131 triggers apoptosis in lymphoblastic and myeloblastic leukemia cells.20 WHI-P131 was well tolerated by cynomolgus monkeys, and plasma concentrations of WHI-P131 cytotoxic to human leukemia cells in vitro could be achieved in monkeys at nontoxic dose levels.21 Intriguingly, JAK3 is not required for the production of myeloid precursors in bone marrow,37 and WHI-P131 did not cause any myelosuppression in mice or monkeys.21 Antileukemic activity and lack of significant systemic toxicity of WHI-P131 suggested that this JAK3 inhibitor may be useful in the treatment of patients with relapsed or therapy-refractive leukemia. The primary purpose of the present study was to examine the effectiveness of targeting JAK3 with WHI-P131 for the prevention and treatment of lethal GVHD across the MHC barrier in mice. Here, we show that WHI-P131 exhibits potent in vivo biologic activity in an aggressive acute GVHD model using BALB/c (H-2d) donor bone marrow–spleen cells and H-2 disparate C57BL/6 (H-2b) recipient mice. TBI-conditioned, vehicle-treated control C57BL/6 mice receiving bone marrow–splenocyte grafts from BALB/c mice survived the acute TBI toxicity, but they all developed histologically confirmed severe multi-organ GVHD and died after a median survival time of 37 days. WHI-P131 treatment significantly improved the survival outcome of the BMT recipients even when the therapy was delayed until the third week after BMT. Our present study indicates that this JAK3 inhibitor could be useful as a dual-function anti-GVHD agent with potent antileukemic activity. Notably, the combination regimen WHI-P131 plus MTX (10 mg/m2 per day) was more effective than WHI-P131 alone or MTX alone. More than half the C57BL/6 recipients receiving this most effective GVHD prophylaxis remained alive throughout the 85-day observation period.
Taken together, these results indicate that targeting JAK3 in alloreactive donor lymphocytes with a chemical inhibitor such as WHI-P131 may be useful in the prevention of severe GVHD after BMT. The H-2d typing of nucleated peripheral blood cells from WHI-P131–treated mice confirmed that more than 90% of the circulating cells are of donor origin. Thus, the attenuation of GVHD in WHI-P131–treated recipient mice was not due to lack of donor cell engraftment with concomitant autologous recovery.
The effect of WHI-P131 on donor cell engraftment remains to be studied and compared with the effect of standard T-cell depletion techniques on engraftment using varying doses of bone marrow cells and T cells in the inoculum and in different conditioning regimens. Such studies are required to determine whether WHI-P131 impairs the ability of donor T cells in the graft to prevent rejection and, hence, whether additional immunosuppression would be necessary to ensure long-term engraftment in the context of WHI-P131 therapy for GVHD prophylaxis after BMT, especially when less toxic conditioning regimens or fewer donor bone marrow cells are used. Similarly, the effects of WHI-P131 on the graft-versus-leukemia function of the bone marrow allografts remains to be determined in appropriate experimental model systems.
Our future studies in GVHD models will examine whether WHI-P131 plus MTX is as effective as CSA + MTX and whether the efficacy of CSA + MTX can be enhanced by the addition of WHI-P131. The minimum duration of WHI-P131 therapy required for its anti-GVHD effects also must be studied because prolonged WHI-P131 treatment may impair the immune reconstitution after BMT.
M.C.-C. is a Michael Boyum Scholar in Bone Marrow Transplantation. F.M.U. is a Parker Hughes Chair in Oncology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Fatih M. Uckun, Parker Hughes Institute, Ste 300, 2665 Long Lake Rd, St Paul, MN 55113; e-mail: fatih_uckun@ih.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal