Several transgenic murine models for sickle cell anemia have been developed that closely reproduce the biochemical and physiological disorders in the human disease. A comprehensive characterization is described of hematologic parameters of mature red blood cells, reticulocytes, and red cell precursors in the bone marrow and spleen of a murine sickle cell model in which erythroid cells expressed exclusively human α, γ, and βS globin. Red cell survival was dramatically decreased in these anemic animals, partially compensated by considerable enhancement in erythropoietic activity. As in humans, these murine sickle cells contain a subpopulation of phosphatidylserine-exposing cells that may play a role in their premature removal. Continuous in vivo generation of this phosphatidylserine-exposing subset may have a significant impact on the pathophysiology of sickle cell disease.
Introduction
Sickle cell disease (SCD) is characterized by hemoglobin polymerization and sickling of the red blood cells (RBCs) that, in turn, result in changes in the RBC plasma membrane. One of these changes is the exposure of phosphatidylserine (PS) on the surface of a subpopulation of sickle RBCs.1,2 PS exposure on the RBC surface may lead to a prothrombotic state in patients with SCD3 and to the recognition and removal of RBCs by macrophages.4-6 This PS-induced removal could contribute to the markedly reduced RBC survival observed in sickle cell anemia.7
In contrast to the anemic state in thalassemia that is largely due to ineffective erythropoiesis (premature cell death of erythroblasts), reduced RBC survival is thought to be the major contributor to anemia in SCD. On the other hand, stress erythropoiesis in SCD may be responsible for the membrane defects found in sickle erythrocytes and reticulocytes. PS-exposing RBCs are found at all stages of the RBC life in SCD, and a significant number of (young) reticulocytes expose PS on their surface.8 Defects in erythroid cells can be expected as soon as erythroblasts with sickle hemoglobin are exposed to hypoxic conditions. Because hemoglobin synthesis begins in the early stages of erythropoiesis,9 this situation could arise during erythroid differentiation in hematopoietic organs. This defective erythropoiesis could result in premature cell death and removal of erythroblasts and in the appearance of abnormal reticulocytes in the circulation. To further study this defective erythropoiesis, PS exposure, and RBC survival, we used transgenic sickle mice developed by Paszty et al10 as a model for the human disorder.
Several transgenic murine models for sickle cell anemia have been developed that closely reproduce the biochemical and physiological disorders in the human disease (summarized in 11). Mice used in this study express only human α, γ, and βSglobin (knockout for murine globin) to overcome the inhibitory effect that murine globins have on hemoglobin polymerization. Various hematologic and erythrocytic perturbations in these mice parallel those in the human disorder, such as the presence of irreversible sickle cells (ISCs), RBC rigidity, and extensive organ damage. Thus, these mice provide a useful and realistic model for the hematologic aspects of human SCD and make it possible to investigate RBC turnover and erythroid precursor development more easily than in human patients. We report the hematologic characterization of these mice, including RBC survival studies, and compare this with an established strain of thalassemic mice.12 RBC survival studies using biotinylation and analysis by flow cytometry and fluorescence microscopy enabled us to determine the number of surviving cells as a function of time and to characterize some of the features of these prematurely aging RBCs. As in the human disease, we found that PS was exposed on a subset of mature murine sickle RBCs and on their precursors, and we present evidence that PS exposure resulted in the premature removal of these cells, which contributed to the development of anemia.
Materials and methods
Mice
The founder mice of our murine sickle colony were generated at Lawrence Berkeley National Laboratory as previously described by Paszty et al,10 and a colony was bred at the Children's Hospital Oakland Research Institute animal facility. Homozygous sickle mice expressing exclusively human sickle hemoglobin were bred from homozygous hemoglobin SS males (Tg(Hu-miniLCRα1GγAγδβS) Hba0//Hba0 Hbb0//Hbb0) that were mated with nonsickle females (Tg(Hu-miniLCRα1GγAγδβS) Hba0//Hba0 Hbb0//+). Hemoglobin typing was routinely performed by cellulose acetate gel electrophoresis of peripheral blood samples. β-Thalassemic Hbth−3/+ mice have a heterozygous deletion of both β-major and β-minor globin on one chromosome.12 This murine strain is anemic and is a good model of human β-thalassemic intermedia.
Hematologic characterization
RBC indices and reticulocyte count were derived using a prototype hematology analyzer H3 (Bayer Diagnostics, Tarrytown, NY). This system provides histograms of volume, hemoglobin content, and hemoglobin concentration of reticulocytes and mature cells. Murine blood (1 μL) was incubated at room temperature in 1 mL sphering reagent, adjusted to 330 mOsm with NaCl containing 11 μg/mL oxazine to stain RNA-containing cells, and was analyzed. Cell deformability was monitored by ektacytometry using a Technicon ektacytometer (Bayer Diagnostics), as described previously.13 Data were acquired on an Apple Macintosh (Cupertino, CA) computer for automated data analysis.
Erythrocyte labeling and flow cytometric analysis
Murine erythrocytes from tail vein or heart punctures were washed by suspension in HBSM (10 mM HEPES, 165 mM NaCl, pH 7.4) and centrifugation. PS exposure on the cell surfaces was determined by labeling with various fluorescent annexin V conjugates (see figure legends) in buffers containing 2 mM CaCl2 as described earlier.14 For flow cytometric analysis, RBCs were routinely suspended at 1 to 5 × 106 cells/mL in HBSM containing 2 mM CaCl2.
Flow cytometric analysis of the reticulocyte fraction was performed by staining of the RNA-containing subset for 30 minutes at room temperature with 50 ng/mL thiazole orange (Aldrich, Milwaukee, WI) in HBSM containing 2 mM CaCl2. This dye was combined with labeling with biotin–annexin V (40 ng/mL) and 2 μg/mL phycoerythrin (PE)–conjugated streptavidin to identify the PS-exposing reticulocytes.
Flow cytometry was carried out with a Becton Dickinson FACScalibur (4-color studies) or FACScan (Becton Dickinson, San Jose, CA), and data were analyzed using CellQuest software (Becton Dickinson). As described earlier,14 intact cells were selected by forward and side scatter parameters, and the percentage of positive intact cells was determined from the fluorescence signal in excess of that obtained with a negative (unlabeled) control for each sample.
Electron microscopy
Human sickle RBCs were obtained after informed consent from a patient with SCD (hemoglobin SS, 12.5% hemoglobin F) and washed in HBS (10 mM HEPES, 145 mM NaCl, pH 7.4). Murine sickle RBCs were washed in HBSM. Cells were suspended at 10% hematocrit in the appropriate HEPES buffers, subjected to deoxygenation under nitrogen gas for 3 minutes, and immediately fixed with 2% glutaraldehyde in 0.1 M sodium cacodylate buffer containing sucrose (pH 7.4, 350 mOsm). Control samples were fixed similarly without prior deoxygenation. Then the samples were washed in cacodylate buffer and fixed in 1% OsO4 in cacodylate buffer with sucrose, added just before use, for 30 minutes. After washing in buffer, fixed samples were dehydrated in ethanol. Cells were suspended in hexamethyldisilazane and dried on a glass coverslip. Then cells were sputter-coated with approximately 150 Å platinum and imaged in the secondary electron mode at 20 kV in an Amray 1000A scanning electron microscope (KLA Tencor, Bedford, MA).
Survival studies
RBC turnover was studied in sickle and normal mice using biotinylation of the entire RBC cohort at t = 0 and determination of the number of biotinylated cells by flow cytometry. This method provided direct measurement of the RBC replacement per day. Complete biotinylation of all RBCs was achieved by tail vein injection of 100 to 200 μL of 30 mg/mL sulfo-N-hydroxysuccinimide-biotin (sulfo-NHS-biotin; Pierce, Rockford, IL). For flow cytometric analysis, a few microliters blood was obtained by tail vein puncture, and 3 × 106 cells were labeled with 3 μg/mL PE-streptavidin in 0.25 mL HBSM. The small sample size was essential to allow for daily determinations without significant changes in circulating blood mass. Alternatively, to detect the number of PS-exposing cells in the biotinylated subpopulation, we added 75 ng/mL green fluorescence protein (GFP)– annexin V (a kind gift of Joel Ernst, University of California at San Francisco) in addition to PE-streptavidin in HBSM containing 2 mM CaCl2 and analyzed 20 000 of the labeled cells by dual-color flow cytometry.
RBC morphology studies were conducted by comparing the number of surviving (biotinylated) discocytes and ISCs with the corresponding unlabeled population. The biotinylated fraction was labeled by incubating the samples with 2μg/mL Oregon green–conjugated streptavidin (Molecular Probes, Eugene, OR) in 0.25 mL HBSM so that the surviving cells could be identified in fluorescent micrographs taken on a model TMD inverted optical microscope (Nikon, Garden City, NY). Corresponding bright-field and fluorescent image fields were obtained by switching between green (546 nm)-filtered transillumination and epi-illumination, respectively, with a filter cube in the epi-light path incorporating a 480-nm excitation filter, a 520-nm emission filter, and a 510-nm dichroic mirror. Relative percentages of ISCs and biotinylated cells were determined by counting at least 600 cells per sample in the bright-field images and identifying biotinylated ISC and discocytes in the corresponding fluorescent images.
Bone marrow analysis
Precursors were harvested from murine femora and tibia by flushing out the bone marrow in HBSGM (10 mM HEPES, 165 mM NaCl, 5 mM glucose, pH 7.4) containing 1 U/mL heparin and 1 mM EDTA. Spleen cells were suspended by gentle pressing of the tissue, and all cells were filtered through a 70-μm cell strainer.
To determine the prevalence of erythroblasts in bone marrow and spleen, we had to distinguish these cells from nonerythroid cells and from mature erythrocytes. We used multicolor flow cytometry combining the RNA dye thiazole orange, PE-conjugated Ter119 (Pharmingen, San Diego, CA), an erythroid marker, and CyChrome-conjugated CD45 (Pharmingen), a probe for early progenitors and white blood cell lineages. Bone marrow or spleen cells at 5 × 106 cells/mL were labeled with 0.32 μg/mL PE-Ter119 and 0.16 μg/mL CyChrome-CD45 in HBSGM for 30 minutes at room temperature, followed by resuspension of the cells in HBSGM containing 25 ng/mL thiazole orange less than 1 hour before analysis by flow cytometry. Intact cells were gated by selection of forward- and side-scatter parameters, and the intact Ter119-positive (erythroid) population was analyzed for the presence of RNA by comparing the thiazole orange fluorescence level to an unlabeled control.
To determine PS exposure in the erythroblasts, we used allophycocyanin-conjugated streptavidin in combination with biotin–annexin V and PE-Ter119 and thiazole orange to distinguish the immature erythroid cell population. Annexin V has been commonly used to detect PS exposure in apoptotic cells.15 Bone marrow or spleen cells at 5 × 106 cells/mL were labeled with 0.32 μg/mL PE-Ter119 in HBSGM with 2 mM CaCl2 for 30 minutes at room temperature, followed by resuspension of the cells in HBSGM containing 25 ng/mL thiazole orange and 2 mM CaCl2. In addition, 7-amino actinomycin (7 AAD, 0.6 μg/mL) was added immediately before flow cytometric analysis to exclude dead cells from the population. Intact cells were defined by forward- and side-scatter patterns, and the intact, 7-AAD–negative population was further gated for erythroid (Ter119-positive) and RNA-containing (thiazole orange-positive) events. The percentage of annexin V–binding cells in the RNA-containing erythroblast selection was determined from the fluorescence signal in excess of that obtained with unlabeled control.
Results
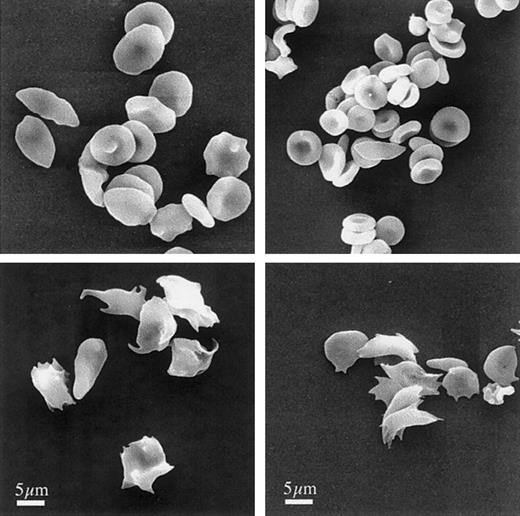
The morphology of human sickle cells and RBCs from our murine sickle model, shown in Figure 1, illustrates the similarities between human and murine sickle cells at high and low partial oxygen pressures. Although the murine RBCs are smaller than their human counterparts, the morphologic features are indistinguishable. At oxygen tensions of approximately 160 mm Hg (room air), most cells are discoid, but typical abnormal sickle RBC morphology, including the presence of ISC, is also observed in both the human and the murine samples. After deoxygenation, both human and murine RBCs exhibit markedly distorted cell shapes because of sickle hemoglobin polymerization.
Morphology of murine sickle cells mimics human sickle cells.
Human (left) and murine (right) RBCs were fixed under oxygenating (top) or during deoxygenating conditions (bottom), as described in “Materials and methods.” After processing, the cells were examined by scanning electron microscopy. Bar indicates 5 μm.
Morphology of murine sickle cells mimics human sickle cells.
Human (left) and murine (right) RBCs were fixed under oxygenating (top) or during deoxygenating conditions (bottom), as described in “Materials and methods.” After processing, the cells were examined by scanning electron microscopy. Bar indicates 5 μm.
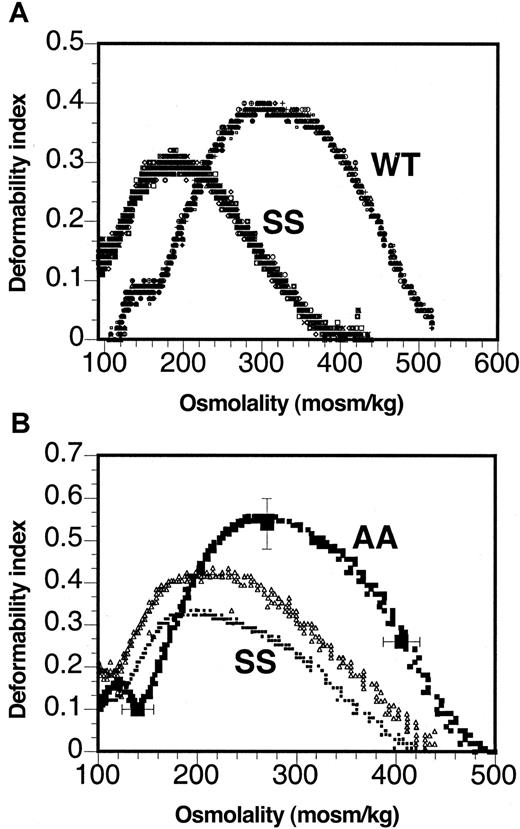
The osmotic deformability profile as determined by ektacytometry (Figure 2) was shifted to a lower osmolality range, and the maximal value of the deformability index attained was significantly lower for murine sickle RBCs than for RBCs from wild-type mice (Figure 2A). The shift to the left of the osmotic deformability profile implied that murine sickle cells exhibited decreased osmotic fragility compared to normal mouse RBC, whereas the decreased maximal deformability index implied increased dynamic rigidity of murine sickle RBCs. Similar osmotic deformability profiles were also seen with human sickle cells (Figure 2B). However, though the osmotic deformability profiles were virtually identical between different mice (the overlay of RBC from 4 different mice is shown in Figure 2A), individual differences were apparent between samples from different sickle cell patients, as illustrated by the deformability profiles of 2 patients with SCD shown in Figure 2B. It is important to note that these 2 patients were neither transfused nor treated with hydroxyurea because such treatment would have affected the osmotic deformability profile.
Cell deformability is altered in murine sickle cells.
Osmotic deformability profiles of 4 murine sickle (SS) and wild-type RBCs (WT) (A) and 2 patients with sickle cell anemia (SS) and the range of normal RBCs (AA) (B).
Cell deformability is altered in murine sickle cells.
Osmotic deformability profiles of 4 murine sickle (SS) and wild-type RBCs (WT) (A) and 2 patients with sickle cell anemia (SS) and the range of normal RBCs (AA) (B).
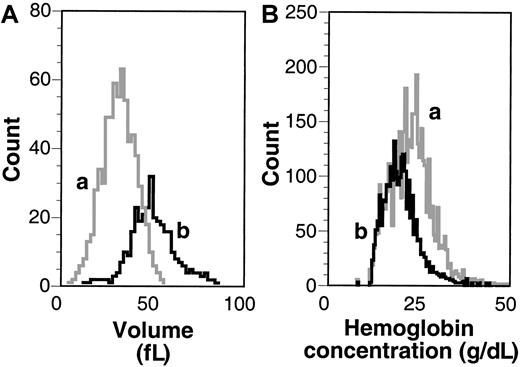
Using a prototype H3 Technicon hematology analyzer (Bayer Diagnostics), we determined the reticulocyte count and the distribution of cell volume and hemoglobin concentration of reticulocytes and mature RBCs. Figure 3shows typical volume and hemoglobin concentration histograms, and Table1 indicates the mean values of various cell parameters derived for 40 control and 22 sickle mice, confirming the earlier reported preliminary data.10 Hematocrit levels of sickle mice were significantly decreased compared to wild-type mice, and the reticulocyte counts of sickle mice were increased 10-fold compared to wild-type mice, which was confirmed by flow cytometry using thiazole orange (data not shown). We did not find a difference in the distribution of reticulocyte staining intensity between sickle and normal mice. Volume histography (Figure 3A) showed that mature sickle cells were smaller than their reticulocyte counterparts, whereas the hemoglobin concentration histogram showed that the mature RBCs had higher hemoglobin concentrations than reticulocytes. Furthermore, the broad distribution of hemoglobin concentration of mature RBC implied dehydration of sickle cells in the circulation.
MCV and MCH.
Volume (A) and hemoglobin concentration (B) histograms of RBCs and reticulocytes. Indicated are typical curves as assessed by the H3 hematology analyzer for mature sickle RBCs (a) and sickle reticulocytes (b) from the same sickle mouse.
MCV and MCH.
Volume (A) and hemoglobin concentration (B) histograms of RBCs and reticulocytes. Indicated are typical curves as assessed by the H3 hematology analyzer for mature sickle RBCs (a) and sickle reticulocytes (b) from the same sickle mouse.
Hematologic parameters of murine sickle anemia RBCs
| . | SS mice (n = 22) . | Control (n = 40) . |
|---|---|---|
| Reticulocytes (%) | 38 ± 10 | 3.4 ± 1.2 |
| Reticulocytes/mL | 2.7 × 109 | 0.3 × 109 |
| Hematocrit (%) | 30.0 ± 4.7 | 46.1 ± 0.4 |
| Reticulocytes | ||
| MCH (pg) | 9.8 ± 1.5 | 17.0 ± 2.6 |
| MCHC (g/dL) | 20.4 ± 0.7 | 27.1 ± 3.0 |
| MCV (fL) | 50.4 ± 6.7 | 66.0 ± 8.3 |
| Mature RBCs | ||
| MCH (pg) | 8.4 ± 1.3 | 14.2 ± 1.1 |
| MCHC (g/dL) | 23.6 ± 1.5 | 32.1 ± 3.6 |
| MCV (fL) | 37.5 ± 2.9 | 45.5 ± 3.0 |
| . | SS mice (n = 22) . | Control (n = 40) . |
|---|---|---|
| Reticulocytes (%) | 38 ± 10 | 3.4 ± 1.2 |
| Reticulocytes/mL | 2.7 × 109 | 0.3 × 109 |
| Hematocrit (%) | 30.0 ± 4.7 | 46.1 ± 0.4 |
| Reticulocytes | ||
| MCH (pg) | 9.8 ± 1.5 | 17.0 ± 2.6 |
| MCHC (g/dL) | 20.4 ± 0.7 | 27.1 ± 3.0 |
| MCV (fL) | 50.4 ± 6.7 | 66.0 ± 8.3 |
| Mature RBCs | ||
| MCH (pg) | 8.4 ± 1.3 | 14.2 ± 1.1 |
| MCHC (g/dL) | 23.6 ± 1.5 | 32.1 ± 3.6 |
| MCV (fL) | 37.5 ± 2.9 | 45.5 ± 3.0 |
Mean ± standard deviation given for 40 control and 22 sickle mice.
Although enhanced erythropoietic activity in sickle mice was obvious from the high reticulocyte count, additional evidence was obtained by enumerating erythroblasts in the hematopoietic organs. In contrast to humans, hematopoietic compensation for anemia in mice takes place largely in the spleen rather than in the bone marrow. We found that the sickle mouse spleens were grossly enlarged (1.2-3.0 g) compared with spleens of normal mice (0.08 g) and contained on average 16-fold more cells (n = 6) than normal spleens (n = 8). We used multicolor flow cytometry to estimate the increase in erythropoietic activity in the sickle mice by assessing the total number of erythroblasts (Table2). Erythroid cells were defined as those cells that could be labeled with Ter119, an erythroid marker that labels murine glycophorin A.16 Erythroblasts were defined as those cells that labeled with both Ter119 and thiazole orange, an RNA stain. Because the spleen was analyzed in its entirety, the total number of erythroid cells in this organ represented the total spleen-derived erythroid cells. Bone marrow was harvested from femora and tibia. Although this did not represent all bone marrow, these 2 sources accounted for most marrow-derived erythroblasts. The amount of erythroid cells was slightly increased in the sickle bone marrow, with a 1.6-fold increase in relative presence of erythroblasts over cells of other lineages. On the other hand, the sickle spleens contained on average 60-fold more immature erythroid cells than normal spleens. Average total number of immature erythroid cells was 35-fold higher in sickle cell mice, indicating a massive increase in erythropoiesis to compensate for anemia. Despite this large increase in erythroid cells, anemia persisted in these animals, implying that either peripheral RBC destruction was high in these animals or that not all erythroid cells completed their maturation cycle, or both.
Enumeration of erythroid cells in the hematopoietic organs of sickle and wild-type mice
| . | . | Total cells no. cells . | Erythroid % of total . | Erythroblasts . | |
|---|---|---|---|---|---|
| % of total . | No. cells . | ||||
| Bone marrow | WT (n = 8) | 1.0 × 108 ± 0.3 × 108 | 58 ± 8 | 30 ± 5 | 3.1 × 107 ± 0.9 × 107 |
| SS (n = 6) | 0.8 × 108 ± 0.3 × 108 | 68 ± 7 | 47 ± 9 | 3.7 × 107 ± 1.6 × 107 | |
| Spleen | WT (n = 8) | 3.8 × 108 ± 1.9 × 108 | 54 ± 10 | 13 ± 7 | 4.2 × 107 ± 1.8 × 107 |
| SS (n = 6) | 62.0 × 108 ± 29 × 108 | 88 ± 7 | 42 ± 11 | 250 × 107 ± 140 × 107 | |
| . | . | Total cells no. cells . | Erythroid % of total . | Erythroblasts . | |
|---|---|---|---|---|---|
| % of total . | No. cells . | ||||
| Bone marrow | WT (n = 8) | 1.0 × 108 ± 0.3 × 108 | 58 ± 8 | 30 ± 5 | 3.1 × 107 ± 0.9 × 107 |
| SS (n = 6) | 0.8 × 108 ± 0.3 × 108 | 68 ± 7 | 47 ± 9 | 3.7 × 107 ± 1.6 × 107 | |
| Spleen | WT (n = 8) | 3.8 × 108 ± 1.9 × 108 | 54 ± 10 | 13 ± 7 | 4.2 × 107 ± 1.8 × 107 |
| SS (n = 6) | 62.0 × 108 ± 29 × 108 | 88 ± 7 | 42 ± 11 | 250 × 107 ± 140 × 107 | |
We determined the survival times of murine sickle cells in the circulation by labeling the entire RBC cohort with biotin and monitoring the disappearance of the biotinylated cells using streptavidin labeling at several time intervals after biotinylation. Survival curves plotted in Figure 4 show that normal mouse RBC removal was linear, as reported earlier.17 The number of biotinylated cells decreased approximately 2.5% per day, with 50% surviving at 20 days and complete disappearance of all labeled cells by 42 days, indicating that on average 2.5% of the senescent RBC mass was replaced per day. In contrast, sickle RBCs (Figure 4, SS) showed a rapid exponential decline in biotinylated cells. The exponential curve was fitted to the equation A(t) = A0[1− (t/T)]e−kt,18where A(t) is the number of biotinylated cells in the RBC mass at time (t), A0 is the initial number of biotinylated cells in the RBC mass at t = 0, and T is the time of senescent death of RBC (extinction time). Data indicate a destruction rate of 45% of the cells each day and essentially complete replacement of the RBC mass within a week. Similarly, we determined the survival of RBC in β-thalassemic mice (Figure 4, β-thal). Thalassemic RBCs also demonstrated exponential removal, with both age-dependent and -independent processes. Excellent fit was found to the equation A(t) = A0[1−(t/T)]e−kt, indicating the rapid removal (k = 0.04) of a subpopulation of RBCs compared to normal. In addition, in contrast to normal RBCs, by day 20 only 25% of the RBCs of the β-thalassemic mice survived. These data indicate that some β-thalassemic cells are removed prematurely, whereas others exhibit normal survival, which is not seen with sickle cells. Taken together, our data show that the rate of RBC destruction is extremely rapid in sickle mice compared with control or thalassemic animals. Based on the rate of RBC replacement and the total number of RBCs in the circulation, effective erythropoiesis can be calculated. In normal mice, RBC replacement per day amounts to 4.6 × 108cells/d, whereas in sickle mice this replacement rate is 65 × 108 cells/d to 100 × 108 cells/d, a 14- to 22-fold increase. Because we showed that there is a 35-fold increase in immature erythroid cells in spleen and marrow, which is larger than the replacement rate, these findings may suggest some ineffective erythropoiesis. However, the spleen also contains a large percentage (45%) of peripheral blood that contains a large percentage (35%) of thiazole orange–staining reticulocytes. Therefore, the erythroblast number in the spleen (which includes these reticulocytes) may be overestimated. When the total number of erythroid cells in the spleen is corrected for the presence of these reticulocytes, erythropoiesis in sickle mice is close to 100% effective.
Reduced RBC survival in murine sickle cell anemia and thalassemia.
At t = 0, murine RBCs were biotinylated by tail vein injection of sulfo-NHS-biotin. At indicated time intervals, streptavidin labeling and flow cytometric analysis measured the number of biotinylated cells. Survival data were fitted to A(t) = A0[1−(t/T)]exp−kt, in which A(t) is the number of biotinylated RBCs at time (t), A0 is the initial number of biotinylated cells at t = 0, and T is the time of senescent death of RBCs. Data are depicted for 6 different sickle (SS) mice, 4 different β-thalassemic (β-thal) mice, and 3 different control (WT) mice.
Reduced RBC survival in murine sickle cell anemia and thalassemia.
At t = 0, murine RBCs were biotinylated by tail vein injection of sulfo-NHS-biotin. At indicated time intervals, streptavidin labeling and flow cytometric analysis measured the number of biotinylated cells. Survival data were fitted to A(t) = A0[1−(t/T)]exp−kt, in which A(t) is the number of biotinylated RBCs at time (t), A0 is the initial number of biotinylated cells at t = 0, and T is the time of senescent death of RBCs. Data are depicted for 6 different sickle (SS) mice, 4 different β-thalassemic (β-thal) mice, and 3 different control (WT) mice.
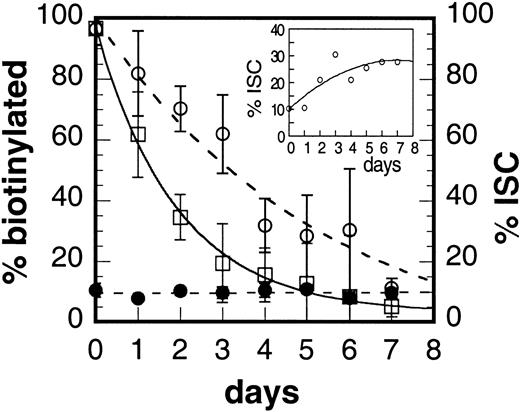
Biotinylation of the cells, in conjunction with fluorescent streptavidin labeling, provided a tag to identify the surviving fraction in flow cytometric and microscopic studies. We used fluorescence microscopy to visualize the morphology of the biotinylated cells (Figure 5) and to track the surviving populations of ISC and non-ISC relative to the overall cell count. Figure 6 shows the time-dependent reduction in the total number of fluorescent (biotinylated) cells as determined by microscopic observation (solid line). These data are in excellent agreement with the flow cytometry data shown in Figure 4. We defined ISCs as cells at least twice as long as wide (typical ISCs are indicated by arrows in Figure 5A). Total percentage of ISCs in the blood of the sickle mice was constant at approximately 9% (Figure 6, solid circles). Although the number of biotinylated ISCs decreased in time (Figure 6, open circles), the rate of this decrease was slower than the loss of all biotinylated RBCs (Figure 6, open squares). The inset in Figure 6 depicts the same data differently to indicate that the percentage of ISCs in the biotinylated RBC population increases with time. These data indicate an enrichment of ISCs in the surviving population of RBCs and suggest that the formation of ISCs is related to the extent of time that a sickle cell spends in the circulation.
Morphologic comparison of biotinylated cells.
Corresponding visual fields from bright-field (A) and fluorescent (B) micrographs of Oregon green streptavidin–labeled RBCs recorded at 3 days after biotinylation. Typical ISCs are indicated by arrows in panel A.
Morphologic comparison of biotinylated cells.
Corresponding visual fields from bright-field (A) and fluorescent (B) micrographs of Oregon green streptavidin–labeled RBCs recorded at 3 days after biotinylation. Typical ISCs are indicated by arrows in panel A.
Irreversible sickle cell formation during the lifespan of the murine sickle cell.
Percentage of fluorescent cells in the population as measured by fluorescence microscopy (□, solid line), total percentage of ISCs in the population as counted by microscopy (●), and percentage of ISCs that are fluorescent (○). Inset shows the percentage of biotinylated cells with ISC morphology.
Irreversible sickle cell formation during the lifespan of the murine sickle cell.
Percentage of fluorescent cells in the population as measured by fluorescence microscopy (□, solid line), total percentage of ISCs in the population as counted by microscopy (●), and percentage of ISCs that are fluorescent (○). Inset shows the percentage of biotinylated cells with ISC morphology.
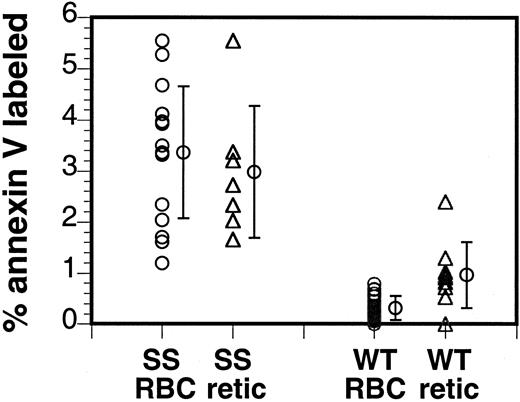
Human sickle cells show a subpopulation of RBCs that expose PS.1,2 This membrane defect is typical for SCD. Similarly, we observed the presence of a subpopulation of PS-exposing RBCs in the sickle mouse. In the human patient with SCD, the subpopulation of PS-exposing RBCs can vary from a low of 0.2% to up to 10%.1,2 In contrast, the size of this subpopulation varied less in the murine sickle cell population, with all sickle mice showing a significant increase in PS-exposing cells (4.4% ± 1.9%, n = 11) compared to control mice (0.4% ± 0.2%, n = 19; Figure7). In addition, double labeling with the RNA stain thiazole orange showed that reticulocytes bound annexin V in sickle and in control mice, indicating that PS exposure can also be a feature of immature circulating RBCs (Figure 6). Similar to the situation in human sickle cells, the PS-exposing cells form a heterogeneous population, with some cells entering the circulation already with PS exposed on the surface while others acquire this defect after entering the circulation.8
PS is exposed on a subpopulation of murine sickle cells.
Murine RBCs were labeled with fluorescent annexin V and analyzed by flow cytometry. Average of 11 sickle mouse samples (SS RBC) and 19 control samples (WT RBC) are indicated. To assess the percentage of annexin V–binding reticulocytes, the cells were double- labeled with biotin–annexin V–PE-streptavidin and thiazole orange to indicate the presence of RNA. Averages of 8 sickle mouse samples (SS retic) and 9 control samples (WT retic), subgroups of the annexin V–labeled samples, are indicated.
PS is exposed on a subpopulation of murine sickle cells.
Murine RBCs were labeled with fluorescent annexin V and analyzed by flow cytometry. Average of 11 sickle mouse samples (SS RBC) and 19 control samples (WT RBC) are indicated. To assess the percentage of annexin V–binding reticulocytes, the cells were double- labeled with biotin–annexin V–PE-streptavidin and thiazole orange to indicate the presence of RNA. Averages of 8 sickle mouse samples (SS retic) and 9 control samples (WT retic), subgroups of the annexin V–labeled samples, are indicated.
To determine a possible role of PS exposure in premature removal of the cells from the circulation, we used double-label flow cytometric analysis with fluorescent annexin V and streptavidin during survival studies of biotinylated RBCs. Annexin V labeling indicated the PS exposure on RBCs, and streptavidin labeling determined RBC survival as shown in Figure 4. Although the total number of annexin V–binding cells remained constant in time, the relative percentage of annexin V–binding cells in the biotinylated fraction decreased more rapidly with time than the total biotinylated fraction (Figure8). This indicates that PS-exposing cells are continuously formed in the sickle mouse circulation and are rapidly removed from the circulation.
Reduced RBC survival time of PS-exposing sickle cells.
Murine RBC population was biotinylated on day 0, and at the indicated time intervals, RBCs were sampled and double-labeled with PE-streptavidin and GFP–annexin V to assess the survival of PS-exposing cells. Data were fitted to A(t) = A0[1−(t/T)]exp−kt, in which A(t) is the number of biotinylated RBCs at time (t), A0 is the initial number of biotinylated cells at t = 0, and T is the time of senescent death of RBC. Data are depicted for 6 different mice; the annexin V–binding population is indicated as filled circles representing the mean ± SD, whereas the biotinylated population is depicted as open symbols representing each individual mouse. Curve fits are indicated for the total population (solid line) and the annexin V–binding population (dashed line).
Reduced RBC survival time of PS-exposing sickle cells.
Murine RBC population was biotinylated on day 0, and at the indicated time intervals, RBCs were sampled and double-labeled with PE-streptavidin and GFP–annexin V to assess the survival of PS-exposing cells. Data were fitted to A(t) = A0[1−(t/T)]exp−kt, in which A(t) is the number of biotinylated RBCs at time (t), A0 is the initial number of biotinylated cells at t = 0, and T is the time of senescent death of RBC. Data are depicted for 6 different mice; the annexin V–binding population is indicated as filled circles representing the mean ± SD, whereas the biotinylated population is depicted as open symbols representing each individual mouse. Curve fits are indicated for the total population (solid line) and the annexin V–binding population (dashed line).
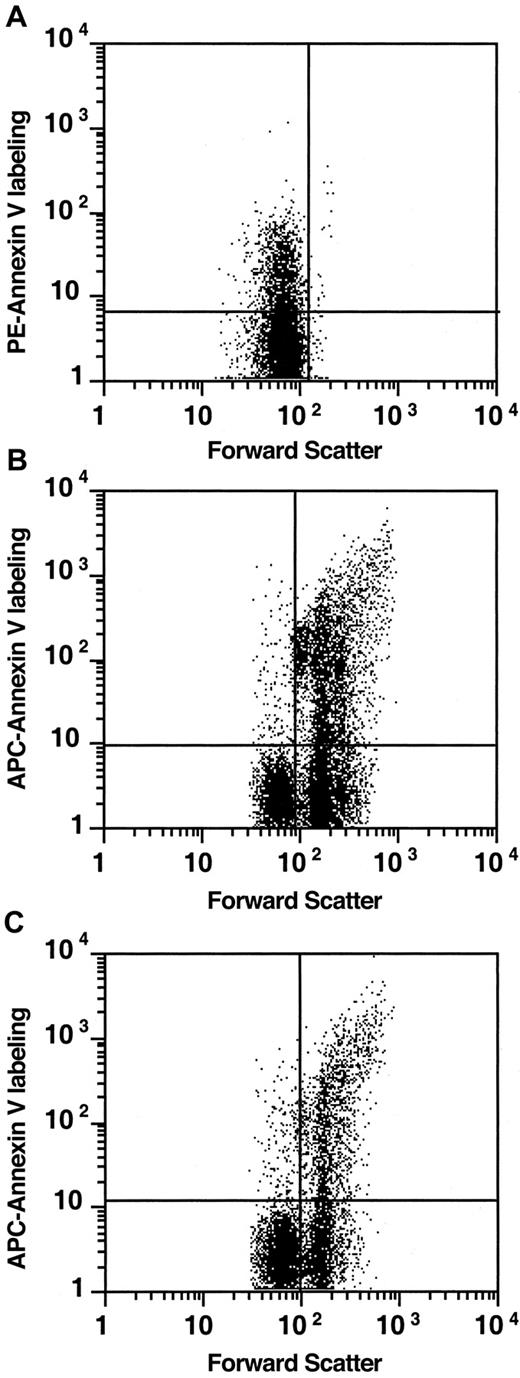
The observations that PS-exposing cells are present in reticulocytes and that they are in fact less prevalent among the old fraction of cells suggest that these cells are present as a subpopulation of the erythroblasts of marrow, spleen, or both. In Figure9, we show that such a subpopulation of annexin V–binding, PS-exposing cells is indeed present in RBC precursors of spleen and marrow. PS exposure is also recognized as an early event in apoptosis,15 and we cannot exclude that a fraction of the precursors expose PS as a result of the activation of the apoptotic cascade during harvest—ie, after release from their natural environment. However, comparison of the average percentages of annexin V–binding cells among sickle precursors (37% ± 17% in bone marrow, 31% ± 10% in spleen; n = 5) to normal precursors (18% ± 13% in bone marrow, n = 8; 16% ± 12% in spleen, n = 5) suggests that the sickle precursor population either contains a higher subpopulation of these PS-exposing cells or is more susceptible to the induction of PS exposure.
PS exposure in murine sickle cell bone marrow.
RBCs (A) were labeled with biotin–annexin V–PE-streptavidin and thiazole orange. Bone marrow (B) and spleen (C) cells were labeled with Ter119-PE, biotin–annexin V–allophycocyanin-conjugated–streptavidin, thiazole orange, and 7-AAD. Projected events were gated for intact thiazole orange staining (immature), non–7-AAD staining (nonnecrotic), erythroid (Ter119-labeled) cells. The quadrant marker was placed to distinguish between enucleated (left) and larger nucleated (right) cells and was annexin-labeled (top) from unlabeled cells (bottom). Graphs represent examples of data obtained from one mouse but typical for 5 mice.
PS exposure in murine sickle cell bone marrow.
RBCs (A) were labeled with biotin–annexin V–PE-streptavidin and thiazole orange. Bone marrow (B) and spleen (C) cells were labeled with Ter119-PE, biotin–annexin V–allophycocyanin-conjugated–streptavidin, thiazole orange, and 7-AAD. Projected events were gated for intact thiazole orange staining (immature), non–7-AAD staining (nonnecrotic), erythroid (Ter119-labeled) cells. The quadrant marker was placed to distinguish between enucleated (left) and larger nucleated (right) cells and was annexin-labeled (top) from unlabeled cells (bottom). Graphs represent examples of data obtained from one mouse but typical for 5 mice.
Discussion
Several transgenic murine models for sickle cell anemia have been developed that closely reproduce the biochemical and physiological disorders in the human disease (summarized in 11). In this study, we describe characteristics of the transgenic sickle mice developed by Paszty et al10 that express only human α, γ, and βS globin (knockout for murine globin). Various hematologic and erythrocytic perturbations in these mice parallel those in the human disorder. The animals are anemic, and murine cells exhibit similar morphology at normal- and low-oxygen tension compared with human sickle cells. Murine sickle cells show a decreased deformability and altered density distribution. RBC survival is significantly reduced, and erythropoiesis is dramatically increased to compensate for the anemic state. ISCs are continuously formed during the life of the sickle cell in the circulation. A subpopulation of sickle cells exposes PS, similar to the human sickle RBCs. Together with the reported extensive organ damage,10 our studies show that these mice provide a useful and realistic model for human SCD.
We find reduced mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) values in the mature RBC population that are distinctly different from those in the reticulocyte fraction. This indicates that the RBC are rapidly remodeled after their release into the circulation to reduce their volume by approximately 30% (from 50.4 to 37.5 fL). Previously, it was suggested that the low mean corpuscular hemoglobin concentration (MCHC), in combination with the slight imbalance in globin synthesis observed in these mice (α/β ratio of 1.26 ± 0.02),10 signifies a thalassemic phenotype that may lessen disease severity and permit survival.11 Although this may be the case, the severe sickling propensity of the cells under low oxygen and other hematologic parameters characteristic of sickle cell anemia suggest that the thalassemic features are not a dominant characteristic of the phenotype of these mice.
Access to early erythroblasts and RBC survival studies has enabled us to examine the contributions to the anemia of reduced RBC survival or ineffective erythropoiesis. Ineffective erythropoiesis is thought to play a minor role in human sickle cell anemia. A recent report describes ineffective erythropoiesis in SAD mice: transgenic mice that express human α-globin and a modified human β-globin gene that contains HbS, HbS Antilles, and HbD Punjab mutations.19Defects in erythroid cells can be expected as soon as sickle hemoglobin is formed and hypoxic conditions prevail. Hemoglobin synthesis starts during the early erythroblast stage,9 around the same time that glycophorin A is expressed.20 Ter119, an antibody widely used as a marker for murine erythroid cells, has recently been shown to bind glycophorin A,16 and we have used this probe to identify hemoglobin-containing erythroid cells at all stages of development. Combination of this probe with an RNA stain, thiazole orange, allowed us to enumerate the erythroblasts in the bone marrow and spleen (Table 2). We found enhanced erythropoietic activity in the sickle mice, and as expected in mice, this compensatory erythropoiesis mainly took place in the enlarged spleen, which is a major difference between mice and humans. However, the erythropoietic activity appeared to be unable to compensate for the anemic state of these mice (31% hematocrit level compared to 46% in normal mice), indicating either that RBC destruction must be faster than erythropoiesis or that cells do not survive to maturity in the erythropoietic organs (ineffective erythropoiesis). To address this question, we measured the RBC destruction rate directly by determination of the cells surviving after they were tagged, ie biotinylated. Although normal wild-type mouse RBCs showed a normal linear survival pattern with a 2.5% turnover per day, as reported earlier,17 sickle mice showed a rapid disappearance of biotinylated cells, with the exponential decline indicating random destruction of 45% of the RBC pool each day. This dramatic turnover rate puts a large burden on erythropoiesis, as indicated by the 35-fold increase in the number of erythroblasts. When the total number of erythroblasts in the spleen and marrow samples was corrected for the reticulocytes in these samples because of the presence of peripheral blood, it appears that the excess of precursors matches the excess of RBC turnover. In other words, ineffective erythropoiesis is not a feature of these sickle mice.
As a comparison, we determined RBC survival in a well-established strain of thalassemic mice. These β-thalassemic, Hbth−3/+, mice have a heterozygous deletion of both β-major and β-minor globin on one chromosome12 and are a good model of human β-thalassemic intermedia. The RBC survival curve in these mice was distinctly different from that in sickle and normal mice. Although some RBCs showed normal senescent survival of approximately 40 days, other cells in the population were removed prematurely. The survival curve of these mice was similar to that for other thalassemic mice reported to have ineffective erythropoiesis.21 Heterogeneity was also observed in the sickle RBC survival, yet none of the cells survived to the normal termination time.
Although erythropoiesis in the sickle mice may be mostly effective, reticulocytes show signs of defects during RBC maturation. In normal cells, PS is located exclusively in the inner lipid monolayer of the plasma membrane. In human SCD, a subpopulation of RBCs is found that exposes PS on its surface.1,2 Similarly, approximately 4% of the murine sickle cells show the same feature. Reticulocytes show a comparable percentage of PS-exposing RBCs, suggesting that these cells exposed PS before they entered the circulation. Indeed, when erythroblasts were analyzed, they also showed an increase in PS exposure. Interestingly, exposure of PS on the cell surfaces is a general feature of apoptosis,15 leading to recognition and removal by macrophages.4-6 PS exposure has been linked to ineffective erythropoiesis, or apoptosis of erythroid cells, in thalassemia.22 Although PS exposure is apparent in sickle erythroblasts, it does not seem to result in premature elimination of precursors and ineffective erythropoiesis. The machinery for recognition and removal of such cells may be simply overwhelmed by the highly increased turnout of defective erythroid cells.
The RBC survival study using biotinylation allowed us to determine the number of surviving cells as a function of time and to characterize certain features of these cells, including morphologic changes and the presence of PS on the surfaces. Analysis of the number of ISCs surviving over time shows that there is an enrichment of these cells in the older cell fraction. This indicates that the formation of ISCs is correlated with the extent of time in the circulation, which is in line with the hypothesis that these cells are formed because of repeated sickling. Our data do not allow the conclusion that ISC are removed at a faster rate than other cells. However, because reductions in membrane deformability are thought to lead to the removal of RBCs from the circulation, we cannot exclude the possibility that the formation rate of ISCs is even faster than the removal rate.
In time, the number of PS-exposing cells is constant (approximately 4%), but PS-exposing cells show a faster turnover than other cells, as indicated by the decreasing fraction of annexin V–positive cells in the biotinylated RBC population. In humans, PS-exposing cells from the dense fraction were not removed faster than the remainder of this population in 8 hours.23 However, the difference in removal rate may have gone undetected using their method. Our data suggest that PS-exposing cells are continuously formed, either before their release into the circulation or during their life in the circulation. PS-exposing cells are more rapidly removed from the circulation than other cells, which is in agreement with the reported role of PS exposure as a recognition signal and a trigger for removal by macrophages.4-6 Apoptosis could be the mechanism underlying PS exposure in precursors, but the mechanism that leads to PS exposure on enucleated, circulating sickle cells is thus far unclear. Although not addressed in our studies, it is possible that similar mechanisms responsible for ISC formation play a role in the generation of PS-exposing cells in older cell populations. It could be triggered by repeated sickling leading to alterations in the membrane that are of a similar nature, as observed in the apoptotic cascade. Hence, RBCs could enter the circulation with PS on the surface, or they could expose PS during their life. Because it is not possible to assess the formation rate of PS exposure in these 2 populations in vivo, the actual removal rate of these cells could be much higher than our data indicate. The high turnover of these cells suggests that the number of PS-exposing cells measured at a given time could reflect the steady state of a short-lived population of RBCs. The presence of the PS-exposing RBCs in sickle cell anemia could have important implications for the physiology of the disease. Recognition and removal4-6 will contribute to anemia. However, it is evident that the phagocyte system is unable to remove PS-exposing cells at the rate that they are formed. Therefore, remaining PS-exposing RBCs are continuously present in the circulation and could lead to an imbalance in hemostasis or altered cell–cell interactions. It is firmly established that PS-exposing surfaces propagate the proteolytic reactions that result in thrombin formation by promoting the assembly of coagulation factors on their surfaces.24,25 PS exposure also plays a role in the feedback inhibition of thrombin formation by activating the protein C pathway. Although these processes are essential for platelet functionality, they are not restricted to this cell type. In human sickle cell anemia, we found a correlation between the risk for stroke and the presence of PS-exposing cells3and an increase in binding of PS-exposing cells to endothelial cells.26 In addition, significant alterations in other coagulative abnormalities, such as decreased activity of protein C and S and increased presence of anti-PS antibodies,27 may result from the presence of PS-exposing cells in the circulation.
In summary, our data show that RBC survival is markedly reduced in sickle cell mice, which is in part compensated by a considerably enhanced erythropoiesis. Furthermore, we show that the PS-exposing RBCs in these mice may play a role in their premature removal from the circulation. The continuous presence of this subset of PS-exposing cells in the circulation could contribute to the hemostatic imbalance and may have a significant impact on the pathophysiology of SCD.
We thank Evelyn Clausnitzer for valuable help with the electron microscopy study and Joel Ernst for the kind gift of GFP–annexin V.
Supported by National Institutes of Health grants HL66355, DK32094, HL20985, and HL31579.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Frans A. Kuypers, Children's Hospital Oakland Research Institute, 5700 Martin Luther King Jr Way, Oakland, CA 94609; e-mail: fkuypers@chori.org.

![Fig. 4. Reduced RBC survival in murine sickle cell anemia and thalassemia. / At t = 0, murine RBCs were biotinylated by tail vein injection of sulfo-NHS-biotin. At indicated time intervals, streptavidin labeling and flow cytometric analysis measured the number of biotinylated cells. Survival data were fitted to A(t) = A0[1−(t/T)]exp−kt, in which A(t) is the number of biotinylated RBCs at time (t), A0 is the initial number of biotinylated cells at t = 0, and T is the time of senescent death of RBCs. Data are depicted for 6 different sickle (SS) mice, 4 different β-thalassemic (β-thal) mice, and 3 different control (WT) mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/5/10.1182_blood.v98.5.1577/5/m_h81711472004.jpeg?Expires=1767733249&Signature=1nRaDK80LHF5m5XlfLPohm6-zBuZ0v0qjo9WQWaPBGYSAV8Yb-IZT6MaF5nLBXQYvUc2YKGKLJSUJv~BjGPNnWdmZiINBhR33MOU4uub0rY4jMb78BCVCQCqOG5jdMkGQD6OlrUNuXM8fTNE-td4FSJ9PBby5-uVjyDTUqkW0MZ8ZYcjoZS0V8mHgJbBeEzFtIRp4d~FHpSUKYIDl-TlCrFexBzwVI9IDaH9Uzk~wJtb2V4sUIOmhqf0TLqWPs3iraorxmVuAXl~EXODOUQc6Pruf3rpA5qwMEvlGtCuW2yBqcIKUQ2KDvDnUYAUeI4AKVXb-8TEJqE5ozz6J7wEDQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 8. Reduced RBC survival time of PS-exposing sickle cells. / Murine RBC population was biotinylated on day 0, and at the indicated time intervals, RBCs were sampled and double-labeled with PE-streptavidin and GFP–annexin V to assess the survival of PS-exposing cells. Data were fitted to A(t) = A0[1−(t/T)]exp−kt, in which A(t) is the number of biotinylated RBCs at time (t), A0 is the initial number of biotinylated cells at t = 0, and T is the time of senescent death of RBC. Data are depicted for 6 different mice; the annexin V–binding population is indicated as filled circles representing the mean ± SD, whereas the biotinylated population is depicted as open symbols representing each individual mouse. Curve fits are indicated for the total population (solid line) and the annexin V–binding population (dashed line).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/5/10.1182_blood.v98.5.1577/5/m_h81711472008.jpeg?Expires=1767733249&Signature=ysKcyxU~tgvMK8rwx7NcixTWwvNon48GvutXuU25mJ8dM1KzYWKnfvM5NLmdPlYPdLfJMgpKsi6-~pmwhC5fqa~clwK3WBbEV0ARgUr3fUPmIYbprZobTTu5YaGZNttCSP-eCNYQxpATDNTvM7WGgDlvRveYE5Mu9CNAVU4v6r~E4aBvT8G5lkPNMsPPvdGqYO5THMN2KNBbQ1hWXHR2ryaTAv825uwA6m6JlwqYXWEOe~YmDYe3WEBQ7d5KZXg~StnCtZcMaX24BzZXW1Hh9c4qjI2wZZu1G8CWJQhbgZcf3JuHkduK99F24BOCt46GUD~E0FtFhHBO3FA93dboSQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal