Although it is known that dendritic cells (DCs) produce cytokines, there is little information about how cytokine synthesis is regulated during DC development. A range of cytokine mRNA/proteins was analyzed in immature (CD86−) or mature (CD86+) murine bone marrow (BM)- derived DCs. Highly purified, flow-sorted, immature DCs exhibited higher amounts of interleukin-1α (IL-1α), IL-1β, tumor necrosis factor-α (TNF-α), transforming growth factor β1 (TGF-β1), and macrophage migration inhibitory factor (MIF) mRNA/protein than mature DCs. After differentiation, DC up-regulated the levels of IL-6 and IL-15 mRNA/protein and synthesized de novo mRNA/protein for IL-12p35, IL-12p40, and IL-18. Although immature BM-derived DCs did not stimulate naive allogeneic T cells, mature DCs elicited a mixed population of T helper (Th) 1 (mainly) and Th2 cells in 3d-mixed leukocyte reactions. CD86+ BM DCs switched to different cytokine patterns according to whether they were terminally differentiated by lipopolysaccharide (LPS) or CD40 ligation. Although both stimuli increased IL-6, IL-12p40, IL-15, and TNF-α mRNA/protein levels, only LPS up-regulated transcription of IL-1α, IL-1β, IL-12p35, and MIF genes. Although LPS and CD40 cross-linking increased the T-cell allostimulatory function of BM DCs, only LPS stimulation shifted the balance of naive Th differentiation to Th1 cells, a mechanism dependent on the up-regulation of IL-12p35 and not of IL-23. These results demonstrate that, depending on the stimuli used to terminally mature BM DCs, DCs synthesize a different pattern of cytokines and exhibit distinct Th cell–driving potential.
Introduction
Myeloid dendritic cells (DCs) are crucial antigen-presenting cells (APCs) for primary T-cell responses. They arise from bone marrow (BM) precursors that colonize peripheral tissues through the blood or lymph.1,2 Tissue-resident immature DCs are excellent at internalizing and processing antigen, but they exhibit low ability to stimulate naive T cells. Exposure to allergens, bacterial (lipopolysaccharide [LPS], CpG DNA motifs) or viral (dsRNA) components, proinflammatory cytokines (interleukin-1β [IL-1β], tumor necrosis factor-α [TNF-α], interferon α [IFN-α], granulocyte-macrophage colony-stimulating factor (GM-CSF), and cognate T-cell interactions are some of the stimuli that trigger DC differentiation.1 2 The capacity of mature DCs to prime naive T lymphocytes and to promote their differentiation into different T-cell subsets is attributed to the up-regulation of surface major histocompatibility complex (MHC), costimulatory and adhesion molecules, and the ability to secrete IL-1, IL-6, IL-7, IL-12, IL-15, and IL-18.
Although the capacity of DCs to produce an ample repertoire of cytokines is documented in humans and rodents,3-27 there is little information on how cytokine genes are expressed during DC ontogeny.4 In this study, we analyzed a range of cytokine transcripts and their respective proteins in highly purified mouse BM-derived myeloid DCs (BM DCs) at different stages of cell differentiation. Immature BM DCs expressed higher levels of IL-1α, IL-1β, TNF-α, transforming growth factor β1 (TGF-β1), and macrophage migration inhibitory factor (MIF) transcripts/protein. After spontaneous differentiation in culture, the DCs up-regulated the levels of IL-6 and IL-15 mRNA and transcribed mRNA for IL-12p35, IL-12p40, and IL-18 de novo. Similar findings were found at the protein level by flow cytometry or enzyme-linked immunoabsorbent assay (ELISA).
We also investigated the changes in the cytokine repertoire of BM DCs terminally differentiated with LPS or after CD40 cross-linking. Both stimuli increased the levels of IL-6, IL-12p40, IL-15, and TNF-α transcripts/intracellular protein. However, only LPS markedly up-regulated the transcription of IL-1α, IL-1β, IL-12p35, and MIF genes. Although LPS or CD40 ligation augmented the T-cell allostimulatory capacity of DC, only LPS shifted the balance of naive T helper (Th) from a mixed Th1/Th2 population to Th1 cells, a result that agrees with the fact that only LPS was able to up-regulate the transcription of IL-12p35 in BM DCs. These results also demonstrate that, depending on the stimuli that induce the terminal differentiation of DCs, their T-cell stimulatory activity (signal 2) can be dissociated from their Th cell–driving ability (signal 3).28 It appears that one of the key factors that regulates the Th cell–driving ability of myeloid DCs is the ability of the maturation-inducing stimulus to differentially regulate the transcription of the IL-12p35 gene.
Materials and methods
Experimental animals
Ten- to 12-week-old C57BL/10 (B10; H2Kb, IAb, IE−) and C3H/He (C3H; H2Kk, IAk, IEk) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in the pathogen-free Central Animal Facility of the University of Pittsburgh Medical Center.
Reagents
Mouse (m) rGM-CSF was a gift from the Schering-Plough Research Institute (Kenilworth, NJ), and mrIL-4 was purchased from R&D Systems (Minneapolis, MN). Low endotoxin hamster IgM anti-CD40 monclonal antibody (mAb) (HM40-3), control hamster IgM, neutralizing rat anti–mouse IL-12p40/p70 mAb (C17.8), and control rat IgG were from BD Pharmingen (San Diego, CA). Neutralizing hamster anti–mouse IL-12p35 mAb (Red-T) was from Santa Cruz Biotechnology (Santa Cruz, CA). Fluorescein isothiocyanate (FITC)-dextran (MWt 42 000), FITC-bovine albumin (FITC-albumin), LPS (Escherichia coli serotype 026:B6), and brefeldin A were purchased from Sigma (St Louis, MO); 7-amino-actinomycin D (7-ADD) was purchased from Calbiochem (San Diego, CA). E1−E3−-deleted recombinant adenovirus (rAd) encoding no transgene or transpromoter (Add1434) was kindly provided by Dr A. Shaked (Department of Surgery, University of Pennsylvania, Philadelphia).29
Generation of bone marrow dendritic cells
The method for generating BM DCs was modified from that described originally by Inaba et al30 and has been described in detail.31 Briefly, BM cells were removed from femurs of B10 mice and depleted of erythrocytes by hypotonic lysis. Erythroid precursors, T and B lymphocytes, natural killer (NK) cells, granulocytes, and MHC-II+ cells were removed by complement depletion using a cocktail of monoclonal antibodies (mAbs) (anti–TER-119, anti-CD3ε, anti-B220, anti–NK-1.1, anti-Gr1, and anti-IAb; BD Pharmingen), followed by incubation with rabbit complement (Cedarlane, Ontario, Canada). BM cells were cultured in RPMI-1640 (Life Technologies, Grand Island, NY) in 75-cm2 flasks (5 × 106 cells/flask) with 10% heat-inactivated fetal calf serum (FCS; Life Technologies), glutamine, nonessential amino acids, sodium pyruvate, HEPES, 2-ME, and penicillin–streptomycin, supplemented with mrGM-CSF (1000 U/mL) and rmIL-4 (1000 U/mL). Inclusion of mIL-4 reduced the development of granulocytes (from approximately 35% to less than 5%) and monocytes. Culture medium supplemented with cytokines was replaced at day 3. At day 5, nonadherent cells were removed, and fresh medium with cytokines was added. Two days later (day 7) 65% ± 15% of the new population of nonadherent cells was CD11c+ DCs (CD11c is a relatively restricted DC marker in the mouse; Figure1A) with similar numbers of immature (CD86−) and mature (CD86+) DCs.
Stages of cell differentiation of murine BM DCs generated in GM-CSF + IL-4.
(A-B) Phenotype of BM DCs analyzed by flow cytometry. Cells were labeled with Cy-Chrome anti-CD11c, PE anti-CD86, and one of the following FITC-labeled mAbs: anti–MHC-I, anti–MHC-II, anti-CD40, anti-CD80, anti-CD11b, anti-CD54, or anti–OX-40L. Flow profiles illustrate the expression of specific markers on CD86− DC (gray profiles) and on CD86+ DC (open profiles, thick line). Isotype controls are represented by open profiles in dashed lines. (C) FITC-dextran and FITC-albumin uptake by CD11c+immunobead-sorted CD86− and CD86+ DC. Only CD86− DCs internalized FITC-dextran and FITC-albumin at 37°C, a phenomenon down-regulated at 0°C. Results are representative of 3 independent experiments. (D) Allostimulatory activity of γ-irradiated, FACS-sorted CD86− (▾), or CD86+ B10 DC (▿), assessed using C3H splenic T cells as responders. B10 (H2b) DC (d 7) were set up at graded concentrations with 2 × 105 responder naive C3H (H2k) T cells, and the cultures were maintained for 72 hours. [3H]TdR was added 18 hours before harvesting. The MLR stimulatory activity of freshly isolated allogeneic (B10 [○]) or syngeneic (C3H [●]) bulk spleen cells is also shown. Results are expressed as mean cpm ± 1 SD and are representative of at least 3 separate experiments.
Stages of cell differentiation of murine BM DCs generated in GM-CSF + IL-4.
(A-B) Phenotype of BM DCs analyzed by flow cytometry. Cells were labeled with Cy-Chrome anti-CD11c, PE anti-CD86, and one of the following FITC-labeled mAbs: anti–MHC-I, anti–MHC-II, anti-CD40, anti-CD80, anti-CD11b, anti-CD54, or anti–OX-40L. Flow profiles illustrate the expression of specific markers on CD86− DC (gray profiles) and on CD86+ DC (open profiles, thick line). Isotype controls are represented by open profiles in dashed lines. (C) FITC-dextran and FITC-albumin uptake by CD11c+immunobead-sorted CD86− and CD86+ DC. Only CD86− DCs internalized FITC-dextran and FITC-albumin at 37°C, a phenomenon down-regulated at 0°C. Results are representative of 3 independent experiments. (D) Allostimulatory activity of γ-irradiated, FACS-sorted CD86− (▾), or CD86+ B10 DC (▿), assessed using C3H splenic T cells as responders. B10 (H2b) DC (d 7) were set up at graded concentrations with 2 × 105 responder naive C3H (H2k) T cells, and the cultures were maintained for 72 hours. [3H]TdR was added 18 hours before harvesting. The MLR stimulatory activity of freshly isolated allogeneic (B10 [○]) or syngeneic (C3H [●]) bulk spleen cells is also shown. Results are expressed as mean cpm ± 1 SD and are representative of at least 3 separate experiments.
Flow cytometric analysis of cell surface phenotype
BM-derived cells were blocked with normal goat serum and then incubated with biotin-conjugated anti-CD11c mAb (HL3; all mAbs were from BD Pharmingen), phycoerythrin (PE)-conjugated anti-CD86 mAb (GL1), and one of the following FITC-conjugated mAbs: anti-H2Kb(AF6-88.5), anti-IAb β chain (25-9-17), anti-CD40 (3/23), anti-CD80 (16-10A1), anti-CD11b (M1/70), or anti-CD54 (3E2). Incubation with primary mAbs was followed by Cy-Chrome-streptavidin (BD Pharmingen). For OX-40 ligand (L) labeling, BM cells were incubated successively with (1) rat anti–OX-40L (RM134L) and biotin-conjugated anti-CD11c mAbs; (2) FITC-conjugated F(ab′)2 donkey anti–rat immunoglobulin (Jackson ImmunoResearch Laboratory, West Grove, PA) and Cy-Chrome-streptavidin; (3) irrelevant rat IgG to block any residual rat immunoglobulin-binding sites of the FITC donkey anti–rat immunoglobulins; and (4) PE-conjugated anti-CD86 mAb. Cells were fixed in 2% paraformaldehyde and analyzed using an EPICS Elite flow cytometer (Coulter, Hialeah, FL). Fluorochrome-conjugated species- and isotype-matched irrelevant mAbs were used as negative controls.
Dendritic cell morphology and ultrastructure
CD11c+CD86− and CD11c+CD86+ flow-sorted DCs (purity, 92%-96%) were used for cytospins or for transmission electron microscopy (TEM) or scanning electron microscopy (SEM). For cytospins, DC were spun onto glass slides using a Shandon cytocentrifuge (Cheshire, England) (230g) and were stained with May-Grünwald-Giemsa. For TEM, DCs were fixed in 2.5% glutaraldehyde–1% osmium tetroxide, dehydrated, and embedded in Epon 812. Sections were stained with uranyl acetate–lead citrate and were analyzed using a JEOL 1210 transmission electron microscope (JEOL, Chicago, IL). For SEM, DCs attached to glass coverslips pretreated with 0.1% poly-L-lysine were fixed with 2.5% glutaraldehyde, dehydrated, coated with 20 nm evaporated carbon, and analyzed in a JEOL 35 scanning electron microscope.
Endocytosis assay
Day 7 BM DCs were purified by labeling with bead-conjugated anti-CD11c mAb (Miltenyi Biotec, Auburn, CA) followed by positive selection through paramagnetic columns (Miltenyi Biotec) (DC purity, 90%-93%). Immunobead-sorted DC were incubated with 5 μg/mL FITC-albumin or 0.1 mg/mL FITC-dextran, at 37°C or at 4°C for 1 hour. Uptake was stopped by 3 washes with ice-cold 0.1% sodium azide–1% FCS–phosphate-buffered saline (PBS). After staining with PE anti-CD86 mAb (30 minutes on ice), cells were fixed with 2% paraformaldehyde and analyzed by flow cytometry.
RNase protection assay
The procedure adopted for RNase protection assay (RPA) has been described.31 Briefly, RNA was isolated from 5 × 106 snap-frozen, flow-sorted DCs using a total RNA Isolation Kit (BD Pharmingen). RPA was performed using the RiboQuant Multi-Probe RPA System (BD Pharmingen). Four kits containing cDNAs encoding mouse IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-11, IL-12p35, IL-12p40, IL-13, IL-15, IL-18, IFN-α, IFN-β, IFN-γ, TNF-α, TGF-β1, TGF-β2, TGF-β3, MIF, and the housekeeping genes L32 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as templates for the T7 polymerase-directed synthesis of 32P-UTP–labeled antisense RNA probes. Hybridization (16 hours at 56°C) of 5 μg each target mRNA with the antisense RNA probes sets was followed by RNase and proteinase K treatment, phenol-chloroform extraction, and ammonium acetate precipitation of protected RNA duplexes. In each RPA, the corresponding antisense RNA probe set was included as molecular weight standard. Mouse RNA and RNA degradation controls were included. Yeast tRNA served as negative control. Samples were electrophoresed on acrylamide-urea sequencing gels, and dried gels were exposed on x-ray film at −80°C. Quantification of bands was performed by densitometry followed by assessment using Image QuantNT software (Molecular Dynamics, Sunnyvale, CA). The signals from specific mRNA were normalized to signals from housekeeping genes (L32 andGADPH) run on each lane to adjust for loading differences.
Intracellular cytokine staining
BM DCs were treated with brefeldin A (10 μg/mL; 5 hours at 37°C), fixed in 4% paraformaldehyde, and permeabilized with 0.1% saponin–1% FCS–PBS. DCs were incubated with one of the following PE-coupled mAbs (all from BD Pharmingen): anti–mIL-4 (BVD4-1D11), anti–mIL-5 (TRFK5), anti–mIL-6 (MP5-2OF3), anti–mIL-10 (JES5-16E3), anti–mIL-12 p40/p70 (C15.6), anti–mTNF-α (MP6-XT22), or anti–mIFN-γ (XMG1.2). DCs were also incubated with either rat anti–mIL-1α (MAB500; R&D), anti–mIL-1β (MAB401; R&D), anti–mIL-12p70 mAbs (9A5; BD Pharmingen), or anti–mIFN-α (RMMA-1; PBL Biomedical Laboratories, New Brunswick, NJ); or rabbit anti–mIL-15 (H-114) or goat anti–mIL-18 (C-18, both from Santa Cruz Biotechnology) polyclonal IgG. In a second step, cells were incubated with PE goat F(ab′)2 anti–rat IgG, PE goat F(ab′)2anti–rabbit IgG, or PE swine anti–goat IgG (Caltag Laboratories, Burlingame, CA). After cytokine staining, DCs were labeled with FITC anti-CD86 or FITC anti-CD11c mAbs, fixed in 2% paraformaldehyde, and analyzed by flow cytometry.
Cytokines were detected in responder T cells after 3-day MLR (stimulator–responder cell ratio, 1:10) as described.32Briefly, T cells were restimulated with plate-bound anti-CD3ε and soluble anti-CD28 (37.51; BD Pharmingen) mAbs (10 μg/mL, 5 hours at 37°C), in the presence of brefeldin A. Thereafter, the cells were fixed in 2% paraformaldehyde, permeabilized with 0.1% saponin–1% FCS–PBS, and incubated simultaneously with Cy-Cychrome anti-CD3ε mAb (145-2-C11), FITC anti-CD4 mAb (GK1.5), and PE anti-mIFNγ (XMG1.2), PE anti–mIL-10 (JES5-16E3), or PE anti–mIL-4 (BVD4-1D11). Cells were fixed in 2% paraformaldehyde and analyzed by FACS. Fluorochrome-conjugated, isotype- and species-matched irrelevant mAbs were used as negative controls.
Cytokine quantitation
IL-4, IL-10, and TGF-β1 were quantified in 24-hour supernatants of DC cultures using an ELISA kit (OptEIA; BD Pharmingen) according to the manufacturer's protocol.
Allostimulatory activity
B10 BM DCs were flow-sorted into CD11c+CD86− or CD11c+CD86+ DCs, γ-irradiated, and used as stimulators in 72-hour primary MLR using nylon-wool column purified allogeneic (C3H) splenic T cells or naive CD4+ T cells as responders.32 Naive CD4+CD62L+CD44low T cells were purified by negative selection (purity 94% or greater) using T-cell enrichment columns (R&D). As controls, allogeneic (B10) or syngeneic (C3H) splenocytes were used as stimulators.
Statistical analysis
Results are expressed as means ± 1 SD. Comparisons between different means were performed by analysis of variance and then by the Newman-Keuls test. Comparison between 2 means was performed by the Student t test. P < .05 was considered significant.
Results
Stages of differentiation of bone marrow dendritic cells in vitro
BM DCs generated in vitro with GM-CSF + IL-4 exhibited (day 7) a mixed population of CD86− and CD86+CD11c+ DCs (Figure 1A). A third population of nonadherent, CD11c−CD86− cells was also detected. CD11c+ DCs were negative for monocyte–macrophage (CD14, F4/80), T-cell (CD3ε), B-cell (B220, CD19), NK-cell (NK1.1), and granulocyte (Gr-1) markers. The lack of CD8α (expressed by “lymphoid-related” DCs in the mouse) was consistent with the myeloid lineage of CD11c+ DCs generated with GM-CSF + IL-4. As expected, CD86− DCs corresponded to immature APCs (MHC-Ilo, MHC-IIlo, CD40−/lo, CD80lo, CD11bhi, CD54lo, OX40L−) that induced minimal proliferation of naive T cells (Figure 1B,D) in a 72-hour MLR. Classification of CD86− DCs as immature APCs was further confirmed by their capacity to internalize exogenous FITC-dextran or FITC-albumin, a function down-regulated at 0°C, or when DC expressed CD86 on the surface (Figure 1C). By contrast, CD86+ DCs exhibited the phenotype of mature DCs (MHC-Ihi, MHC-IIhi, CD40+, CD80hi, CD11blo, CD54hi, OX40L+), lacked endocytic capacity, and triggered a potent allogeneic naive T-cell response (Figure1).
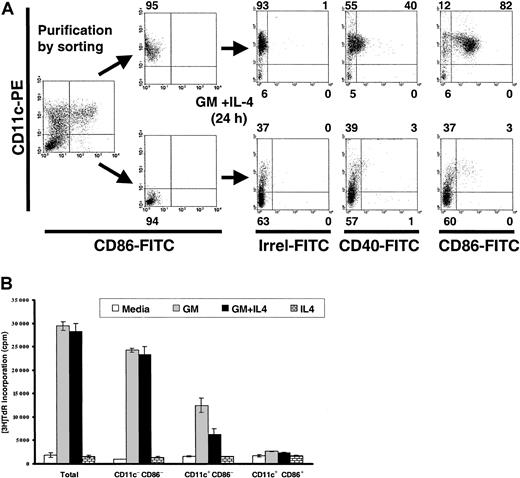
To confirm that CD86− DCs were the immediate precursors of CD86+ DCs and that double-negative cells (CD11c−CD86−) were the precursors of the CD11c+CD86− DCs, flow-sorted CD11c+CD86− DCs or double-negative cells were cultured with GM-CSF + IL-4. After 24-hour culture, CD11c+CD86− DCs acquired CD86 and CD40, and double-negative cells became mainly CD11c+CD86− DCs (Figure 2A).
Pathway of cell differentiation and proliferative capacity of BM DCs.
(A) CD11c+CD86− BM DCs are the immediate precursors of CD11c+CD86+ DCs, whereas double-negative cells are the progenitors of CD11c+CD86− DCs. BM cells cultured in GM-CSF + IL-4 (day 7) were labeled with PE anti-CD11c and FITC anti-CD86, FACS-sorted into CD11c+CD86− DCs (upper row) and into double-negative cells (lower row), and recultured in GM-CSF + IL-4 for 24 hours. Numbers outside quadrants indicate percentages of cells. (B) Proliferative capacity of CD11c− CD86−, CD11c+CD86−, and CD11c+CD86+ BM-derived cells. BM cells cultured with GM-CSF + IL-4 (day 7) were labeled, FACS-sorted, and grown with medium, GM-CSF (GM), GM-CSF + IL-4 (GM + IL-4), or IL-4 (3 × 104 cells/well). Proliferation was assessed by3H-TdR incorporation after 3 days of culture. Results are representative of 3 experiments.
Pathway of cell differentiation and proliferative capacity of BM DCs.
(A) CD11c+CD86− BM DCs are the immediate precursors of CD11c+CD86+ DCs, whereas double-negative cells are the progenitors of CD11c+CD86− DCs. BM cells cultured in GM-CSF + IL-4 (day 7) were labeled with PE anti-CD11c and FITC anti-CD86, FACS-sorted into CD11c+CD86− DCs (upper row) and into double-negative cells (lower row), and recultured in GM-CSF + IL-4 for 24 hours. Numbers outside quadrants indicate percentages of cells. (B) Proliferative capacity of CD11c− CD86−, CD11c+CD86−, and CD11c+CD86+ BM-derived cells. BM cells cultured with GM-CSF + IL-4 (day 7) were labeled, FACS-sorted, and grown with medium, GM-CSF (GM), GM-CSF + IL-4 (GM + IL-4), or IL-4 (3 × 104 cells/well). Proliferation was assessed by3H-TdR incorporation after 3 days of culture. Results are representative of 3 experiments.
The proliferative capacity of FACS-sorted double-negative cells, CD86− and CD86+ DCs was evaluated by3H-TdR incorporation after 3-day culture without growth factors or with GM-CSF or IL-4. In agreement with the differentiation pathway: CD11c−CD86− cells → CD11c+CD86− DCs → CD11c+CD86+ DCs, double-negative cells proliferated extensively in response to GM-CSF ± IL-4, whereas CD11c+CD86− DCs exhibited a reduced replicative capacity. DNA synthesis was virtually absent in CD11c+CD86+ DCs (Figure 2B).
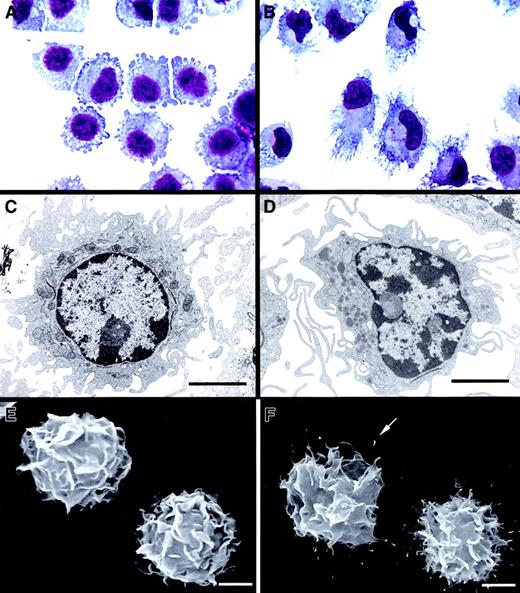
FACS-sorted CD86− DCs exhibited intracytoplasmic multivesicular bodies, numerous mitochondria, few lysosomes, short veillike cytoplasmic extensions, and an eccentric oval nucleus with prominent nucleoli (Figure 3A,C,E). By contrast, FACS-sorted CD86+ DCs showed a reduced endocytic compartment, abundant lysosomes, well-developed veillike processes, and an eccentric lobulated nucleus (Figure 3B,D,F). Analyzed by SEM, CD86+ DCs displayed filamentous surface membrane projections ending in a knoblike tip (Figure 3F) in addition to the veils (as in immature DCs). Birbeck granules were absent in both DC populations.
Morphology and ultrastructure of CD86− BM DCs and CD86+ BM DCs.
CD86− DCs exhibited short, blunt prolongations (A), a round nucleus with prominent nucleoli, multiple cytoplasmic vesicles, mitochondria, few lysosomes (C), and typical “veils” (E). After maturation, CD86+ DCs showed a typical dendritic morphology, with an eccentric, indented nucleus (B, D) and a veiled surface with delicate filamentous projections with knoblike tips (F, arrow). (A-B) May-Grünwald-Giemsa. (C-D) TEM ×6000. (E-F) SEM ×3500). Bar, 5 μm.
Morphology and ultrastructure of CD86− BM DCs and CD86+ BM DCs.
CD86− DCs exhibited short, blunt prolongations (A), a round nucleus with prominent nucleoli, multiple cytoplasmic vesicles, mitochondria, few lysosomes (C), and typical “veils” (E). After maturation, CD86+ DCs showed a typical dendritic morphology, with an eccentric, indented nucleus (B, D) and a veiled surface with delicate filamentous projections with knoblike tips (F, arrow). (A-B) May-Grünwald-Giemsa. (C-D) TEM ×6000. (E-F) SEM ×3500). Bar, 5 μm.
Immature (CD86−) dendritic cells exhibit a different pattern of cytokine mRNAs than mature (CD86+) dendritic cells
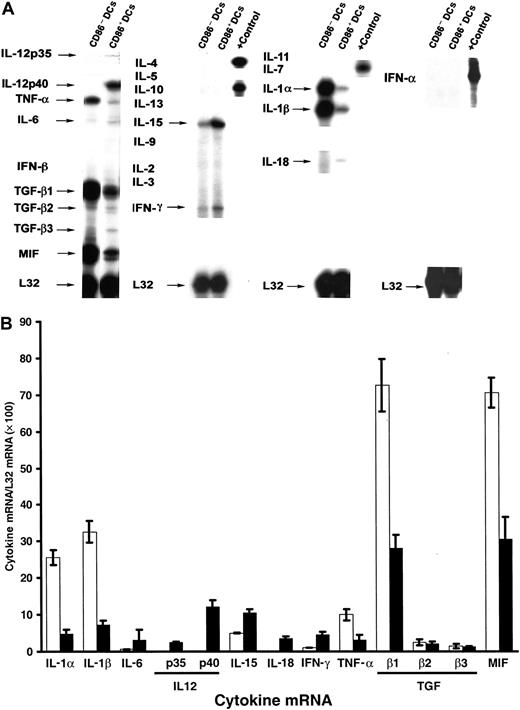
The pattern of cytokine gene transcription of myeloid DCs at different stages of cell development was analyzed by RPA. To minimize cell cross-contamination, only highly purified, FACS-sorted CD86− or CD86+ DC (purity, approximately 95%) were used for RNA extraction. CD86− DCs expressed higher levels of IL-1α, IL-1β, TNF-α, TGF-β1, and MIF mRNA than CD86+ DCs (Figure 4A-B). Both DC subpopulations showed weak but similar signals for TGF-β2 and TGF-β3. Production of IL-6 and IL-15 mRNA, and to a lesser extent, the level of IFN-γ mRNA, increased during DC differentiation. CD86+ DCs showed de novo expression of IL-12p35, IL-12p40, and IL-18 mRNA (Figure 4A-B). We were unable to detect mRNA for IL-2, IL-3, IL-4, IL-5, IL-7, IL-9, IL-10, IL-13, IFN-α, and IFN-β, even after increasing the x-ray film exposure time (up to 4 days) or the amount of total RNA used in the hybridization (up to 6 μg).
Comparative RPA analysis of cytokine mRNA expression of BM DCs at different stages of cell differentiation.
mRNA isolated from FACS-sorted DCs was analyzed by RPA. Data are from a single experiment representative of 3 separate experiments. (B) Quantitative analysis of mRNA cytokine gene expression. Densitometric analysis of each lane was performed on scanned autoradiographs, and all values are expressed relative to corresponding housekeeping gene transcripts (L32). ■, CD11c+CD86− DCs; ▪, CD11c+CD86+ DCs. Densitometric values were pooled from 3 separate experiments.
Comparative RPA analysis of cytokine mRNA expression of BM DCs at different stages of cell differentiation.
mRNA isolated from FACS-sorted DCs was analyzed by RPA. Data are from a single experiment representative of 3 separate experiments. (B) Quantitative analysis of mRNA cytokine gene expression. Densitometric analysis of each lane was performed on scanned autoradiographs, and all values are expressed relative to corresponding housekeeping gene transcripts (L32). ■, CD11c+CD86− DCs; ▪, CD11c+CD86+ DCs. Densitometric values were pooled from 3 separate experiments.
In view of conflicting reports of the effect of IL-4 on synthesis of IL-12 by DCs,16 33-37 we compared cytokine mRNA of CD86− and CD86+ DCs cultured in GM-CSF + IL-4 with those from similar subpopulations of DC grown in GM-CSF alone. In the absence of IL-4, similar levels and patterns of cytokine mRNA were obtained (data not shown).
Immature dendritic cells exhibit a different pattern of cytokine protein synthesis than mature dendritic cells
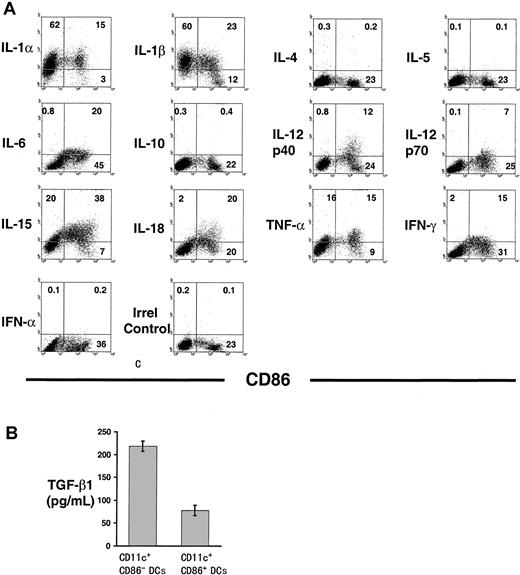
The pattern of cytokine expression during DC ontogeny (with the exception of TGF-β1) was assessed by intracellular staining, followed by flow cytometry analysis. This methodology circumvented some of the following obstacles related to the detection of cytokines in a dynamic population of cells at different stages of differentiation: (1) purified immature DCs differentiate rapidly into mature DCs; hence, the ultimate cellular source of a secreted cytokine cannot be identified by analysis of culture supernatants by ELISA; (2) some cytokines exhibit a short half-life after being released into the medium; and (3) some of the cytokines analyzed are consumed by DCs (ie, TNF-α, IL-1, IL-4, IL-12). The production of IL-1α, IL-1β, IL-15, and TNF-α protein by CD86− and CD86+ immunobead-sorted CD11c+ DCs and the synthesis of IL-6, IL-12p40, IL-12p70, IL-18, and IFN-γ protein almost exclusively by CD86+ DC were confirmed by FACS (Figure 5A). In agreement with the RPA results, expression of IL-4, IL-5, IL-10, and IFN-α was absent in DC by FACS analysis and confirmed by ELISA (not shown).
Intracellular expression of cytokines in BM DCs at different stages of cell differentiation.
(A) Detection of intracellular cytokines by flow cytometry in CD86− and CD86+ BM DCs. CD11c+immunobead-sorted DCs were double labeled with FITC anti-CD86 and PE anticytokine mAbs. Figures within quadrants indicate percentages of cells. (B) Detection of TGF-β1 by ELISA in 24-hour culture supernatants of FACS-sorted CD86− DCs and CD86+ DCs (2 × 106 cells/well). Results are representative of 4 separate experiments.
Intracellular expression of cytokines in BM DCs at different stages of cell differentiation.
(A) Detection of intracellular cytokines by flow cytometry in CD86− and CD86+ BM DCs. CD11c+immunobead-sorted DCs were double labeled with FITC anti-CD86 and PE anticytokine mAbs. Figures within quadrants indicate percentages of cells. (B) Detection of TGF-β1 by ELISA in 24-hour culture supernatants of FACS-sorted CD86− DCs and CD86+ DCs (2 × 106 cells/well). Results are representative of 4 separate experiments.
Despite the TGF-β1 mRNA expression in DCs, no signal for TGF-β1 protein was detected by FACS (3 mAbs were tested). Therefore, TGF-β1 concentration was assessed by ELISA in 24-hour culture supernatants of CD86− and CD86+ FACS-sorted DC. Consistent with the mRNA level, the amount of TGF-β1 in supernatants of CD86− DCs was significantly higher than that secreted by CD86+ DCs (Figure 5B).
Terminal maturation of dendritic cells by distinct stimuli regulates differentially the levels and pattern of cytokine mRNA and protein
To analyze whether the pattern of cytokines produced by murine DCs was determined by the molecular pathway used for terminal differentiation, the levels of cytokine mRNA and protein were assessed in CD86+ DCs before and after activation by either a T-cell–independent (LPS) or a T-cell–dependent (CD40 ligation) pathway. Total RNA was extracted from CD11c+CD86+ FACS-sorted DCs incubated with either LPS (0.5 μg/mL) or IgM anti-CD40 (10 μg/mL), or irrelevant IgM (10 μg/mL). To avoid the exhaustion of cytokine production, as reported recently for human DCs,38 and based on our preliminary observations that the maximum expression of most cytokine mRNAs analyzed was within 6 and 16 hours of stimulation, the final experiments were carried out after 10 hours of LPS or anti-CD40 treatment. Terminal differentiation of CD86+ DCs induced by LPS or CD40 cross-linking was confirmed by increases in the surface levels of MHC-I and MHC-II, CD40, CD80, CD86, and in the cells' allogeneic T-cell stimulatory capacity (not shown).
LPS up-regulated significantly the levels of IL-1α, IL-1β, and IL-6 transcripts and, to a lesser extent, the amount of IL-15, TNF-α, and MIF mRNA, compared to control DCs (Figure6A-B). Increase of the intracellular content of IL-1α, IL-1β, IL-6, IL-15, and TNF-α in DCs after LPS treatment was confirmed by FACS (Figure7A).
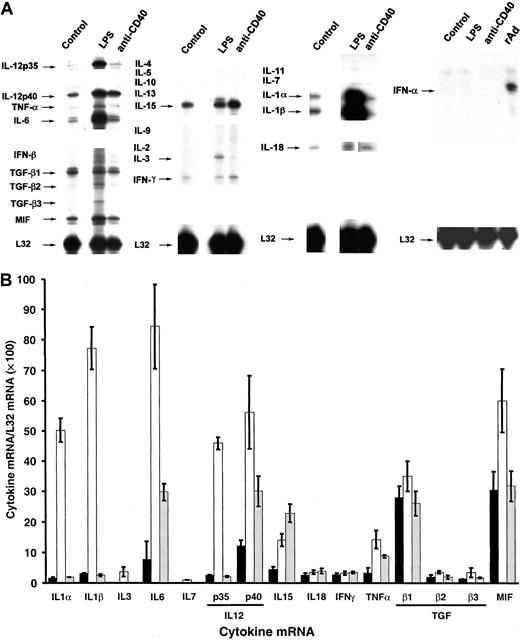
Comparative RPA analysis of cytokine mRNA expression of BM DCs terminally differentiated by LPS or CD40 ligation.
(A) Comparison of cytokine mRNA expression in FACS-sorted CD86+ BM DCs (control) and DCs terminally differentiated with LPS or anti-CD40 IgM mAb. Expression of IFN-α mRNA was also investigated in CD86+ BM DCs infected with a rAd encoding no transgene (MOI = 100) used as positive control. mRNA was analyzed by RPA. DCs treated with irrelevant IgM exhibited a pattern similar to that of the control group (not shown). Data are from a single experiment representative of 3 separate experiments. (B) Quantitative analysis of mRNA cytokine gene expression. Densitometric analysis of each lane was performed on scanned autoradiographs, and all values are expressed relative to corresponding housekeeping gene transcripts (L32). ▪, control DC; ■, DC + LPS; ░, DC + anti-CD40. Densitometric values (means ± 1 SD) were pooled from 3 separate experiments.
Comparative RPA analysis of cytokine mRNA expression of BM DCs terminally differentiated by LPS or CD40 ligation.
(A) Comparison of cytokine mRNA expression in FACS-sorted CD86+ BM DCs (control) and DCs terminally differentiated with LPS or anti-CD40 IgM mAb. Expression of IFN-α mRNA was also investigated in CD86+ BM DCs infected with a rAd encoding no transgene (MOI = 100) used as positive control. mRNA was analyzed by RPA. DCs treated with irrelevant IgM exhibited a pattern similar to that of the control group (not shown). Data are from a single experiment representative of 3 separate experiments. (B) Quantitative analysis of mRNA cytokine gene expression. Densitometric analysis of each lane was performed on scanned autoradiographs, and all values are expressed relative to corresponding housekeeping gene transcripts (L32). ▪, control DC; ■, DC + LPS; ░, DC + anti-CD40. Densitometric values (means ± 1 SD) were pooled from 3 separate experiments.
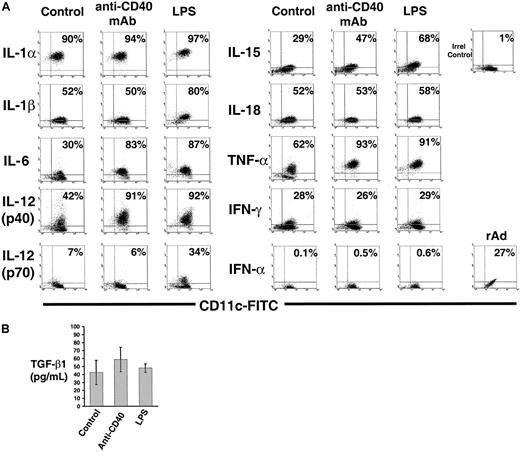
Intracellular expression of cytokines in BM DCs terminally differentiated by LPS or CD40 ligation.
(A) Detection of intracellular cytokines by flow cytometry in CD11c+ immunobead-sorted CD86+ BM DCs (control) or terminally differentiated with LPS or anti-CD40 IgM mAb. DCs treated with irrelevant IgM exhibited a pattern similar to that of the control group (not shown). Expression of IFN-α was also investigated in CD86+ BM DCs infected with a rAd encoding no transgene (MOI = 100) used as positive control. DC were double labeled with FITC anti-CD11c and specific PE anticytokine mAbs. Figures inside quadrants indicate percentages of cells. (B) Detection of TGF-β1 by ELISA in 24-hour culture supernatants of FACS-sorted CD86+mature DC (2 × 106 cells/well) incubated with LPS, or anti-CD40 IgM mAb, or irrelevant IgM (control). Results are representative of 3 independent experiments.
Intracellular expression of cytokines in BM DCs terminally differentiated by LPS or CD40 ligation.
(A) Detection of intracellular cytokines by flow cytometry in CD11c+ immunobead-sorted CD86+ BM DCs (control) or terminally differentiated with LPS or anti-CD40 IgM mAb. DCs treated with irrelevant IgM exhibited a pattern similar to that of the control group (not shown). Expression of IFN-α was also investigated in CD86+ BM DCs infected with a rAd encoding no transgene (MOI = 100) used as positive control. DC were double labeled with FITC anti-CD11c and specific PE anticytokine mAbs. Figures inside quadrants indicate percentages of cells. (B) Detection of TGF-β1 by ELISA in 24-hour culture supernatants of FACS-sorted CD86+mature DC (2 × 106 cells/well) incubated with LPS, or anti-CD40 IgM mAb, or irrelevant IgM (control). Results are representative of 3 independent experiments.
Unlike LPS, CD40 ligation of DCs induced more discrete changes in the levels of cytokine mRNA. Only IL-6, IL-12p40, IL-15, and TNF-α transcripts augmented compared to control DCs (Figure 6A-B), whereas the signals for IL-1α, IL-1β, IL-18, IFN-γ, TGF-β1-3, and MIF mRNAs remained at the level of control DCs (Figure 6A-B). The up-regulation of IL-6, IL-12p40, IL-15, and TNF-α mRNA by CD40 ligation was confirmed at the protein level by FACS analysis (Figure7A).
The level of intracellular IL-18 and IFN-γ detected by FACS and the amount of TGF-β1 detected by ELISA in 24-hour supernatants of LPS- or anti–CD40-stimulated mature DCs were similar to control DC (Figure 7A-B), a fact that correlated with the absence of effect of LPS or CD40L on the respective mRNA levels (Figure 6A-B).
IFN-α transcripts and intracellular IFN-α were detected exclusively in CD86+ DCs infected with a control rAd encoding no transgene (used as positive control at a multiplicity of infection, or MOI = 100).
Transcription of IL-12 genes in CD86+ dendritic cells is affected differentially by lipopolysaccharide and CD40 cross-linking
DCs secrete IL-12p70, a heterodimeric cytokine composed of p35 and p40 chains, covalently linked and encoded by different genes. Secretion of IL-12p70 by DCs during the early stages of antigen presentation directs the differentiation of naive Th cells into Th1 lymphocytes. CD86+ DC express IL-12p40 mRNA and low levels of IL-12p35 mRNA (Figures 4A, 6A). Either LPS or anti-CD40 stimulation of DCs increased significantly the amount of IL-12p40 transcripts (Figure 6A-B) and intracellular IL-12p40 protein detected by FACS (Figure 7A). Interestingly, unlike CD40 ligation, LPS stimulation augmented IL-12p35 transcripts markedly, and the levels of IL-12p70 protein detected by FACS using a mAb specific for the p35/p40 heterodimer, a fact indicative of an increased synthesis of IL-12p35 protein (Figure 7A). Similar results were obtained with DCs generated in the absence of IL-4 (not shown).
Mature dendritic cells exhibit a DC1/DC2 phenotype (Th1/Th2 driving capacity) maintained after CD40 cross-linking, but they shift toward DC1 after terminal differentiation with LPS
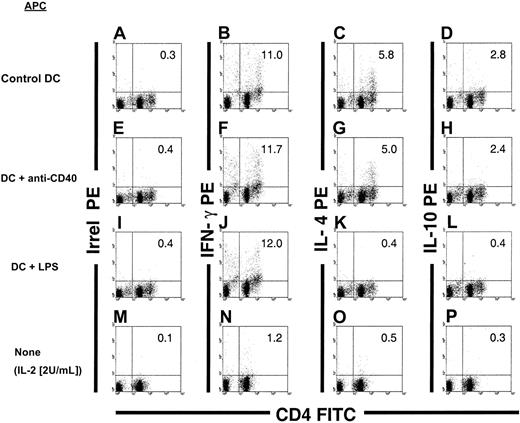
The type of allogeneic Th response elicited by CD86+ DCs, before and after terminal differentiation with LPS or CD40 cross-linking, was studied by flow cytometry. FACS-sorted CD86+ (B10) DCs, either nonstimulated or after treatment with LPS, anti-CD40 IgM mAb or irrelevant IgM was used as a stimulator of (C3H) splenic T cells in 3d-MLR. Responder T cells were triple labeled with Cy-Cychrome anti-CD3ε, FITC anti-CD4, and PE anti–mIFN-γ or anti–mIL-10 or anti-mIL-4 mAbs. Allogeneic DCs were gated out of the mixed cell population from the MLR (C3H T cells and B10 DC at a ratio of 10:1) according to their lack of CD3 expression. When CD86+ DCs or anti-CD40 treated-DCs were used as stimulators, a substantial percentage of CD4+ splenic T cells (approximately 11%-12%; Figure8B,F) produced IFN-γ, and a lower proportion synthesized Th2 cytokines (approximately 5%-6% and approximately 2%-3% produced IL-4 or IL-10, respectively; Figure 8). Similar results were obtained with control DCs incubated with irrelevant IgM (not shown). By contrast, when DCs were differentiated with LPS, CD4+ splenic T cells produced exclusively IFN-γ (Figure 8J).
Th-cell–driving potential of BM DCs terminally differentiated by LPS or CD40 ligation.
Detection of Th1 (IFN-γ) and Th2 cytokines (IL-4 and IL-10) in C3H CD3+CD4+ splenic T cells after in vitro stimulation with γ-irradiated allogeneic (B10): (1) CD86+BM DCs (A-D); (2) CD86+ BM DCs differentiated with anti-CD40 IgM mAbs (E-H); or (3) CD86+ BM DCs incubated with LPS (I-L). After 3-day MLR, the T cells were harvested and restimulated with anti-CD3ε + anti-CD28 mAbs in the presence of brefeldin A. Thereafter, the cells were labeled with anti-CD3, FITC anti-CD4, and PE anti–IFN-γ, anti–IL-4, or anti–IL-10 mAbs. DCs were gated out according to their lack of expression of CD3. As controls, C3H T cells were maintained for 3 to 4 days in low-dose rIL-2 (2 U/mL) and then restimulated with anti-CD3 + anti-CD28 mAbs (M-P). Irrel indicates irrelevant. Figures in quadrants denote percentages. Data are representative of 3 separate experiments.
Th-cell–driving potential of BM DCs terminally differentiated by LPS or CD40 ligation.
Detection of Th1 (IFN-γ) and Th2 cytokines (IL-4 and IL-10) in C3H CD3+CD4+ splenic T cells after in vitro stimulation with γ-irradiated allogeneic (B10): (1) CD86+BM DCs (A-D); (2) CD86+ BM DCs differentiated with anti-CD40 IgM mAbs (E-H); or (3) CD86+ BM DCs incubated with LPS (I-L). After 3-day MLR, the T cells were harvested and restimulated with anti-CD3ε + anti-CD28 mAbs in the presence of brefeldin A. Thereafter, the cells were labeled with anti-CD3, FITC anti-CD4, and PE anti–IFN-γ, anti–IL-4, or anti–IL-10 mAbs. DCs were gated out according to their lack of expression of CD3. As controls, C3H T cells were maintained for 3 to 4 days in low-dose rIL-2 (2 U/mL) and then restimulated with anti-CD3 + anti-CD28 mAbs (M-P). Irrel indicates irrelevant. Figures in quadrants denote percentages. Data are representative of 3 separate experiments.
IL-12 p70 (IL-12p35/IL-12p40), and not IL-23 (p19/IL-12p40), is mainly involved in Th1-driving capacity of lipopolysaccharide-treated dendritic cells
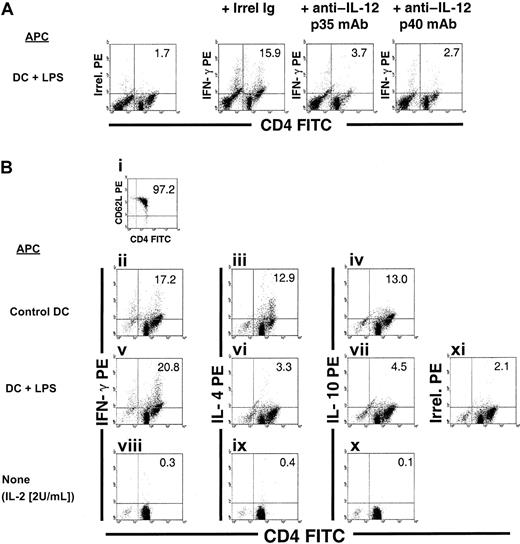
IL-23 is a cytokine that combines the IL-12p40 subunit with p19.39 Like IL-12 p70, IL-23 induces proliferation and IFN-γ production by T cells.39 In the mouse, IL-23 induces exclusively the proliferation of memory T cells, whereas IL-12p70 only affects naive T lymphocytes.39 Because it was not possible to analyze expression of the recently defined IL-23p19 mRNA/protein, 2 alternative approaches were used to address the role of IL-23 in the Th1-driving potential of CD86+ DCs in our system. First, untreated control, anti–CD40-treated, or LPS-treated CD86+ (B10) DCs were used as stimulators of (C3H) splenic T cells in 3d-MLR in the presence of neutralizing anti–IL-12p35 mAbs (20 μg/mL) to establish a role for IL-12p70. Alternatively, neutralizing anti–IL-12p40 mAbs (20 μg/mL) were used to rule out a role for IL-23. In all cases, there was a substantial decrease in the number of IFN-γ+ CD4+ T cells (Figure9A). In a second approach, highly purified allogeneic (C3H) CD4+CD62L+CD44low naive splenic T cells were used as responders in 3d-MLR. Control or anti–CD40-treated CD86+ DCs induced a mixed Th1/Th2 response, whereas LPS-treated CD86+ DCs generated predominantly Th1 cells (Figure 9B). These results confirm that the Th1 shift caused by LPS was caused mainly by the increased production of IL-12p35 by DCs.
Role of IL-12p70 (IL-12p35/IL-12p40) and IL-23 (p19/IL-12p40) in the Th1-driving ability of LPS-treated DCs.
Addition of 20 μg/mL neutralizing anti–IL-12p35 or anti–IL-12p40 mAb reduces substantially the ability of LPS-treated DCs to generate IFN-γ+ CD4+ T cells when a mixed population of naive and memory responder T cells was used in 3d-MLR. The partial Th1-inducing potential of control and anti-CD40–treated DCs was also reduced by anti–IL-12p35 or anti–IL-12p40 mAb treatment (not shown). Irrel indicates irrelevant. (B) Detection of IFN-γ, IL-4, and IL-10 in highly purified (C3H) splenic CD4+CD62L+CD44low naive T cells (i) after in vitro stimulation with γ-irradiated allogeneic (B10) CD86+ control DCs (ii-iv) or LPS-treated DCs (v-vii) in a 3d-MLR. Anti-CD40–treated DCs showed similar results to control DCs (not shown). As controls, (C3H) naive T cells maintained for 3 to 4 days in low-dose rIL-2 (2 U/mL) were included (viii-x). After 3d MLR, the T cells were harvested and restimulated with anti-CD3ε + anti-CD28 mAbs in the presence of brefeldin A. Cells were labeled with Cy-Cychrome anti-CD3, FITC anti-CD4, and PE anti–IFN-γ, anti–IL-4, or anti–IL-10 mAbs. DCs were gated out according to their lack of expression of CD3. Figures in quadrants denote percentages. Data are representative of 3 separate experiments.
Role of IL-12p70 (IL-12p35/IL-12p40) and IL-23 (p19/IL-12p40) in the Th1-driving ability of LPS-treated DCs.
Addition of 20 μg/mL neutralizing anti–IL-12p35 or anti–IL-12p40 mAb reduces substantially the ability of LPS-treated DCs to generate IFN-γ+ CD4+ T cells when a mixed population of naive and memory responder T cells was used in 3d-MLR. The partial Th1-inducing potential of control and anti-CD40–treated DCs was also reduced by anti–IL-12p35 or anti–IL-12p40 mAb treatment (not shown). Irrel indicates irrelevant. (B) Detection of IFN-γ, IL-4, and IL-10 in highly purified (C3H) splenic CD4+CD62L+CD44low naive T cells (i) after in vitro stimulation with γ-irradiated allogeneic (B10) CD86+ control DCs (ii-iv) or LPS-treated DCs (v-vii) in a 3d-MLR. Anti-CD40–treated DCs showed similar results to control DCs (not shown). As controls, (C3H) naive T cells maintained for 3 to 4 days in low-dose rIL-2 (2 U/mL) were included (viii-x). After 3d MLR, the T cells were harvested and restimulated with anti-CD3ε + anti-CD28 mAbs in the presence of brefeldin A. Cells were labeled with Cy-Cychrome anti-CD3, FITC anti-CD4, and PE anti–IFN-γ, anti–IL-4, or anti–IL-10 mAbs. DCs were gated out according to their lack of expression of CD3. Figures in quadrants denote percentages. Data are representative of 3 separate experiments.
Discussion
Our results demonstrate that BM-derived myeloid DCs transcribe different spectra of cytokines at distinct stages of development. CD86− DCs transcribed high levels of TNF-α, IL-1α, IL-1β, TGF-β1, and MIF mRNAs that were down-regulated during cell maturation. There is evidence that though TNF-α and IL-1β induce murine DC maturation,40 TGF-β1 arrests the development of DCs.41 Autocrine or paracrine secretion of TNF-α and IL-1β by CD86− DCs may play a role in the maturation from CD86− to CD86+ DCs in cultures grown with GM-CSF + IL-4, a process that may be counterregulated by the release of TGF-β1 by the same cells. Whether the high level of MIF (a glucocorticoid-induced modulator of cytokine production) mRNA present in immature DCs is translated into a functional protein that may act as an endogenous regulator of DC differentiation remains to be determined.42 Besides the low expression of costimulatory molecules on the surface of CD86− DCs, the low level of mRNA/protein for cytokines involved in T-cell activation (ie, IL-6, IL-12, or IL-15), combined with the high production of TGF-β1, contributed to the known low-stimulatory activity of immature DCs for naive T cells. As part of their maturation into CD86+cells, DCs expressed IL-12p35, IL-12p40, and IL-18 transcripts/protein de novo and significantly increased the levels of IL-15 and, to a lesser extent, IL-6 and IFN-γ. CD86+ DCs maintained considerable levels of other T-cell stimulatory cytokines (such as IL-1α, IL-1β, and TNF-α).
IL-15 shares with IL-2 the capacity to stimulate T-cell proliferation and induction of cytotoxic T lymphocyte and lymphokine-activated killer cells.43 Because IL-2 is not produced by DCs, the secretion of IL-15, whose mRNA/protein levels increased during DC maturation, may provide alternative cytokine costimulation for T cells.21 IL-15 is also chemotactic for T cells.44 Thus, secretion of IL-15 by mature DCs may serve to recruit T cells and, hence, facilitate the initial cell contact during antigen presentation. In line with the role of IL-15 in T-cell costimulation by DCs, the IL-15 mRNA/protein levels increased after terminal differentiation of mature DCs induced by LPS or CD40 cross-linking. Other bacterial products (Staphylococcus aureus Cowan I strain: SAC), and other members of the TNF family (such as TRANCE, TNF-related activation-induced cytokine) increase IL-15 levels in human and mouse DCs.11
IL-12 is a 70-kd heterodimeric cytokine composed of 2 covalently linked chains, p40 and p35, encoded by 2 separate genes regulated independently. Release of IL-12p70 by DCs is the key factor that drives the differentiation of naive T lymphocytes to Th1 cells (so-called DC1).12,15,28 The present work demonstrates that transcription of both IL-12 mRNAs occurs late during DC development, at the CD86+ stage. Similarly, the level of IL-12p40 mRNA is augmented in Langerhans cells or Langerhans cell lines during maturation in culture,14,45 and IL-12p70 secretion correlates with the maturation of splenic DCs.40
Our results show that CD40 ligation increased the level of IL-12p40 mRNA/protein in BM DCs but did not affect p35 mRNA/protein expression. This indicates that CD40 ligation triggers activation signals for IL-12p40 mRNA transcription, but not for IL-12p35. It is known that CD40 cross-linking induces the up-regulation of IL-12p40 mRNA in monocytic and B-cell lines and in human monocyte–derived DCs6,46 through activation of the transcription factor NF-κB,47,48 without affecting levels of p35 mRNA.47 More recently, Snijders et al49demonstrated that CD40 cross-linking is sufficient to induce the secretion of IL-12p40 in human monocyte–derived DCs but not to augment the production of IL-12p70, an effect suppressed by type 1 IFNs.46 Interestingly, interactions of other members of the TNF/TNFR superfamily, such as TRANCE/TRANCE-R, or OX40L(CD134L)/OX40, selectively increased the levels of IL-12p40 in DCs without affecting IL-12p35 mRNA.11,50 In apparent contrast to these results, initial studies on the regulation of IL-12 synthesis by DCs indicated that CD40 ligation alone increased IL-12p40/p35 secretion.4,16,17 This discrepancy can be explained by the presence of an additional signal for IL-12p35 up-regulation, such as IFN-γ,33,49,51,52 secreted by the CD40L-transfected cells, Th clones, or memory T cells used in these experiments4,16,17 or by the stimulation of the APCs through MHC class II–TCR interactions.53
In contrast to CD40 cross-linking, LPS stimulation increased the levels of IL-12p35 mRNA/protein in CD86+ DCs. The presence of a small amount of IFN-γ (produced by the CD86+ DCs) might have primed the DCs for the IL-12p70–inducing effect of LPS.54 The augmentation of IL-12p40/p35 mRNA and IL-12p70 protein levels correlated with the shift of CD86+ DCs (originally composed of DCs with Th1- and Th2-driving capacity [DC1 + DC2]) into Th1-driving DC (DC1) (Figures 8, 9). As has been demonstrated in LPS-stimulated human monocytes,33 the increase in expression of IL-12 p35 mRNA/protein appears to be the key step in the IL-12p70–producing and Th1-driving potential of BM DCs. However, in our model, the effect of LPS on the Th1-driving potential of DCs may also be attributed to an increase in IL-23p19 subunit production. IL-23 is a novel heterodimeric cytokine, produced by DCs, that shares the IL-12p40 molecule bound to a recently described subunit termed p19.39 Similar to IL-12p70, though to a lower extent, IL-23 induces IFN-γ production of naive and memory human T cells.39 However, in the mouse, and unlike IL-12p70, IL-23 does not have known effects on naive T cells.39 Our finding that the Th1-driving potential of LPS-treated DCs was inhibited after IL-12p35 neutralization, and our observation that naive CD4+ T cells also shifted to Th1 cells after allogeneic LPS-treated DC stimulation, rule out a significant role of IL-23 in our system. Besides LPS, other bacterial or parasite products (CpG DNA,Mycobacterium tuberculosis, Borrelia burgdorferi,Toxoplasma gondii, Leishmania major) have been proven to increase the secretion of IL-12p40 or IL-12p70 in mouse and human DCs, either directly15,55-61 or through the release of chemotactic factors or CD40–CD40L interactions.62-64 The influence of LPS on other costimulation pathways involved in Th1 differentiation, such as SLAM (signaling lymphocytic activation molecule) cannot be completely excluded.65 Interestingly, SLAM (CDw150, IPO-3) is rapidly induced on naive T cells after activation and on APCs (B cells) after LPS stimulation.66
Other cytokines involved in T-cell priming (IL-1-α, IL-1-β, IL-6, and TNF-α) were regulated differentially in DCs by different stimuli. Our results demonstrated that CD86+ DCs augmented levels of IL-1–α, IL-1–β, IL-6s, and TNF-α mRNA (and their respective intracellular protein products) in response to LPS, the major component of the outer membrane of gram-negative bacteria. Paracrine or autocrine activation of DCs by IL-1–β and TNF-α at the time of microorganism invasion represents an important link in the transition from an innate to an adaptive immune response. In fact, LPS and other bacterial products, such as lipoteichoic acid (component of gram-positive bacteria), S aureus, M tuberculosis, andBacillus Calmette-Guerin, have been shown to induce the secretion of IL-1–α, IL-1-β, IL-6, or TNF-α in human monocyte–derived DCs.46,57,59,64 Unlike LPS, CD40 cross-linking increased the levels of IL-6 and TNF-α mRNA/protein, with no effect on IL-1–α or IL-1–β transcripts. In agreement with our results, CD40L–CD40 interaction increased TNF-α and IL-6 secretion in human monocyte–derived DCs.46,49 Interaction between other members of the TNF/TNFR superfamily (such as TRANCE/TRANCE-R and OX40/OX40L) also augments levels of IL-6 and TNF-α transcripts/protein in mouse and human DCs.11 50
It is interesting that, after differentiation into CD11c+CD86+ cells, mature BM-derived DCs express IL-18 (IFN-γ–inducing factor) mRNA/protein de novo. IL-18 stimulates T-cell proliferation and potentiates the Th1-driving capacity of IL-12p70.67 Unlike IL-12p35 or IL-12p40, the levels of IL-18 mRNA were not affected by LPS or CD40 cross-linking.
There has been controversy as to whether DCs, like macrophages, are able to produce IFN-γ.4 Recent evidence demonstrated that both myeloid-derived (CD8α−) and lymphoid-related (CD8α+) mouse splenic DCs produced IFN-γ in response to IL-12p70,23 an effect that was potentiated by the addition of IL-4 or IL-18.24 In the present work, BM DCs acquired detectable levels of IFN-γ mRNA/protein during differentiation into CD86+ DCs, and this expression was not influenced by LPS or CD40 ligation. Whether IFN-γ production is associated, at the cellular level, with the synthesis of IL-12p70 or IL-18 and with the Th1-driving capacity of an individual DC is unknown.
We were unable to detect IFN-α transcripts in BM DCs even after LPS or CD40 L stimulation. In humans, plasmacytoid monocytes (also known as precursor DC2) and monocyte-derived DCs secrete IFN-α after infection with herpes simplex virus or influenza virus.68-70Interestingly, infection with rAds, the most efficient vectors for DC transduction, triggered IFN-α transcription. IFN-α is a powerful inducer of DC maturation; thus, it is likely that at least part of the effect that rAd exerts on DC differentiation can be ascribed to autocrine secretion of type 1 IFNs.31 71
In conclusion, we have demonstrated that mouse BM DCs modulate their repertoire of cytokine mRNAs/proteins at different stages of development. Although CD40 ligation or LPS stimulation induced similar changes in phenotype and allostimulatory activity in mature myeloid DCs, these stimuli generated different cytokine patterns in DCs and induced distinct naive Th-cell–driving potential (Th1/Th2 vs Th1).
Inflammatory cytokines (IFN-γ) released in the DC microenvironment and bacterial (SAC) and viral (unmethylated CpG motifs) components have been implicated as inducers of IL-12p70 secretion and subsequent Th1 differentiation by human monocyte–derived DCs.52,54 Our results support, at the cytokine mRNA level, the recently proposed concept28 that DCs are able to regulate independently their T-cell costimulatory function (signal 2) and their capacity to direct the differentiation of naive Th cells into distinct Th subsets (signal 3). This appears to be mediated through transcription of a different repertoire of cytokine mRNAs in response to distinct external stimuli.28
We thank Mr Jan Urso and Ms Nancy Zurowski for skillful assistance with cell culture techniques, Dr Donna Stolz for assistance with electron microscopy, and Mr Cipriano Almonte and Dr Simon Watkins for image processing. We thank the Schering-Plough Research Institute for the gifts of cytokines.
Supported by National Institutes of Health grants DK 49745 and AI 41011 (A.W.T.) and DC Program Project grant CA 73743. A.E.M. is the recipient of an American Heart Association Scientist Development Grant, and A.T.L. is the recipient of a Dermatology Foundation Research Career Development Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Adrian E. Morelli, Department of Surgery, University of Pittsburgh Medical Center, W1540 Biomedical Science Tower, 200 Lothrop St, Pittsburgh, PA 15213; e-mail:morelli@imap.pitt.edu.

![Fig. 1. Stages of cell differentiation of murine BM DCs generated in GM-CSF + IL-4. / (A-B) Phenotype of BM DCs analyzed by flow cytometry. Cells were labeled with Cy-Chrome anti-CD11c, PE anti-CD86, and one of the following FITC-labeled mAbs: anti–MHC-I, anti–MHC-II, anti-CD40, anti-CD80, anti-CD11b, anti-CD54, or anti–OX-40L. Flow profiles illustrate the expression of specific markers on CD86− DC (gray profiles) and on CD86+ DC (open profiles, thick line). Isotype controls are represented by open profiles in dashed lines. (C) FITC-dextran and FITC-albumin uptake by CD11c+immunobead-sorted CD86− and CD86+ DC. Only CD86− DCs internalized FITC-dextran and FITC-albumin at 37°C, a phenomenon down-regulated at 0°C. Results are representative of 3 independent experiments. (D) Allostimulatory activity of γ-irradiated, FACS-sorted CD86− (▾), or CD86+ B10 DC (▿), assessed using C3H splenic T cells as responders. B10 (H2b) DC (d 7) were set up at graded concentrations with 2 × 105 responder naive C3H (H2k) T cells, and the cultures were maintained for 72 hours. [3H]TdR was added 18 hours before harvesting. The MLR stimulatory activity of freshly isolated allogeneic (B10 [○]) or syngeneic (C3H [●]) bulk spleen cells is also shown. Results are expressed as mean cpm ± 1 SD and are representative of at least 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/5/10.1182_blood.v98.5.1512/5/m_h81711484001.jpeg?Expires=1767812634&Signature=Ecye2eBWZsQFG4MElRiEY8jI7PJmLqO9PPvkvk5C8GbZWrqF7T8zy~Y9vfquwhnqVn7wSI-qdVmTaDMrUxvf0PQewm1MNVQ4H8BJ5K-AqO97LleJwoLWgTxqJow8fwslpS2RofxTYkS6c-~S--mLyQcV4mgq951JKN5llPpEn8kqLEX2vdmkpMsYnft7hCaWQeRz9gEdx0tnYXVZv9emg3sHFD3f9urydoKl7OWPTHZ8QsXD42TRH9pN3JrjFcJIgs0gOCWDGjdQmUAMNvGedsK0KLXh03NQDwUmfQbrdWncBz3wDVrOemexnwNOsd9Fbjt5xHjLljVuuOmw4XDJpw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal