Data were analyzed from 23 patients with Sézary syndrome (defined by erythroderma, more than 10% circulating atypical mononuclear cells, and peripheral blood T-cell clone) undergoing monthly extracorporeal photopheresis as the sole therapy for up to 1 year. The cohort showed a significant reduction of skin scores during treatment (P = .001). Thirteen patients (57%) achieved a reduction in skin score greater than 25% from baseline at 3, 6, 9, or 12 months (responders). Reduction in skin score correlated with reduction in the Sézary cell count as a percentage of total white cell count (P = .03). Responders and nonresponders were compared. None of the measured parameters was significantly different between the 2 groups. It was assessed whether any of the baseline parameters predicted outcome. A higher baseline lymphocyte count was significantly associated with a decrease in skin score at 6 months (P < .05). A higher baseline Sézary cell count as a percentage of total white cell count predicted a subject was more likely to be a responder after 6 months of treatment (P = .021). No other parameters predicted responder status. These data show that the modest falls in CD4, CD8, and Sézary cell counts were seen in all patients and might have resulted from lymphocyte apoptosis. This mechanism could explain the more favorable response seen in patients with higher percentages of Sézary cells in the peripheral blood. Alternatively, minimum tumor burden might be required for the induction of a cytotoxic response. Analysis of tumor-specific cytotoxic T cells is needed to investigate these possibilities further.
Introduction
Extracorporeal photopheresis (ECP) has been used to treat cutaneous T-cell lymphoma (CTCL) for more than 10 years. In ECP, photosensitized peripheral blood lymphocytes are exposed to ultraviolet A radiation in an extracorporeal circuit and reinfused into the patient. Photosensitization of the lymphocytes is achieved by administering 8 methoxypsoralen (8-MOP) by mouth to the patient 2 hours before leukopheresis or by exposing the lymphocytes to another photosensitizing agent, such as Uvadex (Therakos, West Chester, PA) (soluble 8-MOP), during the extracorporeal circuit. The efficacy of ECP in CTCL was first reported by Edelson et al in 1987.1Since then, studies have reported the therapeutic benefit of ECP in CTCL,2-10 though the response data have been variable.11 This is thought to result from differences in the entry criteria, patient selection, and intervals between diagnosis and treatment. The ECP mode of action has been the subject of considerable interest.12,13 Release of tumor necrosis factor (IFN)-α from monocyte and lymphocyte apoptosis occurs at an early stage during the ECP procedure.14-16
Additional work by Edelson's group13 has shown that photoactivated 8-MOP treatment causes an increase in antigen display by transformed lymphocytes. This increase in the expression of immunogenic peptides at the cell surface is thought to be driven by the degradation of cytoplasmic proteins into small peptides and the subsequent transport of these peptides to the major histocompatibility complex class I molecules in the endoplasmic reticulum, a process that is enhanced by 8-MOP. It is suggested that ECP increases the immunogenicity of tumor-derived peptides and that the combination of ECP-damaged neoplastic cells with antigen-presenting cells induces an enhanced cytotoxic response by autologous CD8 lymphocytes against the CD4+ T-cell clones. Although Berger et al17have shown that tumor-derived peptides are capable of eliciting a cytotoxic response in vitro, the same process has not been demonstrated in vivo, and the immunogenicity of tumor-derived peptides is likely to vary among patients. Most patients with Sézary syndrome who receive ECP have been pretreated with chemotherapy, and many receive concurrent treatment with IFN-α. This can influence their responses to ECP and their eventual outcomes. Limited data exist on patients in whom ECP was used as the sole therapy for CTCL.
In addition, this is the first study to correlate laboratory data with the clinical response to ECP of patients with erythrodermic CTCL in whom a clonal population has been confirmed in all cases by T-cell receptor (TCR) gene analysis of peripheral blood samples. Vonderheid et al9 measured serum soluble interleukin (IL)-2 receptor levels in 36 patients with erythrodermic CTCL during therapy with ECP, but in 8 of these patients no peripheral blood T-cell clone was demonstrated using Southern blot analysis of the T-cell receptor gene. Although serum-soluble IL-2 receptor levels correlated with disease activity, they did not predict response to ECP. By contrast, Gottlieb et al4 reported that an absence of Sézary cells was a factor predicting nonresponse to ECP, whereas Heald et al18 reported a poor clinical response in patients with a low initial CD8 count. However, neither study used clonality as an entry criterion, and there is no agreed upon or reproducible biologic assay for predicting response to ECP. We correlated skin score with laboratory parameters and examined which baseline variables might predict a favorable response to ECP.
Patients and methods
We analyzed data on a cohort of 23 patients with Sézary syndrome, defined by erythroderma with compatible skin histology, more than 10% circulating atypical lymphocytes, and peripheral blood T-cell clone. TCR gene analysis of peripheral blood samples was undertaken using polymerase chain reaction single-strand conformational polymorphism analysis of the TCR-γ gene with different Vγ consensus primers as previously described.19 Other investigations performed included an automated total white cell count, lymphocyte count, and T-cell subset analysis. Sézary cell counts were performed in all patients by one experienced observer (A.D.) on a May-Gruenwald-Giemsa–stained peripheral blood smear.
There were 23 patients (15 men, 8 women) whose mean age was 69.3 years (range, 43-83 years). Five patients had previously undergone intravenous chemotherapy, and 5 others had received oral chemotherapy with chlorambucil. All systemic treatment had been discontinued at least 6 weeks before photopheresis was started. The same treatment protocol was used in all patients. Patients had been given ECP as a sole systemic therapy on 2 consecutive days each month for at least 6 months using a UVAR photopheresis system (Therakos). The amount of blood removed from the patient and put through the extracorporeal circuit was calculated by machine and was based on the patient's hematocrit level. Soluble 8-MOP (Uvadex; Therakos) was used as a photosensitizing agent, in a concentration of 20 μg/mL, and was mixed with the patient's blood during the ECP circuit, averting the need for measuring serum levels. The volume of Uvadex (Therakos) required was calculated according to the following formula: [Vol Uvadex (20 μg/mL) (mL) = Vol blood collected from patient (mL) × 0.017].
Patients were assessed before therapy and at 3, 6, 9, and 12 months after its initiation. On each of these occasions, skin score was recorded as previously described.1 Responders were defined as those who achieved at least a 25% reduction in skin score from baseline, and nonresponders were defined as those who did not. Peripheral blood samples were also taken and analyzed for total white cell count, lymphocyte count, CD4 count, CD8 count, and Sézary count, as described above.
Statistical methodology
Statistical analysis was performed in 3 different ways. First, trends in the measured parameters over time, and in relation to each other and in particular skin score, were calculated using pooled within-subject linear regression analysis. Second, unpairedt tests were used to compare patients who responded to treatment and those who did not. Third, backward step-wise logistic regression was used to assess whether any of the baseline parameters predicted outcome of any other variables or responder status at 3, 6, 9, or 12 months.
Results
Twenty-three patients completed 6 months of ECP as a sole therapy, and 8 were classified as responders at this stage. Twelve patients completed 12 months of ECP without adjuvant therapy, and 8 of these were responders at this stage. Overall, 13 of the 23 patients who entered the trial were responders at 3, 6, 9, or 12 months. Three different analyses were performed. In the first analysis, changes in skin score were correlated with different parameters over the course of the study. The cohort showed a significant reduction in skin score (P = .001) during treatment with ECP, with a mean of 6.4 U/mo. Reduction in skin score was positively and significantly associated with a reduction in the Sézary count as a percentage of total white cell count (P = .03). Positive, but not statistically significant, correlations were found between reduction in skin score and reduction in absolute CD4 count (P = .24), CD8 count (P = .085), and absolute Sézary cell count (P = .082).
The second analysis undertaken was to compare responders and nonresponders. Neither the absolute change in total white cell count, lymphocyte count, CD4 count, CD8 count, or absolute Sézary count was significantly different between responders and nonresponders at 6 months. Data on the absolute and proportional Sézary counts during treatment for responders and nonresponders are shown in Table1. Neither age nor sex of the patients was significantly associated with responder status. Mean age of responders was 68.2 years compared with 63.3 years in nonresponders. The difference of 4.9 years was not statistically significant (unpaired t test P = .24; 95% confidence interval [CI], 3.5 to 13.4 years). The percentage of responders among male patients was 47% (7 of 15), and among female patients it was 75% (6 of 8). The difference (28%) was not statistically significant (P = .19; 95% CI, 11.5% to 68%). Previous administration of chemotherapy was not associated with responder status. Ten patients had undergone prior chemotherapy, and 6 were classified as responders. Other clinical parameters, such as lymphadenopathy and presence of coexistent tumors, were not related to responder status.
Change in absolute Sézary cell count and Sézary cell count as a percentage of total white cell count during extracorporeal photopheresis
| . | Time (months) . | ||||
|---|---|---|---|---|---|
| 0 . | 3 . | 6 . | 9 . | 12 . | |
| Absolute Sézary cell count (×109) | |||||
| Responders | 7.54 | 4.94 | 6.83 | 4.88 | 3.33 |
| Nonresponders | 5.45 | 5.56 | 3.51 | 3.41 | 4.17 |
| Sézary cell count as a percentage of total white cell count | |||||
| Responders | 46.2 | 38.1 | 36.1 | 39.3 | 27.9 |
| Nonresponders | 26.8 | 26.5 | 23.0 | 31.8 | 35.8 |
| . | Time (months) . | ||||
|---|---|---|---|---|---|
| 0 . | 3 . | 6 . | 9 . | 12 . | |
| Absolute Sézary cell count (×109) | |||||
| Responders | 7.54 | 4.94 | 6.83 | 4.88 | 3.33 |
| Nonresponders | 5.45 | 5.56 | 3.51 | 3.41 | 4.17 |
| Sézary cell count as a percentage of total white cell count | |||||
| Responders | 46.2 | 38.1 | 36.1 | 39.3 | 27.9 |
| Nonresponders | 26.8 | 26.5 | 23.0 | 31.8 | 35.8 |
In the third analysis, we assessed whether any baseline variable predicted change in skin score or responder status at 6 or 12 months. The only baseline variable that had a significant association with skin score at 6 months was the baseline lymphocyte count. A 1 U increase in baseline lymphocyte count (× 109/L) was associated with a mean reduction in skin score of 6 U at 6 months (P = .046; 95% CI, 0.1-12.0). No baseline variable was significantly associated with skin score at 12 months, though there were only 12 subjects in this analysis compared to 23 in the 6-month analysis, so statistical significance was harder to achieve.
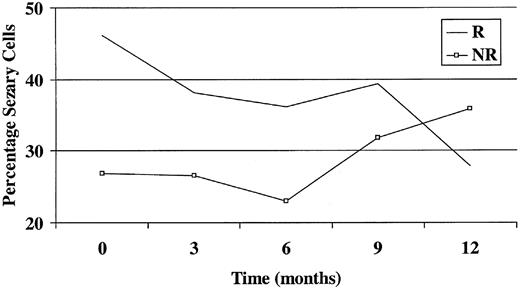
The only baseline variable that significantly predicted responder status at 6 months was the baseline Sézary count as a percentage of total white cell count (Figure 1). A 1% increase in the Sézary count as a percentage of total white cell count at baseline was associated with an odds ratio of 1.07 of becoming a responder (P = .021; 95% CI, 1.01-1.14). None of the parameters was significantly associated with responder status at 12 months, though again the numbers here were smaller.
Graph showing Sézary count as a percentage of total white cell count against time. R indicates responder; NR, nonresponder.
Graph showing Sézary count as a percentage of total white cell count against time. R indicates responder; NR, nonresponder.
Discussion
Limited data exist on patients with Sézary syndrome in whom a T-cell clone has been demonstrated and who have been treated with ECP alone. The use of chemotherapy or adjuvant therapy with IFN-α during ECP makes it difficult to recruit a large enough group of patients to provide sufficient power in statistical analysis. Patients who undergo photopheresis have widely different outcomes, and there is no reliable method of predicting clinical response. This can be a problem because ECP is expensive and time consuming, and it requires considerable input from highly trained staff. It is not widely available, and patients may have to travel long distances to a specialist center for treatment.
We monitored a number of laboratory parameters during treatment and correlated these with clinical outcome. We also attempted to find baseline variables that would predict a favorable response to ECP.
Response rates found in this study were lower than those reported previously in erythrodermic disease.1-6 However, our response rates were consistent with more recent studies that have demonstrated T-cell clones in all or most patients treated.8,9 Edelson et al1 classified their patients on the basis of reduction in skin score as follows: responders had a 25% or more reduction in skin score from baseline, whereas those with a less than 25% reduction in skin score from baseline were classified as treatment failures or as unchanged. On this basis, Edelson et al1 reported that 83% of patients with erythrodermia responded to photopheresis. In our study, 13 of 23 patients (57%) were classified as responders (more than 25% reduction in skin score from baseline) at 3, 6, 9, or 12 months. This compares with a complete or partial response (more than 50% reduction in skin score from baseline) rate of 33% reported by Vonderheid et al9 and a previous study from our own group8that reported 53% of patients achieved more than 25% reduction in skin score from baseline. The latter study included 11 of the patients involved in this study; the other patients were not included in this study because of incomplete laboratory data or concurrent chemotherapy during treatment with ECP.
We also found that a greater clinical response is achieved in those patients with a higher proportion of neoplastic cells. A higher baseline Sézary cell count as a percentage of total white cell count predicted responder status at 6 months, and a higher baseline lymphocyte count was significantly associated with a decrease in skin score. This would support the concept of a minimum tumor burden necessary for ECP to be successful, but it does not clarify its mode of action. Edelson et al's hypothesis1 that ECP induces an autologous cytotoxic T-cell response against the CD4+neoplastic clone is consistent with results of previous studies showing that low baseline CD8 counts predict a poor response to ECP.18 In our cohort, however, the mean CD8 count at baseline was 0.38, and we found no significant difference in baseline CD8 count between responders and nonresponders and no correlation between CD8 count and skin score. Studies in which it was found to be predictive did not use the presence of a circulating T-cell clone as an entry criterion, and this might have led to the inclusion of patients with reactive erythroderma and circulating Sézary cells.
Responders and nonresponders had decreases in CD4 and CD8 counts that were not significantly different from each other and occurred independently of clinical response. Blaydon and Taylor16have shown that all patients undergo lymphocyte apoptosis to some degree while undergoing ECP and that this begins in the machine itself and is presumably independent of any host immune response. This might be expected to produce modest drops in lymphocyte counts in all patients.
If one accepts the mode of action proposed for photopheresis by Edelson et al and others,12,13 17 there are several reasons ECP might fail to induce an immune response. First, the mixing of large numbers of tumor cells and antigen-presenting cells occurs only during the ECP procedure, which lasts for only 3 hours on 2 successive days each month. Thereafter, the processing of apoptotic tumor cells by dendritic cells occurs in vivo and is likely to be highly variable and dependent on the integrity of the host immune response.
Second, reinfusion of antigen directly into the peripheral blood is probably not the most effective method of inducing a cytotoxic response. Certainly, studies in malignant melanoma demonstrate a better response if tumor lysate is injected directly into tissue such as skin or lymph node.20
Third, not all tumor-derived peptides will be equally immunogenic, and some may not elicit a cytotoxic response at all. The fact that cytotoxicity assays are positive with selected clones in vitro17 does not imply that the same process occurs in every patient in vivo. This may in fact explain why so few patients develop flulike symptoms after the ECP procedure. Our data do not exclude the possibility that a cytotoxic response is induced in a minority of patients undergoing ECP, but this would require the identification of expanded tumor-specific CD8 subsets before and after the ECP procedure.
Our group is focusing on sequencing the TCR-β chain gene from the neoplastic cell population of individual patients and identifying potentially immunogenic peptide sequences. Although these can be shown to induce the release of IFN-α in an enzyme-linked immunosorbent cytotoxicity assay,21 this effect has not yet been correlated with a therapeutic response to ECP in vivo. In addition, the immunogenic response may not be directed against TCR peptides but against another tumor-associated cell surface antigen. Other mechanisms need to be considered. Lymphocyte apoptosis might explain why patients with a greater proportion of neoplastic cells within the vascular compartment respond better to ECP. However, it is unlikely to account for the dramatic improvement seen in complete responders unless one invokes the indirect effects of apoptosis on CD4 counts, seen, for example, in patients with human immunodeficiency virus.22Whatever the precise mechanism, our data indicate that a minimum tumor burden in the blood is necessary for ECP to be clinically effective and that treatment should not be offered to patients with CTCL who have no evidence of hematologic involvement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
R. Russell-Jones, Skin Tumour Unit, St John's Institute of Dermatology, St Thomas' Hospital, Lambeth Palace Rd, London SE1 7EH, United Kingdom; e-mail: russelljones@btinternet.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal