Mobilized progenitor cells currently represent the most commonly used source of hematopoietic progenitor cells (HPCs) to effect hematopoietic reconstitution following myeloablative chemotherapies. Despite their widespread use, the molecular mechanisms responsible for the enforced egress of HPCs from the bone marrow (BM) into the circulation in response to mobilizing agents such as cytokines remain to be determined. Results of this study indicate that expression of vascular cell adhesion molecule-1 (VCAM-1) is strongly reduced in vivo in the BM during HPC mobilization by granulocyte colony-stimulating factor (G-CSF) and stem cell factor. Two serine proteases, namely, neutrophil elastase and cathepsin G, were identified, which cleave VCAM-1 and are released by neutrophils accumulating in the BM during the course of immobilization induced by G-CSF. The proposal is made that an essential step contributing to the mobilization of HPCs is the proteolytic cleavage of VCAM-1 expressed by BM stromal cells, an event triggered by the degranulation of neutrophils accumulating in the BM in response to the administration of G-CSF.
Introduction
Mobilization is the redistribution of hematopoietic precursor/progenitor cells (HPCs) from the bone marrow (BM) into the peripheral blood (PB). The number of autologous transplants performed with mobilized HPCs currently exceeds those performed with BM.1 Earlier studies used PB progenitor cells (PBPCs) collected during the recovery phase following myelosuppressive chemotherapy.2 More recently, it has become evident that a variety of hematopoietic growth factors (HGFs) such as granulocyte colony-stimulating factor (G-CSF), interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), stem cell factor (SCF), Flt-3 ligand, c-MPL ligand, IL-1, or chemokines such as IL-8 administered to rodents or humans can further enhance the recovery of PBPCs.1 By far, the most commonly used agent to elicit mobilization of HPCs in patients and normal donors is G-CSF with about 30 000 transplantations a year performed with G-CSF–mobilized PB cells. Mobilization induced by G-CSF is time- and dose-dependent in rodents and in humans, the prominent features being a rapid neutrophilia followed by a delayed increase of HPC numbers in the blood peaking at levels 10- to 100-fold above baseline between 4 and 7 days after initiation of G-CSF administration. Despite the now almost universal use of PBPCs, the mechanisms responsible for the enforced egress of HPCs from the BM into PB in response to cytokines remain, to a large extent, unknown.
Recent findings suggest an important role for neutrophils in HPC mobilization induced by G-CSF, cytotoxic agents, and chemokines. Mice carrying a targeted homozygous deletion of the G-CSF receptor gene(G-CSFR−/−) exhibit a marked neutropenia despite normal numbers and function of HPCs and are refractory to HPC mobilization induced by the administration of either cyclophosphamide (CY), G-CSF, or IL-8.3 Mobilization by either CY or G-CSF is, however, restored in these G-CSFR−/− animals by prior transplantation of HPCs expressing G-CSFR. Of note, in G-CSFR−/− animals transplanted with both G-CSFR−/− and G-CSF+/+ HPCs, G-CSFR−/− and G-CSFR+/+ HPCs are mobilized in equivalent amounts,4 suggesting that mobilization is not dependent on G-CSFR expression by HPCs but rather on the number of functional neutrophils. This view is supported by studies in which mice made neutropenic by infusion of the anti–GR-1 monoclonal antibody (mAb) subsequently fail to mobilize in response to IL-8 during the neutropenic phase but do so during the subsequent neutrophilic recovery phase.5 6
In common with many groups, we have hypothesized that the retention of HPCs in hematopoietic organs is controlled by adhesive interactions.7-11 Experiments in gene-deleted mice have demonstrated the major role of the interaction between the β1-integrin, α4β1, or very late antigen 4 (VLA-4) expressed at the surface of HPCs and its counterreceptor vascular cell adhesion molecule-1 (VCAM-1/CD106), which is constitutively expressed by BM stromal cells,12 in the retention of HPCs into hemopoietic organs during development.13-15 Similarly, an important role of these 2 cell adhesion molecules (CAMs) in the mobilization of HPCs has emerged from studies in rodents and nonhuman primates demonstrating that the inhibition of VLA-4/VCAM-1 interaction following administration of function-blocking anti–VLA-4 or anti–VCAM-1 mAbs results in HPC mobilization.8 16-18
Although providing an important “proof of principle” that the perturbation of this single cell adhesive interaction can result in mobilization of primitive HPCs, these data do not provide clear insights into the physiologic mechanisms responsible for mobilization resulting from the administration of cytokines. We now report that VCAM-1 expression is reduced in the BM stroma of mice mobilized either with G-CSF alone, SCF alone, and G-CSF in combination with SCF. Moreover, we identify 2 serine proteases, neutrophil elastase (NE) and cathepsin G (CG), that both cleave VCAM-1 and are released by neutrophils accumulating in the BM during the course of G-CSF–induced mobilization.
Materials and methods
Proteases and HGFs
Human NE, CG, and proteinase-3 (P3) purified from human sputum and the rabbit antihuman NE serum were from Elastin Products (Owensville, MO). Recombinant human matrix metalloproteinase-9 (rhuMMP-9) was from R & D Systems (Minneapolis, MN). Active recombinant human granzymes B, H, and M were generously provided by Drs J. A. Trapani and M. J. Smyth (Peter MacCallum Cancer Institute). Recombinant human (rhu) G-CSF, IL-6, and SCF, and rat SCF were kindly provided by Amgen Biologicals (Thousands Oaks, CA). Human IL-3 and GM-CSF were from Sandoz Pharmaceuticals (Basel, Switzerland). Human IL-1α was a gift from Hoffman-La Roche (Nutley, NJ). The source of murine IL-3 was medium conditioned by a genetically altered mouse mammary cell line C127 expressing the mouse IL-3 complementary DNA (cDNA).19 Partially purified pregnant mouse uterus extract was used as a source of mouse CSF-1.
Cell adhesion assays
Cell adhesion assays were performed as previously described20 with the following modifications. Microwells were coated overnight at 4°C with 40 μL phosphate-buffered saline (PBS) containing 10 μg/mL rhuVCAM-1 corresponding to the extracellular domain of the mature protein (R & D Systems). When assays were performed on a monolayer of human BM stromal cells, wells were seeded with 8000 stromal cells in α-minimal essential medium (α-MEM) with 10% fetal calf serum (FCS), and grown until confluent. CD34+ HPCs were purified from BM aspirates, starved overnight at 37°C in serum-deprived medium, labeled with calcein-am and added to coated wells as previously described.20 Adhesion mediated by β1-integrin was stimulated by addition of rhuIL-3, IL-6, G-CSF, GM-CSF, and SCF as previously described.21 After a 30-minute incubation at 37°C, nonadherent cells were removed by 3 washes20,21 before addition of either conditioned media or purified proteases. Following an additional 1-hour incubation at 37°C, wells were then gently washed thrice, adherent cells were lysed, and fluorescence associated with adherent cells was quantified as previously described.20
Generation of FDCP-1 cells stably expressing rhuVCAM-1
A 2.3-kb fragment containing the full coding sequence of huVCAM-1 was generated by high-fidelity polymerase chain reaction (PCR) from a full-length VCAM-1 cDNA inserted in the pCDM8 vector (provided by Dr R. R. Lobb, Biogen, Cambridge, MA) with the primers 5′ GTGTGTGTGTGCGGCCGCAGCAACTTAAAATGCCTGGG 3′ and 5′ GTGTGTGTGTGTTAACGGAAGTCTGCCTCTCAGCTC 3′ that introduce NotI and HpaI sites at the 5′ and 3′ ends of the VCAM-1 fragment, respectively. The purified product was cut with HpaI andNotI and ligated into the pRUFNeo(NotI) retroviral expression plasmid.22 Following amplification in JM109, 2 μg purified pRUFNeo-VCAM-1 plasmid was transfected into the ecotropic packaging cell line Ψ2.23Infection of the GM-CSF–dependent myeloid murine cell line FDCP-1 and selection of transduced cells is described elsewhere.23 A clone expressing high levels of huVCAM-1 was selected by immunofluorescence staining with the mouse anti–huVCAM-1 mAb 6G10.24
Mobilization of mice
Eight-week-old female balb/c mice were mobilized by twice daily subcutaneous injections for 6 days of either huG-CSF at 250 μg/kg body weight, rat SCF alone at 100 μg/kg, or huG-CSF in combination with rat SCF. Unless specified, mice were killed for PB, spleen, and BM collection on the day following the last injection. The number of PBPCs was estimated by performing clonogenic assays with PB. Approximately 300 μL PB was aspirated into 100 μL of 10% (wt/vol) EDTA pH 7.0. Red blood cells were lysed in 0.84% NH4Cl at room temperature for 10 minutes. The white blood cells were then washed twice in PBS-5% heat-inactivated FCS, counted, and plated in a double-layer nutrient agar culture system using a combination of 4 HGFs comprising human IL-1α, mouse IL-3, mouse CSF-1, and rat SCF.19 25
Immunohistochemical staining of mouse BM
Femurs were immediately taken from killed mice and the BM was directly flushed with a syringe and a needle onto glass slides. The BM plug was then covered by a coverslip and gently spread by gentle tapping of the coverslip to prepare a monolayer. Each slide together with its coverslip was then plunged into liquid N2 for 1 minute, coverslips were removed by flicking, and the BM monolayers left on the slides were immediately fixed by immersion in 100% acetone for 10 minutes. Slides were rehydrated in PBS for 2 hours and then blocked for 2 hours in PBS containing 0.05% Tween 20 and 5% goat serum (PBSTGS). Slides were then incubated overnight at 4°C with either neat M/K2-7 hybridoma supernatant (American Type Culture Collection, Manassas, VA) or with nonimmune rat IgG1 (Pharmingen, San Diego, CA) diluted at 2 μg/mL in hybridoma growth medium. Slides were washed 5 times with Tris-buffered saline 0.05% Tween-20 (TBST) between each step. Slides were incubated for 1 hour with biotinylated goat anti–rat IgG diluted 1:250 in PBSTGS and then for 1 hour with alkaline phosphatase–conjugated streptavidin diluted 1:400 in TBST. Leukocyte alkaline phosphatase was blocked by a 10-minute incubation in TBST with 10 mmol/L levamisole. Staining was revealed by a 20-minute incubation in 0.1 mol/L Tris-HCl pH 9.0, 5 mmol/L MgCl2, 10 mmol/L levamisole, 0.25 mg/mL fast red TR, and 0.01% naphthol AS-MX phosphate. Slides were finally rinsed with water before counterstaining with hematoxylin.
Preparation of mobilized mouse BM extracellular extracts
Balb/c mice were injected for 6 days with G-CSF or saline as described above. At specified times, femurs were immediately taken from killed mice and the BM was directly flushed with a syringe and a 21-gauge needle into 1 mL ice-cold PBS without serum to prevent immediate protease inhibition by serum protease inhibitors. Following dispersion of BM cells by repetitive flushing through the needle, cells were pelleted at 400g, 4°C for 5 minutes. Supernatants containing BM extracellular fluids were taken, aliquoted, and stored at −80°C until further analysis.
Purification of human neutrophils and BM CD34+ and CD34− cells, preparation of conditioned media
Human neutrophils were purified from the PB of normal donors. Blood was mixed with 0.6 volume of a 6% (wt/vol) dextran 70 saline solution (Baxter Healthcare, Thetford, United Kingdom) and incubated for 30 minutes at 37°C to sediment erythrocytes. Leukocytes were washed twice in Hanks balanced saline containing 10 mmol/L Hepes and 5% newborn calf serum (HHN). Cells were layered over Ficoll-Paque and centrifuged at 400g for 30 minutes. Neutrophils contained in the pellet were washed twice in HHN and incubated for 10 minutes at 37°C in 0.83% NH4Cl to lyse residual erythrocytes. Neutrophils were then washed 3 times in Iscoves modified Dulbecco medium (IMDM) without serum or cytokine, resuspended at 107cells/mL, and incubated for 24 hours at 37°C to condition the medium.
CD34+ and CD34− cells were purified from BM aspirates collected from healthy donors using CD34−Dynabeads as previously described.20 Medium was conditioned by 107 BM CD34− cells/mL as described above.
Normal human bone (NHB) cells were isolated from explants of trabecular bone and maintained as previously described.26 BM stromal cells were isolated from human BM aspirates and maintained as previously described.27 BM stromal and NHB cells were grown to confluence in T75 flasks, washed 3 times with PBS before addition of 6 mL IMDM per flask. Media were conditioned for 24 hours at 37°C.
Following cell removal by centrifugation at 400g, conditioned media were stored at −80°C.
Assessment by flow cytometry of CAM shedding
The FCDP1–huVCAM-1 cells were washed 3 times in α-MEM supplemented with 0.2% bovine serum albumin (BSA). Cell aliquots of 50 μL were incubated at 37°C with 50 μL conditioned medium or purified proteases for 1 hour. In some experiments, neutrophil-conditioned medium was preincubated for 20 minutes at room temperature with protease inhibitors before addition of the cells. Protease inhibitors were used at the following concentrations: 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 50 μg/mL aprotinin, 50 μg/mL pepstatin A, 50 μmol/L leupeptin, 40 μg/mL E-64, 1 mg/mL α2-macroglobulin, and 2 μmol/L BB-94 (British Biotechnology, Oxford, United Kingdom). Proteolytic cleavage was stopped by addition of HHN with 5% goat serum. All subsequent steps were performed on ice. Cells were labeled with 10 μg/mL 6G10 mAb or the mouse IgG1 isotypic control 1B5 followed by incubation with fluorescein isothiocyante (FITC)-conjugated goat anti–mouse IgG antibody before analysis by flow cytometry. Significant differences were determined using Student t test on mean fluorescence intensities (MFIs).
The proteolytic cleavage of other CAMs such as VLA-4, VLA-5, and platelet-endothelial cell adhesion molecule-1 (PECAM-1) was assessed using the human IL-3–dependent myeloid cell line MO7e. Cells were washed and incubated with neutrophil-conditioned medium or purified proteases as described above. Treated cells were stained as described but using the mouse function blocking mAbs P4C228(anti–VLA-4), PHM229 (anti–VLA-5), and B2B130(anti–PECAM-1) or 1B5 (IgG1 control) as primary antibodies.
NE and CG enzymatic activity determination
The activity of each protease was quantified using the chromogenic substrates MeOSuc-Ala-Ala-Pro-Val-pNA and Suc-Ala-Ala-Pro-Phe-pNA (Calbiochem-Novabiochem, San Diego, CA) specific for elastase and CG, respectively.31 Purified NE was diluted to 2 μg/mL in 0.1 mol/L Tris-HCl, pH 7.5, 0.5 M NaCl, 0.01% NaN3 (NE buffer), whereas CG was diluted to 5 μg/mL in 0.1 mol/L Tris-HCl, pH 8.3, 0.01% NaN3 (CG buffer). Aliquots of 50 μL were incubated for 20 minutes at room temperature in the presence or absence of protease inhibitors at concentrations specified above. An equal volume of specific substrate at 0.4 mmol/L in the same buffer was added to each aliquot. Negative controls were performed in identical conditions but without enzyme. After 30 minutes at 25°C, reactions were stopped by addition of 0.2 mmol/L PMSF and absorbance of free pNA was read at 405 nm on an enzyme-linked immunosorbent assay (ELISA) plate reader. Reciprocal cross-contamination of purified NE and CG was examined by diluting CG in NE buffer with the NE-specific substrate and conversely diluting NE in the CG buffer with the CG-specific substrate. This experiment demonstrated no cross-contamination of either purified CG by NE or the converse.
The activity NE and CG in the BM extracellular fluid of mobilized mice was measured using 20 μL/well of mouse BM extracts instead of purified proteases. Plates were read after a 3-hour incubation at 37°C. Calibration was performed with wells containing serial dilutions of purified NE or CG.
Proteolytic digestion of rhuVCAM-1 and fragment analysis
The recombinant extracellular domain of human VCAM-1 (rhuVCAM-1) was diluted to 10 μg/mL in PBS. Aliquots of 20 μL were mixed with an equal volume of conditioned media, mouse BM extracts, or PBS containing either 2 μg/mL human NE, 2 μg/mL P3, 5 μg/mL CG, 2 μg/mL aminophenylmercuric acid–activated rhuMMP-9, 2 μg/mL granzyme B, H, or M, or no enzyme and were incubated at 37°C. At specified times, reactions were stopped by addition of an equal volume of 125 mmol/L Tris-HCl, pH 6.8, 20% glycerol, and 4% sodium dodecyl sulfate and boiling for 5 minutes. Samples were separated by electrophoresis on 10% polyacrylamide gels, transferred onto nitrocellulose, and immunoblotted with a goat antihuman VCAM-1 serum (R & D Systems) at a 1:3000 dilution and a horseradish peroxidase (HRP)-conjugated donkey F(ab)′2 fragment anti–goat IgG (Jackson Immunoresearch Laboratories, West Grove, PA) at a 1:20 000 dilution.
Mobilization of human patients
Mobilization protocols using rhuG-CSF alone or rhuG-CSF in combination with rhuSCF together with patients' profiles have been described elsewhere.32 Five female and 5 male patients, median age 50 years (range, 10-64 years), with non-Hodgkin lymphoma, who had previously had a relapse after initial chemotherapy, received rhuIL-3, 5 μg/kg per day, for 5 days followed by rhuGM-CSF, 5 μg/kg per day, for an additional 5 days. Sera were collected before and during cytokine treatment and stored at −80°C. Collected PBPCs were quantitated by plating light density cells in clonogenic assays as previously described.32
ELISAs for human soluble VCAM-1 and NE
Serum samples were diluted 100-fold in PBS supplemented with 0.1% BSA and analyzed for soluble VCAM-1 (sVCAM-1) concentration by ELISA using the R & D Systems kit.
Neutrophil elastase concentrations were measured using an in-house ELISA test. Briefly, 96-well polyvinyl assay plates (Costar, Cambridge, MA) were coated overnight at 4°C with affinity-purified goat anti–mouse IgG (H+L) immunoglobulins (Caltag Laboratories, San Francisco, CA) diluted to 5 μg/mL in 50 mmol/L NaHCO3, pH 9.4, buffer. Following blocking with 2% BSA in PBS, wells were incubated overnight at 4°C with mouse anti–human NE mAb AHN-10 (Pharmingen) diluted to 1 μg/mL in PBS 0.1% BSA. Elastase was captured for 2 hours from 50-μL patients' sera aliquots diluted 100-fold in PBS with 0.1% BSA. Wells were then incubated for 1 hour with 50 μL rabbit anti–human NE antibody at 1:9000 in PBST plus 3% BSA followed by a 1-hour incubation with 50 μL HRP-conjugated donkey F(ab)′2 fragments anti–rabbit IgG at 1:20 000. Reactions were revealed by addition of 50 μL/well K-Blue TMB substrate (ELISA Technologies, Lexington, KY) and stopped by addition of 25 μL/well 1M H2SO4, and optical densities (ODs) were measured on an ELISA plate reader at 450 nm. Calibration curves using aliquots of purified NE diluted in PBS 0.1% BSA gave a linear relationship within the range 0 to 100 ng/mL NE (r2 = 0.996).
Results
BM expression of VCAM-1 is reduced during mobilization in mice
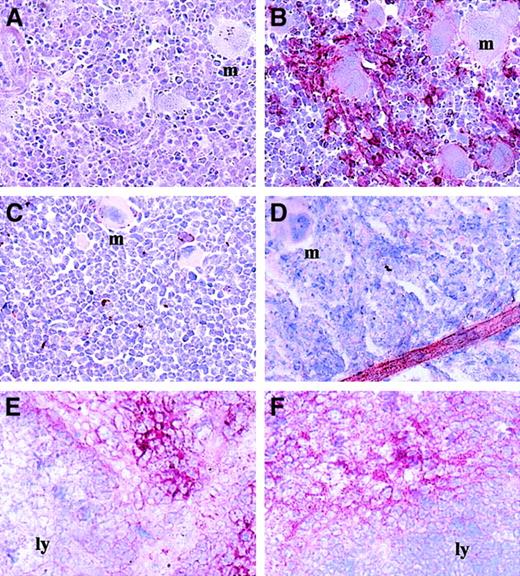
We first investigated the expression of VCAM-1 in the BM of mice mobilized with either human G-CSF, rat SCF, or a combination of both. The BM samples were processed for immunohistologic staining with the function-blocking rat anti–mouse VCAM-1 mAb M/K2-7 that binds to the VLA-4 binding site located in the first Ig-like domain of VCAM-1.33 In mice treated for 6 days with G-CSF, M/K2-7 staining in the BM stroma was dramatically decreased, whereas control mice treated with saline or untreated exhibited high expression levels of M/K2-7 functional epitope at the periphery of BM cells (Figure1). Interestingly, the expression of VCAM-1 was not reduced on blood vessels within the BM of mobilized mice (Figure 1D). Similar reduction of VCAM-1 expression was observed in the BM stroma of mice mobilized with either SCF alone or SCF in combination with G-CSF (data not shown). When PBPCs were quantitated in clonogenic assays, the decrease of M/K2-7 staining in the BM coincided with increased mobilized PBPCs.
Expression of VCAM-1 is reduced in the BM of mice mobilized with G-CSF.
BM monolayers were stained with the mAb M/K2-7 (B-F) or with an isotypic control (A). BM were isolated from mice infused for 6 days with saline (A,B) or G-CSF (C,D). Panels E and F show VCAM-1 staining on frozen sections of spleens from mice infused for 6 days with saline (E) or G-CSF (F). Some of the megakaryocytes present in these BM samples are indicated with the letter “m”; the lymphoid areas of the spleen are indicated by “ly.” Each micrograph is representative of BM monolayers from 15 to 27 different mice in each group. The red color shows VCAM-1 staining (× 250 magnification).
Expression of VCAM-1 is reduced in the BM of mice mobilized with G-CSF.
BM monolayers were stained with the mAb M/K2-7 (B-F) or with an isotypic control (A). BM were isolated from mice infused for 6 days with saline (A,B) or G-CSF (C,D). Panels E and F show VCAM-1 staining on frozen sections of spleens from mice infused for 6 days with saline (E) or G-CSF (F). Some of the megakaryocytes present in these BM samples are indicated with the letter “m”; the lymphoid areas of the spleen are indicated by “ly.” Each micrograph is representative of BM monolayers from 15 to 27 different mice in each group. The red color shows VCAM-1 staining (× 250 magnification).
The reduction of VCAM-1 expression occurred specifically in the BM. The pattern of VCAM-1 expression in the spleen is spatially arranged with circular lymphoid areas that do not express VCAM-1 with VCAM-1bright spaces between these areas (Figure 1E). After 6 days of G-CSF administration, an identical pattern of VCAM-1 expression was exhibited in enlarged spleens (Figure 1F) demonstrating that the reduction of VCAM-1 expression during G-CSF–induced mobilization is restricted to the BM.
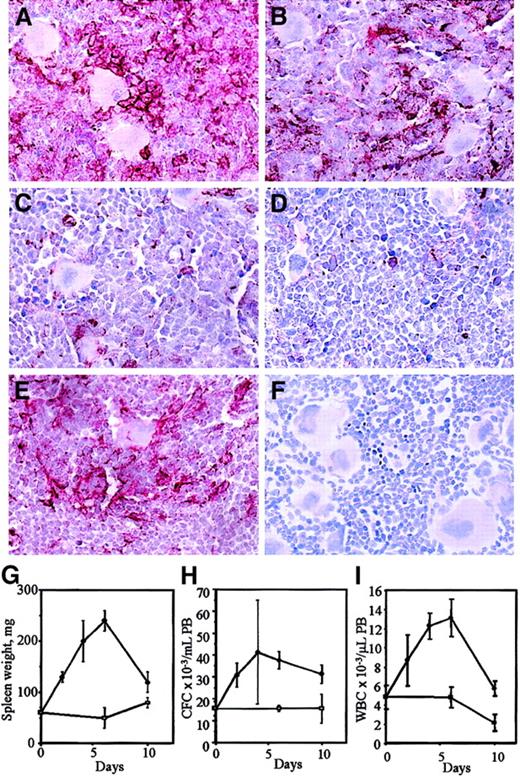
We next analyzed VCAM-1 expression in the BM during the course of G-CSF injection (days 0, 2, 4, 6) and 4 days following cessation of G-CSF administration (day 10; Figure 2). VCAM-1 expression decreased progressively from day 0 to day 4 during G-CSF injection and this decrease was maximal between days 4 and 6. This fits exactly with the progressive mobilization of HPCs as measured by spleen enlargement, the number of colony-forming cells, and leukocytes circulating in the PB. Four days after cessation of G-CSF administration, VCAM-1 expression returned to levels seen in noninjected animals. This corresponds to the normalization of spleen weights and leukocyte numbers in the PB although the number of circulating PBPCs was still elevated due to the transit of HPCs back into the BM via the circulation.
VCAM-1 expression in the BM during the course of G-CSF administration.
Mice were injected with either saline for 6 days (A) or G-CSF for 2 days (B), 4 days (C), or 6 days (D). In panels E and F, BM was taken 4 days after cessation of a 6-day course of G-CSF injection. Marrows were stained with MK/2-7 (A-E) or with an isotypic control (F). The red color shows VCAM-1 staining (× 250 magnification). Spleen weights from G-CSF– (●) and saline- (■) treated mice and the number of circulating CFCs and white blood cells (WBC) in the PB are shown in panels G, H, and I. Data represent mean ± SEM (6 mice/time point).
VCAM-1 expression in the BM during the course of G-CSF administration.
Mice were injected with either saline for 6 days (A) or G-CSF for 2 days (B), 4 days (C), or 6 days (D). In panels E and F, BM was taken 4 days after cessation of a 6-day course of G-CSF injection. Marrows were stained with MK/2-7 (A-E) or with an isotypic control (F). The red color shows VCAM-1 staining (× 250 magnification). Spleen weights from G-CSF– (●) and saline- (■) treated mice and the number of circulating CFCs and white blood cells (WBC) in the PB are shown in panels G, H, and I. Data represent mean ± SEM (6 mice/time point).
Proteases cleaving VCAM-1 are produced in the BM of G-CSF–mobilized mice
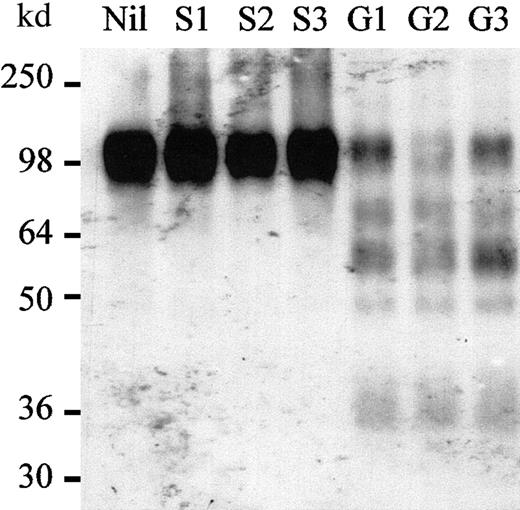
Because cells were permeabilized during the immunostaining procedure, internalized VCAM-1 should have been detected by MK/2-7. Therefore, these results suggest that VCAM-1 was shed from the surface of BM stromal cells during mobilization induced by G-CSF, SCF, or CY. This was assessed by measuring the ability of extracellular BM fluids to cleave VCAM-1 in vitro. Because purified murine VCAM-1 was not available to perform these studies, we used a commercial preparation of a 98-kd recombinant protein corresponding to the entire extracellular domain of mature human VCAM-1, which is 75.2% identical to the corresponding domain of murine VCAM-1 and promotes specific adhesion of murine cells expressing mouse VLA-4 (data not shown). The BM of femurs from G-CSF–mobilized mice was flushed into PBS and then centrifuged to remove cells. The resulting supernatants containing the extracellular fluid of the BM were incubated with purified rhuVCAM-1 and then analyzed by Western blot with a polyclonal goat antiserum raised against the extracellular domain of huVCAM-1 (Figure3). Extracts from the BM of 3 different G-CSF–mobilized mice cleaved rhuVCAM-1 in smaller fragments, whereas the extracts from 3 different saline-injected mice left rhuVCAM-1 intact. These experiments demonstrate that active proteases cleaving VCAM-1 are produced and accumulate in the BM of G-CSF–mobilized mice but remain undetectable in the BM of noninjected and saline-treated mice.
Proteases cleaving rhuVCAM-1 accumulate in BM of G-CSF–mobilized mice.
rhuVCAM-1 was incubated for 15 minutes at 37°C in the presence of either PBS (Nil), BM extracts from 3 different mice injected for 6 days with saline (S1, S2, S3), or from 3 different mice injected for 6 days with G-CSF (G1, G2, G3), and analyzed by Western blot.
Proteases cleaving rhuVCAM-1 accumulate in BM of G-CSF–mobilized mice.
rhuVCAM-1 was incubated for 15 minutes at 37°C in the presence of either PBS (Nil), BM extracts from 3 different mice injected for 6 days with saline (S1, S2, S3), or from 3 different mice injected for 6 days with G-CSF (G1, G2, G3), and analyzed by Western blot.
Normal human neutrophils produce proteases cleaving rhuVCAM-1
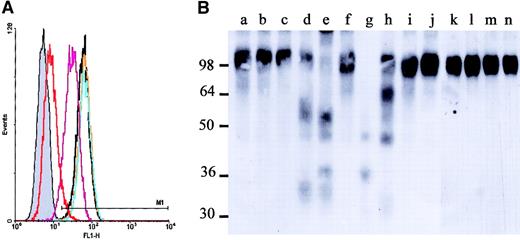
To determine which cell types secrete an activity responsible for rhuVCAM-1 cleavage and shedding, culture medium was conditioned for 24 hours either with purified normal human BM CD34− cells, BM stromal cells, bone cells, or PB neutrophils. The activities of these conditioned media were first evaluated by incubating a murine myeloid cell line FDCP-1 clone stably expressing human VCAM-1 (FDCP-1–huVCAM-1) with the different conditioned media followed by flow cytometry analyses with the function-blocking mouse antihuman VCAM-1 mAb 6G10.24 Whereas media conditioned by either human BM stromal cells or bone cells did not alter the expression of VCAM-1, media conditioned by either human BM CD34− cells (P = .003, n = 3) or PB neutrophils (P < .001, n = 6) significantly reduced the binding of 6G10 to FDCP-1–huVCAM-1 (Figure 4A). This activity was completely inhibited by preincubating these conditioned media with either the pleiotropic protease inhibitor α2-macroglobulin or the serine protease and cysteine protease inhibitor PMSF, but was not inhibited following treatment with either the cysteine protease inhibitor E-64, the aspartate protease inhibitor pepstatin A, or the MMP inhibitor BB-94 (data not shown), suggesting that this activity was due to the proteolytic cleavage of VCAM-1 by a serine protease.
Human neutrophils release proteases cleaving rhuVCAM-1.
(A) FDCP1–huVCAM-1 cells were incubated for 1 hour at 37°C in the presence of an equivalent volume of nonconditioned medium (black), NHB-conditioned media (orange), human BM stromal cell–conditioned media (BMS; blue), human BM CD34− cell–conditioned media (purple), or human PB neutrophil–conditioned media (Neut; red) and were stained with the mAb 6G10. The gray histogram is the staining with an isotypic control. Media conditioned by cells from 6 healthy donors gave similar results. (B) Western blot analysis of rhuVCAM-1 incubated for 15 minutes at 37°C in the presence of medium conditioned either by human BM stroma (lane b), NHB (lane c), human BM CD34− cells (lane d), or human PB neutrophils (lane e), or of purified human NE (lane g), CG (lane h), P3 (lane i), MMP-9 (lane j), and granzymes B (lane l), H (lane m), or M (lane n). Undigested rhuVCAM-1 is shown in lanes a, f, and k.
Human neutrophils release proteases cleaving rhuVCAM-1.
(A) FDCP1–huVCAM-1 cells were incubated for 1 hour at 37°C in the presence of an equivalent volume of nonconditioned medium (black), NHB-conditioned media (orange), human BM stromal cell–conditioned media (BMS; blue), human BM CD34− cell–conditioned media (purple), or human PB neutrophil–conditioned media (Neut; red) and were stained with the mAb 6G10. The gray histogram is the staining with an isotypic control. Media conditioned by cells from 6 healthy donors gave similar results. (B) Western blot analysis of rhuVCAM-1 incubated for 15 minutes at 37°C in the presence of medium conditioned either by human BM stroma (lane b), NHB (lane c), human BM CD34− cells (lane d), or human PB neutrophils (lane e), or of purified human NE (lane g), CG (lane h), P3 (lane i), MMP-9 (lane j), and granzymes B (lane l), H (lane m), or M (lane n). Undigested rhuVCAM-1 is shown in lanes a, f, and k.
Purified rhuVCAM-1 was then incubated with the different conditioned media and analyzed by Western blot. This analysis confirmed that both PB neutrophil- and BM CD34− cell-conditioned media contained proteases cleaving rhuVCAM-1, whereas rhuVCAM-1 remained intact when incubated with either BM stromal cell– or bone cell– conditioned media (Figure 4B). Interestingly, the patterns of VCAM-1 fragments produced by mobilized mouse BM extracts and human PB neutrophils were identical, showing that the 2 species produced proteases able to cleave rhuVCAM-1 in an identical manner.
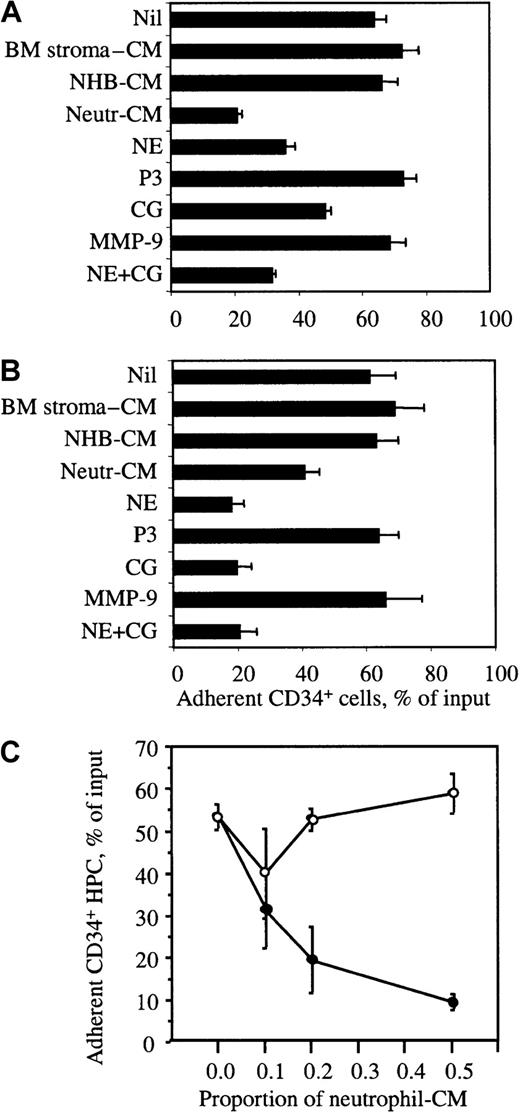
PB neutrophil– and BM CD34− cell–conditioned media release CD34+ HPCs attached to immobilized VCAM-1 or BM stromal cell monolayers
We performed an in vitro mock mobilization to determine whether conditioned media were able to release BM CD34+ HPCs adhered to immobilized rhuVCAM-1 or to pre-established human BM stromal cell monolayers. VLA-4–mediated attachment of BM CD34+HPCs was first stimulated by HGF as described in “Materials and methods.” Wells were washed to remove nonadherent cells and further incubated with conditioned media for 1 hour. Following washes to remove nonadherent cells, the number of CD34+ HPCs remaining adherent was quantified. Conditioned media from both BM CD34− cells and PB neutrophils induced the detachment of BM CD34+ HPCs previously adhered to rhuVCAM-1 or BM stromal cells (Figure5), whereas media conditioned by either BM stromal cells or bone cells did not display such activity. This effect was dose-dependent and inhibited by α2-macroglobulin, demonstrating that the in vitro release of adherent HPCs was due to the proteolytic cleavage of VCAM-1 (Figure5C).
Release of adherent human BM CD34+ HPCs.
The release of human BM CD34+ HPCs adhered to human BM stromal cell monolayers (A) or immobilized rhuVCAM-1 (B) is shown. CD34+ HPC were adhered for 30 minutes at 37°C and then incubated for 1 hour in the presence of either conditioned medium or purified proteases. The percentage of cells remaining adherent was quantified. (C) Neutrophil-conditioned medium was preincubated without (●) or with 1 mg/mL α2-macrobulin (○) before addition to CD34+ HPC adhered to BM stromal cell monolayers. Results are expressed as the mean of triplicates ± SD. The experiment was repeated 3 times. CM indicates conditioned medium.
Release of adherent human BM CD34+ HPCs.
The release of human BM CD34+ HPCs adhered to human BM stromal cell monolayers (A) or immobilized rhuVCAM-1 (B) is shown. CD34+ HPC were adhered for 30 minutes at 37°C and then incubated for 1 hour in the presence of either conditioned medium or purified proteases. The percentage of cells remaining adherent was quantified. (C) Neutrophil-conditioned medium was preincubated without (●) or with 1 mg/mL α2-macrobulin (○) before addition to CD34+ HPC adhered to BM stromal cell monolayers. Results are expressed as the mean of triplicates ± SD. The experiment was repeated 3 times. CM indicates conditioned medium.
NE and CG cleave VCAM-1 and release CD34+ HPCs attached to immobilized VCAM-1 or BM stromal cell monolayers
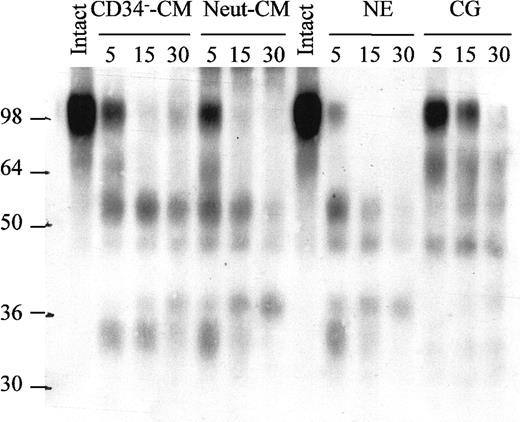
The major proteases released by neutrophils are MMP-9, CG, NE, and P3.34 rhuVCAM-1 was incubated with each of these enzymes and analyzed by Western blot. Both NE and CG cleaved rhuVCAM-1, whereas MMP-9 and P3 did not (Figure 3B). As a further control, VCAM-1 was also incubated in the presence of recombinant human serine proteases produced by cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells such as granzymes B, H, and M. None of these enzymes cleaved rhuVCAM-1 in vitro. When the pattern of VCAM-1 fragments generated by NE and CG was examined in more detail by performing a time-course, media conditioned by PB neutrophils or BM CD34− cells, purified NE and CG generated the same pattern of fragments (Figure 6). To further document the role of NE and CG, we performed enzymatic colorimetric assays with chromogenic substrates specific for each enzyme. In accord with experiments performed with neutrophil-conditioned medium, pretreatment of CG or NE with PMSF inhibited the cleavage of each of the specific substrates, whereas leupeptin, E-64, pepstatin A, or BB-94 did not (data not shown).
Time-course of huVCAM-1 degradation by NE, CG, BM CD34− cell– and PB neutrophil–conditioned media.
rhuVCAM-1 was incubated at 37°C for 0, 5, 15, or 30 minutes in the presence of either human CD34− cell– or neutrophil-conditioned media, purified human NE, or purified human CG and analyzed by immunoblotting.
Time-course of huVCAM-1 degradation by NE, CG, BM CD34− cell– and PB neutrophil–conditioned media.
rhuVCAM-1 was incubated at 37°C for 0, 5, 15, or 30 minutes in the presence of either human CD34− cell– or neutrophil-conditioned media, purified human NE, or purified human CG and analyzed by immunoblotting.
Cell adhesion assays on immobilized rhuVCAM-1 and BM stromal cell monolayers were performed as described above but using purified human NE, CG, MMP-9, and P3. As anticipated, both NE and CG induced the release of BM CD34+ HPCs previously attached to immobilized rhuVCAM-1 or BM stromal cells, whereas MMP-9 or P-3 had no effect (Figure 5A,B).
NE and CG do not cleave β1-integrins VLA-4, VLA-5, nor PECAM-1
Previous studies have suggested that cytokine-induced mobilization in humans is accompanied by alterations of the level of cell surface expression of a number of CAMs on HPCs.35 The current study suggests the possibility that the apparent reduction in the expression of these CAMs may be due to proteolytic degradation. To investigate this possibility, the CD34+ cytokine-dependent human myeloid cell line MO7e that expresses a similar pattern of CAMs as normal HPCs,21 36 was treated for 1 hour at 37°C with neutrophil-conditioned medium or purified NE or CG, following which the cells were analyzed by flow cytometry for their expression of VLA-4, VLA-5, and CD31 using mAbs to function-blocking epitopes. Whereas the expression of the 6G10 functional epitope of VCAM-1 on FDCP-1–huVCAM-1 cells was strongly reduced following exposure to either neutrophil-conditioned medium, NE, or CG, none of these enzymes altered the expression of VLA-4, VLA-5, and PECAM-1 functional epitopes (Table1).
NE and CG cleave VCAM-1 but neither VLA-4, VLA-5, nor PECAM-1
| Antigens . | No treatment . | Neutrophil-CM . | NE . | CG . | NE + CG . |
|---|---|---|---|---|---|
| VCAM-1 | 39.6 | 6.4* | 6.2* | 12.7* | 5.6* |
| VLA-4 | 186.8 | 190.6 | 183.3 | 173.3 | 184.4 |
| VLA-5 | 111.6 | 115.8 | 99.8 | 101.2 | 98.1 |
| PECAM-1 | 153.9 | 158.1 | 134.5 | 149.0 | 135.9 |
| Antigens . | No treatment . | Neutrophil-CM . | NE . | CG . | NE + CG . |
|---|---|---|---|---|---|
| VCAM-1 | 39.6 | 6.4* | 6.2* | 12.7* | 5.6* |
| VLA-4 | 186.8 | 190.6 | 183.3 | 173.3 | 184.4 |
| VLA-5 | 111.6 | 115.8 | 99.8 | 101.2 | 98.1 |
| PECAM-1 | 153.9 | 158.1 | 134.5 | 149.0 | 135.9 |
FDCP1-huVCAM-1 (VCAM-1 staining) and MO7e cells (VLA-4, VLA-5, and PECAM-1 staining) were incubated for 1 hour at 37°C in the presence of neutrophil-conditioned medium, NE, or CG and were labeled with specific mAbs before analysis by flow cytometry. Settings were optimized to obtain MFI values between 1.4 and 1.6 with nonbinding isotype-matched control antibodies. Values are expressed as MFI and significant differences with untreated cells (P < .01, n = 3) are shown with an asterisk. CM indicates conditioned medium.
Increase of proteolytically active NE and CG in the BM of G-CSF–mobilized mice
Having identified 2 proteases cleaving VCAM-1 in humans, we measured the amount of catalytically active NE and CG present in mobilized mouse BM extracellular fluids. Proteolytic activities were measured in a colorimetric assay using chromogenic substrates specific for each enzyme. The assays were calibrated using dilutions of purified human NE and CG. Whereas active NE was below detection levels (< 0.01 μg/femur) in the BM of saline-treated mice, it increased up to 0.96 ± 0.36 μg/femur following 6 days of G-CSF administration before returning to basal levels 4 days following the last injection of G-CSF (Table 2). Following the same pattern, active CG increased 15-fold in the BM of G-CSF–treated mice before returning to basal levels 4 days following the last injection of G-CSF. Considering that the BM volume in the femur of an 8-week-old mouse is approximately 10 μL and that less than 10% of this volume is made of extracellular fluid, these amounts correspond to estimated concentrations of 1 mg/mL NE and 4 mg/mL CG in the BM extracellular fluid of G-CSF–mobilized mice. These concentrations are 500 times higher than those used in the in vitro rhuVCAM-1 digestions.
Catalytically active NE and CG in the BM extracellular fluid of G-CSF–mobilized mice
| . | Active NE, μg/femur . | Active CG, μg/femur . |
|---|---|---|
| Saline 6 d | 0.01 ± 0.02 (n = 6) | 0.26 ± 0.22 (n = 6) |
| G-CSF 2 d | 0.95 ± 0.59* (n = 3) | 1.52 ± 1.03* (n = 3) |
| G-CSF 4 d | 1.00 ± 0.17† (n = 3) | 3.27 ± 0.19† (n = 3) |
| G-CSF 6 d | 1.04 ± 0.32† (n = 6) | 4.04 ± 0.92† (n = 6) |
| G-CSF 6 d + 4 | 0.06 ± 0.01 (n = 3) | 0.69 ± 0.07 (n = 3) |
| . | Active NE, μg/femur . | Active CG, μg/femur . |
|---|---|---|
| Saline 6 d | 0.01 ± 0.02 (n = 6) | 0.26 ± 0.22 (n = 6) |
| G-CSF 2 d | 0.95 ± 0.59* (n = 3) | 1.52 ± 1.03* (n = 3) |
| G-CSF 4 d | 1.00 ± 0.17† (n = 3) | 3.27 ± 0.19† (n = 3) |
| G-CSF 6 d | 1.04 ± 0.32† (n = 6) | 4.04 ± 0.92† (n = 6) |
| G-CSF 6 d + 4 | 0.06 ± 0.01 (n = 3) | 0.69 ± 0.07 (n = 3) |
The number of mice analyzed in each group is indicated between brackets. Each measurement was performed in duplicate.
P values between .05 and .01 compared to saline 6 days values (Student t test).
P values below 10−4 compared to saline 6 days values (Student t test).
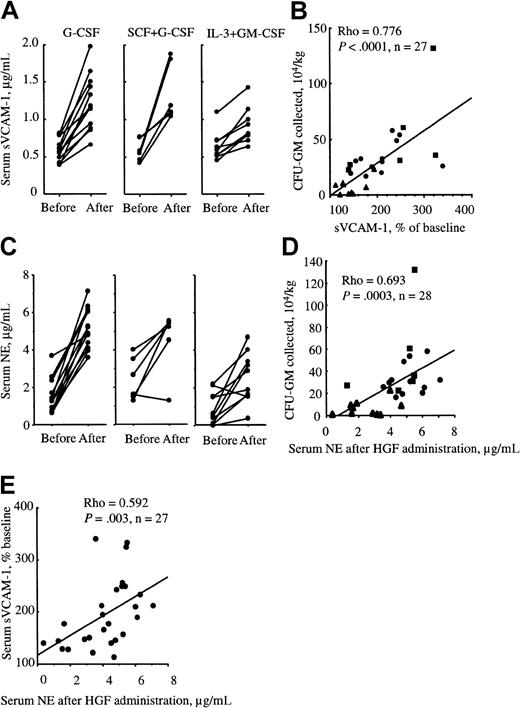
Increase of sVCAM-1 and NE in serum is correlated with the number of mobilized HPCs in humans
To determine whether VCAM-1 is also cleaved during mobilization in humans, we measured the concentration of sVCAM-1 in the serum of 3 cohorts of patients. Patients were mobilized with either G-CSF alone, G-CSF in combination with SCF, or IL-3 in combination with GM-CSF. The concentrations of sVCAM-1 were measured just before the start of mobilizing treatment and on the last day of treatment before collection of PBPCs. Serum concentrations of sVCAM-1 significantly increased in patients undergoing the 3 mobilization protocols (Figure7A). Moreover, when the relative increase of sVCAM-1 serum concentration was plotted versus the number of collected granulocyte-macrophage colony-forming units (CFU-GMs), we found a significant correlation between these 2 parameters irrespective of the means used to elicit mobilization (Figure 7B). This observation is therefore consistent with the possibility that VCAM-1 is also cleaved or shed in response to an agent produced during mobilization in humans. Similarly, serum concentrations of NE on collection day were significantly increased compared to basal levels and correlated significantly to the number of mobilized CFU-GMs (Figure 7C,D). Interestingly, the serum concentrations of NE were also significantly correlated to sVCAM-1 concentrations on collection day (Figure7E).
The concentration of sVCAM-1 and NE is enhanced in the serum of mobilized patients.
(A) sVCAM-1 concentration in the serum of patients mobilized with either rhuG-CSF alone (●), rhuG-CSF in combination with rhuSCF (▪), or rhuIL-3 in combination with rhuGM-CSF (▴) taken just prior to the commencement of HGF administration and on blood collection day. Each pair of dots represents a different patient. (B) These results are expressed versus the number of mobilized CFUs collected by apheresis on collection day. (C) NE concentration in the serum of patients mobilized with either rhuG-CSF alone, rhuG-CSF in combination with rhuSCF, or rhuIL-3 in combination with rhuGM-CSF taken just before the start of HGF administration and on blood collection day. NE concentrations on collection day are plotted versus the number of CFUs collected in PB (D) or versus the increase of sVCAM-1 plasma concentration (E). Correlations and significances were determined with the nonparametric Spearman correlation statistic.
The concentration of sVCAM-1 and NE is enhanced in the serum of mobilized patients.
(A) sVCAM-1 concentration in the serum of patients mobilized with either rhuG-CSF alone (●), rhuG-CSF in combination with rhuSCF (▪), or rhuIL-3 in combination with rhuGM-CSF (▴) taken just prior to the commencement of HGF administration and on blood collection day. Each pair of dots represents a different patient. (B) These results are expressed versus the number of mobilized CFUs collected by apheresis on collection day. (C) NE concentration in the serum of patients mobilized with either rhuG-CSF alone, rhuG-CSF in combination with rhuSCF, or rhuIL-3 in combination with rhuGM-CSF taken just before the start of HGF administration and on blood collection day. NE concentrations on collection day are plotted versus the number of CFUs collected in PB (D) or versus the increase of sVCAM-1 plasma concentration (E). Correlations and significances were determined with the nonparametric Spearman correlation statistic.
Discussion
Previous attempts to identify the molecular mechanisms of cytokine-induced mobilization focused on possible alterations in HPC adhesive properties. We now report that mobilization induced by cytokines results in a dramatic change in the hematopoietic microenvironment in the BM. Specifically, the expression of VCAM-1 is dramatically reduced in vivo in the BM of mice mobilized either by G-CSF alone, SCF alone, or SCF in combination with G-CSF. This decrease in VCAM-1 expression followed the same kinetics as those of HPC mobilization and was restricted to the extravascular compartment of the BM. VCAM-1 expression in the spleen, an alternative hematopoietic organ in mice in which hematopoiesis relocates following HPC mobilization from the BM, was not altered. A number of investigators have demonstrated that the inhibition of the adhesive interaction between VCAM-1 and its receptor VLA-4 by infusion of either function-blocking anti–VCAM-1 or anti–VLA-4 mAbs is sufficient to promote mobilization in vivo in rodents and primates.8 16-18 Therefore, a strong reduction of VCAM-1 expression in the BM should, by the same token, be sufficient to promote HPC mobilization.
We then investigated the molecular mechanisms responsible for the reduction of VCAM-1 expression in the BM. In mice, administration of G-CSF induced the production and accumulation in the BM of proteases with the ability to directly cleave VCAM-1. In human patients, sVCAM-1 concentrations increased in the plasma during mobilization and this increase correlated with the number of mobilized CFU-GMs suggesting that VCAM-1 is shed by proteolytic cleavage during mobilization in both mice and humans. We next identified 2 serine proteases, NE and CG, that in common with media conditioned by either human PB neutrophils or BM CD34− cells, induced the detachment of human BM CD34+ HPCs adhered to either immobilized rhuVCAM-1 or BM stromal cell monolayers and cleaved rhuVCAM-1 generating an identical pattern of fragments. Consistent with these data, we found that the concentration of NE in the plasma of mobilized patients increased during mobilization induced by either G-CSF, G-CSF plus SCF, or IL-3 plus GM-CSF and that this increase was significantly correlated to both the number of CFU-GMs mobilized in PB and the rise of the sVCAM-1 plasma concentration. Finally, the concentrations of both active NE and CG increased dramatically in the extracellular fluid of the BM of mice mobilized with G-CSF.
We therefore propose that an important step of HPC mobilization by G-CSF is the disruption of the adhesive interaction between VCAM-1 and VLA-4 as a consequence of VCAM-1 cleavage by neutrophil proteases such as NE and CG, released by neutrophils accumulating in the extravascular compartment of the BM following cytokine administration. This is consistent with the view that neutrophils play a key role in mobilization and with previous data showing neutrophil degranulation37-39 and a burst of IL-8 in the plasma,40 a chemokine inducing neutrophil degranulation, during the course of G-CSF administration in humans in vivo. Finally, our proposition is consistent with the observation that HPCs relocate to the spleen of mobilized mice because after G-CSF administration, high levels of VCAM-1 expression are maintained in this organ. Therefore, PBPCs may be sequestered in the spleen due to persistent VCAM-1 expression.
The restriction of VCAM-1 cleavage to the extravascular compartment of the BM may be due to the presence of large amounts of α2-macroglobulin and α1-antitrypsin in the blood (3 and 1.5 mg/mL, respectively) that both irreversibly inhibit proteases such as NE and CG thus preventing VCAM-1 cleavage in the blood and from the lumen of blood vessels. Irreversible inhibition in the blood also prevents active proteases to reach the spleen or any distant organ via the circulation thus allowing persistent VCAM-1 expression in the spleen. Conversely, the concentration of these protease inhibitors in the extravascular compartment of the BM is not sufficient to inhibit VCAM-1 cleavage by the large amount of active enzymes released by neutrophils generated in the latter compartment (Table 2).
Finally, our model does not exclude a role for other players in mobilization. We have identified NE and CG as the major proteases in normal mice and humans, with the ability to cleave VCAM-1. This does not, however, exclude the possibility that other cell surface or secreted proteases may cleave VCAM-1 and other CAMs relevant to HPC homing and mobilization. Moreover, the administration of HGFs may alter the function of the BM stroma and the production by this tissue of proteases and chemokines such as SDF-141,42 whose role in the retention and mobilization of HPCs has been demonstrated. Similarly, other CAMs such as PECAM-1/CD31, which is not cleaved by neutrophil proteases, are likely to play an active role in the transendothelial migration of CD34+ HPCs.43Previous studies, however, have unambiguously demonstrated that the inhibition of the VLA-4/VCAM-1 interaction with antibodies results in mobilization.8 16-18 Therefore, the disruption of VCAM-1/VLA-4 interaction by proteolysis of VCAM-1 may be one of the essential steps contributing to the mobilization of HPCs following G-CSF administration.
In conclusion, in addition to this new role for bactericidal proteases in the regulation of hematopoietic precursor trafficking, this mechanism may be essential to the down-regulation of inflammation by dampening neutrophil recruitment and extravasation through VCAM-1–expressing inflamed vasculature. Mobilization could therefore be a supraphysiologic manifestation of this negative feedback following systemic administration of agents promoting the expansion of neutrophils residing in the BM.
We thank Dr Ivan Bertoncello, Dr Ingrid G. Winkler, and Ms Brenda Williams for their grateful help.
Supported in part by the National Health and Medical Research Council of Australia (grants no. 080193 and 145711), Medvet Science (Adelaide, Australia), and Kirin Breweries (Y.T.) (Tokyo, Japan).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jean-Pierre Lévesque, Peter MacCallum Cancer Institute, Stem Cell Biology Laboratory, Locked Bag 1, A'Beckett St, Melbourne, VIC 8006, Australia; e-mail:jp.levesque@pmci.unimelb.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal