Despite being inert and nontoxic, implanted biomaterials often trigger adverse foreign body reactions such as inflammation, fibrosis, infection, and thrombosis. With regard to the inflammatory responses to biomaterial implants, it was previously found that a crucial precedent event was the spontaneous adsorption and denaturation of fibrinogen on implant surfaces. It was further found that interactions between the phagocyte integrin Mac-1 (CD11b/CD18) and one short sequence within the fibrinogen D domain (γ190-202; P1) at least partially explained phagocyte accumulation on implant surfaces. However, the reason that adsorbed fibrinogen is proinflammatory—while soluble fibrinogen clearly is not—remained obscure. In this study, therefore, the question of how fibrinogen is converted to a proinflammatory state when adsorbed to biomaterial surfaces is investigated. In soluble fibrinogen, the 13 amino acid P1 sequence was found to be hidden. However, the adsorption and denaturation of fibrinogen on the surfaces of commonly used biomaterials lead to the exposure of P1 and a second neo-epitope, γ377-395 (P2), which also interacts with Mac-1 and is similarly occult in the soluble protein. The extent of biomaterial-mediated P1 and P2 exposure appears directly related to the severity of inflammatory responses to a test panel of biomaterials. Finally, thrombin-mediated conversion of fibrinogen to fibrin also exposes both P1 and P2 epitopes. These observations may help explain both the inflammation caused by many types of implanted biomaterials and that which occurs naturally following thrombotic events.
Introduction
In general, commonly used biomaterials are physically and chemically stable, nonimmunogenic, and nontoxic. Despite this, implanted and blood-contact biomaterials trigger a wide variety of unwanted responses, including inflammation,1-5thrombosis,6,7 infection,8,9 and fibrosis.10-13 In many cases, these adverse responses are associated with the rapid accumulation of large numbers of phagocytic cells.2,3,14 15 Given the inert and nontoxic nature of these materials, it is not clear how the host detects and then responds to the presence of biomaterial implants.
Implanted materials very quickly acquire a layer of host proteins, well before the arrival of inflammatory cells. Therefore, it is generally believed that phagocytes interact with the spontaneously adsorbed proteins rather than with the material itself. Consequently, we and others have hypothesized that the species and state of adsorbed proteins probably influence or absolutely dictate subsequent phagocyte responses.14-18 To test this hypothesis, we had earlier implanted protein-precoated polyethylene terephthalate (PET) disks intraperitoneally in Swiss Webster mice and quantified the numbers of adherent phagocytes to estimate the influence of surface protein type on acute inflammatory responses.14,15 Our results indicated that adsorbed fibrinogen is primarily responsible for the accumulation of phagocytes on implant surfaces. In support of this, implants precoated with serum or with hypofibrinogenemic plasma fail to exhibit phagocyte accumulation, although the addition of physiological concentrations of fibrinogen to these coatings fully restores their proinflammatory properties. Perhaps the most direct supporting evidence is that profoundly hypofibrinogenemic mice do not mount inflammatory responses to implanted PET unless the material is precoated with purified fibrinogen or the mouse peritoneal fluid is made to contain fibrinogen before implantation.15
To determine which domain(s) of the very large (approximately 340 000 d) fibrinogen molecule might be important in triggering this inflammatory response, we precoated implants with purified fragments of fibrinogen and determined that the proinflammatory sequences reside within the fibrinogen D30 domain.19 A peptide sequence, P1 (γ190-202), within D30, was previously shown to interact with the phagocyte integrin Mac-1 (CD11b/CD18). The importance of Mac-1 in these responses is supported by the observation that both CD11b knockout and CD18 knockout mice, which exhibit normal phagocyte recruitment to the sites of experimental implants, fail to accumulate phagocytes on implant surfaces.20 21
Despite this limited progress, the reasons that adsorbed, but not solution-phase, fibrinogen is proinflammatory remained unknown. Therefore, we have investigated the events involved in the surface-mediated conversion of fibrinogen to a proinflammatory state and have attempted to determine whether the interactions of fibrinogen with different biomaterials might explain why they trigger inflammatory responses of varying severity. Overall, the results indicate that fibrinogen adsorption to biomaterial surfaces exposes 2 normally occult epitopes, P1 (γ190-202) and P2 (γ377-395), as does thrombin-mediated conversion of fibrinogen to fibrin. Phagocytes may recognize fibrinogen adherent to medical implants as fibrin and respond by launching a series of inflammatory and wound-healing responses commonly initiated by fibrin clot formation.
Materials and methods
Materials
Human albumin (AlbumarC 25%) was purchased from Baxter Healthcare (Glendale, CA). Horseradish peroxidase, guaiacol (o-methoxyphenol), hydrogen peroxide (30% solution), eserine (physostigmine), o-nitrophenyl butyrate, β-nicotinamide adenine dinucleotide, reduced form (NADH) (from yeast), sodium pyruvate, Triton X-100 (octyl phenoxy polyethoxyethanol), EDTA, glass beads (0.3 cm diameter), 2-(N-morpholino)ethane sulfonic acid (MES), N-hydroxysulfosuccinimide (NHS), and complete and incomplete Freund adjuvant were obtained from Sigma (St Louis, MO). We purchased 1-ethyl-3-(3-dimethyl-aminopropyl)carbodiimide hydrochloride (EDC) from Pierce (Rockford, IL). Human 125I-fibrinogen was purchased from Amersham Pharmacia Biotech UK (Buckinghamshire, England). Monoclonal antibody (clone 4-2) against fibrinogen P2 sequence was purchased from Accurate Chemical and Scientific (Westbury, NY).
This study used 5 commonly used biomaterials.1Polyethylene terephthalate (“Mylar”; PET) film (type A, 0.005-mm thick) was obtained from Cadillac Plastic and Chemical (Birmingham, MI).2 Low-density polyethylene (LDPE), a well-characterized NHLBL reference material, was purchased from Abiomed R&D (Danvers, MA).3 Films of polydimethyl siloxane (PDMS) were obtained from Dow Corning (Midland, MI).4Polyether urethane (PEU) 55D films were generously donated by Dr James R. Keogh of Medtronic (Minneapolis, MN).5 Polyvinyl chloride (PVC) films were kindly provided by Dr Yeong Hua Huang of Sherwood-Davis & Geck (St Louis, MO).
Purification of fibrinogen and generation of proteolytic fragments
For reasons of economy, human fibrinogen was used in both in vitro and in vivo experiments reported here. This was possible because our earlier results indicated that no immune response to human fibrinogen occurs in our short-term in vivo mouse implantation model.15 Fibrinogen was purified from fresh human blood by differential ethanol precipitation.19,22 Plasmin degradation fragments D100 (105 kd molecular weight) and E50 (50 kd molecular weight) were prepared by digestion of fibrinogen in the presence of 5 mM CaCl2 and plasmin (0.013 U/mg fibrinogen). The D and E fragments were separated by ion exchange chromatography (CM sephadex C-50). In the presence of 5 mM EDTA and 2 M urea, fragment D100 was further degraded to fragment D30 (30 kd molecular weight) by plasmin and purified by ion exchange chromatography.19These fragments were not cross-contaminated as judged by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. All of these proteins were stored at −70°C and thawed at 37°C before use.
Peptide synthesis and characterization
Fibrinogen P1 (γ190-202), P2 (γ377-395), and scrambled peptides were made by solid-phase synthesis by means of Fmoc (9-fluorenylmethoxycarboxyl) chemistry in a peptide synthesizer (model 431A) (Applied Biosystems, Foster City, CA). After synthesis, the peptides were deprotected and cleaved from the resin with 95% trifluoroacetic acid. The structure of the peptides was verified by amino acid analysis and by molecular mass analysis with electrospray mass spectrometry. Lyophilized crude peptides were purified by preparative reverse-phase high-pressure liquid chromatography on a C-18 column. Variant peptides of both P1 and P2 peptides were synthesized with the addition of the residues K-Y (single-letter amino acid codes) at the NH2 terminus to provide a site for cross-linking to albumin. The amino acid sequences of P1 and P2 are G(190)WTVFQKRLDGSV(202) and Y(377)SMKKTTMKIIPFNRLTIG(395),19,23 24 respectively (single-letter amino acid codes). Two scrambled control peptides were produced on the basis of the constituent amino acids of P1 and P2 having the sequences FRLGWVQTSVDKG and RFSRLKGWTSVGDKITMSG, respectively.
Conjugation of the peptides to albumin
The synthetic peptides, P1, P2, and the scrambled variants were chemically conjugated to human albumin by means of EDC. Earlier investigations indicate that the coupling of synthetic peptides to larger carrier proteins improves their interactions with cells.25-27 The conjugation of the peptides to albumin was done by means of a modified 2-step coupling procedure described earlier.19,28 29 Briefly, equal amounts (wt/wt) of the peptides and human albumin were solubilized in activation buffer (0.1 M MES, 0.5 M NaCl, pH 6.0). The reaction was started by addition of EDC (final concentration, 2 mM) and NHS (final concentration, 5 mM). After reaction at room temperature for 3 hours, the peptide-albumin complexes were dialyzed extensively against hypertonic phosphate-buffered saline (PBS) (100 mM sodium phosphate plus 0.8% NaCl, pH adjusted to 7.3) to remove excess linker and uncoupled peptides.
Preparation of biomaterial disks
Disks of 1.2 cm diameter were cut from the test materials PET, PVC, LDPE, PEU, and PDMS. The disks were stirred for longer than 96 hours in multiple changes of 70% ethanol to remove dust and sterilize the surface and were then stored in 100% ethanol. Prior to use, the disks were hydrated by immersion in sterile, pyrogen-free saline for at least 1 hour. To generate stable protein coatings, test surfaces were incubated with human albumin (1000 μg/mL), fibrinogen (200 μg/mL), or peptide-albumin (200 μg/mL) at room temperature on a rotary shaker (25 rpm) overnight (approximately 16 hours) under sterile conditions. Any unoccupied surface on the coated disks was blocked by subsequent incubation with unmodified human albumin (100 μg/mL) for 3 hours. Our recent results have demonstrated that, in such conditions, most surface-bound proteins (more than 60%) are irreversibly adsorbed (or denatured) onto the surfaces15,19 30 and will therefore remain on material surfaces for the duration of the experiments. These disks were directly used for in vitro measurements of exposed fibrinogen epitopes by enzyme-linked immunosorbent assays (ELISAs) and in vivo inflammatory responses in animal implantation experiments. Control studies employing125I-labeled albumin indicated that the amounts of adsorbed protein were about 300 to 500 ng/cm2, roughly equivalent to a continuous monolayer.
Preparation of fibrin-coated glass beads
Fibrin-coated glass beads were produced by incubating 6 glass beads with 200 μL fibrinogen (4000 μg/mL in isotonic PBS; 50 mM sodium phosphate plus 100 mM NaCl, pH 7.4) in individual wells of a 48-well plate. Following the addition of fibrinogen, 0.5 μL (1 U/μL) thrombin was added to each well, and the glass beads were rolled in the wells to generate a homogeneous fibrin coating. Surface fibrin was then allowed to fully polymerize at 37°C for 5 minutes. After removal of the nonadherent fibrin, the glass beads were transferred to new wells for ELISA measurements of exposed fibrin epitopes as described below.
Immunization and preparation of monoclonal antibody against P1 peptide
An immunoglobulin-M monoclonal antibody (mAb) against P1 (mAb D8D8) was prepared according to procedures described by Kohler and Milstein.31 Briefly, female Balb/c mice were injected intraperitoneally with 200 μg bovine serum albumin (BSA)–conjugated synthetic P1 peptide (BSA-P1) in Freund complete adjuvant and twice more at 2-week intervals with 200 μg BSA-P1 in Freund incomplete adjuvant. An intraperitoneal injection of 200 μg BSA-P1 in 0.15 M NaCl was given 3 days before fusion. Spleen cells were harvested from immunized mice and fused with myeloma cells in the presence of 40% (wt/vol) polyethylene glycol 4000. Using an ELISA described below, we isolated a hybridoma cell line (D8D8) that produced monoclonal antibody against the P1 sequence.
Measurement of the amounts of surface-exposed P1 and P2 peptides
An ELISA procedure was developed to measure the extent of P1 and P2 exposure on various surfaces and fibrin(ogen) preparations. Test surfaces (disks or beads) precoated with different proteins, peptide-albumin conjugates, or fibrin preparations were incubated with the monoclonal antibodies (1:1000 dilution) in blocking buffer (isotonic PBS containing 0.5% BSA plus 0.05% Tween-20) at 37°C for 2 hours. After rinsing 3 times with washing solution (isotonic PBS containing 0.05% Tween-20), test surfaces were immersed in solutions of secondary antibody conjugated with horseradish peroxidase at 37°C for 2 hours. Unbound secondary antibodies were removed by washing 3 times in PBS, and test samples were transferred to new plates before color development. Color was developed by means of 1-Step Turbo TMB-ELISA (Pierce) and read at 450 nm with a Spectra Max 340 plate reader (Molecular Devices, Sunnyvale, CA).
Implantation of biomaterial disks
As an in vivo model for assessing the extent of inflammatory responses to various materials, sterile biomaterial disks were implanted intraperitoneally in male Swiss Webster mice (20 to 25 g body weight) (Taconic Farms, Germantown, NY) as described earlier.14 15 To reduce possible experimental variations due to age, different shipment batches, or even the season of the year, only mice from the same shipment were used in individual experiments, and control groups were included in every experiment for comparison. The values shown in all graphs represent the results of single implantation experiments using 5 animals per treatment. It should be noted that all experiments shown were performed at least 3 times with very similar results.
Explantation of biomaterial disks
Explantation was performed at 16 hours after implantation (earlier found to be the time of maximal phagocyte accumulation). After careful removal from the peritoneal cavity, explanted disks were gently washed with isotonic PBS. Adherent cells were then lysed by incubating the explanted disks with 0.6 mL of 1% (vol/vol) Triton X-100 for 1 hour (to release cytosolic and granular contents of adherent cells). The numbers of adherent phagocytes were then estimated by enzyme activities (myeloperoxidase for neutrophils [polymorphonuclear neutrophils, or PMNs] and nonspecific esterase for macrophages/monocytes [Mφs]).
Measurement of enzyme activities
Myeloperoxidase (MPO), largely from PMNs, was measured by a guaiacol reaction.32 Our preliminary experiments showed that more than 95% of explant-associated peroxidase activity was from MPO as opposed to eosinophil peroxidase,15 so we did not perform separate assays for the latter. Control studies on purified PMNs from mice indicated that the MPO activity of mouse peripheral PMN is about 23 nU/cell.15
Nonspecific esterase (NSE) is relatively restricted to Mφs,33 and the activity of this enzyme was used as a measure of the numbers of adherent Mφs. NSE activity was determined by following the rate of hydrolysis of o-nitrophenyl butyrate34 in the presence of eserine (10 mM final concentration), which will eliminate possible interference by plasma cholinesterase.35 Enzyme assays on nonelicited mouse peritoneal Mφs indicated that the NSE activity is about 11 nU/cell.15
Determinations of endotoxin contamination
Subsamples of variously treated disks were assayed for endotoxin contamination by the chromogenic limulus amebocyte lysate test (BioWhittaker, Walkersville, MD). In no case was the reading above minimal detectable concentration (ie, no samples contained more than 0.01 ng endotoxin per square centimeter of material surface).
Statistical analyses
The significance of difference among surfaces coated with different proteins was assessed by means of a one-factor analysis of variance. A Bonferroni multiple comparison test was then used to compare pairwise means where the omnibus test was significant. Linear regression was used to analyze the correlations between biomaterial-mediated inflammatory responses and fibrinogen P1/P2 exposure.
Results
The proinflammatory activity of adsorbed fibrinogen resides in the D domain
Fibrinogen degradation products were purified, coated on PET disks, and then implanted in Swiss Webster mice for 16 hours. Quantification of the numbers of adherent phagocytes revealed, as previously reported,19 that the proinflammatory sequences reside within the fibrinogen D domain; PET disks coated with fibrinogen D30 and D100—but not fibrinogen E50—fragments prompt the same degree of inflammatory responses as disks coated with purified fibrinogen (Table 1).
Phagocyte accumulation on the surfaces of variously treated polyethylene terephthalate disks after implantation for 16 hours in Swiss Webster mice
| Sample coating . | Peroxidase activity (mU/cm2) . | PMNs* (× 104/cm2) . | NSE activity (mU/cm2) . | Mφs* (× 104/cm2) . |
|---|---|---|---|---|
| Fibrinogen | 2.42 ± 1.32 | 10.52 ± 5.74 | 2.10 ± 0.17 | 19.09 ± 1.54 |
| D100 | 2.27 ± 0.58 | 9.87 ± 2.52 | 2.19 ± 0.72 | 19.91 ± 6.55 |
| D30 | 2.44 ± 1.45 | 10.61 ± 6.30 | 3.52 ± 0.87 | 32.00 ± 7.91 |
| E | 0.042 ± 0.074† | 0.18 ± 0.32† | 0.094 ± 0.041† | 0.85 ± 0.37† |
| Albumin | 0.026 ± 0.040† | 0.11 ± 0.17† | 0.14 ± 0.10† | 1.27 ± 0.91† |
| Sample coating . | Peroxidase activity (mU/cm2) . | PMNs* (× 104/cm2) . | NSE activity (mU/cm2) . | Mφs* (× 104/cm2) . |
|---|---|---|---|---|
| Fibrinogen | 2.42 ± 1.32 | 10.52 ± 5.74 | 2.10 ± 0.17 | 19.09 ± 1.54 |
| D100 | 2.27 ± 0.58 | 9.87 ± 2.52 | 2.19 ± 0.72 | 19.91 ± 6.55 |
| D30 | 2.44 ± 1.45 | 10.61 ± 6.30 | 3.52 ± 0.87 | 32.00 ± 7.91 |
| E | 0.042 ± 0.074† | 0.18 ± 0.32† | 0.094 ± 0.041† | 0.85 ± 0.37† |
| Albumin | 0.026 ± 0.040† | 0.11 ± 0.17† | 0.14 ± 0.10† | 1.27 ± 0.91† |
D100, D30, and E are purified plasmin degradation fragments of fibrinogen.
PMN indicates polymorphonuclear neutrophils; NSE, nonspecific esterase; Mφs, macrophages/monocytes.
Phagocyte numbers calculated on the basis of measured activities of MPO for PMN and NSE for Mφs.
Differs from fibrinogen coating at P < .01.
Both P1 and P2 motifs are responsible for triggering inflammatory responses to implanted biomaterials
As previously mentioned, the phagocyte Mac-1 integrin mediates phagocyte adherence to fibrinogen-bearing material implants, and the proinflammatory activity of adsorbed fibrinogen resides in the D30 domain. Thus, the motifs that reside within D30 and interact with Mac-1 are likely to cause phagocyte accumulation on biomaterial implants. On the basis of available results, only P1 (γ190-202) and P2 (γ377-395) fulfill both criteria.23,24 Using an animal implantation model, we tested the potency of both P1 and P2 sequences in triggering inflammatory responses to material implants in vivo. The results (Figure 1) indicate that these peptides are roughly equipotent with adsorbed fibrinogen in triggering inflammatory responses. In contrast, PET disks coated with the scrambled peptide-albumin conjugates had no such effect (Figure 1). To evaluate whether the exposure of these P1 and P2 sequences might be sufficient to explain inflammatory cell accumulation on implants, fibrinogen-coated PET disks were incubated with the Fab portions of antibodies against P1 or P2 and then implanted. The results indicate that Fab blockade of either P1 (Figure 2) or P2 (not shown) significantly reduces the numbers of adherent phagocytes after implantation. It should be noted that both P1 and P2 sequences—despite their distance in the linear amino acid sequence—are close together in the folded protein.24Thus, Fab blockade of either epitope might hinder integrin access to the other and help explain why either antibody almost totally prevents phagocyte accumulation. Overall, these results indicate that both P1 and P2 sequences participate in the accumulation of phagocytes on biomaterial implants.
Phagocyte accumulation on the surfaces of PET disks.
Disks were preincubated with human fibrinogen (200 μg/mL), P1-albumin (200 μg/mL), P2-albumin (200 μg/mL), scrambled P1 peptide–albumin (200 μg/mL), scrambled P2 peptide–albumin (200 μg/mL), and albumin (200 000 μg/L [200 μg/mL]) and implanted in Swiss Webster mice for 16 hours. Vertical lines denote ± 1 SD (n = 5 in all cases). (**Significance vs fibrinogen-coated implants is P < .01.) Estimated numbers of Mφs (cells per square centimeter): 425 000 ± 45 000 on fibrinogen-coated disks; 278 000 ± 85 000 on P1-albumin–coated disks; 305 000 ± 115 000 on P2-albumin–coated disks; 17 300 ± 7300 on scrambled P1 peptide-albumin–coated disks; 29 100 ± 8000 on scrambled P2 peptide-albumin–coated disks; and 13 600 ± 11 800 on albumin-coated disks. Estimated numbers of PMNs (cells per square centimeter): 145 700 ± 43 900 on fibrinogen-coated disks; 146 500 ± 73 000 on P1-albumin–coated disks; 131 700 ± 21 300 on P2-albumin–coated disks; 33 900 ± 9100 on scrambled P1 peptide-albumin–coated disks; 25 700 ± 11 300 on scrambled P2 peptide-albumin–coated disks; and 22 200 ± 13 500 on albumin-coated disks.
Phagocyte accumulation on the surfaces of PET disks.
Disks were preincubated with human fibrinogen (200 μg/mL), P1-albumin (200 μg/mL), P2-albumin (200 μg/mL), scrambled P1 peptide–albumin (200 μg/mL), scrambled P2 peptide–albumin (200 μg/mL), and albumin (200 000 μg/L [200 μg/mL]) and implanted in Swiss Webster mice for 16 hours. Vertical lines denote ± 1 SD (n = 5 in all cases). (**Significance vs fibrinogen-coated implants is P < .01.) Estimated numbers of Mφs (cells per square centimeter): 425 000 ± 45 000 on fibrinogen-coated disks; 278 000 ± 85 000 on P1-albumin–coated disks; 305 000 ± 115 000 on P2-albumin–coated disks; 17 300 ± 7300 on scrambled P1 peptide-albumin–coated disks; 29 100 ± 8000 on scrambled P2 peptide-albumin–coated disks; and 13 600 ± 11 800 on albumin-coated disks. Estimated numbers of PMNs (cells per square centimeter): 145 700 ± 43 900 on fibrinogen-coated disks; 146 500 ± 73 000 on P1-albumin–coated disks; 131 700 ± 21 300 on P2-albumin–coated disks; 33 900 ± 9100 on scrambled P1 peptide-albumin–coated disks; 25 700 ± 11 300 on scrambled P2 peptide-albumin–coated disks; and 22 200 ± 13 500 on albumin-coated disks.
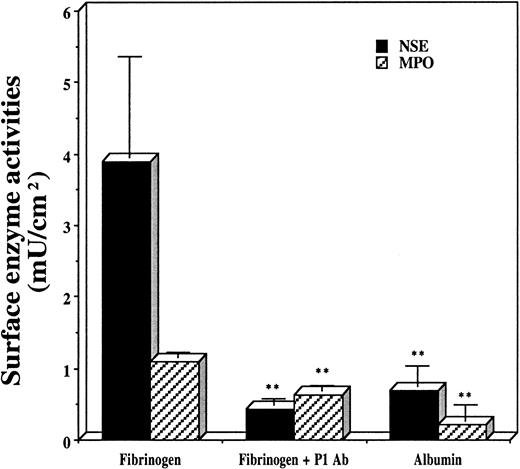
Effect of anti-P1 Fab preincubation.
Anti-P1 Fab preincubation prevents phagocyte accumulation on surfaces of PET disks coated with human fibrinogen (200 μg/mL) and albumin (1000 μg/mL) after 16 hours of implantation in Swiss Webster mice. Vertical lines denote ± 1 SD (n = 5 in all cases). (**Significance vs fibrinogen-coated implants isP < .01.) Estimated numbers of Mφs (cells per square centimeter): 355 000 ± 128 000 on fibrinogen-coated disks; 39 100 ± 8200 on P1 antibody-blocked fibrinogen-coated disks; and 62 700 ± 26 400 on albumin-coated disks. Estimated numbers of PMNs (cells per square centimeter): 47 400 ± 3500 on fibrinogen-coated disks; 27 400 ± 3500 on P1 antibody-blocked fibrinogen-coated disks; and 9600 ± 9100 on albumin-coated disks.
Effect of anti-P1 Fab preincubation.
Anti-P1 Fab preincubation prevents phagocyte accumulation on surfaces of PET disks coated with human fibrinogen (200 μg/mL) and albumin (1000 μg/mL) after 16 hours of implantation in Swiss Webster mice. Vertical lines denote ± 1 SD (n = 5 in all cases). (**Significance vs fibrinogen-coated implants isP < .01.) Estimated numbers of Mφs (cells per square centimeter): 355 000 ± 128 000 on fibrinogen-coated disks; 39 100 ± 8200 on P1 antibody-blocked fibrinogen-coated disks; and 62 700 ± 26 400 on albumin-coated disks. Estimated numbers of PMNs (cells per square centimeter): 47 400 ± 3500 on fibrinogen-coated disks; 27 400 ± 3500 on P1 antibody-blocked fibrinogen-coated disks; and 9600 ± 9100 on albumin-coated disks.
Fibrinogen-biomaterial interactions enhance the exposure of both P1 and P2 motifs
Because soluble fibrinogen is not proinflammatory, it seemed likely that fibrinogen-biomaterial interactions might cause the unmasking of P1 and P2 motifs. Using monoclonal antibodies specifically against either P1 or P2 epitopes, we therefore measured the exposure of P1 and P2 on both surface-adsorbed and soluble fibrinogen. Indeed, using an ELISA assay, we find that fibrinogen-coated and plasma-coated PET surfaces display both epitopes (Table2). Interestingly, the addition of large amounts (1000-fold excess; 1 mg soluble fibrinogen vs 1 μg adsorbed fibrinogen) of soluble fibrinogen to these assays did not affect the detection of either epitope (Table 2), suggesting that soluble fibrinogen does not expose either of these epitopes. On the other hand, the specificity of both antibodies is ensured by the fact that the addition of an excess of free peptides blocks the detection of P1 and P2 exposed by surface-bound fibrinogen (Table 2). Thus, adsorption of fibrinogen, whether in pure form or from plasma, to the surfaces of at least some kinds of biomaterials causes the simultaneous appearance of both P1 and P2 epitopes.
Fibrin(ogen) P1 and P2 exposure on plasma-coated and fibrinogen-coated polyethylene terephthalate disks and fibrin-coated glass beads (n = 4 in all cases)
| Sample coating . | First antibody buffer . | P1 . | P2 . |
|---|---|---|---|
| (OD/cm2) . | |||
| Albumin-PET | PBS | 0.000 ± 0.005 | 0.000 ± 0.003 |
| Fibrinogen-PET | PBS | 0.105 ± 0.023 | 0.196 ± 0.030 |
| 1 mg/mL fibrinogen* | 0.109 ± 0.009 | 0.190 ± 0.041 | |
| 1 mg/mL P1 or P2† | 0.003 ± 0.002 | 0.011 ± 0.005 | |
| Plasma-PET | PBS | 0.040 ± 0.010 | 0.059 ± 0.015 |
| Fibrin-glass | PBS | 0.630 ± 0.046 | 0.493 ± 0.066 |
| Sample coating . | First antibody buffer . | P1 . | P2 . |
|---|---|---|---|
| (OD/cm2) . | |||
| Albumin-PET | PBS | 0.000 ± 0.005 | 0.000 ± 0.003 |
| Fibrinogen-PET | PBS | 0.105 ± 0.023 | 0.196 ± 0.030 |
| 1 mg/mL fibrinogen* | 0.109 ± 0.009 | 0.190 ± 0.041 | |
| 1 mg/mL P1 or P2† | 0.003 ± 0.002 | 0.011 ± 0.005 | |
| Plasma-PET | PBS | 0.040 ± 0.010 | 0.059 ± 0.015 |
| Fibrin-glass | PBS | 0.630 ± 0.046 | 0.493 ± 0.066 |
OD indicates optical density; PET, polyethylene terephthalate; PBS, phosphate-buffered saline.
Soluble fibrinogen (1 mg/mL) was added to test whether soluble fibrinogen possesses P1 or P2 epitope.
Soluble P1 and P2 peptides (1 mg/mL) were added in enzyme-linked immunosorbent assays to test the specificity of P1 and P2 antibodies.
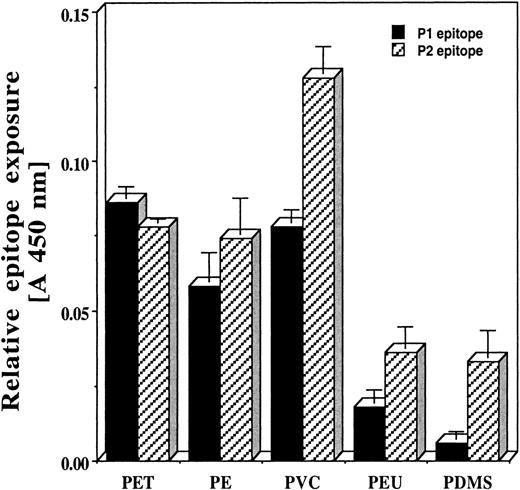
Material surface properties affect the extent of epitope exposure by adsorbed fibrinogen
Material surface properties are known to influence fibrinogen adsorption and denaturation.18,20,36 37 The extent of conformational changes that occur in adsorbed fibrinogen might govern the degree of exposure of hidden epitopes such as P1 and P2. We therefore thought it likely that materials with different surface properties would vary in the extent to which they cause such neo-epitope exposure. Indeed, in a limited series of fibrinogen-coated biomaterials, we find that PET, PVC, and polyethylene (PE) are very effective in exposing both P1 and P2 epitopes. On the other hand, fibrinogen adsorbed to PEU and PDMS disks exhibits substantially less immunoreactive P1 or P2 (Figure 3).
Quantitative measurement of P1 and P2 epitope exposure on surfaces of PET, PE, PVC, PEU, and PDMS disks precoated with human fibrinogen.
Vertical lines denote ± 1 SD (n = 4 in all cases).
Quantitative measurement of P1 and P2 epitope exposure on surfaces of PET, PE, PVC, PEU, and PDMS disks precoated with human fibrinogen.
Vertical lines denote ± 1 SD (n = 4 in all cases).
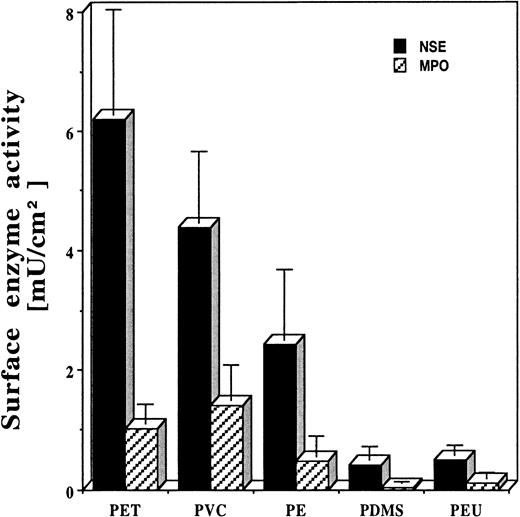
The degree of P1 and P2 exposure correlates with the extent of biomaterial-mediated acute inflammatory responses
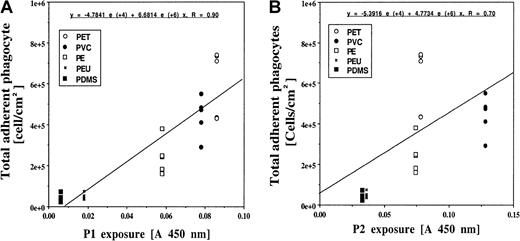
As shown above, both P1- and P2-coated surfaces prompt strong inflammatory responses. Therefore, those surfaces that induce the greatest exposure of these epitopes might prompt maximal inflammatory cell accumulation in vivo. In support of this, we find large differences in the extent of inflammatory cell accumulation on implants composed of these different test surfaces in the following order: (1) PET, (2) PVC, (3) PE (4) PDMS and PEU (Figure4). The possible importance of surface adsorption–mediated P1 and P2 exposure in this reaction is indicated by the significant correlation (P < .05) between the extent of P1/P2 exposure and the total numbers of adherent PMNs and Mφs (Figure 5A-B). Overall, the foregoing results support the proposition that biomaterial-mediated P1 and P2 exposure on adsorbed fibrinogen is required for the accumulation of inflammatory cells on biomaterial implants.
Phagocyte accumulation on the surfaces of commonly used biomaterials, including PET, PVC, PE, PDMS, and PEU, after 16 hours of implantation in Swiss Webster mice.
Vertical lines denote ± 1 SD (n = 5 in all cases). Estimated numbers of Mφs (cells per square centimeter): 565 000 ± 159 000 on PET disks; 398 000 ± 108 000 on PVC disks; 221 000 ± 106 000 on PE disks; 39 000 ± 20 000 on PDMS disks; and 45 800 ± 14 300 on PEU disks. Estimated numbers of PMNs (cells per square centimeter): 44 800 ± 14 300 on PET disks; 61 700 ± 24 800 on PVC disks; 21 200 ± 14 400 on PE disks; 1700 ± 900 on PDMS disks; and 5500 ± 4100 on PEU disks.
Phagocyte accumulation on the surfaces of commonly used biomaterials, including PET, PVC, PE, PDMS, and PEU, after 16 hours of implantation in Swiss Webster mice.
Vertical lines denote ± 1 SD (n = 5 in all cases). Estimated numbers of Mφs (cells per square centimeter): 565 000 ± 159 000 on PET disks; 398 000 ± 108 000 on PVC disks; 221 000 ± 106 000 on PE disks; 39 000 ± 20 000 on PDMS disks; and 45 800 ± 14 300 on PEU disks. Estimated numbers of PMNs (cells per square centimeter): 44 800 ± 14 300 on PET disks; 61 700 ± 24 800 on PVC disks; 21 200 ± 14 400 on PE disks; 1700 ± 900 on PDMS disks; and 5500 ± 4100 on PEU disks.
Correlation between biomaterial-mediated inflammatory responses and fibrinogen P1/P2 epitope exposure.
The numbers of implant-associated phagocytes (both Mφs and PMNs) are graphed against the measurement of the exposure of P1 (panel A) and P2 (panel B) epitopes. The correlations between P1/P2 exposure and inflammatory responses are significant at P < .05 in both cases.
Correlation between biomaterial-mediated inflammatory responses and fibrinogen P1/P2 epitope exposure.
The numbers of implant-associated phagocytes (both Mφs and PMNs) are graphed against the measurement of the exposure of P1 (panel A) and P2 (panel B) epitopes. The correlations between P1/P2 exposure and inflammatory responses are significant at P < .05 in both cases.
Foreign body reactions may represent a variation on the theme of fibrin clot–mediated inflammatory and wound-healing responses
The foregoing results suggested to us that phagocyte responses to fibrinogen adsorbed to biomaterial surfaces might be analogous to those normally triggered by the formation of fibrin clots. In other words, phagocytes might recognize fibrinogen adsorbed to biomaterials as fibrin clot and initiate a series of responses meant to ward off infection and initiate wound healing at a site of vascular injury. If fibrinogen adsorbed to biomaterial surfaces resembles fibrin, then fibrin should also display both P1 and P2 motifs. We therefore analyzed the appearance of the P1 and P2 neo-epitopes on fibrin coated on glass beads. Indeed, the results indicate that, following the conversion of fibrinogen to fibrin, substantial amounts of both P1 and P2 epitopes appear (Table 2).
Discussion
Despite the increasing importance of implanted medical devices in the practice of medicine, the synthetic materials of which they are made often induce iatrogenic effects, such as acute and chronic inflammatory responses. Typically, biomaterial surfaces attract substantial numbers of adherent phagocytes. These implant-associated activated phagocytes are thought to be responsible for a variety of biomaterial-mediated adverse responses, including1inflammation surrounding many types of implants1-3; implant degradation and “stress cracking”10,38-41; tissue fibrosis surrounding mammary prostheses, joint implants, and many other types of implants11-13,42-44; and device-centered infection.45-47 Such adverse responses to biomaterials may lead to implant failure and, in cases such as the oxidative degradation of pacemaker lead insulation,37 fatality. However, the mechanisms underlying biomaterial-mediated inflammatory responses remain largely unknown.
In searching for the proximate trigger of inflammatory responses to biomaterial implants, we earlier observed that, after implantation, serum-coated, as well as albumin-coated, PET disks accumulate far fewer phagocytes (PMNs and Mφs) than do plasma-coated surfaces or uncoated surfaces (which spontaneously acquire a layer of host proteins). The possible involvement of fibrinogen in biomaterial-mediated inflammatory responses is supported by the observation that disks precoated with fibrinogen-repleted serum attract as many phagocytes as do disks coated with fresh, minimally heparinized plasma. Most importantly, ancrod-treated (severely hypofibrinogenemic) mice show almost no phagocyte accumulation on uncoated disks, but do so if the disks are precoated with purified murine fibrinogen.15
In an attempt to determine the fibrinogen sequence(s) critical for attracting phagocytes, fibrinogen degradation products were produced with plasmin digestion and purified with gel-filtration chromatography. By implanting surfaces precoated with different fibrinogen degradation products and then measuring the numbers of adherent phagocytes, we found that the fibrinogen fragment D30 appeared to contain the proinflammatory sequence(s).19 We also found that the phagocyte integrin Mac-1 (CD11b/CD18) is required for phagocyte adhesion to fibrinogen-bearing implants, because both CD18 knockout and CD11b knockout mice fail to accumulate phagocytes on either untreated or fibrinogen-precoated PET implants.20 21 Thus, interactions between motifs within the fibrinogen D30 domain and the phagocyte Mac-1 integrin appear particularly important in the accumulation of phagocytes on implant surfaces and, therefore, in early biomaterial-mediated inflammatory responses.
Two segments of D30, P1 (γ190-202) and P2 (γ377-395), are known to interact with phagocyte Mac-1,23 24 suggesting that both might be involved in phagocyte accumulation on biomaterial implants. In agreement with this, implant surfaces precoated with (albumin-conjugated) P1 or P2 peptides, but not scrambled peptides, trigger substantial phagocyte accumulation, roughly equivalent to that caused by fibrinogen-coated surfaces. Furthermore, Fab blockade of either neo-epitope on adsorbed fibrinogen almost completely prevents the accumulation of phagocytes following subsequent implantation. Overall, these results indicate that both P1 and P2 are critical mediators of the accumulation of phagocytes on implanted biomaterials. In fact, using several commonly employed biomaterials, we find a strong correlation between the extent of surface-mediated P1 or P2 exposure in vitro and the degree of material-mediated inflammatory responses (reflected by the numbers of adherent phagocytes) in vivo.
It is remarkable that the simple adsorption of fibrinogen to biomaterial surfaces evidently converts an abundant coagulation protein, fibrinogen, to a proinflammatory state. There is, however, some precedent for such a phenomenon. For example, binding of fibrinogen to platelet glycoprotein IIb-IIIa has been found to result in conformational changes in both the occupied receptor and the bound ligand.48-51 By the same token, the present results suggest that material-induced conformational changes of adsorbed fibrinogen are critical in the early phases of the foreign body reaction to biomaterials. This general concept is further supported by several earlier observations. Adsorbed proteins, especially on hydrophobic surfaces (which are typical of the majority of implanted biomaterials), undergo conformational changes, becoming tightly adherent and—at least in a sense—denatured.52-55Furthermore, the unfolding of adsorbed fibrinogen (perhaps via loss of the normal sphere of hydration encouraged by contact with the material surfaces),56 can lead to changes in antibody binding or epitope exposure of adsorbed fibrinogen.57-60 This is probably due to changes in the tertiary structure of surface-bound protein leading to the exposure of previously hidden epitopes that, in turn, help initiate adverse reactions such as coagulation and inflammation.61,62 Finally, it has been documented that surface properties may influence protein adsorption/denaturation and, indirectly, affect cellular/tissue responses to biomaterials.61 63-66
The inflammatory responses to adsorbed fibrinogen may derive from a resemblance between the surface denatured protein and fibrin, the latter being a well-known participant in inflammatory reactions. Indeed, fibrin formation is one of the earliest events following wounding and appears to be an important precedent to subsequent wound healing.67 Furthermore, like fibrinogen adsorbed to biomaterial surfaces, fibrin deposition has been associated with subsequent adverse reactions, including inflammatory conditions and fibrosis.68-70 Adsorbed fibrinogen in itself appears sufficient to prompt foreign body reactions. We have found that the in vivo modification of fibrinogen adsorbed to biomaterial surfaces (eg, by further coagulation or proteolytic attack) is not required for ensuing inflammatory responses, because the blockade of either coagulation or proteolysis (fibrinolysis) in vivo does not affect phagocyte accumulation on implanted biomaterials (L.T. et al, unpublished results, December 2000). Furthermore, on the basis of calorimetric determinations, the major conformational changes of both fibrin and surface-denatured fibrinogen occur within the D domain,71,72 which contains both P1 and P2 epitopes. Using monoclonal antibodies against either fibrinogen or fibrin, earlier studies have revealed that the D domains of fibrinogen and fibrin are immunologically distinct.73-75 The central region of the gamma chain is inaccessible to antibodies in native fibrinogen73,74 but hidden epitopes within it are exposed following platelet binding and/or enzyme degradation.75 76Finally, and directly pertinent to the present results, we find that the critically important P1 and P2 epitopes are displayed on both fibrin and surface-bound fibrinogen but are occult in native fibrinogen.
This is, to the best of our knowledge, the first solid evidence for a critical opsonic activity of adsorbed fibrinogen in prompting inflammatory reactions to biomaterial implants and, possibly, other types of foreign bodies. Although the detailed mechanisms are still not clear, on the basis of available information we believe that several steps might participate in these reactions. First, after intrusion or implantation, foreign bodies or biomaterials spontaneously acquire a layer of adsorbed host proteins, including fibrinogen. Second, interactions between adsorbed fibrinogen and the implant surface—particularly involving the fibrinogen D domain, which is highly folded and stacked with α-helices—cause conformational changes, denaturation, and very tight adherence of the adsorbed fibrinogen.15,18,20,35 37 In fact, these conformational events may well resemble those occurring upon thrombin-mediated fibrinogen conversion to fibrin. Third, during the unfolding/denaturation processes, the P1 and P2 motifs within the fibrinogen D domain are exposed. Finally, the interaction between P1/P2 motifs and phagocyte Mac-1 mediate the adhesion and activation of phagocytes.
If the foregoing is correct, then fibrinogen may spontaneously convert to a fibrinlike conformation on foreign surfaces, such as those of hydrophobic polymers. In this form, the adsorbed fibrinogen—perhaps like fibrin within a coagulum—facilitates the binding and activation of inflammatory cells. In the case of implanted biomaterials, the ensuing somatic responses may have detrimental effects on both the recipient and the implant.
Supported by grants R01-HL56187 and R01-HL60787 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Liping Tang, Biomedical Engineering Program, Box 19138, University of Texas at Arlington, Arlington, TX 76019; e-mail: ltang@uta.edu.

![Fig. 1. Phagocyte accumulation on the surfaces of PET disks. / Disks were preincubated with human fibrinogen (200 μg/mL), P1-albumin (200 μg/mL), P2-albumin (200 μg/mL), scrambled P1 peptide–albumin (200 μg/mL), scrambled P2 peptide–albumin (200 μg/mL), and albumin (200 000 μg/L [200 μg/mL]) and implanted in Swiss Webster mice for 16 hours. Vertical lines denote ± 1 SD (n = 5 in all cases). (**Significance vs fibrinogen-coated implants is P < .01.) Estimated numbers of Mφs (cells per square centimeter): 425 000 ± 45 000 on fibrinogen-coated disks; 278 000 ± 85 000 on P1-albumin–coated disks; 305 000 ± 115 000 on P2-albumin–coated disks; 17 300 ± 7300 on scrambled P1 peptide-albumin–coated disks; 29 100 ± 8000 on scrambled P2 peptide-albumin–coated disks; and 13 600 ± 11 800 on albumin-coated disks. Estimated numbers of PMNs (cells per square centimeter): 145 700 ± 43 900 on fibrinogen-coated disks; 146 500 ± 73 000 on P1-albumin–coated disks; 131 700 ± 21 300 on P2-albumin–coated disks; 33 900 ± 9100 on scrambled P1 peptide-albumin–coated disks; 25 700 ± 11 300 on scrambled P2 peptide-albumin–coated disks; and 22 200 ± 13 500 on albumin-coated disks.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/4/10.1182_blood.v98.4.1231/6/m_h81611411001.jpeg?Expires=1769107387&Signature=Dkg81XyTgY7oDl8lSyxd8Phoc4k~ZB9qvakfG-WrEFj2L-qGiD9rhan33GAsCKdwTa8Dvgz6pumSVpqtF0VFYH~2rckIlU~fhtPrVssn6uq-GXYau7HGLXqeRK0Y17xOb4laRWe28EcEF9E15CBTbBVsygx98OJppOAZWQtr3IebhioyowzjSbWMJ7654OxrxY-APIe6zVGnfol9Bfiy8a25CvYppZSIIf2Mg~mrsAaudfmMhQZESykq6HYMIh7LHHappy33om1SOrGAse2a~obtW95JDTJi7F~PCkZCdhlwkgGVjywyKTzBgfuPXYeORTWhLa39qqsHTbA2tH9WtA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal