Adult T-cell leukemia (ATL) is an aggressive malignancy of CD4+ T cells caused by the human T-cell leukemia virus type 1 (HTLV-1). The viral leukemogenesis is critically dependent on its oncoprotein Tax because the protein as well as the virus can immortalize primary human lymphocytes to permanent growth. As a transcriptional transactivator, Tax can stimulate the expression of distinct cellular genes. Alterations in the expression levels of unknown growth-relevant genes may contribute to the changed growth properties of Tax-immortalized and leukemic cells. To identify genes that are linked to Tax transformation and ATL leukemogenesis, this study systematically compared the gene expression of cultured cells from patients with acute ATL with that of stimulated peripheral blood T lymphocytes. Several overexpressed RNAs that encode signal transduction functions were identified. These include a dual-specific protein phosphatase (PAC1), an interferon-inducible factor (ISG15), a basic helix-loop-helix transcription factor (DEC-1), and the secreted antiapoptotic chemokine I-309. The ATL cell culture supernatants contained an antiapoptotic activity that could be specifically inhibited by antibodies directed against I-309. Inhibition of I-309 receptor (CCR8) signaling by pertussis toxin increased the apoptosis rate of ATL cell cultures in the presence and absence of external apoptotic stimuli. Both the I-309–specific antiapoptotic activity and the proapoptotic effect of inhibitors of I-309 signaling suggest the existence of an antiapoptotic autocrine loop in ATL cells. Thus, the overexpression of this chemokine may inhibit apoptosis in ATL cells and could substantially contribute to their growth.
Introduction
Adult T-cell leukemia (ATL) is an aggressive leukemic disorder of CD4+ T cells caused by the human T-cell leukemia virus type 1 (HTLV-1).1,2 It develops in 1% to 3% of infected individuals after more than 2 decades of viral persistence.3,4 The contribution of the virus to the pathology of the disease is believed to involve 2 mechanisms. First, the virus directly stimulates the proliferation of the infected T cells independently of antigen.5 Second, the HTLV-1 infection leads to chromosomal abnormalities and an increased mutation rate in infected cells.6-8 In addition to its oncogenic properties, HTLV-1 induces a chronic neurologic disorder, termed HTLV-1–associated myelopathy or tropical spastic paraparesis (HAM/TSP),9-11 which primarily develops in patients with a specific HLA subtype.12
As a complex retrovirus, HTLV-1 encodes the regulatory proteins Tax and Rex in addition to structural proteins. Rex is essential for the expression of the viral structural proteins,13-15 and Tax acts as a transactivator of the viral promoter.16-18 In addition, Tax influences multiple cellular functions including gene expression and proliferation and it increases the mutation rate.19 Several lines of evidence indicate that the HTLV-1 regulatory protein p40tax is responsible for the leukocyte transforming features of the virus.20-23 The growth of primary human lymphocytes conditionally immortalized by Tax depends on Tax expression. The proliferation of these immortalized cells was reversibly arrested in the G1 phase of the cell cycle by suppression of tax transcription, thus demonstrating that Tax stimulates the G1-to-S phase transition in immortalized T lymphocytes.24
The mechanism by which Tax influences G1-to-S phase transition and the growth of transformed primary human T cells is not well understood and different Tax functions may cooperate in the stimulation of cell proliferation. This includes the property of Tax to directly interfere with the function of cell cycle–controlling proteins.25-28 For instance, Tax inhibits the transactivating functions of the tumor suppressor p5329,30and activates cyclin-dependent kinases CDK4 and CDK6; these CDKs are essential for the control of the G1 phase progression.31,32 Tax has also been shown to interfere with DNA repair and in this way it possibly contributes to increase the cellular mutagenesis rate.33 Thus, also this Tax function may be crucial for leukemogenesis. Tax-induced mutations, which accumulate during the decades of viral persistence, might affect growth-relevant genes and as a consequence of dysregulated signaling result in overexpressed genes.8
Its function as a modulator of cellular transcription is believed to play a pivotal role in the stimulation of host cell proliferation, because Tax affects the gene expression of a variety of growth-relevant genes. It activates genes encoding proto-oncogenes,34,35the α-chain of the interleukin-2 (IL-2) receptor,36-38cytokines,39,40 stimulators (cyclin D2),41,42and repressors of cell cycle kinases (p21).43 On the other hand, the Tax protein represses the expression of DNA polymerase β, an enzyme important for DNA repair,44 the CDK inhibitor p18,27 and the proapoptotic Bax protein.45Additionally, further genes involved in signal transduction and glycoprotein synthesis have been found to be overexpressed in HTLV-1–infected T cells.28,46 Several of the mechanisms by which Tax influences the transcription of these genes are well characterized. However, for many genes that are up-regulated in HTLV-1–infected cells, including several signal transduction genes, the mechanism is not yet clear.47 The altered gene expression in HTLV-1–transformed cells is probably an important prerequisite for ATL induction. The malignant progression of the HTLV-1–infected cell into a leukemia cell is likely to affect the expression of growth-relevant genes. Hence, the identification of differentially expressed cellular genes is crucial for understanding the regulation of ATL leukemic cell proliferation.
In an attempt to identify genes that are linked to Tax transformation or ATL leukemogenesis, we systematically compared the gene expression patterns of cultured ATL cells with normal T lymphocytes using subtractive hybridization.48 By this approach multiple overexpressed messenger RNAs (mRNAs) coding for signal transduction proteins were identified, among them the antiapoptotic chemokine I-309. As reported here, the ATL cell cultures secreted an antiapoptotic activity and expressed the I-309 receptor CCR8. The Fas-mediated apoptosis rate of ATL cells was significantly increased when I-309 signaling was blocked. Taken together our data provide compelling evidence for an antiapoptotic autocrine loop in ATL cells.
Materials and methods
Cell culture and stimulation of primary human T cells
The CD4+ HTLV-1− T-cell lines (Jurkat, HuT-78, Molt-4), the HTLV-1+ T-cell lines (C91-PL, MT-2, HuT-102), and the Tax-immortalized T-cell line (TAXI-1) were cultured as previously described.49 HTLV-1+ Mondi cells are derived from a HAM/TSP patient and were cultured in RPMI 1640 medium supplemented with 40% Panserin medium (PAN Biotech, Aidenbach, Germany), 20% fetal calf serum (FCS), 2 mM glutamine, antibiotics, and 40 U/mL IL-2 (Roche Diagnostics, Mannheim, Germany). BW5147C cells are murine thymomic lymphoma cells and were cultured in Iscoves medium supplemented with 10% FCS, 1.5 mM l-glutamine, 0.24 mMl-asparagine, 0.55 mM l-arginine, and 50 μM β-mercaptoethanol (Sigma, Deisenhofen, Germany).50 The murine T-cell hybridoma cell line DO-Cep4 was kept in RPMI 1640 supplemented with 10% FCS, 2 mM glutamine, and 50 μg/mL gentamycin.51 To establish cell cultures from patients with acute ATL, peripheral blood lymphocytes from patients Champ, PaBe, StEd, and JuanaW were isolated by a Ficoll Hypaque gradient (Biochrom, Berlin, Germany) and stimulated with 5 μg/mL phytohemagglutinin P (PHA; Sigma) and 20 U/mL IL-2. The HTLV-1+ ATL cells were propagated in RPMI 1640 medium supplemented with 10% to 20% FCS, 2 mM glutamine, antibiotics, 50 μM β-mercaptoethanol, and 20 U/mL IL-2. The experiments were performed after 3 to 6 weeks in culture. At the same time aliquots were cryopreserved and used for repetition of the experiments at later times. In addition, the cells were kept in permanent culture for more than 6 months and 2 years (JuanaW) with no sign of change in growth behavior. Human peripheral blood mononuclear cells (PBMCs) from healthy blood donors were prepared and cultured as described above. Under these conditions T-cell growth was selectively stimulated (PBMCPHA). After 1 week the cell culture consisted of T cells to more than 99% and total RNA was isolated.52
Subtractive hybridization
Suppressive subtractive hybridization (SSH) is a polymerase chain reaction (PCR)-based method that was applied to systematically compare mRNA populations of ATL cells (JuanaW) and activated postmitotic T cells (PBMCPHA). It resulted in complementary DNA (cDNA) clones representing genes that are overrepresented in JuanaW cells. As a first step, poly(A)+-RNA was prepared from total RNA by oligo(dT)25-Dynabeads (Dynal, Hamburg, Germany) and cDNA was generated by using avian myeloblastosis virus (AMV) reverse transcriptase and oligo(dT)30-primers (Clontech, Heidelberg, Germany). The second cDNA strand was produced with T4 DNA-polymerase. Double-stranded cDNA was then digested withRsaI to produce small blunt-ended cDNA fragments. The JuanaW cDNA was subdivided into 2 portions and each was ligated with a different cDNA adaptor. The subtractive cDNA hybridization was performed in 2 successive rounds with an excess of PBMC cDNA as described.48 Unhybridized cDNA, which represents differentially expressed genes, was amplified by nested PCR using Advantage KlenTaq polymerase (Clontech). The adaptors ligated to JuanaW cDNA served as primer annealing sites in these PCR reactions. Subsequently, the cDNAs were cloned into pT-Adv vectors (Clontech) and sequenced using the dye didesoxy terminator method (ABI, Weiterstadt, Germany). The sequences were then subjected to database analysis using the Wisconsin GCG sequence analysis software package (Genetics Computer Group, Madison, WI).
Northern blotting
For the verification and quantification of RNA expression, Northern blot analyses were performed. Total RNA was prepared from various cells, separated (10 μg/lane) on 1% formaldehyde denaturing agarose gels, and blotted as previously described.14 For the generation of probes the cDNA inserts of pT-Adv vectors were recovered by EcoRI digestion, purified by gel electrophoresis, and extracted from the agarose gel by silica-gel particles (Qiagen, Hilden, Germany). DNA fragments were radioactively labeled with α[32P]-dATP (deoxyadenosine triphosphate) by the random priming method. The hybridized mRNA was quantified by phosphorimaging using the BAS2000 bioimaging analyzer (Fuji, Tokyo, Japan). Signal analysis was done using the program TINA2.0 (Raytest, Straubenhardt, Germany).
Cell proliferation assays
The antiapoptotic activity of ATL cell culture supernatants was tested using BW5147C and DO-Cep4 cells, which were treated to undergo apoptosis. These and all other apoptosis- and cell proliferation–related experiments were done in triplicate in microtiter plates. Cells (2 × 103 cells/well) were incubated with 62.5 or 250 nM apoptosis-inducing dexamethasone (DEX; Sigma) and with serial dilutions of ATL cell culture supernatants in a final volume of 200 μL. Alternatively, apoptosis was induced using cisplatin (Sigma) at final concentrations ranging from 50 pg/mL to 5 ng/mL. To selectively block I-309 receptor signaling, 2.5 ng/mL pertussis toxin (Biomol, Hamburg, Germany) was added. To test the specificity of the antiapoptotic activity in the supernatants, sodium azide-free anti–I-309 antibody (R&D Systems, Wiesbaden, Germany) or a nonspecific control antibody (anti–human CD95; Becton Dickinson, Heidelberg, Germany) was added at final concentrations ranging from 1.25 ng/mL to 5 μg/mL. Purified I-309 (30 pg/mL to 20 ng/mL) and IL-9 (25 pg/mL to 2 ng/mL)50 served as controls. To assess cell viability and activity under the conditions above, MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide; Sigma) dye assays were carried out. After 48 hours, 250 μg/mL MTT was added and cells were incubated for further 4 hours. In viable cells MTT is converted into formasan dye granules. Cells were lysed by the addition of 180 μL 0.01 N HCl in isopropanol and the formasan absorbance was determined in a microplate reader set at 630 nm. To calculate total cell survival, absorbance in the presence of ATL cell culture supernatant and DEX (ODDEX+sup) was standardized to the background (absorbance in the presence of DEX only: ODDEX), the latter being arbitrarily set 1. Additional cell survival (ACS) was determined by subtraction of the background (ODDEX).
To study the influence of I-309–containing culture supernatants on ATL cell growth in the presence of a proapoptotic stimulus, [3H]-thymidine incorporation assays were carried out. The ATL cells (3 × 103/well) were incubated in the presence of homologous 1:2.5 diluted supernatant, supplemented with anti-Fas antibody (10-500 ng/mL anti–human CD95; Becton Dickinson) and 12 μg/mL anti–I-309 or isotypic control antibody (monoclonal anti-IgG1; R&D Systems). In some experiments pertussis toxin (200 ng/mL) was added to abrogate intracellularly triggered I-309 signaling. After 24 hours [3H]-thymidine (Amersham Pharmacia, Freiburg, Germany) was added at a final concentration of 0.2 Ci/μmol. Cells were incubated for further 24 hours and harvested on glass fiber filters (Packard, Groningen, Netherlands). Bound radioactivity was quantified by phosphorimaging.
Analysis of apoptosis
One method for the detection of apoptotic cells was annexin V staining. Briefly, ATL-derived and control cells were cultured under the conditions described above in 48-well plates (4 × 103 cells/well in 400 μL). After 48 hours, the cells were harvested, washed in phosphate-buffered saline (PBS), and incubated with fluorescein isothiocyanate (FITC)-conjugated recombinant annexin V (Bendermedsystems Vienna, Austria) for 15 minutes. After staining, the cells were washed in PBS and measured by flow cytometry (FACScalibur; Becton Dickinson). Alternatively, cell death rate was measured using a modified propidium iodide (PI) staining assay, which allows the distinction between apoptotic, necrotic, and viable cells.53 Cells were treated with 25 μg/mL PI (Sigma) in 2% Triton-X100 and 0.05% sodium citrate and subjected to multiparameter flow cytometry analysis.
Enzyme-linked immunosorbent assay
The I-309 concentration in cell culture supernatants was determined by antigen capture enzyme-linked immunosorbent assay (ELISA) using monoclonal anti–I-309 capture and biotinylated anti–I-309 detection antibodies according to the manufacturer's recommendations (R&D Systems). Briefly, 96-well microtiter plates were coated overnight with capture antibodies. The plates were incubated with serially diluted cell culture supernatants and biotinylated anti–I-309 detection antibody (R&D Systems). A standard curve was obtained using serial dilutions of recombinant human I-309 (R&D Systems). To detect the chemokine, streptavidin-conjugated horseradish peroxidase (HRP; Zymed, San Francisco, CA) and substrate solution (H2O2:tetramethylbenzidine = 1:1; Kirkegaard & Perry Laboratories, Gaithersburg, MD) were added. After terminating the reaction with 1 M H2SO4, the optical density was determined using a microplate reader set to 540 nm and 570 nm.
Results
Identification of differentially expressed cDNAs in ATL-derived cells
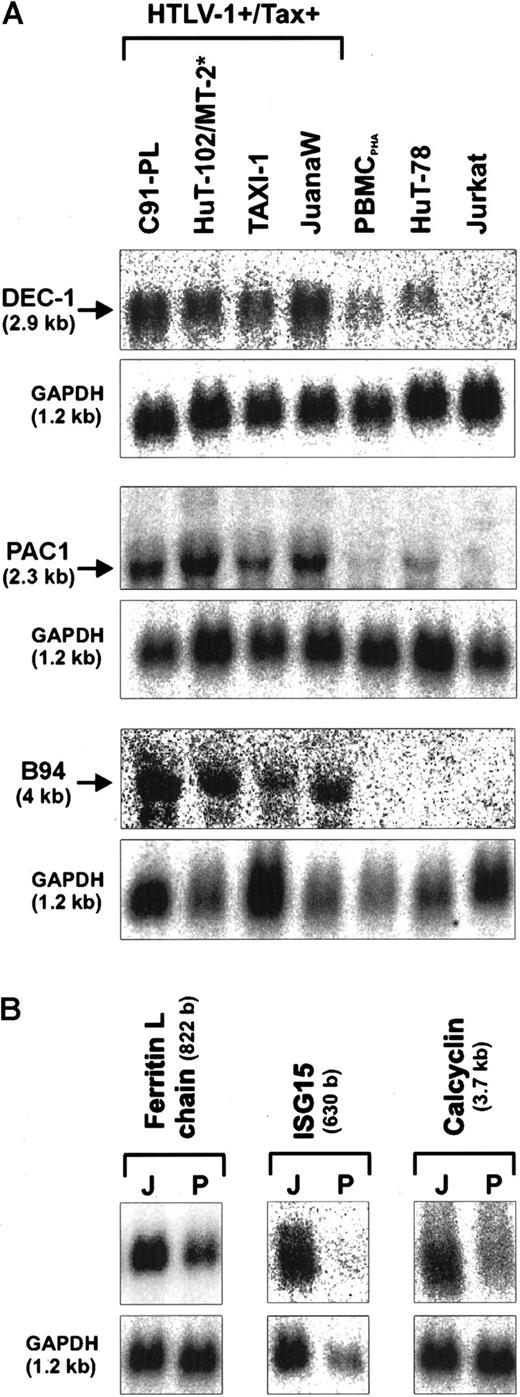
Dysregulated gene expression frequently indicates or causes aberrant activation of growth-relevant signal transduction pathways. In ATL cells such changes may result from transactivation of cellular promoters mediated by viral proteins or mutagenesis or both. To identify genes linked to the malignant growth, the gene expression pattern of acute ATL cells was systematically compared with that of nontransformed normal postmitotic T lymphocytes using the PCR-based SSH. This approach resulted in the cloning of cDNAs derived from transcripts, which are overexpressed in the cultured leukemia cells. In contrast to gene array techniques,46 SSH allows the isolation of novel sequences derived from unknown genes. In total, the nucleotide sequences of 346 cDNA clones were determined. The subsequent database analysis revealed that about one third of the sequences were derived from genes involved in gene regulation and signal transduction pathways. Two sequences were novel (GenBank accession numbers AJ276510and AJ272002) and many were homologous to uncharacterized cDNAs from the expressed sequence tag (EST) database (Table1). A variety of cDNAs corresponded to HTLV-1 or genes that have already been shown to be up-regulated in HTLV-1–infected cells due to Tax transactivation. These included the IL-2 receptor α-chain and the CDK inhibitor p21WAF1/CIP1.38,43 The genes for the transferrin receptor and thymosin β-4 have previously been found to be considerably overexpressed in ATL cells.54,55 The isolation of known HTLV-1–up-regulated genes confirmed that our experiment had resulted in cloning of differentially expressed sequences. To quantify the RNA expression, Northern blots were performed with following genes selected according to their biologic function in transcription control and cellular signaling. The basic helix-loop-helix transcription factor DEC-1 and the tumor necrosis factor-α (TNF-α)–inducible B94 protein are putatively associated with differentiation.56,57 The dual-specific protein phosphatase PAC1 and the interferon-induced ISG15 gene were selected because of their contribution to cellular signaling.58,59 Calcylin, a calcium-binding protein, is expressed in a cell cycle–specific manner and dysregulated in various malignancies.60 The ferritin L chain and the chemokine I-309 play a role in cell proliferation and apoptosis control, respectively.50 61
Cellular genes isolated by suppressive subtractive hybridization
| Gene encoding . | HTLV . | n . | GenBank no. . |
|---|---|---|---|
| Transcription factors | |||
| EGR2 (early growth response protein) | 1 | J04076 | |
| JunB (component of AP1-complex) | * | 1 | M29039 |
| ESE-1a (Ets-like transcription factor) | 2 | U73844 | |
| B94 (TNF-α–inducible response protein) | 4 | M92357 | |
| BBC1 (breast basic conserved protein 1) | 2 | X64707 | |
| DEC1 (bHLH motif containing protein) | 2 | AB004066 | |
| Surface receptors | |||
| IL-2 receptor α-chain | * | 6 | X01057 |
| Ferritin L-chain | 1 | M11147 | |
| Transferrin receptor (T9) | * | 1 | X01060 |
| T-cell receptor α-chain | 1 | M13052 | |
| Tumor necrosis factor receptor II | 1 | S63368 | |
| HLA-DR | * | 7 | V00523 |
| β2-microglobulin | * | 2 | V00567 |
| Cytokines | |||
| I-309 (CC chemokine) | 30 | M57502 | |
| IFN-γ | * | 1 | U10360 |
| IL-13 | 2 | L13029 | |
| ISG15 (IFN-induced 15-kd protein) | 2 | M13755 | |
| Signal transduction proteins | |||
| Plk-1 (serin/threonin protein kinase) | 1 | X73458 | |
| PI3 kinase (phosphatidylinositol 3-kinase) | 1 | Z46973 | |
| hVH-3 (tyrosine protein phosphatase) | 2 | U16996 | |
| PAC1 (tyrosine protein phosphatase) | 2 | U23853 | |
| Cyclin G1 (apoptosis regulator) | 1 | X77794 | |
| p21WAF1/CIP1(CDK-inhibitor) | * | 3 | U24170 |
| DDB2 (damage-specific DNA protein) | 2 | U18300 | |
| Ly-GDI (GDP-dissociation inhibitor) | 1 | L20688 | |
| Rab9 (GTPase) | 1 | Z97074 | |
| e3B1 (Eps8-binding protein) | 2 | AF006516 | |
| SGN2 (signalosome subunit 2) | 2 | AF084260 | |
| D123 (S-phase entry regulator) | 1 | D14878 | |
| Calcyclin (Ca2+ binding protein) | 1 | J02763 | |
| A20 (TNF-α inducible protein) | * | 1 | M59465 |
| Thymosin β-4 (immune response modulator) | * | 2 | M17733 |
| HIAP1 (human inhibitor of apoptosis 1) | 1 | U45878 | |
| HTLV-1 transcripts | 16 | ||
| EST | 100 | ||
| Unknown | 2 | ||
| Others | 138 |
| Gene encoding . | HTLV . | n . | GenBank no. . |
|---|---|---|---|
| Transcription factors | |||
| EGR2 (early growth response protein) | 1 | J04076 | |
| JunB (component of AP1-complex) | * | 1 | M29039 |
| ESE-1a (Ets-like transcription factor) | 2 | U73844 | |
| B94 (TNF-α–inducible response protein) | 4 | M92357 | |
| BBC1 (breast basic conserved protein 1) | 2 | X64707 | |
| DEC1 (bHLH motif containing protein) | 2 | AB004066 | |
| Surface receptors | |||
| IL-2 receptor α-chain | * | 6 | X01057 |
| Ferritin L-chain | 1 | M11147 | |
| Transferrin receptor (T9) | * | 1 | X01060 |
| T-cell receptor α-chain | 1 | M13052 | |
| Tumor necrosis factor receptor II | 1 | S63368 | |
| HLA-DR | * | 7 | V00523 |
| β2-microglobulin | * | 2 | V00567 |
| Cytokines | |||
| I-309 (CC chemokine) | 30 | M57502 | |
| IFN-γ | * | 1 | U10360 |
| IL-13 | 2 | L13029 | |
| ISG15 (IFN-induced 15-kd protein) | 2 | M13755 | |
| Signal transduction proteins | |||
| Plk-1 (serin/threonin protein kinase) | 1 | X73458 | |
| PI3 kinase (phosphatidylinositol 3-kinase) | 1 | Z46973 | |
| hVH-3 (tyrosine protein phosphatase) | 2 | U16996 | |
| PAC1 (tyrosine protein phosphatase) | 2 | U23853 | |
| Cyclin G1 (apoptosis regulator) | 1 | X77794 | |
| p21WAF1/CIP1(CDK-inhibitor) | * | 3 | U24170 |
| DDB2 (damage-specific DNA protein) | 2 | U18300 | |
| Ly-GDI (GDP-dissociation inhibitor) | 1 | L20688 | |
| Rab9 (GTPase) | 1 | Z97074 | |
| e3B1 (Eps8-binding protein) | 2 | AF006516 | |
| SGN2 (signalosome subunit 2) | 2 | AF084260 | |
| D123 (S-phase entry regulator) | 1 | D14878 | |
| Calcyclin (Ca2+ binding protein) | 1 | J02763 | |
| A20 (TNF-α inducible protein) | * | 1 | M59465 |
| Thymosin β-4 (immune response modulator) | * | 2 | M17733 |
| HIAP1 (human inhibitor of apoptosis 1) | 1 | U45878 | |
| HTLV-1 transcripts | 16 | ||
| EST | 100 | ||
| Unknown | 2 | ||
| Others | 138 |
HTLV indicates human T-cell leukemia virus type 1; n, number of complementary DNA isolations; TNF-α, tumor necrosis factor-α; IL-2, interleukin-2; IFN-γ, interferon γ; EST, expressed sequence tag.
Tax-/ATL-associated genes.
These expression analyses revealed that the ATL-derived JuanaW cells contained increased mRNA levels of various transcription factor and signal transduction genes compared to PHA-stimulated T cells (PBMCPHA). To determine the fold overexpression of these genes, the specific radioactive signals were normalized to the GAPDH signals. A significant to strong overexpression could be found for the basic helix-loop-helix transcription factor DEC-1 (4-fold), the dual specific phosphatase PAC1 (7-fold), the calcium ion-binding protein calcyclin (3-fold), the ferritin L-subunit (3-fold), the TNF-inducible protein B94 (3-fold), and the interferon (IFN)-induced ISG15gene (9-fold) as well as for the antiapoptotic chemokine I-309 (more than 40-fold) (Figures 1 and2A). Further Northern analyses were carried out to investigate whether the up-regulation observed in JuanaW cells is specific for HTLV-1–infected or Tax-expressing T cells. The RNA samples used were extracted from HTLV-1–transformed cell lines (C91-PL, MT-2), a Tax-transformed cell line (TAXI-1), ATL cells (HuT-102, JuanaW), HTLV− CD4+ leukemia cells (Jurkat, HuT-78), and PBMCPHA. The mRNA levels of DEC-1, B94, and PAC1 were elevated in all HTLV-1–transformed and Tax-immortalized cell lines (Figure 1A). This expression pattern would be expected if the gene was transactivated by the viral transactivator Tax. The genes encoding the ferritin L chain, ISG15, and calcyclin were strongly overexpressed in many Tax-positive cell lines including ATL cultures (Figure 1B and data not shown). In contrast, I-309 mRNA could only be detected in the ATL-derived cell culture JuanaW. These cells contained a remarkably large amount of I-309 transcripts (Figure 2A), which correlated well with the high prevalence of I-309 sequences among SSH cDNA clones (30 of 346).
Up-regulation of transcription factor and signal transduction genes in HTLV-1–infected T lymphocytes.
Total RNA was isolated from HTLV-1+/Tax+(C91-PL, HuT-102, MT-2, TAXI-1, JuanaW) and HTLV-1−/Tax− cell cultures (PBMCPHA, HuT-78, Jurkat), separated on denaturing gels, and subjected to Northern blot analysis. Specific mRNAs of the expected sizes were detected by radioactively labeled cDNAs derived from the transcription factor DEC-1, and signal transduction genes (PAC1, B94, ferritin L-chain, ISG15, and calcyclin). Some of the genes were found to be consistently up-regulated in all Tax+ cells (A); others were preferentially up-regulated in ATL patient-derived cultures (B). Equal RNA loading was controlled by hybridization to an internal standard (GAPDH). Results are representative of 3 independent Northern blots. J indicates JuanaW RNA; P, PBMCPHA RNA. The size of the transcripts is indicated in brackets. *For the B94 expression analysis MT-2 RNA was used instead of HuT-102 RNA.
Up-regulation of transcription factor and signal transduction genes in HTLV-1–infected T lymphocytes.
Total RNA was isolated from HTLV-1+/Tax+(C91-PL, HuT-102, MT-2, TAXI-1, JuanaW) and HTLV-1−/Tax− cell cultures (PBMCPHA, HuT-78, Jurkat), separated on denaturing gels, and subjected to Northern blot analysis. Specific mRNAs of the expected sizes were detected by radioactively labeled cDNAs derived from the transcription factor DEC-1, and signal transduction genes (PAC1, B94, ferritin L-chain, ISG15, and calcyclin). Some of the genes were found to be consistently up-regulated in all Tax+ cells (A); others were preferentially up-regulated in ATL patient-derived cultures (B). Equal RNA loading was controlled by hybridization to an internal standard (GAPDH). Results are representative of 3 independent Northern blots. J indicates JuanaW RNA; P, PBMCPHA RNA. The size of the transcripts is indicated in brackets. *For the B94 expression analysis MT-2 RNA was used instead of HuT-102 RNA.
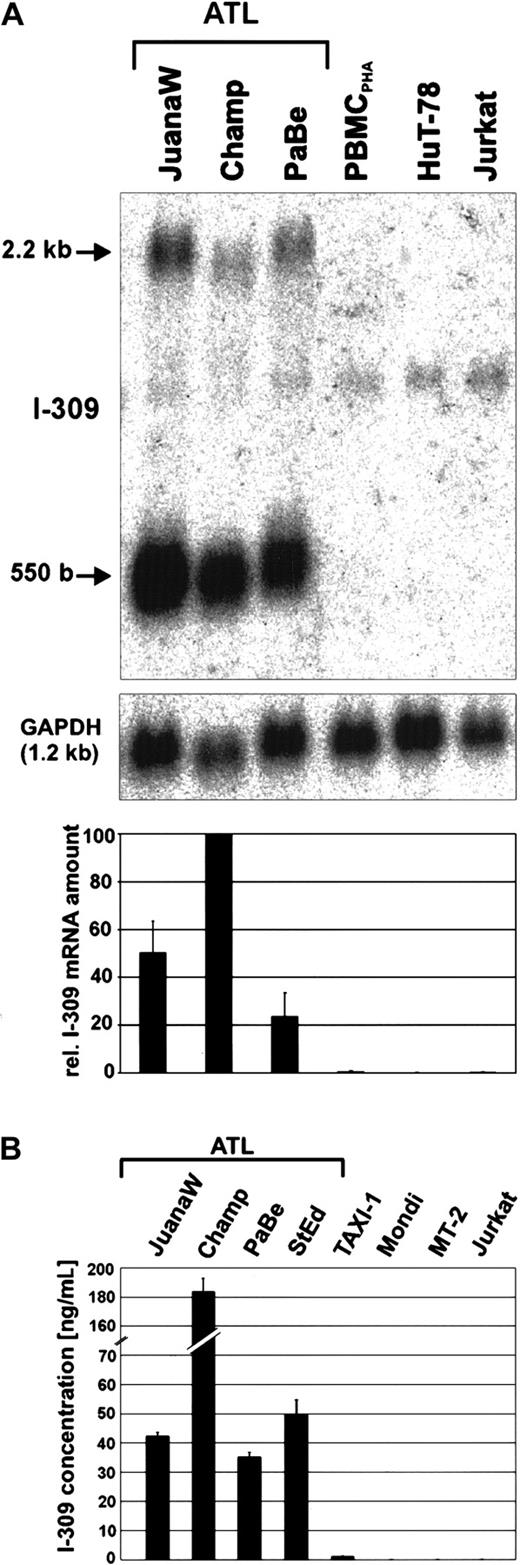
Overexpression of the chemokine I-309 in ATL-derived cells.
(A) Total RNA derived from ATL cultures (JuanaW, Champ, PaBe) and HTLV-1− reference cells (PBMCPHA, HuT-78, Jurkat) was subjected to Northern blot analysis using an I-309–specific cDNA probe. The top panel shows a representative autoradiograph. The 550-base RNA band was quantified by phosphorimaging. The lower panel depicts the mean RNA levels ± SD resulting from 3 independent experiments. (B) Culture supernatant from ATL cultures (JuanaW, Champ, PaBe, StEd) and reference cells (TAXI-1, Mondi, MT-2, Jurkat) were subjected to antigen capture ELISA. Plates were coated with monoclonal anti–I-309 antibodies and incubated with serial dilutions of the supernatants. Bound I-309 was detected with biotinylated secondary I-309 antibodies. I-309 concentrations were determined using recombinant human I-309 as a standard. The columns show the mean ± SD of 3 independent experiments.
Overexpression of the chemokine I-309 in ATL-derived cells.
(A) Total RNA derived from ATL cultures (JuanaW, Champ, PaBe) and HTLV-1− reference cells (PBMCPHA, HuT-78, Jurkat) was subjected to Northern blot analysis using an I-309–specific cDNA probe. The top panel shows a representative autoradiograph. The 550-base RNA band was quantified by phosphorimaging. The lower panel depicts the mean RNA levels ± SD resulting from 3 independent experiments. (B) Culture supernatant from ATL cultures (JuanaW, Champ, PaBe, StEd) and reference cells (TAXI-1, Mondi, MT-2, Jurkat) were subjected to antigen capture ELISA. Plates were coated with monoclonal anti–I-309 antibodies and incubated with serial dilutions of the supernatants. Bound I-309 was detected with biotinylated secondary I-309 antibodies. I-309 concentrations were determined using recombinant human I-309 as a standard. The columns show the mean ± SD of 3 independent experiments.
Strong overexpression of the chemokine I-309 in ATL-derived cells
To discriminate whether the overexpression of I-309 was an individual feature of cultured JuanaW ATL cells or was due to its leukemic origin, permanently growing cultures from 3 other patients with acute ATL were established and checked for I-309 expression. Subsequent Northern blot analyses showed a huge overexpression of theI-309 gene in the ATL-derived cultures Champ, PaBe, JuanaW (Figure 2A), and StEd (data not shown). In contrast, I-309 transcripts could be neither observed in the acute lymphoblastic leukemia (ALL)-derived CD4+ T-cell lines HuT-78 and Jurkat nor in PHA-treated PBMCs. Two specific bands corresponding to the expected mRNA sizes of approximately 550 and 2200 bases were detected. The most abundant transcript observed corresponds to the 550-base mRNA reported to code for the I-309 polypeptide.62 The larger transcript is of unknown function. To determine whether the high amount of RNA leads to the secretion of I-309 protein, cell culture supernatants were analyzed by antigen capture ELISA. As a result, I-309 was detected at concentrations ranging from about 35 ng/mL (PaBe) to more than 180 ng/mL (Champ) (Figure 2B). The same results were obtained after more than 6 months of permanent ATL cell culture. This observation indicates that I-309 overexpression is not due to uninfected activated T cells in the ATL cell preparation. In accordance with Northern blot results (data not shown), the supernatant of TAXI-1 cells, which are transformed by transduction of the Tax gene, contained only minimal amounts of I-309. Neither HAM-derived Mondi cells nor the HTLV-1–transformed MT-2 cells produced any I-309 protein. Because both cell lines are known to express the Tax protein, this suggests that the viral protein alone cannot account for the I-309 overexpression. Yet, the analysis of the I-309 promoter sequence with the MatInspector V2.2 (http://transfac.gbf.de/index.html) program63 revealed binding sites for both CREB and nuclear factor-κB (NF-κB) at upstream positions 393 bp and 272 bp, which exactly match the corresponding consensus sequences. Because Tax can stimulate the transcription via these sites, the transactivator may contribute to the activation of the I-309 gene in ATL cells. Taken together, these data suggest that the production and secretion of I-309 is a characteristic of ATL-derived cells, which is neither shared by HAM-derived nor by other HTLV-1– and Tax-expressing T cells.
Antiapoptotic activity of ATL cell supernatants is mediated by the chemokine I-309
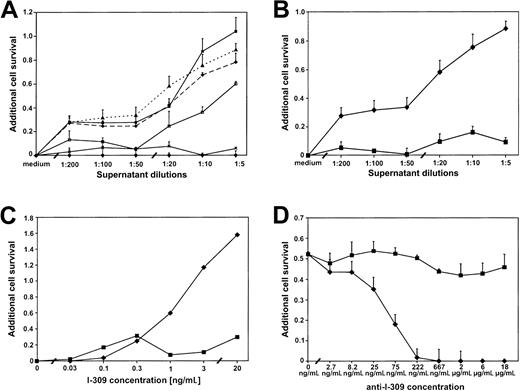
To analyze whether the amount of I-309 secreted into the supernatant is sufficient to exert the antiapoptotic effect,50 ATL cell culture supernatants were titrated onto mouse thymoma BW5147C cells, which had been treated to undergo apoptosis. These cells have been shown to be highly susceptible to apoptosis induction by DEX.50 The addition of supernatants from 4 different ATL cultures (Champ, PaBe, JuanaW, StEd) protected the thymoma cells from DEX-induced apoptosis in a dose-dependent manner, whereas the supernatants of MT-2 and Jurkat had no antiapoptotic effect (Figure 3A). The addition of, for example, Champ supernatant increased cell survival by up to 100% compared with cell survival in the absence of ATL culture supernatant. This is consistent with ELISA results, which showed that Champ cells expressed the largest amounts of I-309 (Figure 2B). Flow cytometric analysis of the DNA content of individual nuclei confirmed that the increased cell survival is largely due to a reduced apoptosis rate (data not shown).
ATL cell culture supernatants contain an antiapoptotic activity mediated by I-309.
BW5147C thymoma cells were treated with dexamethasone (DEX; 62.5 nM) to induce apoptosis (BW5147C/DEX cells) and incubated with increasing dilutions of ATL and control culture supernatants. Cell viability was assessed by MTT assays. Additional cell survival was calculated as described in “Materials and methods.” (A) Antiapoptotic effect of 4 ATL culture supernatants (PaBe, ♦; Champ, ▪; JuanaW, ▴; StEd, ■) in the BW5147C/DEX cells. MT-2 (×) and Jurkat (●) cell culture supernatants served as negative controls. (B) Abrogation of the antiapoptotic effect of the JuanaW cell culture supernatant in BW5147C/DEX cells by the addition of pertussis toxin (2.5 ng/mL) ♦, without pertussis toxin (PT); ▪, with PT. (C) Antiapoptotic capacity of purified I-309 and its susceptibility to pertussis toxin inhibition. The BW5147C/DEX cells were incubated with various concentrations of purified I-30950 in the presence (▪) and absence (♦) of pertussis toxin. (D) Neutralization of the antiapoptotic I-309 effect by monoclonal anti–I-309 antibodies. BW5147C/DEX cells were cultivated in the presence of undiluted ATL supernatant from JuanaW and serial dilutions of anti–I-309 antibodies (♦) or a control antibody (anti-IgG1, ▪). Results represent 3 to 6 independent MTT assays (except for C where only 2 experiments could be done due to limited amounts of purified I-309 protein).
ATL cell culture supernatants contain an antiapoptotic activity mediated by I-309.
BW5147C thymoma cells were treated with dexamethasone (DEX; 62.5 nM) to induce apoptosis (BW5147C/DEX cells) and incubated with increasing dilutions of ATL and control culture supernatants. Cell viability was assessed by MTT assays. Additional cell survival was calculated as described in “Materials and methods.” (A) Antiapoptotic effect of 4 ATL culture supernatants (PaBe, ♦; Champ, ▪; JuanaW, ▴; StEd, ■) in the BW5147C/DEX cells. MT-2 (×) and Jurkat (●) cell culture supernatants served as negative controls. (B) Abrogation of the antiapoptotic effect of the JuanaW cell culture supernatant in BW5147C/DEX cells by the addition of pertussis toxin (2.5 ng/mL) ♦, without pertussis toxin (PT); ▪, with PT. (C) Antiapoptotic capacity of purified I-309 and its susceptibility to pertussis toxin inhibition. The BW5147C/DEX cells were incubated with various concentrations of purified I-30950 in the presence (▪) and absence (♦) of pertussis toxin. (D) Neutralization of the antiapoptotic I-309 effect by monoclonal anti–I-309 antibodies. BW5147C/DEX cells were cultivated in the presence of undiluted ATL supernatant from JuanaW and serial dilutions of anti–I-309 antibodies (♦) or a control antibody (anti-IgG1, ▪). Results represent 3 to 6 independent MTT assays (except for C where only 2 experiments could be done due to limited amounts of purified I-309 protein).
To confirm that the antiapoptotic activity was mediated by the I-309 receptor, CCR8, an inhibitor was added. Pertussis toxin interferes with the CCR8-associated Gi-protein signaling. As a result, the antiapoptotic effect of the ATL cell supernatant was dramatically reduced or even completely abolished in the presence of pertussis toxin (Figure 3B). The same result could be obtained with purified I-309 (Figure 3C). These observations suggested the involvement of a Gi-protein–coupled receptor like CCR8 in this pathway and corroborated the notion that I-309 is the factor in the supernatant that had prevented apoptosis. These data could be confirmed using 2 different methods (MTT and [3H]-thymidine incorporation assays) in 2 murine cell lines (BW5147C, DO-Cep4) (Figure 3 and data not shown). Culturing both tester cells with ATL culture supernatant also increased cell survival by up to 45% in the presence of cisplatin, a DNA intercalating drug leading to strand breaks. Under these conditions, cell viability was considerably reduced by pertussis toxin (data not shown). To add further proof that the I-309 chemokine is the factor mediating the antiapoptotic activity, DEX-treated BW5147C cells were incubated with JuanaW supernatant and increasing amounts of monoclonal antibodies specific to I-309. As shown in Figure 3D, the antiapoptotic effect could be abolished by anti–I-309 antibodies in a dose-dependent manner, whereas the isotypic control antibody had no effect on the growth of the cells. Hence, the I-309 present in the ATL supernatant is capable of preventing apoptosis of T cells.
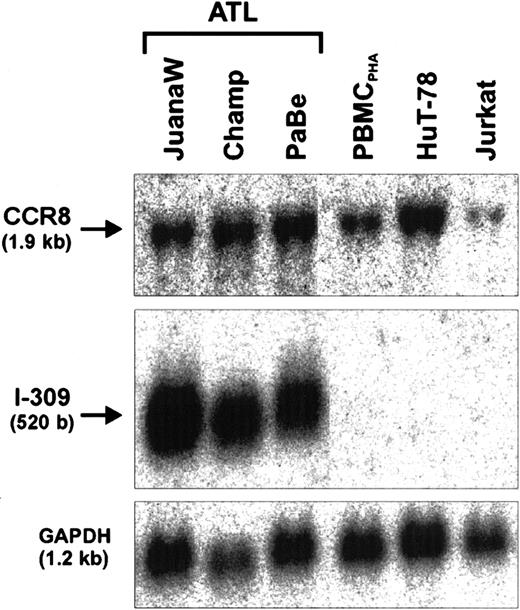
Autocrine antiapoptotic activity of I-309 in an ATL cell culture contributes to cell proliferation
The demonstration of the antiapoptotic activity in the ATL cell supernatant led us to the hypothesis that I-309 may act on the producer cells to increase their resistance to apoptosis. This, however, would require the presence of the cognate receptor (CCR8), which is highly specific for I-309, on these cells. To test for CCR8 gene expression, further Northern blot analyses were performed. As Figure4 shows, CCR8 transcripts are present in high abundance in all ATL-derived cells tested. To obtain further proof for an antiapoptotic autocrine action of I-309, the chemokine signaling was suppressed in PaBe ATL cells that had been treated with monoclonal anti-Fas antibodies to undergo apoptosis (Figure5).64 Increasing the concentration of Fas antibodies resulted in reduced [3H]-thymidine incorporation in ATL (PaBe) and Tax-immortalized (TAXI-1) cells, which is due to an increased apoptosis rate within the culture.65 The level of apoptosis increased with antibody concentrations between 0 and 100 ng/mL and reached a plateau at more than 100 ng/mL, indicating a partial resistance of these cells to apoptosis. This is in good agreement with previous studies.66 At the highest anti-Fas concentration of 500 ng/mL, cell proliferation of both ATL and TAXI-1 cells was reduced by only 39% compared with untreated cells (Figure 5A). However, in the presence of anti–I-309 antibodies the proliferation of the ATL cells was further decreased to 55%, whereas the control (TAXI-1) was unaffected. Similar results were obtained with Champ ATL cells. In contrast, the addition of isotype-matched control antibody did not influence cell growth. The corresponding proliferation rate was identical with the one obtained in the absence of antibody. Interestingly, even in the absence of external apoptotic signals, the anti–I-309 antibodies alone diminished cell proliferation by some 6% (Figure 5A, 0 μg/mL anti-Fas, + anti–I-309 antibody). A triple combination of anti-Fas, anti–I-309, and pertussis toxin did not substantially change the rate of cell proliferation. The moderate additional reduction in cell proliferation is probably due to pertussis toxin–mediated inhibition of I-309 signaling triggered by intracellular ligand-receptor interaction. Thus, this observation also supports the notion that interference with the I-309 receptor and no other G-protein–coupled receptor had caused the reduced cell proliferation. To specifically determine apoptosis, annexin V staining was applied, which detects a marker of early apoptosis.67This analysis revealed that Fas-induced apoptosis in Mondi and TAXI-1 control cells remained unchanged in the presence of I-309–specific antibodies (Figure 5B and data not shown). In contrast, the anti–I-309 antibodies increased early apoptosis in ATL (PaBe) cells (Figure 5B). Together these data corroborate an autocrine antiapoptotic effect of I-309 in the presence of external apoptotic stimuli.
Coexpression of I-309 and its cognate receptor CCR8.
Total RNA derived from ATL cultures (JuanaW, Champ, PaBe) and HTLV-1− reference cells (PBMCPHA, HuT-78, Jurkat) was subjected to Northern blot analyses. The membranes were sequentially incubated with CCR8-, I-309-, and GAPDH-specific cDNA probes. One representative autoradiograph of 3 experiments is shown.
Coexpression of I-309 and its cognate receptor CCR8.
Total RNA derived from ATL cultures (JuanaW, Champ, PaBe) and HTLV-1− reference cells (PBMCPHA, HuT-78, Jurkat) was subjected to Northern blot analyses. The membranes were sequentially incubated with CCR8-, I-309-, and GAPDH-specific cDNA probes. One representative autoradiograph of 3 experiments is shown.
Reduction of Fas-mediated apoptosis in cultured ATL cells by I-309.
(A) ATL cells (PaBe) or Tax-immortalized control T cells (TAXI-1) were cultivated either in the presence of apoptosis-inducing monoclonal anti-Fas antibody alone (♦) or in the presence of anti-Fas and anti–I-309 antibody (▪), anti-Fas, anti–I-309 antibody, and pertussis toxin (●), or anti-Fas and isotypic control anti-IgG1 antibody (▴) for 48 hours. Proliferation was measured by [3H]-thymidine incorporation. The reduced cell proliferation represents the relative [3H]-thymidine incorporation of untreated cells (set to 100%) minus [3H]-thymidine incorporation of antibody/PT-treated cells. Error bars reflect the SEM of 4 to 5 independent experiments. (B) ATL cells (PaBe) or HTLV-1–infected HAM/TSP cells (Mondi) were incubated in the absence or presence of anti-Fas antibody (−/+ anti-Fas) and in the absence (−ab) or presence of anti–I-309 (+a-I-309)/control anti-IgG antibody (+a-IgG). Induction of apoptosis was evaluated by annexin V staining and FACS analysis. One representative of 3 independent experiments is depicted.
Reduction of Fas-mediated apoptosis in cultured ATL cells by I-309.
(A) ATL cells (PaBe) or Tax-immortalized control T cells (TAXI-1) were cultivated either in the presence of apoptosis-inducing monoclonal anti-Fas antibody alone (♦) or in the presence of anti-Fas and anti–I-309 antibody (▪), anti-Fas, anti–I-309 antibody, and pertussis toxin (●), or anti-Fas and isotypic control anti-IgG1 antibody (▴) for 48 hours. Proliferation was measured by [3H]-thymidine incorporation. The reduced cell proliferation represents the relative [3H]-thymidine incorporation of untreated cells (set to 100%) minus [3H]-thymidine incorporation of antibody/PT-treated cells. Error bars reflect the SEM of 4 to 5 independent experiments. (B) ATL cells (PaBe) or HTLV-1–infected HAM/TSP cells (Mondi) were incubated in the absence or presence of anti-Fas antibody (−/+ anti-Fas) and in the absence (−ab) or presence of anti–I-309 (+a-I-309)/control anti-IgG antibody (+a-IgG). Induction of apoptosis was evaluated by annexin V staining and FACS analysis. One representative of 3 independent experiments is depicted.
The high level of I-309 might counteract not only external but also internal proapoptotic stimuli. To elucidate this possibility, ATL cells (JuanaW) were incubated with increasing concentrations of pertussis toxin. As shown in Figure 6A the drug inhibited the proliferation of JuanaW cells in a dose-dependent manner; at the highest pertussis toxin concentration, cell proliferation was reduced by more than 50%. Similar results were obtained with PaBe and Champ ATL-derived cells (data not shown). In contrast, the proliferation of both CD4+ control cell lines, HuT-78 (T-ALL) and MT-2 (HTLV-1–transformed), was not affected. This indicates that pertussis toxin has no unspecific cytotoxic effect on uninfected and HTLV-1–infected T cells. To verify that the reduced proliferation was due to apoptosis, DNA contents of pertussis toxin–treated cells were determined by a modified PI staining assay that allows the simultaneous distinction of viable, apoptotic, and necrotic cells (Figure 6B).53 These analyses showed that pertussis toxin significantly increased apoptosis, whereas necrosis was only minimally affected. These results were confirmed by annexin V staining (Figure 6C). Hence, all 3 assays demonstrate that pertussis toxin induced apoptosis in ATL cells and, therefore, suggest a role for I-309 signaling in counteracting inherent apoptosis. Taken together, the autocrine antiapoptotic CCR8/I-309 signaling may stimulate the proliferation of ATL cells by rendering the leukemic cells less susceptible to internal and external apoptotic signals.
Reduced ATL cell growth and induction of apoptosis by abrogation of I-309 signaling.
To block the I-309 receptor (CCR8), ATL (JuanaW) and control (MT-2, HuT-78) cells were incubated with increasing amounts of pertussis toxin for 48 hours. (A) Reduced cell proliferation was calculated from [3H]-thymidine incorporation experiments as described in the legend to Figure 5. In contrast to ATL cells (JuanaW), the proliferation rates of HTLV-1+ (MT-2) and HTLV-1− (HuT-78) control cells were not altered. (B) Detection of apoptotic cells by modified propidium iodide staining.53 Columns reflect the means of 3 to 4 independent experiments ± SD. (C) Detection of an early apoptosis marker by annexin V staining (one representative of 3 independent experiments is shown).
Reduced ATL cell growth and induction of apoptosis by abrogation of I-309 signaling.
To block the I-309 receptor (CCR8), ATL (JuanaW) and control (MT-2, HuT-78) cells were incubated with increasing amounts of pertussis toxin for 48 hours. (A) Reduced cell proliferation was calculated from [3H]-thymidine incorporation experiments as described in the legend to Figure 5. In contrast to ATL cells (JuanaW), the proliferation rates of HTLV-1+ (MT-2) and HTLV-1− (HuT-78) control cells were not altered. (B) Detection of apoptotic cells by modified propidium iodide staining.53 Columns reflect the means of 3 to 4 independent experiments ± SD. (C) Detection of an early apoptosis marker by annexin V staining (one representative of 3 independent experiments is shown).
Discussion
To link lymphocyte genes to HTLV-1–induced leukemogenesis, we have identified several overexpressed mRNAs in cultured ATL cells. Many of the corresponding proteins have functions in signal transduction and could, thus, play a role in deregulated proliferation of the malignant cells. Up-regulated genes included the dual-specific protein phosphatase PAC1, the bHLH-transcription factor DEC-1, and the TNF-α inducible protein B94. It is conceivable that the overexpression of those genes in all tested HTLV-1–transformed and Tax-immortalized cell lines is mediated by the viral transactivator Tax. For instance, the activation of DEC-1 may be mediated by the CREB pathway. This is suggested by the observation that CREB-stimulating agents have been found to increase DEC-1 transcription56,68 and that Tax stimulates transcription via CREB.39 Likewise, B94 mRNA is rapidly induced by proinflammatory stimuli including TNF-α, IL-1β, and LPS, which induce the activation of NF-κB.69 Because Tax also activates NF-κB,39 Tax presumably stimulates the expression of B94.
The overexpression of the ISG15 and I-309 genes could only be found in JuanaW and other ATL-derived cell cultures. ISG15, a 15-kd protein encoded by an interferon γ (IFN-γ)-stimulated gene (ISG), is secreted from lymphocytes and induces IFN-γ production.70 This property possibly contributes to the increased IFN-γ mRNA levels found in JuanaW cells (Table 1) and in other ATL cells.71
Four of 4 ATL patient–derived cells expressed the protein at strongly increased levels, but none of the in vitro HTLV-1–infected cell lines did so. This restricted expression pattern suggests that I-309 overexpression is a characteristic of ATL cell cultures. Long-term cultivation of one of the used lines indicated that I-309 overexpression can be maintained for a minimum of 2 years in IL-2–dependent cultures. The overexpression of the I-309gene exclusively in ATL cells indicates that viral functions including Tax alone are not sufficient to activate its transcription. However, Tax may contribute to the I-309 overexpression. This is supported by the notion that Tax stimulates the IL-1 gene,73which is required for I-309 gene expression.72Furthermore, the murine homologue (TCA3) carries a NF-κB site in its promoter, which might respond to Tax-mediated NF-κB activation.74 Sequence analysis of the I-309 promoter revealed potentially Tax-responsive upstream CREB and NF-κB binding sites.
The chemokine I-309 and its murine homologue TCA3 were both initially identified in T cells that had been restimulated after a history of previous activation.62,75 I-309 is a member of the family of CC chemokines, which contains 2 disulfide bonds at the N terminus. As a structural peculiarity, I-309 displays a third well-separated disulfide bond.76 I-309, like many other chemokines, induces leukocyte migration. In contrast to all other members of the CC chemokine family, I-309 has an additional biologic function in apoptosis.50 In addition to I-309, all ATL-derived cultures expressed the gene for the cognate receptor, CCR8, a 7-transmembrane domain Gi-protein–coupled receptor.77-79 In contrast to most other chemokine receptors that exhibit a promiscuous binding property to various chemokines, only one high-affinity cellular ligand has been identified for CCR8 (I-309).80 Recently, several tumor viral proteins that bind to CCR8 have been identified.81-83 Both, the high selectivity to its cellular ligand and its function as a viral target indicate that this chemokine has a unique function.
The I-309 protein secreted by our ATL cultures prevented apoptosis of heterologous T cells. The following observations suggest that it also acts on the producer cells and increases their resistance to apoptosis. First, the cognate receptor (CCR8) is expressed in these cells. Second, interference with I-309 signaling considerably augmented the proapoptotic Fas signals in ATL cell cultures. At least partially this autocrine I-309 signaling may be explained by intracellular binding of the ligand to its receptor, a phenomenon observed for various growth factors and cytokines previously.84-87 This is suggested by the moderate increase of apoptosis resulting from addition of pertussis toxin to anti–I-309 antibody–treated ATL cultures. The I-309–mediated counteraction to external proapoptotic stimuli may cooperate with the antiapoptotic function of the death antagonist Bcl-XL, which is up-regulated in ex vivo ATL samples,88 to increase the survival of leukemia cells.89 Both mechanisms may contribute to the high resistance of patients with ATL to chemotherapy. The large amounts of I-309 could not only prevent exogenously induced apoptosis but may also antagonize internal proapoptotic stimuli. This is suggested by our observation that pertussis toxin, an inhibitor of I-309 signaling, significantly increased apoptosis of ATL-derived cells. In summary, these data provide evidence that the CCR8/I-309 signaling may stimulate the proliferation of ATL cells by generating an antiapoptotic autocrine loop, which counteracts inherent apoptotic stimuli. In addition, CCR8/I-309 could render leukemic cells less susceptible to external apoptosis signals and could, hence, contribute to ATL progression.
The technical assistance of Elisabeth Derow and Daniel Romaker is greatly appreciated. We thank Kerstin Haller and Claudia Matteucci for helpful scientific discussions.
Supported by the Deutsche Forschungsgemeinschaft (SFB 466 Lymphoproliferation und virale Immundefizienz), by the Wilhelm Sander-Stiftung, and by HERN (HTLV European Research Network).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ralph Grassmann, Institut für Klinische und Molekulare Virologie, Schlossgarten 4, D-91054 Erlangen, Germany; e-mail: grassmann@viro.med.uni-erlangen.de.

![Fig. 5. Reduction of Fas-mediated apoptosis in cultured ATL cells by I-309. / (A) ATL cells (PaBe) or Tax-immortalized control T cells (TAXI-1) were cultivated either in the presence of apoptosis-inducing monoclonal anti-Fas antibody alone (♦) or in the presence of anti-Fas and anti–I-309 antibody (▪), anti-Fas, anti–I-309 antibody, and pertussis toxin (●), or anti-Fas and isotypic control anti-IgG1 antibody (▴) for 48 hours. Proliferation was measured by [3H]-thymidine incorporation. The reduced cell proliferation represents the relative [3H]-thymidine incorporation of untreated cells (set to 100%) minus [3H]-thymidine incorporation of antibody/PT-treated cells. Error bars reflect the SEM of 4 to 5 independent experiments. (B) ATL cells (PaBe) or HTLV-1–infected HAM/TSP cells (Mondi) were incubated in the absence or presence of anti-Fas antibody (−/+ anti-Fas) and in the absence (−ab) or presence of anti–I-309 (+a-I-309)/control anti-IgG antibody (+a-IgG). Induction of apoptosis was evaluated by annexin V staining and FACS analysis. One representative of 3 independent experiments is depicted.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/4/10.1182_blood.v98.4.1150/6/m_h81611389005.jpeg?Expires=1769091926&Signature=qBaPMr7~xA54RKBoSeSWrzpQjWbhPRR37dObYojl6Fk1QwkEjiUDsy-7y2~38bMjEZWHNPQsePRdTJ65GiIrey0kto6SXk4KB7JjvBR008qyFv65p6IJ5qjBVqLdXuFOLGcx1MzJJrSYhjrj67IoaWc5oPHLwjm4HyWmGU1B2CeC~acpj6fbh8ovqNbQw1abfzsy0zoiXWcxN4PqBaY43mfz670s1UfOinG5W50YWFlr6lXNBhPBCBzw0XWrX3GZI-9NJGzOBQ5objpiJLOHN~gn3m-~Huq4ilIIwelOS~EX3Jq5aERYvoQrI9~8BW15MS6j29dMgS~7laDRnYOP2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Reduced ATL cell growth and induction of apoptosis by abrogation of I-309 signaling. / To block the I-309 receptor (CCR8), ATL (JuanaW) and control (MT-2, HuT-78) cells were incubated with increasing amounts of pertussis toxin for 48 hours. (A) Reduced cell proliferation was calculated from [3H]-thymidine incorporation experiments as described in the legend to Figure 5. In contrast to ATL cells (JuanaW), the proliferation rates of HTLV-1+ (MT-2) and HTLV-1− (HuT-78) control cells were not altered. (B) Detection of apoptotic cells by modified propidium iodide staining.53 Columns reflect the means of 3 to 4 independent experiments ± SD. (C) Detection of an early apoptosis marker by annexin V staining (one representative of 3 independent experiments is shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/4/10.1182_blood.v98.4.1150/6/m_h81611389006.jpeg?Expires=1769091926&Signature=SWoxDgoLk5eSz1sq1n-7cSVGDg3nf8V6hbUwCGbcIVDLUbEGw4R~rO1gTMgSfdVlbu65hHLx9pq9fYm0ygpR7RgoQJc1Vo9BDKPb5NBdRrRfKyVSjf30kcf1aaqLdrROBtSvk5DghP6gph2pD2jnDEPKESxTr9ZGZ4k6p3IWiW7-pf4IeVKFoOPa3TzPGeZ4-ZB2H89oac0a~73Li-a9NQ5Yo02ImydNgLfyIqdx-aoNCpyWmgx27deMj7JUxoPTXeJgnOlp91EnkhWF-KTHO8wAq0nbptol4A2B2zgj2IVRT09S~HfAHFNBsbWJiJSnBQyJoX01FpKMnbfUlxY6WQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal