The cellular mechanisms that configure the cytoskeleton during migration of dendritic cells (DCs) are poorly understood. Immature DCs assemble specialized adhesion structures known as podosomes at their leading edge; these are associated with the localized recruitment of the Wiskott-Aldrich Syndrome protein (WASp) and the actin organizing actin-related protein 2/3 complex. In immature DCs lacking WASp, podosomes are absent, residual dysmorphic lamellipodia and filopodia are nonpolarized, and migration is severely compromised. Microinjection studies indicate that podosome assembly and polarization require concerted action of Cdc42, Rac, and Rho, thereby providing a link between sequential protrusive and adhesive activity. Formation of podosomes is restricted to cells with an immature phenotype, indicating a specific role for these structures during the early migratory phase.
Introduction
Regulated migration of dendritic cells (DCs) is central to the induction of physiological immune responses and possibly to the maintenance of tolerance.1,2 These processes necessitate plasticity of the cytoskeleton, so much so that highly motile cells such as DCs have evolved specialized responses to specific external signals received from the cell's environment. Considerable evidence now exists for the use of sequential chemokine-chemokine receptor pairs for each step in the DC migratory process.2 However, the dynamic organization of the cytoskeleton in DCs is poorly understood.
Cell motility in fibroblasts is controlled by small GTPases Rho, Rac, Cdc42Hs, and Ras.3-5 Cdc42Hs belongs to the Rho family of small guanosine 5′-triphosphate (GTP)–binding proteins and is a regulator of cell polarity, filopodial extension, macrophage chemotaxis, Fc-receptor–mediated phagocytosis, and DC endocytosis.6,7 Activation of the related GTP-binding protein Rac leads to accumulation of an actin network at the cell periphery producing lamellipodia and membrane ruffling.8In Swiss 3T3 fibroblasts and BAC1.2F5 macrophages, Cdc42Hs and Rac have been shown to participate in the formation of cell-substratum focal adhesion complexes distinct from larger Rho-induced focal adhesions.5,9,10 Rho also regulates the formation of contractile actin-myosin filaments to form stress fibers.8Activation of Cdc42Hs in Swiss 3T3 cells leads to sequential activation of Rac, and then Rho, suggesting that multiple aspects of cell locomotion are coordinately regulated. Polarization of Rac activity is also dependent on Cdc42Hs.5 11
Wiskott-Aldrich Syndrome Protein (WASp) is expressed in hematopoietic cells. It belongs to a recently defined family of more widely expressed proteins involved in transduction of signals from receptors on the cell surface to the actin cytoskeleton. The complex multidomain structure of WASp suggests that it plays a central role in the integration of multiple signals and the direction of regulated effector functions. In vitro, WASp binds specifically to the GTP-bound form of Cdc42Hs and less well to GTP-bound Rac.12-14 The interaction with Cdc42Hs/Rac has been shown to be mediated through a Cdc42Hs/Rac small GTPase interactive binding motif (or GTPase-binding domain) in WASp, which is found in many downstream effectors of Cdc42Hs and Rac, although regions outside this motif are necessary for tight interaction.15
The C-terminus of WASp contains a series of acidic residues with net negative charge that are necessary for direct binding of the conserved eukaryotic cytoskeletal organizer known as the actin-related protein (Arp) 2/3 complex.16,17 The function of the complex is to nucleate actin filament assembly and to link the pointed end of new filaments to the side of older filaments, thereby creating a branching network.18,19 Arp2/3 complex is therefore localized to areas of active actin polymerization, such as in lamellipodia, the base of filopodia, and phagocytic cups, specifically at the junctions of Y-branches of polymerizing actin filaments. In vitro, the nucleation of new actin filaments by Arp2/3 complex is considerably enhanced when they are bound to WASp-family proteins. Overexpression of the conserved C-terminal acidic A domain (but not the WH2 domain alone) of WASp and Scar disrupts the localization of the Arp2/3 complex, inhibits lamellipodia and stress-fiber formation in Swiss 3T3 cells, and inhibits endogenous filopodia formation in J774 macrophages, indicating that WASp-related proteins regulate the cytoskeleton through this complex.20 Furthermore, in cell extracts depleted of Arp2/3 complex, WASp-coated microspheres and Listeria monocytogenes are nonmotile and fail to form characteristic actin tails.21 The activity of WASp family proteins in terms of their ability to bind Arp2/3 complex is also subject to regulation. Intramolecular interaction between the GTPase-binding domain and the C-terminus of WASp results in occlusion of the binding site for Arp2/3 complex.22 Binding of GTP-bound Cdc42Hs produces conformational change, disruption of the hydrophobic core, and release of the C-terminus. Additional modulation of this auto-inhibitory mechanism may occur through binding of signaling molecules to other WASp domains.
We have previously shown that DCs lacking WASp exhibit defects of filopodial formation and polarity, suggesting that transduction of signals from activated Cdc42Hs through WASp is abolished. In this study, we show that WASp is necessary for recruitment of Arp2/3 complex to podosomes in DCs and that organization of their cyto-architecture is regulated by Rho-family GTPases Cdc42Hs, Rac, and Rho. We also show that formation of podosomes is developmentally regulated.
Materials and methods
Cell culture
Peripheral blood mononuclear cells were prepared from healthy volunteers and 6 patients with severe WAS, after informed consent was obtained. All patients lacked WASp by Western blotting in mononuclear cell protein extracts and had molecular defects confirmed by DNA sequence analysis. For DC preparations, peripheral blood mononuclear cells were cultured in RPMI supplemented with 10% lipopolysaccharide (LPS)–low fetal calf serum (Myoclone Superplus) (Gibco, Life Technologies, Paisley, United Kingdom), 10 000 IU/mL penicillin and streptomycin (Gibco), 100 ng/mL recombinant human granulocyte-macrophage colony stimulating factor (Leucomax) (Sandoz Pharmaceuticals, Camberley, United Kingdom), and 25 ng/mL recombinant human interleukin-4 (gift from Professor D. Katz, University College London, United Kingdom). Cytokines were refreshed on day 3 or 4 of culture. On day 6 or 7, nonadherent, immature DCs were harvested and purified to approximately 90% extraction of T and B lymphocytes by means of CD3 and CD19 Dynabeads (Dynal Biotech, Wirral, United Kingdom). Immature DCs were matured by addition of 50 ng/mL LPS on day 6 of culture for a total of 24 hours.
Expression and purification of recombinant proteins
V12Rac1, V12Cdc42Hs, N17Rac1, N17Cdc42Hs, and C3 transferase were expressed as glutathione-S-transferase fusion proteins inEscherichia coli and purified on glutathione-sepharose/agarose beads with subsequent thrombin cleavage as described.8 Protein concentrations were assayed by means of a Bradford assay (Bio-Rad Laboratories, Hercules, CA). The plasmid vector p–enhanced green fluorescent protein (pEGFP)–WASp was constructed by amplifying the human WASp coding region by polymerase chain reaction and cloning it into pEGFP-C2 (Clontech Laboratories, Basingstoke, United Kingdom), in frame and downstream of the EGFP coding region. The entire EGFP-WASp coding sequence was then cloned into a Moloney-based retroviral vector plasmid,23-25 downstream of an internal cytomegalovirus promoter.
Microinjection and analysis
We plated 2 × 104 DCs onto 22-mm2 coverslips (Chance Propper, Smethwick, United Kingdom) coated with human fibronectin (Sigma, Poole, United Kingdom) and allowed them to adhere for 2 to 4 hours. We injected 1 mg/mL V12Cdc42Hs, 0.4 mg/mL V12 Rac1, 0.75 mg/mL N17 Rac1, 3.5 mg/mL N17 Cdc42Hs, and 0.1 mg/mL C3 transferase. For coinjection experiments, the concentration of V12Cdc42Hs was reduced to 0.5 mg/mL. The activity of V12Rac1, V12Cdc42Hs, N17 Rac1, and N17Cdc42Hs were verified by injection into BAC1.2F5 cells (as described in Allen et al9) at the same concentrations used for DCs. Injected cells were identified by co-injection of rabbit immunoglobulin (Ig)–G (Sigma) at 0.5 mg/mL with recombinant proteins and labeling with Alexa 488 goat anti–rabbit IgG (Molecular Probes, Leiden, The Netherlands). After injection, DCs were incubated at 37°C in 5% CO2 for 10, 30, or 60 minutes before fixing in 4% paraformaldehyde (PFA)/3% glucose for 20 minutes at room temperature. Plasmid DNA was injected at 10 or 100 ng/μL, and cells were incubated for 2 or 4 hours after injection before fixing and staining.
Time-lapse phase microscopy
DCs were allowed to adhere to fibronectin-coated coverslips as above. Dunn chambers (Weber Scientific International, Hamilton, NJ) were assembled with medium in both inner and outer wells.26 27 For each experiment, 2 Dunn chambers were run in parallel. Cells were visualized with an Olympus inverted microscope (Southall, United Kingdom) and 10 × phase-contrast objectives. Images were acquired from charge-coupled device cameras (model TM-765) (Pulnix Europe, Basingstoke, Hampshire, United Kingdom), captured by a Matrox Magic (Dorval, Quebec, Canada) video capture card, and digitally recorded at a time-lapse interval of 10 minutes for 5 hours. The processed sequences were then replayed as a movie.
Immunofluorescence
Cells were fixed in 4% PFA in phosphate-buffered saline (PBS)/3% glucose for 20 minutes at room temperature, permeabilized by means of 0.5% Triton X-100 in PBS for 5 minutes, and blocked with 1% bovine serum albumin (BSA) in PBS for 30 minutes at room temperature. For localization of filamentous actin, cells were incubated with 0.1 μg/mL tetramethylrhodamine B isothiocyanate (TRITC)–phalloidin (Sigma) for 30 to 45 minutes at room temperature. Localization of phosphotyrosine, vinculin, or the Arp2/3 complex was achieved by incubation for 30 to 45 minutes with a 1:100 dilution of mouse antiphosphotyrosine antibody (Santa Cruz Biotechnology, CA), a 1:500 dilution of mouse anti–human vinculin antibody (Sigma), or 1:250 dilution of rabbit anti-p34 Arc (Arp-related complex) polyclonal antiserum followed by incubation for 45 minutes with 1:100 Alexa 488 goat anti–mouse (Molecular Probes) or 1:200 fluorescein isothiocyanate anti–rabbit antibodies (Jackson Immunoresearch Labs, West Grove, PA). All antibodies were diluted in 1% BSA/PBS. Coverslips were mounted in Mowiol 4-88 (Calbiochem, San Diego, CA) with added p-phenylenediamine anti-fade (Sigma). Confocal images were obtained by means of a confocal laser scanning microscope system (TCS NT) (Leica, St Gallen, Switzerland) fitted with appropriate filter sets. Images were processed for publication by means of Leica TCS Start and Adobe Photoshop software.
Results
Arp2/3 complex localizes to DC podosomes
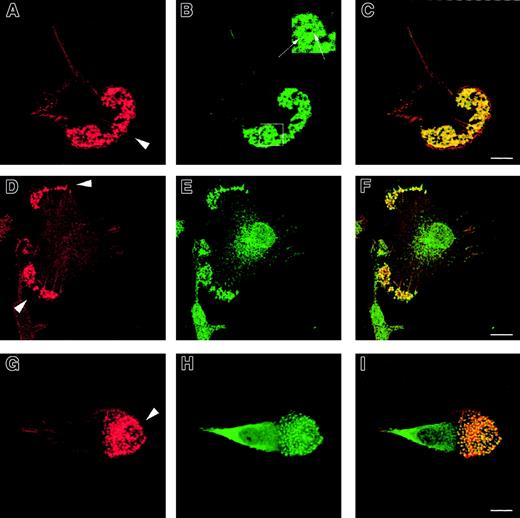
To determine their cytoskeletal architecture, normal immature DCs were fixed on fibronectin-plated coverslips and examined by confocal microscopy. In addition to abundant lamellipodia concentrated mainly at their leading edge, multiple podosomes were present on the ventral surface of polarized cells (Figure1A,D,G). These were clustered predominantly behind the leading edges and were absent from the uropod. Each actin core was surrounded by a rim of vinculin (Figure 1B), distinguishing these structures from smaller focal contacts, which were distributed more widely (not shown). In nonpolarized cells, the clusters of podosomes were also distributed diffusely over the ventral surface. Each podosome colocalized with areas of intense tyrosine phosphorylation (Figure 1E) and the Arp2/3 complex (Figure 1H), suggesting that these participate directly in their formation.
Formation of podosomes by normal immature DCs.
Normal immature DCs plated for 2 hours on fibronectin form podosomes. DCs were stained with TRITC-phalloidin (panels A, D, G) or antivinculin (panel B), antiphosphotyrosine (panel E), or anti–Arp2/3 (panel H) antibodies. Merged images are shown in panels C, F, and I. Under these conditions, the majority of normal DCs adopt a polarized morphology. An abundant f-actin pool is concentrated in punctate condensations at the cell-substratum interface situated just behind the leading edge (arrowheads in panels A, D, G). Rings of vinculin around the actin core confirm that these are podosomes (panel B and insert arrows in panel B). Both tyrosine-phosphorylated proteins (panel E) and the Arp2/3 complex (panel H) colocalize with f-actin in podosomes (panels F, I). Scale bars represent 10 μm.
Formation of podosomes by normal immature DCs.
Normal immature DCs plated for 2 hours on fibronectin form podosomes. DCs were stained with TRITC-phalloidin (panels A, D, G) or antivinculin (panel B), antiphosphotyrosine (panel E), or anti–Arp2/3 (panel H) antibodies. Merged images are shown in panels C, F, and I. Under these conditions, the majority of normal DCs adopt a polarized morphology. An abundant f-actin pool is concentrated in punctate condensations at the cell-substratum interface situated just behind the leading edge (arrowheads in panels A, D, G). Rings of vinculin around the actin core confirm that these are podosomes (panel B and insert arrows in panel B). Both tyrosine-phosphorylated proteins (panel E) and the Arp2/3 complex (panel H) colocalize with f-actin in podosomes (panels F, I). Scale bars represent 10 μm.
Recruitment of Arp2/3 to podosomes requires WASp
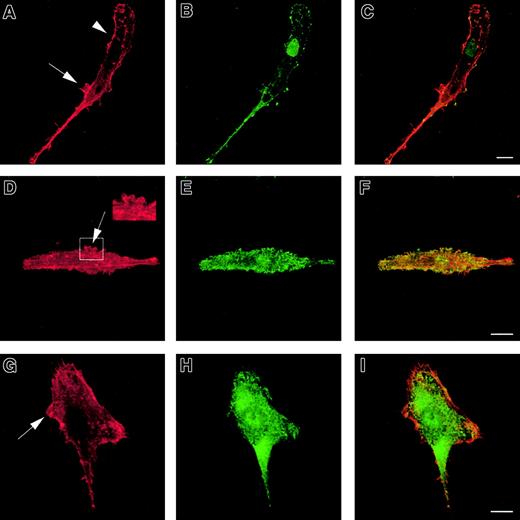
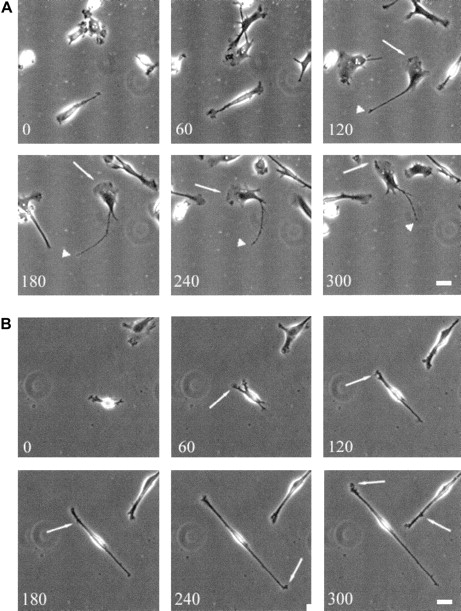
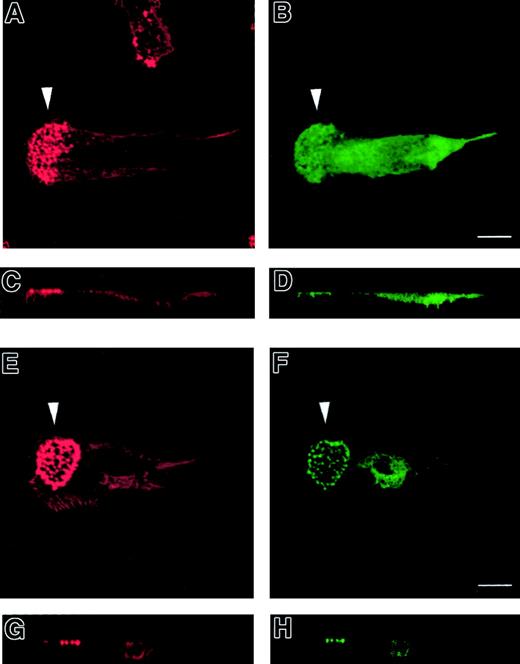
To investigate the role of WASp in the organization of DC cytoskeletal architecture, we derived immature DCs from WAS patients (null mutants). The surface immunophenotype and maturation state of WAS DCs did not differ from those of control cells.28 In spread WAS cells, f-actin was arranged in a cortical distribution, which gives them a ghost-like appearance (Figure2A). Within the cell body, podosomes were completely absent, and vinculin, tyrosine-phosphorylated proteins, and Arp2/3 complex were distributed diffusely (Figure 2A-I). The formation of stable broad lamellipodia was also shown to be disturbed. In WAS cells, lamellipodia were small and dysmorphic and were not polarized to leading edges (Figure 2A,D,G). Many WAS DCs also developed abnormal hyper-elongated morphologies. When observed by time-lapse videomicroscopy, normal immature DCs migrated by selection of a persisting leading edge, uropod retraction, and net cell translocation (Figure 3A). In contrast, WAS immature DCs were shown to develop from rounded cells by bipolar extension, but appeared unable to select a leading edge, retract either pole, or achieve net directional translocation (Figure 3B). As in fixed cells, lamellipodia appeared dysmorphic and were also poorly sustained. We then expressed an EGFP-WASp fusion by microinjection of normal immature DCs with a plasmid vector. Whereas DCs that expressed EGFP alone showed diffuse fluorescence (Figure 4B,D), EGFP-WASp clearly localized to podosomes (Figure 4F,H). Furthermore, expression of EGFP-WASp fusion protein in WAS immature DCs resulted in reconstitution of podosome assembly (Figure5D,F). Therefore, WASp is critical for polarized recruitment of signaling molecules, Arp2/3 complex, and actin-associated proteins to the leading edge of DCs and for effective filopodium, lamellipodium, and podosome formation.
Cytoskeleton of WASp-null immature DCs.
WASp-null immature DCs plated for 2 hours on fibronectin lack podosomes and form abnormal lamellipodia. DCs were stained with TRITC-phalloidin (panels A, D, G) or antivinculin (panel B), antiphosphotyrosine (panel E), or anti–Arp2/3 (panel H) antibodies. Merged images are shown in panels C, F, and I. WASp-null DCs are characterized by diffuse f-actin distribution (panels A, D, G,) with predominantly cortical actin staining (arrowhead in panel A). Podosomes are universally absent. Many cells exhibit a hyper-elongated morphology and form abnormal lamellipodia randomly over the cell surface (arrows in panels A, D, G and insert in panel D). Similarly, a diffuse pattern of staining was seen for vinculin (panel B), phosphotyrosine (panel E), and the Arp2/3 complex (panel H). Scale bars represent 10 μm.
Cytoskeleton of WASp-null immature DCs.
WASp-null immature DCs plated for 2 hours on fibronectin lack podosomes and form abnormal lamellipodia. DCs were stained with TRITC-phalloidin (panels A, D, G) or antivinculin (panel B), antiphosphotyrosine (panel E), or anti–Arp2/3 (panel H) antibodies. Merged images are shown in panels C, F, and I. WASp-null DCs are characterized by diffuse f-actin distribution (panels A, D, G,) with predominantly cortical actin staining (arrowhead in panel A). Podosomes are universally absent. Many cells exhibit a hyper-elongated morphology and form abnormal lamellipodia randomly over the cell surface (arrows in panels A, D, G and insert in panel D). Similarly, a diffuse pattern of staining was seen for vinculin (panel B), phosphotyrosine (panel E), and the Arp2/3 complex (panel H). Scale bars represent 10 μm.
Translocation of normal and WAS-immature DCs.
Translocation of WAS immature DCs on a fibronectin substratum is severely compromised. Scale bars represent 10 μm. Frames were taken at 60-minute intervals for up to 300 minutes. (A) Over time, normal immature DCs become polarized and begin random translocation over the substratum. In general, migrating cells have a well-developed broad and stable leading edge (arrows) with a fine uropod (arrowheads) that retracts into the cell body at sporadic intervals, resulting in net cell translocation. (B) In contrast, many WAS DCs elongate to an extreme degree. Persistent broad, leading-edge lamellipodia do not form, although small, transient extensions are formed randomly around the cell periphery (arrows). Cells fail to select a leading edge or a uropod, and net cell translocation over the substratum is severely compromised.
Translocation of normal and WAS-immature DCs.
Translocation of WAS immature DCs on a fibronectin substratum is severely compromised. Scale bars represent 10 μm. Frames were taken at 60-minute intervals for up to 300 minutes. (A) Over time, normal immature DCs become polarized and begin random translocation over the substratum. In general, migrating cells have a well-developed broad and stable leading edge (arrows) with a fine uropod (arrowheads) that retracts into the cell body at sporadic intervals, resulting in net cell translocation. (B) In contrast, many WAS DCs elongate to an extreme degree. Persistent broad, leading-edge lamellipodia do not form, although small, transient extensions are formed randomly around the cell periphery (arrows). Cells fail to select a leading edge or a uropod, and net cell translocation over the substratum is severely compromised.
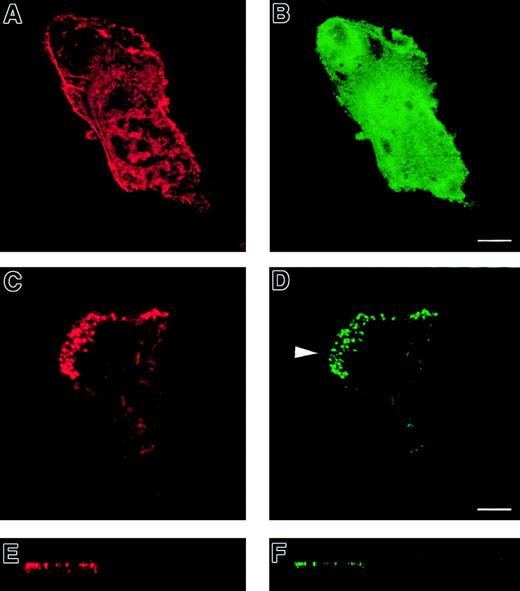
Normal immature DCs expressing a GFP-WASp fusion protein.
GFP-WASp fusion protein localizes to podosomes in normal immature DCs. Confocal images are shown in horizontal (panels A, B, E, F) and vertical (panels C, D, G, H) planes. TRITC-phalloidin staining of microinjected normal DCs is shown in left-hand panels (panels A, C, E, G). Right-hand panels show expression of EGFP (panels B, D) or EGFP-WASp (panels F, H). While EGFP shows a diffuse distribution within the cytoplasm (panels B, D), EGFP-WASp fusion protein in normal DCs is concentrated at sites of podosomal assembly (panels F, H).
Normal immature DCs expressing a GFP-WASp fusion protein.
GFP-WASp fusion protein localizes to podosomes in normal immature DCs. Confocal images are shown in horizontal (panels A, B, E, F) and vertical (panels C, D, G, H) planes. TRITC-phalloidin staining of microinjected normal DCs is shown in left-hand panels (panels A, C, E, G). Right-hand panels show expression of EGFP (panels B, D) or EGFP-WASp (panels F, H). While EGFP shows a diffuse distribution within the cytoplasm (panels B, D), EGFP-WASp fusion protein in normal DCs is concentrated at sites of podosomal assembly (panels F, H).
WASp-null immature DCs expressing a GFP-WASp fusion protein.
WASp-null immature DCs microinjected with a GFP-WASp fusion protein form podosomes and lamellipodia. Confocal images are shown in horizontal (panels A-D) and vertical (panels E-F) planes. TRITC-phalloidin staining of microinjected WASp-null DCs is shown in left-hand panels (panels A, C, E). Right-hand panels show expression of EGFP (panel B) or EGFP-WASp (panels D, F). Confocal images are shown in horizontal (panels A-D) and vertical (panels E-F) planes. Expression of EGFP in WASp-null DCs shows a diffuse distribution within the cytoplasm (panel B). In contrast, expression of EGFP-WASp fusion protein results in the appearance of podosomes at the substratum interface (panels C-F). In addition, EGFP-WASp–expressing cells are capable of forming broad lamellipodia (arrowhead in panel D), behind which the podosomes are located. A normal DC morphology is therefore reconstituted.
WASp-null immature DCs expressing a GFP-WASp fusion protein.
WASp-null immature DCs microinjected with a GFP-WASp fusion protein form podosomes and lamellipodia. Confocal images are shown in horizontal (panels A-D) and vertical (panels E-F) planes. TRITC-phalloidin staining of microinjected WASp-null DCs is shown in left-hand panels (panels A, C, E). Right-hand panels show expression of EGFP (panel B) or EGFP-WASp (panels D, F). Confocal images are shown in horizontal (panels A-D) and vertical (panels E-F) planes. Expression of EGFP in WASp-null DCs shows a diffuse distribution within the cytoplasm (panel B). In contrast, expression of EGFP-WASp fusion protein results in the appearance of podosomes at the substratum interface (panels C-F). In addition, EGFP-WASp–expressing cells are capable of forming broad lamellipodia (arrowhead in panel D), behind which the podosomes are located. A normal DC morphology is therefore reconstituted.
Podosomal assembly requires Cdc42Hs, Rac, and Rho
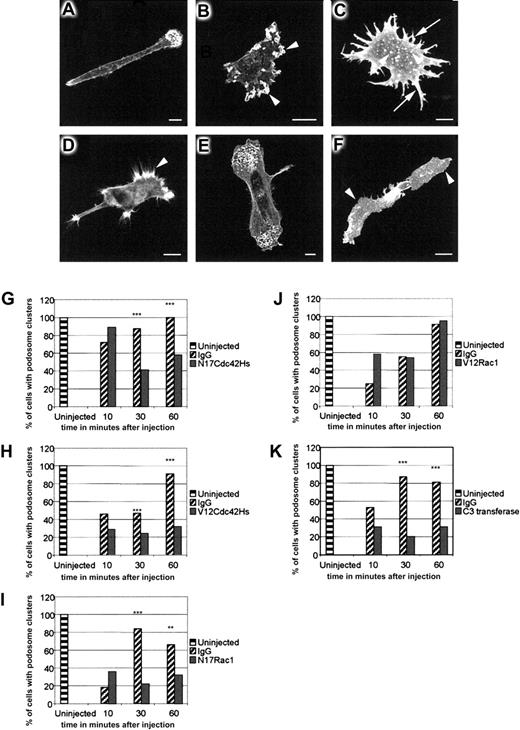
To determine the role of Rho family GTPases in the assembly of DC podosomes, we microinjected dominant-negative N17Cdc42Hs or N17Rac1, and constitutively active V12Cdc42Hs or V12Rac1, into immature DCs adhered to a fibronectin substratum. On the basis of our initial observations that the process of DC microinjection induces membrane ruffling and destabilizes podosomes, we observed the establishment of podosomal structures over a period of 1 hour after injection, by which time they have recovered in both number and distribution (Figure6A). In contrast to uninjected or immunoglobulin-injected cells, many DCs injected with N17Cdc42Hs lost polarity and displayed membrane ruffles around the cell periphery (Figure 6B). Assembly of podosomes was significantly inhibited (Figure6B,G), but where present, podosomes were usually arranged in a nonpolar distribution (not shown). Cells injected with V12Cdc42Hs produced multiple filopodia and nonpolar stellate extensions (Figure 6C). The majority of these cells re-formed some podosomes, but instead of being grouped at the leading edges, they were dispersed over the lower surface (Figure 6C), indicating that their polarity and normal clustering (but not necessarily their formation) had been disturbed (Figure 6H). In contrast, DCs injected with N17Rac1 achieved normal polarity (Figure 6D), but podosome assembly was inhibited (Figure6D,I). Cells injected with V12Rac1 were indistinguishable from control-injected cells (Figure 6E,J). Previous studies in Swiss 3T3 cells have indicated that Rho participates in the formation of focal adhesions by regulating the maturation of focal complexes into focal contacts.29 To determine whether Rho is involved in the formation of podosomes, we injected recombinant C3 transferase.8 In both BAC1.2F5 macrophages9and DCs, this induced striking arborization and spreading (Figure 6F). Recovery of podosomes in DCs was also significantly inhibited (Figure6K). In contrast to immature DCs, LPS-matured cells were completely devoid of podosomes (Figure 7A). Furthermore, it was not possible to reconstitute their assembly by injection of V12Cdc42Hs (Figure 7C), V12Rac1 (Figure 7D), or a combination of the 2 (Figure 7E). Therefore, our findings are consistent with a role for Cdc42Hs, Rac, and Rho in the assembly of podosomes in immature cells, and specifically for Cdc42Hs in their organization into polarized clusters situated near the leading edges.
Role of Rho-family proteins in podosome assembly and distribution.
Rho-family proteins control podosome assembly and distribution in normal immature DCs. DCs were fixed 30 to 60 minutes after microinjection with IgG (panel A), N17Cdc42Hs (panel B), V12Cdc42Hs (panel C), N17Rac1 (panel D), V12Rac1 (panel E), or C3 transferase (panel F) and stained with TRITC-phalliodin. In morphology, the DCs injected with IgG (panel A) are indistinguishable from uninjected cells. N17Cdc42Hs results in a loss of polarity, excessive membrane ruffling (arrowheads in panel B), and loss of podosomes (panel B). Microinjection of V12Cdc42Hs (panel C) stimulates filopodial extension around the periphery of the cell (arrows in panel C), resulting in a characteristic stellate morphology. There are generally fewer podosomes (arrowheads in panel C), and clustering of podosomes is inhibited. Vinculin staining was used to confirm the identity of podosomes (data not shown) in such cells. The leading edge of cells injected with N17Rac1 (panel D) show filopodial extensions (arrowhead in panel D) but lack podosomes. In contrast, V12Rac1 injection causes no morphological change, and normal podosome distribution is retained (panel E). Injection of C3 transferase (panel F) results in cell spreading (arrowheads in panel F), and podosomes are absent. In normal DCs, microinjection of control IgG protein causes partial disruption of podosomes and progressive recovery over 60 minutes (panels G-K). Injection of N17Cdc42Hs (panel G), V12Cdc42Hs (panel H), N17Rac1 (panel I), or C3 transferase (panel K) significantly inhibits podosomal recovery. In contrast, recovery occurs normally in V12Rac1-injected cells (panel J). Data were analyzed by means of the chi-square test. Significant differences between IgG and recombinant protein–injected cells are indicated by asterisks. ** indicates P < .01 and *** indicates P < .05. Each graph represents the combined data from 2 experiments, and each bar represents an average of 102 injected cells.
Role of Rho-family proteins in podosome assembly and distribution.
Rho-family proteins control podosome assembly and distribution in normal immature DCs. DCs were fixed 30 to 60 minutes after microinjection with IgG (panel A), N17Cdc42Hs (panel B), V12Cdc42Hs (panel C), N17Rac1 (panel D), V12Rac1 (panel E), or C3 transferase (panel F) and stained with TRITC-phalliodin. In morphology, the DCs injected with IgG (panel A) are indistinguishable from uninjected cells. N17Cdc42Hs results in a loss of polarity, excessive membrane ruffling (arrowheads in panel B), and loss of podosomes (panel B). Microinjection of V12Cdc42Hs (panel C) stimulates filopodial extension around the periphery of the cell (arrows in panel C), resulting in a characteristic stellate morphology. There are generally fewer podosomes (arrowheads in panel C), and clustering of podosomes is inhibited. Vinculin staining was used to confirm the identity of podosomes (data not shown) in such cells. The leading edge of cells injected with N17Rac1 (panel D) show filopodial extensions (arrowhead in panel D) but lack podosomes. In contrast, V12Rac1 injection causes no morphological change, and normal podosome distribution is retained (panel E). Injection of C3 transferase (panel F) results in cell spreading (arrowheads in panel F), and podosomes are absent. In normal DCs, microinjection of control IgG protein causes partial disruption of podosomes and progressive recovery over 60 minutes (panels G-K). Injection of N17Cdc42Hs (panel G), V12Cdc42Hs (panel H), N17Rac1 (panel I), or C3 transferase (panel K) significantly inhibits podosomal recovery. In contrast, recovery occurs normally in V12Rac1-injected cells (panel J). Data were analyzed by means of the chi-square test. Significant differences between IgG and recombinant protein–injected cells are indicated by asterisks. ** indicates P < .01 and *** indicates P < .05. Each graph represents the combined data from 2 experiments, and each bar represents an average of 102 injected cells.
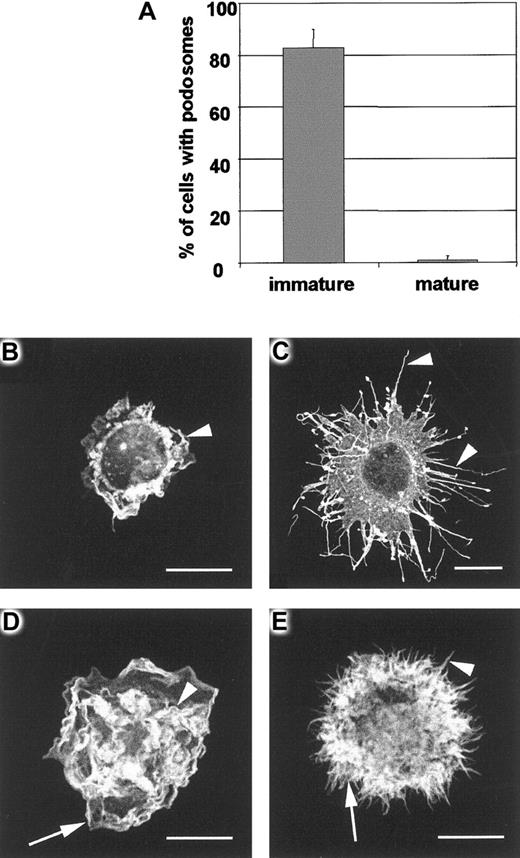
DC maturation and podosome loss.
Maturation of DCs during 24-hour culture with LPS results in almost complete loss of podosomes (panel A). In contrast with immature DCs, mature DCs are rounded cells that are adhere poorly on fibronectin and have little tendency to spread. IgG-injected mature DCs have the same morphology as uninjected cells, remaining unpolarized and unspread despite displaying active peripheral lamellipodial formation (arrowhead in panel B). Microinjection of V12Cdc42 (panel C) causes striking filopodial assembly (arrowheads in panel C) whereas V12Rac injection (panel D) stimulates vigorous ruffling (arrowhead in panel D) and lamellipodial formation (arrow in panel D). When V12Cdc42 and V12Rac are coinjected (panel E), both filopodia (arrowhead in panel E) and lamellipodia (arrow in panel E) are assembled.
DC maturation and podosome loss.
Maturation of DCs during 24-hour culture with LPS results in almost complete loss of podosomes (panel A). In contrast with immature DCs, mature DCs are rounded cells that are adhere poorly on fibronectin and have little tendency to spread. IgG-injected mature DCs have the same morphology as uninjected cells, remaining unpolarized and unspread despite displaying active peripheral lamellipodial formation (arrowhead in panel B). Microinjection of V12Cdc42 (panel C) causes striking filopodial assembly (arrowheads in panel C) whereas V12Rac injection (panel D) stimulates vigorous ruffling (arrowhead in panel D) and lamellipodial formation (arrow in panel D). When V12Cdc42 and V12Rac are coinjected (panel E), both filopodia (arrowhead in panel E) and lamellipodia (arrow in panel E) are assembled.
Discussion
In this study, we have examined the morphology and cytoskeletal organization of human DCs. We have found that clusters of podosomes are polarized to the regions behind the leading edge of immature cells, but are absent in LPS-matured cells. Podosomes are specialized dynamic structures restricted to highly motile cells (monocytes, monocyte-lineage–derived macrophages, osteoclasts, and some transformed cells) and are confined to contact sites between the cell and substratum. Distinct in size and morphology from focal adhesions, podosomes are conical structures composed of a core of actin filaments, α-actinin, fimbrin, gelsolin, and vimentin. These are typically surrounded by a ring of vinculin, talin, and paxillin.30-34 Whereas the formation of filopodia and lamellipodia are clearly linked to the protrusive activity of DCs, the role of podosomes in cell reattachment and motility, and the mechanism of their formation or dissolution, are less well understood.35
As has been noted previously, the formation of filopodia and podosomes in mutant DCs lacking WASp is severely affected.28,33,36These DCs also have significant disturbances in motility, particularly in the initiation and persistence of cell movement. However, the specific roles of, and interactions between, WASp-Arp2/3 and Rho GTPases in these processes have remained unclear. In this study, we have shown that podosome assembly is consistently inhibited when cells are injected with N17Rac1 or C3-transferase and that both their asymmetric localization and their assembly are inhibited in cells injected with dominant-negative N17Cdc42Hs. These data suggest that Cdc42Hs, Rac, and Rho are each involved in their localization and assembly. Consistent with evidence showing that Cdc42Hs is important for establishment and maintenance of polarity in many cell types37,38 and that Cdc42Hs-WASp binds to and activates the Arp2/3 complex,20,22,39 we believe that the role of Cdc42Hs is not in the disassembly of podosomes, as previously reported, but in the regulation of their assembly and polarity.33
The participation of Rac and Rho in podosome assembly or stabilization is reminiscent of their role in the formation of integrin adhesion complexes40-42 and the maturation of focal contacts from focal complexes in Swiss 3T3 cells.29,43,44 In other cell types, engagement of integrins by antibody-mediated clustering or substratum attachment results in activation and translocation of Rho to membrane ruffles at the edges of lamellae.45,46 Podosomes may therefore provide an essential link between protrusive activity and reattachment during DC motility. The way in which Rac and Rho participate with Cdc42Hs-WASp in the assembly of podosomes is less clear. One possibility is that Rac interacts with WASp in vivo (as has been shown in vitro, albeit weakly). Conformational changes in WASp induced by interaction with other signaling molecules could also favor binding and activation by Rac. The interaction between WASp and Rac may also be indirect, as has been shown for other GTPase effectors.6 Furthermore, studies in permeabilized neutrophils have suggested that Rac can act downstream of Cdc42 to initiate actin nucleation by both WASp-Arp2/3–dependent and WASp-Arp2/3–independent pathways, possibly mediated by polyphosphoinositides.47 Rho may contribute to Arp2/3 complex–mediated assembly of the podosome actin core, in much the same way as actin ring formation around phagosomes during complement (CR3) receptor engagement is a Rho-dependent event.48 Overall, we favor the suggestion that Cdc42Hs is responsible for sequential activation of Rac and Rho and that their activity is restricted to the leading edges of the cell.5,10 11
We have also shown that mature DCs are completely devoid of podosomes. This suggests that their presence is necessary for their migratory phase, but not for central antigen presentation. Furthermore, we have been unable to reconstitute podosome assembly in mature cells with Cdc42Hs or Rac1, suggesting that other mechanisms are also involved. This contrasts with a recent study in which developmental suppression of endocytosis in DCs was shown to be directly regulated by Cdc42Hs.43
We conclude that DCs initiate directional movement by Cdc42Hs-mediated polarization and extension of filopodia. We also suggest that sequential polarization of Rac and Rho activity by Cdc42Hs results in formation of lamellipodia and establishment of new podosomal structures at the leading edge of the cell. Perpetuation of cell movement is then dependent on continued signaling and cooperative activation of Cdc42Hs, Rac, and Rho. The formation of podosomes following membrane protrusion therefore allows the DCs to establish new contact sites at the leading edge and, by retraction of the cell body, to achieve translocation. The existence of podosomes in restricted cell types such as DCs may well reflect their size and their requirement for high motility. The role of WASp in cell movement and podosome assembly is more complex than that of a GTPase effector, as it provides a platform for recruitment of tyrosine-phosphorylated signaling molecules; for sequential binding of Cdc42Hs (mediating polarity, filopodial extension, and podosome assembly); for activation of Rac and Rho (mediating lamellipodia protrusion, podosome assembly, and cell contraction); and for localization of the Arp2/3 complex. Recruitment of WASp via its N-terminal EVH1 domain to lipid signals following engagement of chemoattractant receptors49 may be an early event in this process.50 The identification of signaling pathways regulating DC motility provides opportunities for therapeutic manipulation and for modulation of immune responses in vivo.
Supported by grants from the Wellcome Trust (S.B., A.J.T., G.E.J.); the Medical Research Council (L.M., G.E.J.); the Arthritis and Rheumatism Council (A.J.T., G.E.J.); the Primary Immunodeficiency Association (A.J.T., M.P.B.); and in part by the Fifth (EC) Framework Programme, Contract No. QLG1-1999-01090 (A.J.T., M.P.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Adrian J. Thrasher, Molecular Immunology Unit, Institute of Child Health, 30 Guilford St, London, WC1N 1EH, United Kingdom; e-mail: a.thrasher@ich.ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal