Ephrin-B2 is a transmembrane ligand that is specifically expressed on arterial endothelial cells (ECs) and surrounding cells and interacts with multiple EphB class receptors. Conversely, EphB4, a specific receptor for ephrin-B2, is expressed on venous ECs, and both ephrin-B2 and EphB4 play essential roles in vascular development. The bidirectional signals between EphB4 and ephrin-B2 are thought to be specific for the interaction between arteries and veins and to regulate cell mixing and the making of particular boundaries. However, the molecular mechanism during vasculogenesis and angiogenesis remains unclear. Manipulative functional studies were performed on these proteins in an endothelial cell system. Using in vitro stromal cells (OP9 cells) and a paraaortic splanchnopleura (P-Sp) coculture system, these studies found that the stromal cells expressing ephrin-B2 promoted vascular network formation and ephrin-B2+ EC proliferation and that they also induced the recruitment and proliferation of α-smooth muscle actin (α-SMA)–positive cells. Stromal cells expressing EphB4 inhibited vascular network formation, ephrin-B2+ EC proliferation, and α-SMA+ cell recruitment and proliferation. Thus, these data suggest that ephrin-B2 and EphB4 mediate reciprocal interactions between arterial and venous ECs and surrounding cells to form each characteristic vessel.
Introduction
During embryogenesis, vascular development consists of 2 processes: vasculogenesis, whereby endothelial cell (EC) precursors differentiate and proliferate to form the initial vascular plexus,1-3 and angiogenesis, in which the initial vascular plexus forms mature vessels by sprouting, branching, pruning, differential growth of ECs, and recruitment of supporting cells, such as pericytes and smooth muscle cells (SMCs).1,3,4 To date, 3 growth factor systems—vascular endothelial growth factors (VEGFs), angiopoietins, and ephrins—have been identified as critical factors for angiogenesis.2-6
VEGFs and their receptors VEGFR-2/Flk-1, VEGFR-1/Flt-1, and VEGFR-3/Flt-4 are key regulators of vasculogenesis and angiogenesis. VEGF and Flk-1 are required to grow and establish an endothelial lineage,7-10 whereas VEGF and Flt-1 are involved in the organization of ECs into tubelike structures.11 Flt-4 also contributes to angiogenesis and lymphangiogenesis.12Angiopoietins and their EC-specific receptors, TIEs, have been suggested to be important for vascular remodeling and cardiac development.13-15 Platelet-derived growth factor (PDGF) and tumor growth factor (TGF)-β have been reported to be associated with pericyte proliferation and movement.16-19
One of the most important events in vascularization is the development of arteries and veins. Generally, the differences between arteries and veins have been defined by function, anatomy, pressure, and blood flow direction. Recent studies have shown that the arterial and venous ECs are molecularly distinct from the earliest types of blood vessels. This distinction is revealed by a tyrosine kinase receptor, EphB4, that is dominantly expressed on venous ECs and whose cognate ligand, ephrin-B2, is expressed on arterial ECs.20,21 The Eph receptor and ephrin ligand families play important roles in embryonic development.22 They can be grouped into 2 subclasses based on structural homology and binding specificity: ephrin-A ligands (ephrin-A1 to -A5), which are tethered to the cell surface via a glycosylphosphatidylinositol anchor and bind to the EphA receptor subfamily, and ephrin-B ligands (ephrin-B1 to -B3), which are inserted into the plasma membrane via a transmembrane region along with conserved cytoplasmic tyrosine residues and bind to the corresponding EphB receptor subfamily.23-25 These ephrins need to be membrane bound to activate their receptors but do not function in a soluble form.26 Notably, signaling between ligands and receptors in the B subfamily appears to be reciprocal because ephrin-B ligands not only activate their bound receptor but in return are activated by their receptors in a neighboring cell.25 27
A functional analysis of Eph-ephrin signals has been conducted in the circulatory and nervous systems. Targeted disruption of the ephrin-B2 gene leads to embryonic lethality at around E11 because of a defect in both arterial and venous vessel remodeling. This defect was accompanied by a failure of intercalation between the arteries and veins.20 The EphB4 homozygous mutants have a symmetric phenotype with ephrin-B2 homozygous embryos in the cardiovascular system.21 Thus, signaling between arteries and veins mediated by EphB4 and ephrin-B2 may be required for proper morphogenesis of the intervening capillary beds and network as well as for interdigitation and differential growth of arterial and venous vessels.20 28
Other Eph receptors and ephrin ligands are also involved in angiogenesis. Ephrin-B1 promotes the formation of EC capillarylike structures, cell attachment, and sprouting angiogenesis in vitro.29 An in vitro sprouting assay also shows that purified ephrin-B1-Fc induces a significant increase in the number of sprouts in adrenal cortex–derived microvascular endothelial cells, and this activity is completely blocked by EphB1-Fc and EphB2-Fc.30 Ephrin-B1 is coexpressed with ephrin-B2 in ECs. EphB3 and ephrin-B3 are coexpressed with EphB4 in venous ECs. EphB3 is also expressed in some arteries, and EphB2 and ephrin-B2 are coexpressed in mesenchymal cells adjacent to ECs. But this overlapping of expression is unable to compensate for the lack of ephrin-B2 or EphB4. No vascular defects are found in either EphB2 or EphB3 homozygous mutant mice, whereas EphB2 and EphB3 double-mutant mice have shown vascular defects only with 30% penetrance, which is similar but not identical to that of ephrin-B2 mutants.30 It has been suggested that the complex cell-to-cell interaction via Eph receptors and ephrin ligands on ECs is restricted mainly by EphB4/ephrin-B2 but also involves other Eph receptors and ephrin ligands.
In the nervous system, Eph and their ligands regulate topographic map formation in the visual system.31-34 The bidirectional activation of Eph receptor and ephrin-B ligands is important for the patterning of the embryonic structure of the brain and somites24,27,35-37; is implicated in the repulsion that guides the migration of cells and growth cones to specific destinations; and maintains the boundaries between cell groups in the neuronal system.38,39 Recent studies have revealed that a clear border is formed between the Eph- and ephrin-B–expressing cell populations and that bidirectional signaling between Eph/ephrin-B restricts the intermingling of adjacent cell populations and maintenance of the boundaries.40 41
However, the molecular mechanism and how such ligands and receptors work for vasculogenesis and angiogenesis is not clearly understood. To clarify this, we have generated ephrin-B2– and EphB4-overexpressing stromal cell lines OP9 and cocultured P-Sp (paraaortic splanchnopleural mesoderm) explants with transfected OP9 cells. This system provides us with opportunities to observe the interaction of ECs and surrounding cells during vasculogenesis and angiogenesis.42-44
Materials and methods
Cell line and animals
OP9 cells (a gift from Dr H. Kodama, Bayer Yakuhin, Nara, Japan) were maintained in α-modified minimum essential media (Gibco, Grand Island, NY) containing 20% fetal calf serum (FCS) (JRH Biosciences, Lenexa, KS).45 Pregnant C57BL/6 mice (purchased from SLC, Shizuoka, Japan), green mice (green fluorescent protein [GFP] was overexpressed under transcriptional control of a CAG promoter, a gift from Dr Okabe, Osaka University, Japan),46 and ephrin-B2LacZ/+ heterozygous mutant mice (ephrin-B2LacZ/+ mice in which β-galactosidase [LacZ] expression is under the transcriptional control of an ephrin-B2 promoter)47 were housed in environmentally controlled rooms at a facility of Kumamoto University Medical School under the guidelines of Kumamoto University for animal and recombinant DNA experiments.
Transfections
OP9 stromal cells were transfected with expression plasmid pCAGneo–ephrin-B2 containing full-length ephrin-B2 complementary DNA (cDNA) (a gift from Dr John G. Flanagan, Harvard Medical School, Boston, MA) (OP9/ephrin-B2) or expression vector pCAGneo (OP9/vector). Expression vector pRK5-EphB4 (a gift from Dr Axel Ullrich, Max Planck Institute für Biochemie, Martinstried, Germany) containing full-length EphB4 cDNA was cotransfected with expression vector pCAGneo (OP9/EphB4) into OP9 cells by the Effectene Transfection method (Qiagen, Hilden, Germany). The expression clones were selected using 500 μg/mL geneticin disulfate (G418, Life Technologies, Grand Island, NY), and the protein expression was checked by Western blot analysis (Figures 1A,2A). We selected 3 clones, each showing high levels of protein expression, for further use.
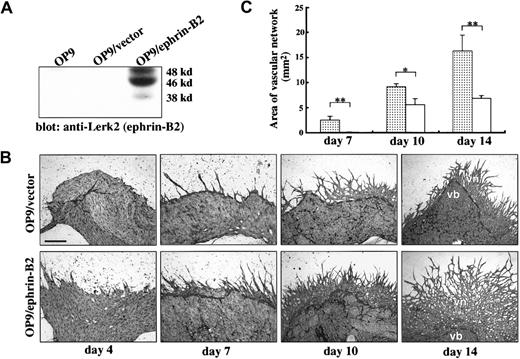
OP9/ephrin-B2 enhanced vascular network formation from E9.5 P-Sp explants.
(A) OP9 cells were stably transfected with expression plasmid DNA pCAGneo–ephrin-B2 (OP9/ephrin-B2) or expression vector pCAGneo alone (OP9/vector), and individual G418-resistant clones were selected. To check protein expression, cells were lysed in a lysis buffer, and an equal amount of each sample was subjected to SDS-PAGE and immunoblotted by anti–Lerk-2 PolyAb. (B) P-Sp explants were cocultured with OP9/ephrin-B2 or OP9/vector stromal cells. After the indicated period, the cultures were immunostained by anti–PECAM-1/CD31 MoAb. The formation of vascular networks was enhanced more on the OP9/ephrin-B2 stromal cells than on OP9/vector stromal cells; vb indicates vascular bed; vn, vascular network. The bar indicates 500 μm (day 4 and day 7) and 200 μm (day 10 and day 14). The dotted line indicates the border of vascular bed and vascular network. (C) Quantitative analysis of vascular network area by NIH Image software. The results represent the mean ± SD of triplicate cultures. ░, OP9/ephrin-B2; ■, OP9/vector; *P < .05; **P < .01.
OP9/ephrin-B2 enhanced vascular network formation from E9.5 P-Sp explants.
(A) OP9 cells were stably transfected with expression plasmid DNA pCAGneo–ephrin-B2 (OP9/ephrin-B2) or expression vector pCAGneo alone (OP9/vector), and individual G418-resistant clones were selected. To check protein expression, cells were lysed in a lysis buffer, and an equal amount of each sample was subjected to SDS-PAGE and immunoblotted by anti–Lerk-2 PolyAb. (B) P-Sp explants were cocultured with OP9/ephrin-B2 or OP9/vector stromal cells. After the indicated period, the cultures were immunostained by anti–PECAM-1/CD31 MoAb. The formation of vascular networks was enhanced more on the OP9/ephrin-B2 stromal cells than on OP9/vector stromal cells; vb indicates vascular bed; vn, vascular network. The bar indicates 500 μm (day 4 and day 7) and 200 μm (day 10 and day 14). The dotted line indicates the border of vascular bed and vascular network. (C) Quantitative analysis of vascular network area by NIH Image software. The results represent the mean ± SD of triplicate cultures. ░, OP9/ephrin-B2; ■, OP9/vector; *P < .05; **P < .01.
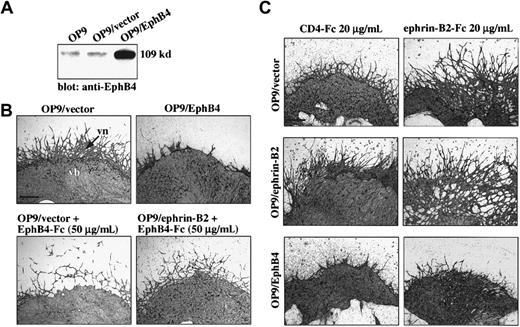
OP9/EphB4 stromal cells inhibited vascular network formation from E9.5 P-Sp explants.
(A) OP9 cells were cotransfected with expression plasmid DNA pRK5-EphB4 (OP9/EphB4) and pCAGneo or expression vector pCAGneo alone (OP9/vector), and individual G418-resistant clones were selected (OP9/EphB4 or OP9/vector). Protein expression was observed by immunoblotting using anti-EphB4 PolyAb as portrayed in Figure 1A. (B) P-Sp explants were cocultured with OP9/EphB4 or OP9/vector stromal cells. After 14 days, the cultures were immunostained by anti–PECAM-1 MoAb. The formation of vascular networks (vn) was inhibited on the OP9/EphB4 stromal cells compared with the OP9/vector stromal cells. Vascular network formation was also inhibited by the addition of EphB4-Fc in both the OP9/vector and OP9/ephrin-B2 stromal cells. The bar indicates 500 μm. (C) P-Sp explants were cocultured with OP9/ephrin-B2, OP9/EphB4, or OP9/vector stromal cells in the presence of chimeric protein ephrin-B2–Fc for 10 days. The vascular EC sprouting and remodeling from P-Sp explants was promoted by the addition of ephrin-B2–Fc, especially in OP9/ephrin-B2 stromal cells. The bar indicates 500 μm.
OP9/EphB4 stromal cells inhibited vascular network formation from E9.5 P-Sp explants.
(A) OP9 cells were cotransfected with expression plasmid DNA pRK5-EphB4 (OP9/EphB4) and pCAGneo or expression vector pCAGneo alone (OP9/vector), and individual G418-resistant clones were selected (OP9/EphB4 or OP9/vector). Protein expression was observed by immunoblotting using anti-EphB4 PolyAb as portrayed in Figure 1A. (B) P-Sp explants were cocultured with OP9/EphB4 or OP9/vector stromal cells. After 14 days, the cultures were immunostained by anti–PECAM-1 MoAb. The formation of vascular networks (vn) was inhibited on the OP9/EphB4 stromal cells compared with the OP9/vector stromal cells. Vascular network formation was also inhibited by the addition of EphB4-Fc in both the OP9/vector and OP9/ephrin-B2 stromal cells. The bar indicates 500 μm. (C) P-Sp explants were cocultured with OP9/ephrin-B2, OP9/EphB4, or OP9/vector stromal cells in the presence of chimeric protein ephrin-B2–Fc for 10 days. The vascular EC sprouting and remodeling from P-Sp explants was promoted by the addition of ephrin-B2–Fc, especially in OP9/ephrin-B2 stromal cells. The bar indicates 500 μm.
Western blot analysis
OP9, OP9/ephrin-B2, OP9/EphB4, and OP9/vector cells were lysed with 1% Triton lysis buffer (50 mM/L HEPES [pH 7.4], 1% Triton X-100, 10% glycerol, 10 mM/L sodium pyrophosphate, 100 mM/L sodium fluoride, 4 mM/L ethylenediaminetetraacetic acid, 2 mM/L sodium orthovanadate, 50 μg/mL aprotinin, 1 mM/L phenylmethylsulfonyl fluoride, 100 μM/L leupeptin, and 25 μM/L pepstatin A) and incubated at 4°C for 30 minutes. Cell lysates were then clarified by centrifugation for 15 minutes at 4°C. An equal amount of each sample was separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to a polyvinylidene diflouride membrane (Millipore, Yonezawa, Japan). After blocking of the residual binding sites by incubation of the membrane with 5% bovine serum albumin (Sigma, St Louis, MO) in PBS-T (0.1% Triton X-100 in phosphate-buffered saline) for 1 hour at room temperature, we performed an immunodetection using a primary antibody, rabbit anti–Lerk-2 polyclonal antibody (PolyAb) (Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit anti-EphB4 PolyAb (made in our laboratory), horseradish peroxidase–conjugated antirabbit secondary antibody, and a chemiluminescence detection system (Amersham, Arlington Heights, IL) according to the manufacturer's instructions.
In vitro coculture of P-Sp explants or single-cell suspensions on stromal cells
The method of P-Sp dissection was performed as described previously.44 Embryos were dissected from pregnant females at 9.0 to 9.5 days postcoitum. Ephrin-B2LacZ/+ embryos were generated from crosses of ephrin-B2LacZ/+ and wild-type mouse. By convention, the morning the vaginal plug was detected was defined as embryonic day 0.5 (E0.5). Ephrin-B2 genotyping was confirmed by LacZ staining in yolk sacs and reverse transcriptase–polymerase chain reaction (RT-PCR) using a LacZ-specific primer. Then the wild-type or ephrin-B2LacZ/+ P-Sp explants were cultured on OP9/ephrin-B2, OP9/EphB4, or OP9/vector stromal cells in RPMI 1640 (Gibco) containing 10% FCS and 10−5 M 2-mercaptoethanol (2ME) (Sigma) and supplemented with interleukin (IL)-6 (20 ng/mL), IL-7 (20 U/mL) (a gift from Dr T. Sudo, Toray Industries, Kamakura, Japan), stem cell factor (SCF, 50 ng/mL) (a gift from Chemo-Sero-Therapeutic, Kumamoto, Japan), and erythropoietin (2 U/mL) (a gift from Snow-Brand Milk Product, Tochigi, Japan) in 12-well plates; the plates were then incubated at 37°C in a humidified 5% CO2 atmosphere for 4 to 14 days.
After isolating the E9.0-9.5 P-Sp regions, we prepared single-cell suspensions with 2.4 U/mL Dispase (Gibco, Grand Island, NY) and passed the tissues through a 23G needle. The single cells were cocultured with OP9/ephrin-B2 or OP9/vector stromal cells in RPMI 1640 medium containing 10% FCS, 10−5 M 2ME, 5 ng/mL recombinant VEGF (Pepro Tech, London, England), and 50 ng/mL SCF for 7 to 14 days in 12-well plates.
Immunohistochemistry
Vascular formation from P-Sp explants was analyzed on OP9/ephrin-B2, OP9/EphB4, and OP9/vector feeder layers. After coculturing the P-Sp with OP9/ephrin-B2, OP9/EphB4, or OP9/vector stromal cells for 4, 7, 10, and 14 days, immunohistochemistry was performed as described.42 48 In brief, the cultures were fixed in situ with 4% paraformaldehyde in PBS for 10 minutes at 4°C, washed twice with PBS, and incubated with 0.3% H2O2 in PBS at room temperature for 30 minutes to block endogenous peroxidase activity. To block nonspecific reactions, cultures were incubated with 1% normal goat serum and 0.2% bovine serum albumin (Sigma) in PBS-T at room temperature for 30 minutes. Then the fixed dishes were incubated with platelet endothelial cell adhesion molecule (PECAM)-1 monoclonal antibody (MoAb) (MEC13.3, rat antimouse monoclonal, PharMingen, San Diego, CA) overnight at 4°C, washed with PBS-T 3 times, and incubated with peroxidase-conjugated antirat immunoglobulin G (BioSource, Camarillo, CA) for 1 hour at room temperature. After 3 more washes, the samples were visualized by using the AEC substrate system (Nichirei, Tokyo, Japan). Alternatively, they were soaked in PBS-T containing 300 μg/mL diaminobenzidine (Dojin Chemical, Kumamoto, Japan) in the presence of 0.05% NiCl2 for 10 to 30 minutes, hydrogen peroxide was added to 0.01%, and the color reactions were visualized. To detect the SMCs, anti–α-smooth muscle actin (α-SMA) antibody (Dako, Glostrup, Denmark) was used for immunohistochemical staining in this culture system.
Immunofluorescence staining, EC sorting, and single EC culture
Single-cell suspensions for cell sorting were prepared from the E9.0-9.5 P-Sp regions. The cells were incubated for 30 minutes on ice with biotin–anti–Flk-1 MoAb (AVAS12, rat antimouse monoclonal, a gift from Dr S.-I. Nishikawa, Kyoto University, Japan) and washed twice with washing buffer (5% FCS/PBS). The cells were subsequently incubated with phycoerythrin-conjugated PECAM-1 antibody (PharMingen) and allophycocyanin-conjugated streptavidin (Caltag Laboratories, South San Francisco, CA) for 30 minutes on ice. Then the cells were washed twice with washing buffer and suspended for cell sorting. The stained cells were sorted by FACSVantage (Becton Dickinson Immunocytometry Systems, San Jose, CA) to obtain Flk-1+PECAM-1−(R2) and Flk-1+PECAM-1+(R3) EC cell populations. Then the sorted R2 and R3 cells were cocultured with OP9/ephrin-B2 or OP9/vector stromal cells. The cell cultures were performed at 300 cells per well in 24-well plates in RPMI 1640 medium containing 10% FCS, 10−5 M 2ME, and 5 ng/mL VEGF. Immunohistochemistry with anti–PECAM-1 MoAb was performed on day 10 to detect the ECs, and the sheetlike or cordlike structures were enumerated.
RT-PCR analysis
Total RNA was isolated from whole yolk sac (as a control), R2 and R3 cells sorted from E9.0-9.5 P-Sp regions using the RNeasy mini kit (Qiagen). Then the RNA was subjected to reverse transcription with an Advantage RT for PCR kit (Clontech Laboratories, Palo Alto, CA). After reverse transcription, the cDNA was amplified using an Advantage polymerase mix PCR kit (Clontech) in a GeneAmp PCR system model 9700 (Perkin-Elmer, Norwalk, CT) for 25 to 30 cycles. To detect the expression of ephrin-B2 and EphB4, the following primers were used: ephrin-B2, 5′-TCTGTGTGGAAGTACTGTTGGGGACTTT-3′ (sense), 5′-TGTACCAGCTTCTAGCTCTGGACGTCTT-3′ (antisense); EphB4, 5′-CGTCCTGATGTCACCTATACCTTTGAGG-3′ (sense), 5′-GAGTACTCAACTTCCCTCCCATTGCTCT-3′ (antisense), for amplification.
Preparation of recombinant fusion protein
To generate cDNA encoding the full-length EphB4 extracellular domain, an expression plasmid containing full-length mouse EphB4 cDNA (pRK5-EphB4) was used as a template for PCR using the Advantage 2 PCR kit (Clontech). Sense and antisense primers that had SalI and EcoRI restriction enzyme sites at their 5′ ends for reconstruction were designed: 5′-ACGCGTCGACATGGAGCTCCGAGCGCTGCTG-3′ (sense), 5′-CGGAATTCTGCT CCCGCCAGCTCTCGCTCTC-3′ (antisense). Amplified DNA fragments were sequenced (ABI Prism 310) and subcloned into the expression vector pCAGneo-human Fc (Fc part of human immunoglobulin G1, EphB4-Fc). EphB4-Fc or CD4-Fc was prepared from COS7 cell supernatant in GIT medium (Wako Pure Chemical, Osaka, Japan) as previously described.49
LacZ staining
For culture plate LacZ staining, the P-Sp cultures were fixed in a solution containing 2% formaldehyde and 0.2% glutaraldehyde in PBS for 10 minutes at room temperature, washed with PBS 3 times, and then stained at room temperature for 30 to 60 minutes in a solution containing 1 mg/mL X-Gal (Nacalai Tesque, Kyoto, Japan), 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, and 2 mM MgCl2 in PBS (X-Gal staining solution). For whole mount LacZ staining of the yolk sac, specimens were fixed in 4% paraformaldehyde in PBS for 5 to 10 minutes at 4°C, washed twice with PBS, and stained with X-Gal staining solution at room temperature for 30 to 60 minutes. For in vivo LacZ/ephrin-B2 expression analysis, the venae cava and aortas, along with surrounding tissues, were removed from adult ephrin-B2LacZ/+ or wild-type mice, and ephrin-B2LacZ/+ or wild-type embryos were removed at E9.0-9.5. The samples were fixed in 4% paraformaldehyde/PBS for 30 to 60 minutes for adult tissues and 5 to 10 minutes for embryos, respectively, at 4°C and washed twice with PBS. The tissues were then stained from 4 hours to overnight at room temperature in a LacZ staining solution. After LacZ staining, the tissues were postfixed in 4% formaldehyde/PBS for 4 hours at 4°C, embedded in polyester wax, and transverse sectioned 8 μm thick. The immunohistochemical staining for PECAM-1 and α-SMA in sections was carried out as previously described.48
Quantitative analysis of vascular network areas
After PECAM-1 staining, the images were integrated using a color camera (Hamamatsu Photonics, Shizuoka, Japan). Image-processing (NIH Image 1.62/Power Macintosh G3, National Institutes of Health, Bethesda, MD) was used to determine alterations in the size of vascular networks.50 In P-Sp cultures, PECAM-1+ ECs formed a sheetlike structure (vascular beds) beside the P-Sp explant. Subsequently, PECAM-1+ ECs sprouted from the sheet and formed a cordlike structure (vascular networks). Because the outside border of the vascular network is not in a regular pattern, we delimited the outside border with curved lines. Then we measured the areas between the outside border of vascular network and the boundary line of vascular bed and network as a vascular network area. Three vascular networks from each P-Sp explant were measured under 12.5 × magnification. The average value and SD for each period was calculated, and the statistical significance was tested using an unpairedt test in Startview.
Results
Function of ephrin-B2 in vasculogenesis and angiogenesis
The OP9 stromal cell line was established from the newborn calvaria of the (C57BL/6xC3H) F2-op/op mouse, which lacks a functional macrophage colony-stimulating factor.51,52According to previous studies,42-44 OP9 stromal cells are suitable for analysis for angiogenesis compared with other stromal cells such as NIH3T3, BALB/c3T3, and PA6. To examine the function of ephrin-B2 in angiogenesis, we transfected full-length ephrin-B2 cDNA into OP9 stromal cells, which did not express ephrin-B2. The predicted molecular weight for ephrin-B2 is 37 kd, but 3 bands of 38 kd, 46 kd, and 48 kd were detected in OP9/ephrin-B2 stromal cells by Western blot analysis. Consistent with a previous paper, this slow electrophoretic mobility may be due to the modification of glycosylation (Figure1A).27
P-Sp explants from E9.0-9.5 embryos were cocultured with the OP9/vector or OP9/ephrin-B2 stromal cells in the presence of IL-6, IL-7, SCF, and erythropoietin. Immunohistochemical staining with PECAM-1 MoAb to detect ECs was performed after culturing for 4, 7, 10, and 14 days. In P-Sp culture, PECAM-1+Flk-1+TIE2low/− ECs formed a sheetlike structure (vascular beds) beside the P-Sp explant. Subsequently, PECAM-1+Flk-1+TIE2+ECs sprouted from the sheet and formed a cordlike structure (vascular networks).44 ECs forming the sheetlike structure are an immature phenotype, which assembles into vascular channels. ECs forming a cordlike structure are the mature phenotype, which expands the vascular network. Early (days 4 and 7) and late (days 10 and 14) vascular network formation was enhanced in all 3 OP9 stromal cell clones that overexpressed ephrin-B2 compared with OP9/vector stromal cell clones (Figure 1B). The vascular formation on the OP9/vector stromal cells was the same intensity as that on the parent OP9 cells (data not shown). Moreover, we measured the area of vascular networks from multiple P-Sp cultures at each time point using NIH Image software. The vascular network areas were 25, 1.6, and 2.4 times higher in OP9/ephrin-B2 stromal cells than that in OP9/vector stromal cells on days 7, 10, and 14, respectively (Figure 1C). It is evident that OP9-expressing ephrin-B2 promotes EC sprouting from an early stage to late stage in this coculture system.
Effect of EphB4 during angiogenesis in P-Sp culture system
Although it has been reported that mesenchymal cells do not express EphB4 in vivo,30 to clarify the interactions between environmental EphB4+ cells and ECs, we transfected EphB4 into OP9 cells and cloned OP9 cells that expressed high levels of EphB4 (OP9/EphB4), while parent OP9 cells expressed little endogenous EphB4 (Figure 2A). To confirm the function of EphB4 in angiogenesis in the OP9 culture system, E9.0-9.5 P-Sp explants were cocultured with OP9/EphB4 or OP9/vector stromal cells as above. After 14 days of coculture, immunohistochemistry with the anti–PECAM-1 antibody was performed to detect the ECs. The level of vascular network formation in all 3 of the EphB4-overexpressing clones was severely suppressed compared with that seen on OP9/vector stromal cell clones; however, the vascular bed formation was not changed (Figure 2B).
When the chimeric protein EphB4-Fc was added to the OP9/ephrin-B2 cocultures, vascular network formation was also inhibited (Figure 2B); however, CD4-Fc as a control did not affect vascular formation (data not shown). On the other hand, when 20 μg/mL ephrin-B2–Fc was added to this coculture system, vascular EC sprouting/remodeling from the P-Sp explant increased in both OP9/EphB4 and OP9/vector stromal cells and was strongly increased in OP9/ephrin-B2 stromal cells (Figure 2C). In contrast, monomer ephrin-B2 chimeric protein (ephrin-B2–FLAG) did not affect vascular formation (data not shown). These results indicate that, contrary to OP9/ephrin-B2, OP9/EphB4 inhibited vascular network formation from P-Sp explants but did not affect development of vascular beds. The signals from a soluble form of ephrin-B2–Fc also promoted EC sprouting/remodeling, but soluble EphB4-Fc inhibited it.
Effect of OP9/ephrin-B2 stromal cells on P-Sp single-cell suspension cultures
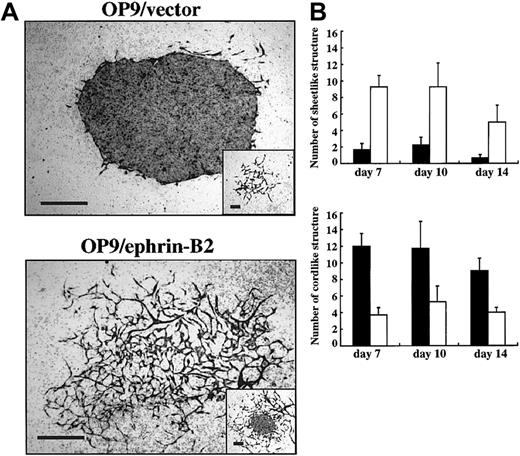
To analyze the effect of ephrin-B2 on endothelial precursor cells, dissociated cells of E9.0-9.5 P-Sp were seeded on OP9/ephrin-B2 or OP9/vector stromal cells in the medium containing VEGF and SCF. ECs proliferated by 2 different means in this condition. One consisted of spreading EC networks and the other of aggregated ECs. We named the former a “cordlike” and the latter a “sheetlike” structure. In the OP9/ephrin-B2 stromal cells, most of the PECAM-1+ ECs formed a cordlike structure, whereas in OP9/vector stromal cells ECs formed a sheetlike structure (Figure 3A). The number of sheetlike structures in OP9/vector stromal cells was about 5 times greater than that in OP9/ephrin-B2 stromal cells. On the other hand, the number of cordlike structures was about 2.5 times higher in OP9/ephrin-B2 stromal cells than in the OP9/vector stromal cells (Figure 3B). These observations further indicated that OP9/ephrin-B2 stromal cells support EC sprouting.
OP9/ephrin-B2 promoted EC sprouting in cultures using dissociated cells from P-Sp explants.
The E9.5 P-Sp region was dissociated enzymatically and cocultured with OP9/ephrin-B2 (▪) or OP9/vector (■) stromal cells in the presence of SCF and VEGF. Immunostaining by anti–PECAM-1 MoAb was performed at the indicated times. (A) The PECAM-1+ ECs formed mostly sheetlike structures on OP9/vector stromal cells (inset shows the cordlike structures in OP9/vector stromal cells), whereas the PECAM-1+ ECs formed cordlike structures on OP9/ephrin-B2 stromal cells (inset shows the sheetlike structure in OP9/ephrin-B2 stromal cells). The bar indicates 500 μm. (B) The number of sheetlike or cordlike structures on OP9/ephrin-B2 or OP9/vector stromal cells. The results represent the mean ± SD of triplicate cultures.
OP9/ephrin-B2 promoted EC sprouting in cultures using dissociated cells from P-Sp explants.
The E9.5 P-Sp region was dissociated enzymatically and cocultured with OP9/ephrin-B2 (▪) or OP9/vector (■) stromal cells in the presence of SCF and VEGF. Immunostaining by anti–PECAM-1 MoAb was performed at the indicated times. (A) The PECAM-1+ ECs formed mostly sheetlike structures on OP9/vector stromal cells (inset shows the cordlike structures in OP9/vector stromal cells), whereas the PECAM-1+ ECs formed cordlike structures on OP9/ephrin-B2 stromal cells (inset shows the sheetlike structure in OP9/ephrin-B2 stromal cells). The bar indicates 500 μm. (B) The number of sheetlike or cordlike structures on OP9/ephrin-B2 or OP9/vector stromal cells. The results represent the mean ± SD of triplicate cultures.
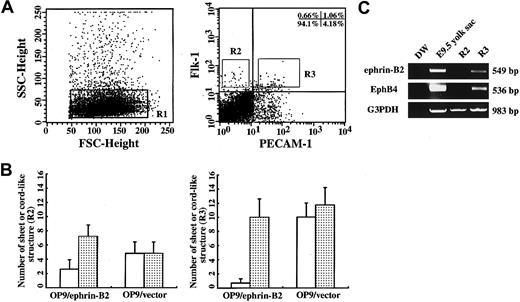
P-Sp explants contain various kinds of cells, such as endothelial precursors, hematopoietic cells, and mesenchymal cells. To examine whether OP9/ephrin-B2 stromal cells affect EC precursors directly, ECs from the E9.5 P-Sp regions were sorted by FACSVantage (Figure4A). Flk-1+PECAM-1−(R2) and Flk-1+PECAM-1+(R3) cells were cocultured with OP9/ephrin-B2 or OP9/vector cells and showed EC growth on these stromal cells. After culturing for 10 days, we counted the number of PECAM-1+ cordlike or sheetlike EC structures. The cordlike structures from R2 and R3 cells were 2.8 times and 14 times higher than sheetlike structures on OP9/ephrin-B2 stromal cells, respectively, but no significant difference was seen between the 2 kinds of vascular structures on OP9/vector stromal cells (Figure 4B). These data indicated that the stromal cells expressing ephrin-B2 could promote EC spreading from a single EC precursor. RT-PCR showed that R2 cells expressed neither ephrin-B2 nor EphB4. R3 expresses both ephrin-B2 and EphB4, according to previous studies that the R3 population may contain ephrin-B2+ arterial ECs and EphB4+ venous ECs (Figure 4C).20,21 47 These results when considered together further indicated that the OP9 expressing ephrin-B2 directly promoted EC sprouting.
OP9/ephrin-B2 promoted ECs sprouting from a single EC.
(A) Flk-1+PECAM-1−(R2) and Flk-1+PECAM-1+(R3) cells were sorted from E9.0-9.5 P-Sp regions stained with Flk-1 and PECAM-1 antibodies. (B) The sorted R2 and R3 cells were cocultured with an OP9/ephrin-B2 or OP9/vector in the presence of VEGF for 10 days, and PECAM-1 immunostaining was performed. The cordlike structures (░) from R2 and R3 cells were higher than the sheetlike structure (■) on OP9/ephrin-B2 stromal cells, but almost no difference was seen between the 2 types of structures on OP9/vector stromsl cells. (C) Expression of ephrin-B2 and EphB4 was detected by RT-PCR analysis. Total RNA was extracted from R2 and R3 cells, and the RNA extracted from E9.5 yolk sac was used as a positive control. R3 cells expressed both ephrin-B2 and EphB4, whereas R2 cells did not express either.
OP9/ephrin-B2 promoted ECs sprouting from a single EC.
(A) Flk-1+PECAM-1−(R2) and Flk-1+PECAM-1+(R3) cells were sorted from E9.0-9.5 P-Sp regions stained with Flk-1 and PECAM-1 antibodies. (B) The sorted R2 and R3 cells were cocultured with an OP9/ephrin-B2 or OP9/vector in the presence of VEGF for 10 days, and PECAM-1 immunostaining was performed. The cordlike structures (░) from R2 and R3 cells were higher than the sheetlike structure (■) on OP9/ephrin-B2 stromal cells, but almost no difference was seen between the 2 types of structures on OP9/vector stromsl cells. (C) Expression of ephrin-B2 and EphB4 was detected by RT-PCR analysis. Total RNA was extracted from R2 and R3 cells, and the RNA extracted from E9.5 yolk sac was used as a positive control. R3 cells expressed both ephrin-B2 and EphB4, whereas R2 cells did not express either.
Effect of ephrin-B2 and EphB4 on ephrin-B2+cells
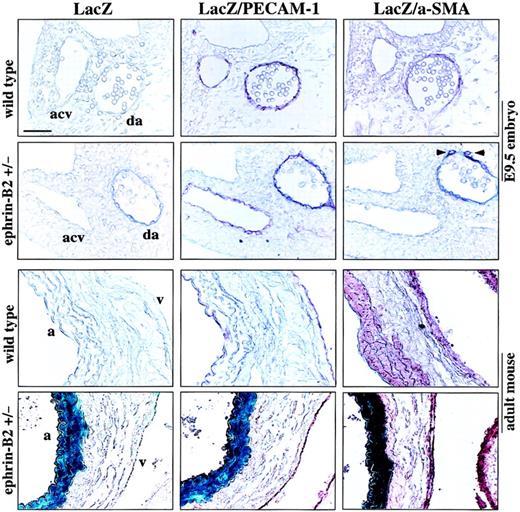
Vascular ECs contain arterial ECs and venous ECs. Recent studies have shown that the 2 kinds of ECs are molecularly distinct, which is revealed by a ligand ephrin-B2 that is dominantly expressed on arterial ECs; and EphB4, a receptor specific for ephrin-B2, is expressed on venous ECs.20,21 47 We analyzed in vivo ephrin-B2 expression on arteries and veins, conducted LacZ and anti–PECAM-1 or anti–α-SMA double staining using wild-type or ephrin-B2LacZ/+ (in which LacZ expression is under the transcriptional control of an ephrin-B2 promoter) embryos, and examined adult abdominal/thoracic aorta and superior/inferior vena cava containing the surrounding tissues. Serial staining of tissue sections revealed that in E9.5 embryos the ephrin-B2 was absolutely expressed in the dorsal aorta ECs and surrounding cells but not in the anterior cardinal vein (Figure 5). Also, in the adult, ephrin-B2 is expressed on arterial ECs and surrounding SMCs. By contrast, no ephrin-B2 was detected in the venous cells (Figure 5).
LacZ/ephrin-B2 expression in mouse embryo and adult mouse vascular tissue.
E9.5 embryos from ephrin-B2 heterozygous and wild-type embryos, abdominal/thoracic aorta, and superior/inferior vena cava with surrounding tissues from adult ephrin-B2 heterozygous or wild-type mice were stained with LacZ staining solution. After LacZ staining, the samples were postfixed, embedded, and sectioned. Then the vascular ECs and surrounding cells/SMCs were examined by immunochemical staining with anti–PECAM-1 and anti–α-SMA antibodies. No LacZ staining was observed in wild-type embryos or in adult tissue. In ephrin-B2 heterozygous embryos, LacZ/ephrin-B2 (blue) was obviously detected in the dorsal aorta (da) ECs and surrounding cells with PECAM-1 (red) and α-SMA (red) (arrowheads) but not in the anterior cardinal vein (acv). In adult tissue the LacZ/ephrin-B2 was coexpressed with PECAM-1 and α-SMA in the arterial ECs and SMCs but not in the venous ECs and SMCs. PECAM-1– and α-SMA–stained cells were identical in expression to the arterial ECs and SMCs in wild-type mice; a indicates aorta; v, vena cava. The bar indicates 25 μm.
LacZ/ephrin-B2 expression in mouse embryo and adult mouse vascular tissue.
E9.5 embryos from ephrin-B2 heterozygous and wild-type embryos, abdominal/thoracic aorta, and superior/inferior vena cava with surrounding tissues from adult ephrin-B2 heterozygous or wild-type mice were stained with LacZ staining solution. After LacZ staining, the samples were postfixed, embedded, and sectioned. Then the vascular ECs and surrounding cells/SMCs were examined by immunochemical staining with anti–PECAM-1 and anti–α-SMA antibodies. No LacZ staining was observed in wild-type embryos or in adult tissue. In ephrin-B2 heterozygous embryos, LacZ/ephrin-B2 (blue) was obviously detected in the dorsal aorta (da) ECs and surrounding cells with PECAM-1 (red) and α-SMA (red) (arrowheads) but not in the anterior cardinal vein (acv). In adult tissue the LacZ/ephrin-B2 was coexpressed with PECAM-1 and α-SMA in the arterial ECs and SMCs but not in the venous ECs and SMCs. PECAM-1– and α-SMA–stained cells were identical in expression to the arterial ECs and SMCs in wild-type mice; a indicates aorta; v, vena cava. The bar indicates 25 μm.
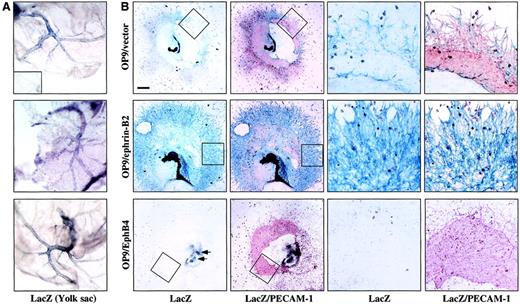
Next, we sought to determine which type of EC (ephrin-B2+or EphB4+ ECs) were supported or inhibited by ephrin-B2 or EphB4 on OP9 cells and how they interact with each other in this system. The ephrin-B2LacZ/+ embryos were obtained from a cross of wild-type and ephrin-B2LacZ/+ mice. After genotypying of the embryo by LacZ staining of the yolk sac (Figure6A), the E9.0-9.5 ephrin-B2+/+ or ephrin-B2LacZ/+ P-Sp was cocultured with OP9/ephrin-B2, OP9/EphB4, or OP9/vector stromal cells. After culturing for 10 to 14 days, we examined the formation of vascular networks by immunohistochemical staining with PECAM-1 MoAb and LacZ staining. Ephrin-B2+ ECs were detected as PECAM-1 and LacZ++ cells. As shown in Figure 6B, the OP9/vector stromal cells form a fine vascular network, which consisted of both PECAM-1+ cells and LacZ/ephrin-B2–PECAM-1++cells. But the vascular networks were formed mainly by LacZ and PECAM-1++ cells on OP9/ephrin-B2 stromal cells. In contrast, OP9/EphB4 stromal cells did not support vascular network formation and almost no LacZ/ephrin-B2+ cells were observed. This indicates that OP9/ephrin-B2 promotes vascular network formation and supports the proliferation and sprouting of ephrin-B2+ cells but that OP9/EphB4 inhibits ephrin-B2+ cell proliferation.
OP9/ephrin-B2 promoted ephrin-B2+ cell proliferation and sprouting, whereas OP9/EphB4 caused inhibition.
(A) Whole-mount LacZ staining of the E9.0-9.5 yolk sac. The blue color in the vascular region indicates LacZ+ staining. The phenotype in each panel corresponds to panel B. The inset shows LacZ-negative staining in the wild-type yolk sac. (B) E9.5 P-Sp explants of mice heterozygous for ephrin-B2–targeted alleles, in which β-galactosidase (LacZ) expression is under the transcriptional control of ephrin-B2 promoter, were cocultured with OP9/ephrin-B2, OP9/EphB4, or OP9/vector stromal cells for 14 days. LacZ and PECAM-1 staining have shown that the vascular network was formed by LacZ/ephrin-B2 (blue) and/or PECAM-1 (red) double-positive ECs on OP9/ephrin-B2 stromal cells, and almost no LacZ/ephrin-B2+ECs were detected on OP9/EphB4 stromal cells. The vascular network was formed by LacZ/ephrin-B2 (blue) and/or PECAM-1 (red) double-positive ECs and PECAM-1+ cells on OP9/vector stromal cells. The right panels are a higher magnification of the area indicated by the box in the left panels. The bar indicates 1 mm (left panels) and 80 μm (right panels). The arrows on the OP9/EphB4 stromal cell plate indicate where some LacZ+ cells are available.
OP9/ephrin-B2 promoted ephrin-B2+ cell proliferation and sprouting, whereas OP9/EphB4 caused inhibition.
(A) Whole-mount LacZ staining of the E9.0-9.5 yolk sac. The blue color in the vascular region indicates LacZ+ staining. The phenotype in each panel corresponds to panel B. The inset shows LacZ-negative staining in the wild-type yolk sac. (B) E9.5 P-Sp explants of mice heterozygous for ephrin-B2–targeted alleles, in which β-galactosidase (LacZ) expression is under the transcriptional control of ephrin-B2 promoter, were cocultured with OP9/ephrin-B2, OP9/EphB4, or OP9/vector stromal cells for 14 days. LacZ and PECAM-1 staining have shown that the vascular network was formed by LacZ/ephrin-B2 (blue) and/or PECAM-1 (red) double-positive ECs on OP9/ephrin-B2 stromal cells, and almost no LacZ/ephrin-B2+ECs were detected on OP9/EphB4 stromal cells. The vascular network was formed by LacZ/ephrin-B2 (blue) and/or PECAM-1 (red) double-positive ECs and PECAM-1+ cells on OP9/vector stromal cells. The right panels are a higher magnification of the area indicated by the box in the left panels. The bar indicates 1 mm (left panels) and 80 μm (right panels). The arrows on the OP9/EphB4 stromal cell plate indicate where some LacZ+ cells are available.
Function of ephrin-B2 and EphB4 in SMC recruitment
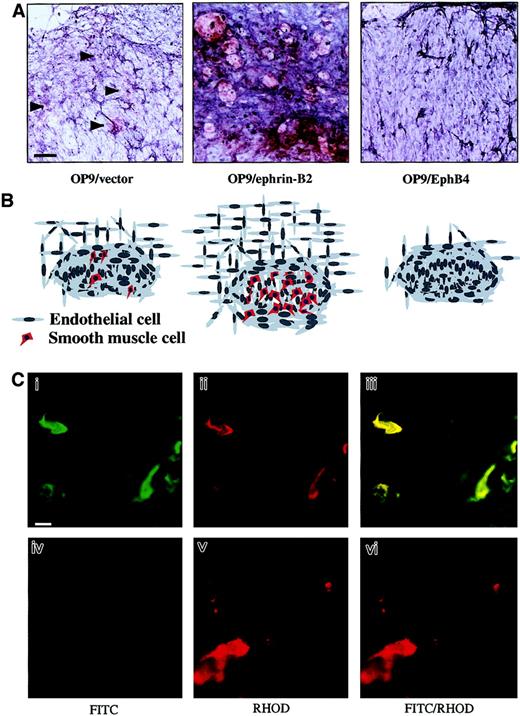
During vascular development, an important event is the formation of vessel walls by recruitment of mural cell precursors, especially in the artery. To investigate SMC recruitment in this culture system, we performed immunohistochemical staining with anti–α-SMA antibody. On day 14 of culturing, the number of α-SMA+ SMCs increased in the surrounding ECs formed on OP9/ephrin-B2 stromal cells. Nevertheless, no α-SMA+ cells were detected in OP9/EphB4 stromal cells, whereas a small number of α-SMA+ cells were detected in OP9/vector stromal cells (Figure7A,B). It has been reported that SMCs develop from mesodermal cells or neural crest cells or transdifferentiate from other cells.53 54 We have further confirmed that the SMA+ cells in the vascular network were derived from P-Sp explants, but not from OP9 stromal cells, by using P-Sp obtained from green mice harboring GFP ubiquitously under the transcriptional control of a CAG promoter in this coculture system (Figure 7C). This result suggested that the ephrin-B2+ ECs and stromal cells have the ability to promote SMC recruitment and proliferation. However, EphB4+ EC might be unable to promote SMC recruitment. This further suggests that ephrin-B2/EphB4 signaling is important for the interaction between the ECs and surrounding cells.
OP9/ephrin-B2 supported SMC recruitment, whereas OP9/EphB4 and EphB4-Fc inhibited it.
(A) The vascular networks in the P-Sp explant cultures were visualized by PECAM-1 staining (blue), and the anti–α-SMA antibody was used to detect the SMCs on the P-Sp culture system after coculture for 14 days. The α-SMA+ (red) cells were located more abundantly in the vascular bed on OP9/ephrin-B2 stromal cells than in OP9/vector stromal cells (arrowheads indicate the α-SMA+ cells), and almost no α-SMA+ (red) cells were detected in the OP9/EphB4 stromal cells. The bar indicates 100 μm. (B) Schematic presentation of SMC recruitment under various conditions. Figures correspond to the upper panels in panel A. (C) SMA+ cells were derived from P-Sp explants. P-Sp explants from embryos of green mice expressing GFP ubiquitously (i-iii) and wild-type embryos (iv-vi) were cocultured with OP9 stromal cells, and the culture plates were immunostained with Cy3-conjugated anti–α-SMA antibodies. Green indicates cells from GFP P-Sp explants; red indicates α-SMA+ cells; and yellow indicates GFP and α-SMA++ cells. Panels Ci and Civ are fluorescein isothiocyanate (FITC) specific, Cii and Cv are rhodamine (RHOD)–specific wavelengths, and the merged configuration is shown in panels Ciii and Cvi. The bar indicates 25 μm.
OP9/ephrin-B2 supported SMC recruitment, whereas OP9/EphB4 and EphB4-Fc inhibited it.
(A) The vascular networks in the P-Sp explant cultures were visualized by PECAM-1 staining (blue), and the anti–α-SMA antibody was used to detect the SMCs on the P-Sp culture system after coculture for 14 days. The α-SMA+ (red) cells were located more abundantly in the vascular bed on OP9/ephrin-B2 stromal cells than in OP9/vector stromal cells (arrowheads indicate the α-SMA+ cells), and almost no α-SMA+ (red) cells were detected in the OP9/EphB4 stromal cells. The bar indicates 100 μm. (B) Schematic presentation of SMC recruitment under various conditions. Figures correspond to the upper panels in panel A. (C) SMA+ cells were derived from P-Sp explants. P-Sp explants from embryos of green mice expressing GFP ubiquitously (i-iii) and wild-type embryos (iv-vi) were cocultured with OP9 stromal cells, and the culture plates were immunostained with Cy3-conjugated anti–α-SMA antibodies. Green indicates cells from GFP P-Sp explants; red indicates α-SMA+ cells; and yellow indicates GFP and α-SMA++ cells. Panels Ci and Civ are fluorescein isothiocyanate (FITC) specific, Cii and Cv are rhodamine (RHOD)–specific wavelengths, and the merged configuration is shown in panels Ciii and Cvi. The bar indicates 25 μm.
Discussion
In this paper, we examined the function of EphB4 receptors and ephrin-B2 ligands in the interaction of ECs with mesenchymal cells using an in vitro P-Sp and stromal cell coculture system. To address this issue, we analyzed angiogenesis using OP9 stromal cells that were transfected with ephrin-B2 or EphB4. Using these sublines of OP9 cells, we observed several pieces of evidence: (1) Environmental ephrin-B2 supports the proliferation of ephrin-B2+ ECs and suppresses the proliferation of ephrin-B2− ECs; (2) Environmental ephrin-B2 supports the proliferation of SMCs; (3) Environmental EphB4 suppresses the proliferation of ephrin-B2+ ECs and does not promote the proliferation of SMCs.
The EphB4 receptor and its cognate ligand, ephrin-B2, are crucial for successful cardiovascular development during embryogenesis.20,21,28 To elucidate the interactions between the ECs and surrounding cells, which express ephrin-B2 and EphB4, we established an in vitro OP9 stromal cell and P-Sp coculture assay for analyses of angiogenesis. Ephrin-B2–transfected stromal cells enhanced the vascular network formation from P-Sp, whereas EphB4-transfected stromal cells inhibited it. RT-PCR analyses have shown that ephrin-B2, ephrin-B1, EphB4, EphB3, and EphB2 are expressed in the PECAM-1+CD45− EC population, which is grown from P-Sp explants in OP9 and P-Sp coculture systems (data not shown). However, other Eph receptors and ephrin ligands may also be involved in angiogenesis. Ephrin-B1, ephrin-B3, EphB2, and EphB3 are expressed in the vascular ECs or the surrounding cells in vivo. But this overlapping of expression is unable to compensate for the lack of ephrin-B2 or EphB4. No vascular defects were seen in either EphB2 or EphB3 homozygous mutant mice. However, EphB2 and EphB3 double-mutant mice have shown the vascular defect only with 30% penetrance.30 In combination, these results suggest that angiogenesis is dependent upon the appropriate expression of ephrin-B2 and EphB4.
In this culture system, we found that both ephrin-B2+ and ephrin-B2− ECs proliferated. For the detection of ephrin-B2 ligand–positive cells, we used the mice in which the lacZ gene is inserted in the ephrin-B2 locus. In the LacZ staining of P-Sp cultures using ephrin-B2LacZ/+ embryos, we found that OP9/ephrin-B2 stromal cells exclusively promoted the proliferation and sprouting of LacZ/ephrin-B2+ ECs, whereas OP9/EphB4 stromal cells inhibited it. This might suggest that overexpression of ephrin-B2 on stromal cells primarily suppresses the proliferation of EphB4+ ECs and subsequently supported the proliferation of arterial ECs. In contrast, the overexpression of EphB4 on stromal cells suppressed ephrin-B2+ EC proliferation and vascular network formation. Indeed, mesenchymal cells surrounding the artery, especially the dorsal aorta, widely expressed ephrin-B2 and might stimulate proliferation or sprouting of ephrin-B2+ ECs, which also express EphB2 or EphB3 (Figure 5).47 The results suggested that vascular development on ephrin-B2– or EphB4-overexpressing stromal cells is mediated by signaling between EphB4 and ephrin-B2 expressed on the endothelial or stromal cells. Also, it may indicate that overexpression of ephrin-B2 might permit the proliferation of ephrin-B2+ ECs through EphB receptors by preventing contact inhibition with EphB4+ EC. Recently, Helbling et al reported that EphB4 and B-class ephrins act as regulators of angiogenesis, possibly by mediating repulsive guidance cues to migrating ECs.55 The interaction between EphB4-expressing ECs and adjacent ephrin-B–expressing somatic tissue is therefore likely to be of a repulsive nature. Also, it suggests that signaling between EphB4 and ephrin-B2 is involved in controlling EC sprouting and vascular boundary formation during vascular development, as is the case in the nervous system.38,39 Mutual repulsive or growth-inhibitory interactions between EphB4 and ephrin-B2+ECs or surrounding cells simultaneously need other Eph receptors and ligand signals to establish a balance between these 2 kinds of ECs to maintain appropriate sprouting and remodeling during vascularization.30
During angiogenesis, one important event is the formation of the vascular wall by the recruitment of pericytes and SMCs from mesenchymal progenitor cells and neural crest cells.56,57 Progress in elucidating the mechanism of SMC recruitment, proliferation, and differentiation was achieved by identification of a number of smooth muscle–specific proteins and their expression in SMC lineage cells.58,59 ECs can modulate phenotypic change, regulate proliferation, and induce migration of SMCs.60,61 These processes are regulated by growth factors such as PDGF and TGF-β. PDGF derived from EC acts as a mitogen for mesenchymal cells,62 whereas TGF-β induces differentiation of neural crest cells into SMCs.63 However, SMC/pericytes also take an active part during vascular development. SMCs are capable of synthesizing angiopoietin-1, an essential factor for angiogenesis.44
Vascular SMCs produce and organize extracellular matrix molecules within the developing vessel wall. In our in vitro assays, OP9/ephrin-B2 promoted not only proliferation and sprouting of ephrin-B2+ ECs but also recruitment and proliferation of α-SMA+ SMCs. Although α-SMA is the earliest marker expressed by SMCs, cardiomyocytes, and skeletal muscle,53,58 its expression is lost in striated muscle and becomes specifically associated with vascular and visceral SMCs. By contrast, OP9/EphB4 inhibited the α-SMA+SMC recruitment and/or proliferation. Moreover, studies by ourselves and others have shown that differences between the artery and vein apply not only to the ECs but also to the SMCs marked by the ephrin-B2 expression (Figure 5).47 In ephrin-B2 knockout mice, it was observed that the mesenchymal cells and pericytes appeared poorly associated with ECs in the yolk sacs and that these cells exhibited a rounded morphology compared with those in wild-type yolk sacs.20 It suggested that the ephrin-B2+arterial ECs may play a crucial role in SMC recruitment and proliferation. It is likely that ephrin-B2–expressing mesenchymal cells surrounding the dorsal aorta are recruited by ephrin-B2–expressing ECs that also coexpress EphB2 and EphB3 during the early stage of angiogenesis. Then the ephrin-B2+ ECs and SMCs stimulate each other during arteriogenesis. Molecular cues as to how such ephrin-B2+ ECs and SMCs interact with each other have not been clarified. However, coexpressions and interactions of ephrin-B1, EphB3, and EphB4 on vein primordia and coexpression of ephrin-B1, ephrin-B2, EphB2, and EphB3 on arteries may also be required for venous and arterial formation.
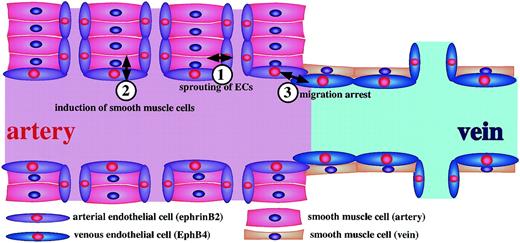
In summary, blood vessels are formed by the recruitment and migration of mesenchymal cells outside the endothelial layer. Therefore, from our results and others, it is reasonable to conclude that the initial commitment of ephrin-B2+ or EphB4+ ECs from angioblasts is the trigger for determining vessels that become arteries or veins.20,21,47 During this process, along with other factors,16-19,64 65 Ephs/ephrins and, in particular, EphB4/ephrin-B2 are the key regulators in the recruitment and migration of SMC precursors in vivo and maintain the balance of proliferation activity. Based on previous studies and our own findings, we propose a model (Figure 8) to account for the role of EphB4/ephrin-B2 signaling in the interaction between endothelial and mesenchymal cells during vasculogenesis, angiogenesis, and vascular morphologic change.
Model of ephrin-B2–EphB4 signals in vasculoangiogenesis.
(1) Ephrin-B2 on SMCs support proliferation and sprouting of arterial ECs. (2) Ephrin-B2+ ECs promote the recruitment of SMCs near ECs. Molecular cues for such induction are unknown; however, PDGF-BB and TGF-β may be involved in this process. (3) When the ephrin-B2+ ECs (arterial ECs) and EphB4+(venous ECs) face each other at the boundary of a capillary, cell proliferation of ECs may be suppressed, and the migratory ability may be arrested there.
Model of ephrin-B2–EphB4 signals in vasculoangiogenesis.
(1) Ephrin-B2 on SMCs support proliferation and sprouting of arterial ECs. (2) Ephrin-B2+ ECs promote the recruitment of SMCs near ECs. Molecular cues for such induction are unknown; however, PDGF-BB and TGF-β may be involved in this process. (3) When the ephrin-B2+ ECs (arterial ECs) and EphB4+(venous ECs) face each other at the boundary of a capillary, cell proliferation of ECs may be suppressed, and the migratory ability may be arrested there.
The authors thank Dr Jon K. Moon for critical reading of this manuscript.
Supported by Grants-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Toshio Suda, Dept of Cell Differentiation, Institute of Molecular Embryology and Genetics, Kumamoto University, 2-2-1 Honjo, Kumamoto 860-0811, Japan; e-mail:sudato@gpo.kumamoto-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal