Abstract
Gain-of-function mutations in c-kit, which appear to contribute to mast cell hyperplasia, have been detected in both limited and aggressive forms of mastocytosis, suggesting that other mutations or polymorphisms may contribute to the clinical phenotype. Because addition of interleukin-4 (IL-4) to mast cell cultures is reported to induce apoptosis, the hypothesis was considered that individuals carrying the gain-of-function polymorphism Q576R in the cytoplasmic domain of the α-subunit of the IL-4 receptor (IL-4R) might be relatively resistant to the gain-of-function mutation in c-kit. To assess this possibility, 36 patients with either cutaneous or systemic mastocytosis were studied for association with the Q576R polymorphism. The Q576R polymorphism was found more frequently in those with disease limited to skin and who exhibited lower levels of surrogate disease markers. These data suggest that the Q576R IL-4R α- chain polymorphism may mitigate disease expression and confer a better prognosis in patients with mastocytosis.
Introduction
Accumulation of excessive numbers of mast cells in tissues is the pathologic basis of mastocytosis. Several distinct clinical presentations of mastocytosis are recognized that differ in their age of onset, physical findings, course, prognosis, and therapy. For example, childhood-onset mastocytosis characteristically is limited to the skin and has a better prognosis than adult-onset disease,1 although a subset has a poorer prognosis because of the coexistence of a hematologic disorder.2
Mastocytosis is often associated with activating mutations in Kit, the receptor for stem cell factor.3 Such mutations have been identified in the lesional skin of adult patients with indolent as well as aggressive mastocytosis and in children with extensive disease.4-6 As such, the presence of activating c-kit mutations alone are not sufficient to account for different clinical forms of the disease, suggesting that other mutations or polymorphisms are likely to influence the clinical outcome of mastocytosis if they occur in genes important for the regulation of mast cell number.
One such candidate gene is the receptor for interleukin 4 (IL-4R) because IL-4 has been shown to down-regulate the growth and differentiation and induce apoptosis of human mast cells.7-9 Polymorphisms in the IL-4R α-chain (IL-4RA) have been investigated in several allergic and inflammatory diseases. Among these, the gain-of- function polymorphism, Q576R (single-letter amino acid codes), appears to have clinical relevance, because it has been shown to be associated with atopy, severe asthma, and renal allograft rejection.10-12 We thus hypothesized that patients expressing the Q576R IL-4RA polymorphism might be relatively protected from severe mast cell hyperplasia resulting from activating mutations in c-kit and other yet-to-be discovered events that promote mast cell hyperplasia. Patients carrying the Q576R polymorphism do appear to have more limited forms of mastocytosis, suggesting that this polymorphism confers relative protection from adverse events that lead to pathologic mast cell hyperplasia.
Study design
Patients
Thirty-six patients with mastocytosis were examined in a study approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases. All patients signed an informed consent document. The diagnosis of mastocytosis was based on the demonstration of characteristic skin or bone marrow biopsy findings. Each patient was assigned a category based on the pattern of the disease: cutaneous mastocytosis (n = 13), indolent systemic mastocytosis as evidenced by characteristic mast cell aggregates in the bone marrow (n = 18), and systemic mastocytosis with an associated nonmast cell hematologic disorder (n = 5). The median age at the time of the study for the patients with mastocytosis was 44 years (range, 2-87 years). Fourteen patients (39%) experienced onset of disease at 18 years of age or younger and thus were classified as having childhood-onset disease. Fourteen patients were male. Buffy coat samples from anonymous healthy adult donors were obtained from the Department of Transfusion Medicine at the National Institutes of Health Clinical Center.
Detection of Q576R polymorphism
Total RNA was extracted from peripheral blood mononuclear cells and reverse transcribed to complementary DNA (cDNA) as reported.6 This cDNA was used as a template to amplify a 307-bp product containing the region encoding the IL-4RA Q576R polymorphism by nested polymerase chain reaction (PCR) as described.10 Presence of the polymorphism and its allelic status were determined by direct PCR sequencing using the Big Dye Terminator kit (PE Biosystems, Foster City, CA) and analysis on a capillary automated sequencer (ABI Prism 310 Genetic Analyzer, PE Biosystems).
Determination of tryptase and soluble CD117
Tryptase levels were measured with the UniCAP system (Pharmacia-Upjohn, Peapack, NJ) using monoclonal antibodies (mAbs) B12 for capture and β-galactosidase-G4 for detection as described.13 Soluble CD117 was measured by enzyme-linked immunosorbent assay (ELISA) using the mAbs L6 and L15 as described (Nichirei Diagnostics, Nichirei, Japan).14 All samples were assayed in duplicate.
Statisticalanalysis
Comparisons for statistical significance were performed using the Wilcoxon rank sum (Mann-Whitney U) test. Contingency table analyses were performed by the Fisher exact test. A Pvalue of less than .05 was considered statistically significant.
Results and discussion
We first determined the frequency of the IL-4RA Q576R polymorphism in individuals with and without mastocytosis. In a randomly selected population of blood bank donors without mastocytosis, the frequency of individuals who carried the polymorphic R576 allele was found to be 28.6% (4 of 14) with an allelic frequency of 17.9%. These values are consistent with the originally reported frequency of this polymorphism.10 Similarly, in a group of 36 patients with mastocytosis, the overall frequency of the patients carrying the polymorphic R576 allele was found to be 30.6%, with an allelic frequency of 19.4%. Thus, the frequency of the IL-4RA Q576R polymorphism in patients with mastocytosis was not different from that of the general population.
We next compared the frequency of the IL-4RA Q576R polymorphism in patients with mastocytosis assigned to different categories based on disease extent and prognosis. Results revealed that the polymorphism was significantly associated with mastocytosis limited to the skin as compared to those with bone marrow involvement or an associated hematologic disorder. Thus, 9 of 13 (69.2%) of the patients with cutaneous disease alone carried the polymorphism, whereas only 2 of 23 (8.7%) with systemic disease had the polymorphic allele (Table1). The odds ratio for having the mild disease in the presence of the polymorphic R576 allele was calculated to be 23.6 (P < .001; 95% confidence interval, 3.6-153). Further, the association of the polymorphism with milder disease persisted when patients are grouped according to the age at onset of disease. Fewer patients with adult-onset disease were found to have the polymorphism as compared to those with childhood-onset disease (18.2% versus 50%, P = .05); however, the polymorphism was more frequently found in patients with limited involvement in both childhood- and adult-onset disease (Table 1).
The IL-4RA Q576R polymorphism in patients with mastocytosis
| . | P . | WT . | % P . |
|---|---|---|---|
| All patients | |||
| Cutaneous | 9 | 4 | 69.2* |
| Systemic | 2 | 21 | 8.7 |
| ISM | 2 | 16 | 11.1 |
| AHD | 0 | 5 | 0 |
| Total | 11 | 25 | 30.6 |
| Adult onset | |||
| Cutaneous | 3 | 1 | 75† |
| Systemic | 1 | 17 | 5.6 |
| ISM | 1 | 13 | 7.1 |
| AHD | 0 | 4 | 0 |
| Total | 4 | 18 | 18.2 |
| Pediatric onset | |||
| Cutaneous | 6 | 3 | 66.7 |
| Systemic | 1 | 4 | 20 |
| ISM | 1 | 3 | 25 |
| AHD | 0 | 1 | 0 |
| Total | 7 | 7 | 50 |
| . | P . | WT . | % P . |
|---|---|---|---|
| All patients | |||
| Cutaneous | 9 | 4 | 69.2* |
| Systemic | 2 | 21 | 8.7 |
| ISM | 2 | 16 | 11.1 |
| AHD | 0 | 5 | 0 |
| Total | 11 | 25 | 30.6 |
| Adult onset | |||
| Cutaneous | 3 | 1 | 75† |
| Systemic | 1 | 17 | 5.6 |
| ISM | 1 | 13 | 7.1 |
| AHD | 0 | 4 | 0 |
| Total | 4 | 18 | 18.2 |
| Pediatric onset | |||
| Cutaneous | 6 | 3 | 66.7 |
| Systemic | 1 | 4 | 20 |
| ISM | 1 | 3 | 25 |
| AHD | 0 | 1 | 0 |
| Total | 7 | 7 | 50 |
P indicates polymorphic; WT, wild type; ISM, indolent systemic mastocytosis; AHD, systemic mastocytosis with an associated nonmast cell hematologic disorder.
P = .0003 versus all systemic, .0014 versus ISM, 0.015 versus AHD.
P = .01 versus all systemic, 0.019 versus ISM.
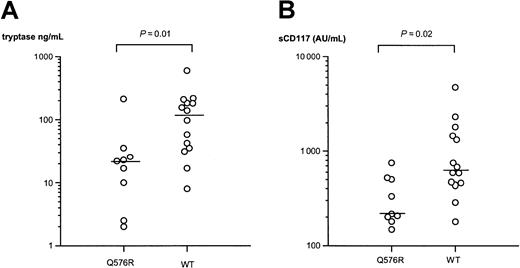
Circulating levels of tryptase and soluble CD117 have been proposed as surrogate markers of disease severity and correlate with bone marrow pathology in mastocytosis.14 We therefore also determined the serum tryptase and plasma soluble CD117 levels in 23 patients with or without the polymorphic allele. Consistent with the association found according to the categorization based on clinical presentation, the patients with the polymorphic R576 allele had significantly lower levels of both tryptase and soluble CD117 (Figure1). Thus, patients with mastocytosis who carried the polymorphism had a median serum tryptase level of 22 ng/mL (range, 2-212 ng/mL), whereas the patients with the wild-type gene had a median serum tryptase level of 118 ng/mL (range, 8-596 ng/mL;P = .01). Similarly, median plasma soluble CD117 values were 216 AU/mL (range, 148-753 AU/mL) and 680 AU/mL (range, 179-4755 AU/mL), respectively, for patients with or without the polymorphism (P = .02). No significant difference was found in serum IgE concentrations between the 2 groups.
Q576R polymorphism and mastocytosis.
IL-4RA Q576R polymorphism is associated with lower levels of surrogate disease markers, tryptase (A) and soluble CD117 (sCD117) (B), in mastocytosis. Each data point represents the result from one patient. Bars represent median values. AU indicates arbitrary units (1 AU = 1.4 ng); WT, wild type.
Q576R polymorphism and mastocytosis.
IL-4RA Q576R polymorphism is associated with lower levels of surrogate disease markers, tryptase (A) and soluble CD117 (sCD117) (B), in mastocytosis. Each data point represents the result from one patient. Bars represent median values. AU indicates arbitrary units (1 AU = 1.4 ng); WT, wild type.
In conclusion, this study shows that the presence of the IL-4RA Q576R polymorphism in a patient with mastocytosis is associated with a lower mast cell burden as determined both by extent of clinical disease and by circulating levels of surrogate disease markers. This finding suggests a protective role for IL-4RA Q576R polymorphism in the limitation of tissue mast cell numbers in patients with mastocytosis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Cem Akin, Laboratory of Allergic Diseases, NIAID/NIH, 10 Center Dr, MSC 1881, Bldg 10, Rm 11C205, Bethesda, MD 20892-1881; e-mail: cakin@niaid.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal