Abstract
Familial Mediterranean fever (FMF) is a recessive disorder characterized by episodes of fever and intense inflammation. FMF attacks are unique in their sensitivity to the microtubule inhibitor colchicine, contrasted with their refractoriness to the anti-inflammatory effects of glucocorticoids. The FMF gene,MEFV, was recently identified by positional cloning; it is expressed at high levels in granulocytes and monocytes. The present study investigated the subcellular localization of the normal gene product, pyrin. These experiments did not support previously proposed nuclear or Golgi localizations. Instead fluorescence microscopy demonstrated colocalization of full-length GFP- and epitope-tagged pyrin with microtubules; this was markedly accentuated in paclitaxel-treated cells. Moreover, immunoblot analysis of precipitates of stabilized microtubules with recombinant pyrin demonstrated a direct interaction in vitro. Pyrin expression did not affect the stability of microtubules. Deletion constructs showed that the unique N-terminal domain of pyrin is necessary and sufficient for colocalization, whereas disease-associated mutations in the C-terminal B30.2 (rfp) domain did not disrupt this interaction. By phalloidin staining, a colocalization of pyrin with actin was also observed in perinuclear filaments and in peripheral lamellar ruffles. The proposal is made that pyrin regulates inflammatory responses at the level of leukocyte cytoskeletal organization and that the unique therapeutic effect of colchicine in FMF may be dependent on this interaction.
Introduction
Familial Mediterranean fever (FMF; MIM249100) is an autosomal recessive inflammatory disorder characterized by apparently unprovoked, debilitating episodes of fever, sterile peritonitis, pleurisy, and arthritis. Attacks are also accompanied by a vigorous acute-phase response, and tissue deposition of serum amyloid A sometimes leads to fatal systemic amyloidosis. Carrier frequencies for FMF mutations in several populations of Mediterranean ancestry rank among the highest known for genetic disorders, suggesting that heterozygosity for FMF mutations may confer protection from one or more as yet unknown infectious agent(s). The gene responsible for FMF,MEFV, was recently identified through positional cloning,1 2 but the function of its product, denoted pyrin (alternatively, marenostrin), has not yet been elucidated.
One of the most striking features of FMF attacks is the massive infiltration of neutrophils into the site of inflammation, hinting that the disease may be the result of increased chemotaxis in response to a normally suboptimal signal. Gene expression studies indicate that FMF is likely to be a disorder of leukocytes, because MEFV RNA has been detected in nearly all branches of the myelomonocytic differentiation pathway1,3,4; similar data have recently been reported for the mouse and rat homologs.5MEFV expression is up-regulated in response to inflammatory activators such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α)3, pointing to a fundamental function for pyrin in the immune response. Indeed, a number of subtle abnormalities of leukocyte function have been noted in patients with FMF.6-10
As is the case for other disorders in which neutrophils play a major role, the inflammatory attacks (as well as the amyloidosis) of FMF are effectively prevented with colchicine.11-13 Colchicine responsiveness distinguishes FMF from the other well-characterized hereditary periodic fevers (TNF receptor–associated periodic syndrome, the hyperimmunoglobulinemia D with periodic fever syndrome, and Muckle-Wells syndrome), and, even in the era of molecular analysis, colchicine responsiveness remains an important diagnostic element. Moreover, in marked contrast with other disorders in which colchicine use may be beneficial, such as Behçet disease, Sweet syndrome, and gout, the serositis and arthritis of FMF are singularly unresponsive to glucocorticoids, either when given prophylactically or at the time of an attack.14 Taken together, these observations suggest that pyrin is part of a uniquely colchicine-sensitive inflammatory pathway. Insofar as daily colchicine prophylaxis is much more effective than treatment after the onset of an attack, it is likely that the drug is blocking a step in the elicitation or amplification of the FMF inflammatory infiltrate, rather than granulocyte function at the site of inflammation.
At high concentrations, colchicine disrupts microtubules by blocking polymerization at the “plus” (growing) terminus,15whereas low concentrations inhibit tubulin exchange at microtubule ends without affecting the amount of polymer.16,17 Drug levels resulting from the usual doses in humans are not sufficient to cause a visible derangement of microtubules even in granulocytes, which accumulate colchicine, but are likely to affect microtubule dynamics and thereby inhibit leukocyte migration.18 For example, the number and distribution of cell surface adhesion molecules is regulated by microtubules, and even low doses of colchicine interfere with leukocyte adhesion by altering the number and distribution of selectins on endothelial cells and neutrophils.19 In contrast, corticosteroids, which have limited effectiveness in FMF, do not bind directly to microtubules; rather they modulate neutrophil migration through their effects on cytokines and their receptors,20-22 adhesion molecules,23-25 and annexin I–mediated pathways.26
Conceptual translation of the MEFV complementary DNA (cDNA) predicts that its product, pyrin, is a 781–amino acid protein with sequence similarities to a number of proteins of apparently disparate functions and cellular localizations. Pyrin can be divided into at least 4 domains: N-terminal, B-box zinc-finger, coiled-coil, and B30.2 (or rfp), arranged in N- to C-terminal order. In addition to its similarity to other known proteins, pyrin contains 2 potential nuclear localization motifs.1 Among the proteins that have similarity with pyrin, several are known to be transcriptional regulators, and this fact together with the presence of nuclear localization signals led to speculation that pyrin may itself be a transcriptional regulator.1,3,27 Recently, the potential for an alternately spliced form of MEFV transcript to code for a nuclear factor has been demonstrated,28 although this transcript form appears to be very rare compared to the predominant “full-length” form.
The recent rapid expansion of gene sequences in the databases has revealed proteins similar to pyrin over one or more domains that reside in nearly every major subcellular location.29-34 In the present study we assessed the subcellular location of recombinant full-length and truncated protein in transfected cells. Fluorescence microscopy demonstrated colocalization of full-length pyrin with microtubules, but failed to support the previously hypothesized nuclear or Golgi localization.35 36 Deletion constructs indicated that the N-terminal portion of pyrin binds microtubules, and, consistent with this finding, FMF-associated mutations in the C-terminal B30.2 domain did not affect binding. Moreover, using a newly developed antipeptide antibody that detects immunoblotted pyrin, we found that recombinant pyrin binds microtubules in vitro. Finally, in phalloidin-stained cells we observed a colocalization of pyrin with actin filaments in the lamellar edges of cells. These data are consistent with the hypothesis that pyrin regulates inflammation by interacting with the cytoskeleton in granulocytes and monocytes. This interaction is likely to be downstream of the anti-inflammatory action of glucocorticoids and sensitive to the effects of colchicine on microtubule dynamics.
Materials and methods
Cell lines and reagents
Cos-7 and HeLa cells were from the American Type Culture Collection (Bethesda, MD). Bovine brain tubulin, paclitaxel, anti–golgin-97, DAPI, TO-PRO-3, Alexa Fluor 488 anti–mouse IgG, and Alexa Fluor 568 phalloidin were from Molecular Probes (Eugene, OR). Complete protease inhibitor tablets, FuGene 6, monoclonal anti–β-tubulin, anti–mouse IgG-rhodamine, and anti–rabbit IgG–horseradish peroxidase were from Roche (Indianapolis, IN). Colchicine, guanosine triphosphate (GTP), monoclonal anti-myc antibody 9E10, Cy3- conjugated monoclonal anti–β-tubulin, anti–mouse IgG–Cy3 Fab2, and other laboratory reagents were from Sigma (St Louis, MO). Cytochalasin D was from Calbiochem (San Diego, CA). Tissue culture reagents were from Biofluids. Lipofectamine was from Life Technologies (Grand Island, NY).
Plasmids
The GFP-tagged pyrin constructs were prepared from polymerase chain reaction (PCR) fragments amplified from pv75-1,1 and pEGFP-N and pEGFP-C vectors. Primers were (N-term GFP): GXF (forward): 5′-GCCTCGAGCGCTAAGACCCCTAGTGACC-3′; and QHR (reverse): 5′-CCTAAGCTTCAGTCAGGCCCCTGACCACCC-3′; and (C-term GFP): GXF2 (forward) 5′-CTGGCTCGAGCCTCTCCTGCTCAGC-3′; and GRR (reverse) 5′-CGTCGAATTCGTCAGGCCCCTGAC-3′. Plasmids were sequenced to select clones with the desired sequences. Ligation ofMEFV-containing insert and pEGFP plasmids yielded pEGFP-MEFV.C (C-term GFP) and pEGFP-MEFV.N (N-term GFP). TheXhoI/EcoRI fragment containing MEFVfrom pEGFP-C was isolated and ligated toXhoI/EcoRI-digested pcDNA3.1/Myc-His(−) plasmid to produce pyrin fused in-frame with the c-myc epitope plus the 6Xhis tag at the C-terminus.
Antipyrinantibodies
Antibodies recognizing pyrin protein were raised by immunization of rabbits with the MAP peptide KSGEEQRSYGEEKAVSF (single-letter amino acid codes; amino acids 435-450) (Research Genetics, Huntsville, AL). Pyrin-specific antibodies were purified from immune serum by affinity chromatography, using full-length pyrin coupled to Affi-Gel 10 support (Biorad, Hercules, CA). Antibodies were demonstrated to be specific by immunoblot against pyrin-containing lysates and by competition for binding with free recombinant pyrin.
Immunofluorescencemicroscopy
Cos-7 or HeLa cells on chamber slides were transfected with plasmids and Lipofectamine or FuGene 6, and incubated 24 hours for expression of recombinant proteins. Slides were fixed in 4% formaldehyde/phosphate-buffered saline (PBS; GFP) for 30 minutes, or 4% paraformadehyde in cytoskeleton buffer (10 mM MES, pH 6.1, 138 mM KCl, 3 mM MgCl2, 2 mM EDTA, 0.32 M sucrose) for 20 minutes (microtubules, actin), or in −20°C methanol (microtubules) for 3 minutes. For removal of free cytoplasmic proteins, slides were first incubated 30 seconds in Brinkley buffer (80 mM PIPES, pH 6.8, 1 mM MgCl2, 5 mM EGTA) + 0.5% Triton X-100, then methanol-fixed. Slides were blocked (30 minutes) and immunostained (1 hour) in 1% bovine serum albumin (BSA), 10% goat serum, and PBS, at room temperature. Nuclei were stained with DAPI or TO-PRO-3. For paclitaxel treatment, cells were chilled to 0°C for 30 minutes, then paclitaxel was added to 1 to 2 μM, and slides were returned to 37°C for 4 hours. For colchicine treatment, colchicine was added to 10 μM and slides were incubated a further 2 or 4 hours before fixing. Brefeldin A treatment was for 2 hours at 1 μM. Cytochalsin D treatment was performed at 1 or 10 μM for 1 to 10 minutes at 37°C to yield varying degrees of actin cytoskeleton collapse.
Confocal microscopy and image analysis
Cells transfected with myc-tagged-pyrin plasmid and labeled with anti-myc, or transfected with pEGFP.MEFV.C plasmid plus phalloidin Alexa Fluor 568 stain, and with the nuclear counterstain TO-PRO-3 were imaged with a Zeiss LSM 410 confocal laser scanning microscope through a 63 ×/1.4 Plan-Apochromat objective. Pinholes were set for a nominal axial resolution of about 700 nm. Eighteen optical sections, averaged twice, were acquired with a spacing of 350 nm along the z-axis. Confocal image stacks were analyzed with the 3-dimensional visualization package Imaris from Bitplane AG (Zurich, Switzerland). Nuclear versus cytoplasmic localization of pyrin was examined in sections along the xz-plane through the cell nucleus. Colocalization of GFP-pyrin and actin was analyzed in confocal image stacks with the software package Colocalisation (Bitplane AG). Colocalized pixels were selected in a 2-dimensional histogram plot generated from the 2-color channels in the confocal image stack and marked as a yellow overlay mask in each section of the image stack.
Preparation of pyrin-containing lysates
Cos-7 cells were transfected using recombinant plasmids and Lipofectamine reagent and incubated for 24 hours. Plates were rinsed twice in cold PBS, then cells were scraped into 1 mL cold assembly buffer (0.1 M PIPES, pH 6.8, 1 mM MgSO4, 2 mM EGTA, 2 mM DTT, 0.1 mM GTP, containing 1 × Complete protease inhibitor solution). Suspended cells were dounced on ice 40 times, then the lysate was centrifuged for 45 minutes at 20 800g at 4°C. Supernatants were aliquoted and frozen at −80°C.
Purification of pyrin from cell lysates
Cos-7 cells were transfected with pcDNA3.1-pyrin-myc/his plasmid using Lipofectamine. After 24 hours, cells were scraped into 50 mM HEPES, pH 7, 300 mM NaCl, 0.5% Triton X-100 plus Complete protease inhibitor without EDTA. Lysates were incubated on ice for 20 minutes, then centrifuged at 20 800g at 4°C. Supernatants were recovered and stored at −80°C. Thawed supernatants were pooled and passed over a Talon affinity resin column (Clontech, Palo Alto, CA) 5 times to allow binding of tagged protein. The column was washed with lysis buffer plus 5 mM imidazole to remove nonspecifically bound proteins, then bound proteins were eluted in lysis buffer plus 150 mM imidazole. Eluted protein purity was assessed by Coomassie staining and immunoblotting with antipyrin antibody.
Microtubulecoprecipitation
Coprecipitation was performed essentially as described.37 Microtubules were polymerized by incubation of 5 mg/mL tubulin in GPEM buffer (80 mM PIPES, pH 6.8, 1 mM MgCl2, 1 mM EGTA, 1 mM GTP), containing 30% glycerol, for 10 minutes at 35°C, followed by addition of paclitaxel to 10 μM. Purified pyrin or lysates containing wild-type or mutant forms of pyrin were made to 1 mM GTP and 10 μM paclitaxel, then added to stabilized microtubules and incubated at 37°C for 30 minutes. Microtubules were pelleted by spinning at 167 000g for 30 minutes at room temperature in an Airfuge (Beckman Coulter, Miami, FL). Pellets were rinsed with GPEM buffer, then dissolved in 2 × sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer.
Results
Pyrin localizes to the cytoplasm but not the nucleus
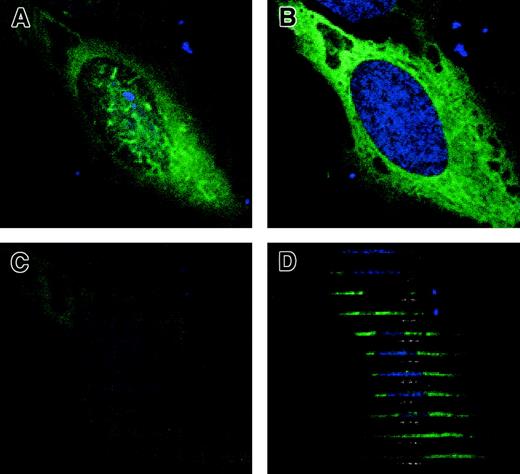
Expression plasmids were constructed with pyrin fused to GFP and to the myc epitope. HeLa cells transfected with myc-tagged pyrin were stained with monoclonal anti-myc 9E10/anti–mouse IgG–Alexa Fluor 488 antibody and counterstained with TO-PRO-3 nuclear stain. Transfected cells showed staining of pyrin-myc over the entire cytoplasm. In some cells, there was scattered wavy staining over the nucleus in a superficial focal plane (Figure 1A), but this staining was not present in deeper focal planes (Figure 1B,C). Z-sections derived from confocal image stacks (Figure 1D) showed that this fluorescence was from pyrin-myc that lies outside the nuclear membrane, rather than from proteins within the nucleus itself. No nuclear staining could be detected in any cells examined. The secondary antibody alone showed no reactivity with the fixed cells.
Cytoplasmic localization of full-length pyrin in HeLa cells by confocal microscopy.
HeLa cells transfected with myc-tagged pyrin plasmid were labeled with anti-myc antibody followed by anti–mouse IgG Alex Fluor 488 (green), plus TO-PRO-3 nuclear dye (blue). (A) Proximal XY-plane (0 μM) of immunofluorescence of myc-tagged pyrin. (B) Median XY-plane (−1.05 μm). (C) Distal XY-plane (−2.1 μm). (D) Computer-generated XZ-sections of the same cell. Sections progress from top to bottom of XY-plane images. Magnification × 1000.
Cytoplasmic localization of full-length pyrin in HeLa cells by confocal microscopy.
HeLa cells transfected with myc-tagged pyrin plasmid were labeled with anti-myc antibody followed by anti–mouse IgG Alex Fluor 488 (green), plus TO-PRO-3 nuclear dye (blue). (A) Proximal XY-plane (0 μM) of immunofluorescence of myc-tagged pyrin. (B) Median XY-plane (−1.05 μm). (C) Distal XY-plane (−2.1 μm). (D) Computer-generated XZ-sections of the same cell. Sections progress from top to bottom of XY-plane images. Magnification × 1000.
Similar experiments using epifluorescence microscopy were carried out using cells transfected with GFP-pyrin in which GFP was fused to the N- and C-terminus of pyrin to control for a position-related effect of GFP on localization. Regardless of the position of GFP relative to pyrin, the GFP signal was confined to the cytoplasm of transfected cells. In most of the cells expressing GFP-pyrin fusion or pyrin-myc, a primarily diffuse signal across the entire cytoplasm was detected, although in highly expressing cells, bright punctate patches or perinuclear aggregates were present. Stimulation with 4β-phorbol 12-myristate 13-acetate or pervanadate did not induce translocation of pyrin to the nucleus; control transfections in which cells expressed only GFP showed diffuse GFP signal in both cytoplasm and nucleus (data not shown). Transfection of U937 (histiocytic) and THP-1 (promonocytic) cells also showed GFP signal only in the cytoplasm (data not shown).
Pyrin associates with microtubules in vivo
On careful examination of cells expressing GFP-pyrin fusions, it was noted that some cells exhibited reticular patterns of GFP staining consistent with microtubules (Figure 2A), above the strong diffuse cytoplasmic fluorescence signal. This effect was not observed in cells transfected with GFP-fused proteins that are normally localized in the Golgi or endoplasmic reticulum (not shown). Counterstaining of GFP-pyrin–transfected cells with antitubulin antibodies plus rhodamine-conjugated secondary antibody confirmed the colocalization of the reticular structures with cytoplasmic microtubules (Figure 2B,C). The secondary antibody alone did not cross-react with any cellular component. Pyrin-microtubule colocalization was more readily visualized in cells preincubated in Triton X-100 in a microtubule-stabilizing buffer (which does not disrupt cytoskeletal scaffolds), by reducing the diffuse cytoplasmic staining background (not shown). In a minority of cells, intense areas of GFP signal were also seen in a juxtanuclear location (Figure 2D), consistent with Golgi or microtubule organizing centers (MTOC); detergent-treated cells retained this pattern of staining.
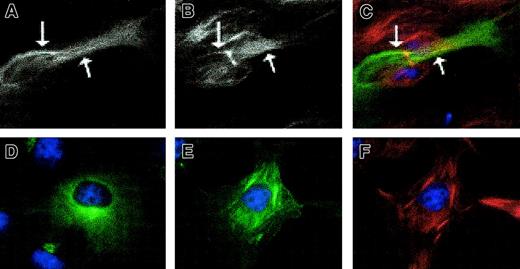
GFP-pyrin localizes with microtubules in untreated and paclitaxel-treated cells.
HeLa cells were transfected with pEGFP-MEFV.N plasmid and immunostained with anti–β-tubulin and Cy3 conjugated goat anti–mouse IgG. (A) A transfected cell without paclitaxel treatment has a filamentlike GFP pattern. (B) Microtubule staining of the same cell. (C) Merge of GFP and microtubule staining demonstrating colocalization of GFP and microtubule staining. Arrows indicate regions where staining overlap is particularly noticeable. (D) A transfected cell with strong perinuclear GFP signal. (E) GFP-pyrin and (F) microtubule staining of a paclitaxel-treated transfected cell in which colocalization is extensive. Magnification × 1000.
GFP-pyrin localizes with microtubules in untreated and paclitaxel-treated cells.
HeLa cells were transfected with pEGFP-MEFV.N plasmid and immunostained with anti–β-tubulin and Cy3 conjugated goat anti–mouse IgG. (A) A transfected cell without paclitaxel treatment has a filamentlike GFP pattern. (B) Microtubule staining of the same cell. (C) Merge of GFP and microtubule staining demonstrating colocalization of GFP and microtubule staining. Arrows indicate regions where staining overlap is particularly noticeable. (D) A transfected cell with strong perinuclear GFP signal. (E) GFP-pyrin and (F) microtubule staining of a paclitaxel-treated transfected cell in which colocalization is extensive. Magnification × 1000.
To further examine the possible association of pyrin with microtubules, localization of GFP-tagged pyrin and β-tubulin was examined in transfected cells in the presence of the microtubule-stabilizing drug paclitaxel. Cells that have been chilled to 4°C and then rewarmed in the presence of paclitaxel form brushlike or bundled microtubules rather than the classic radial morphology usually seen in untreated cells. When GFP-pyrin–transfected cells were treated with cold and paclitaxel, a dramatic colocalization of GFP-pyrin with the brushlike microtubules was seen (Figure 2E,F). This did not appear to be a result of the expression level of GFP-pyrin, because a similar construct in which the N-terminus of pyrin was deleted (see below) was expressed at nearly identical levels, as assessed by flow cytometry, but did not exhibit any colocalization with microtubules. Pyrin colocalization with microtubules was usually greatest near the nucleus, but many transfected cells showed GFP signal on all visible microtubules. As a control for the possibility of “bleedover” of fluorescence signals, examination of paclitaxel-treated cells expressing GFP-pyrin or stained for myc-tagged pyrin showed the same patterns regardless of counterstaining with anti–β-tubulin.
Pyrin is not an intrinsic Golgi protein
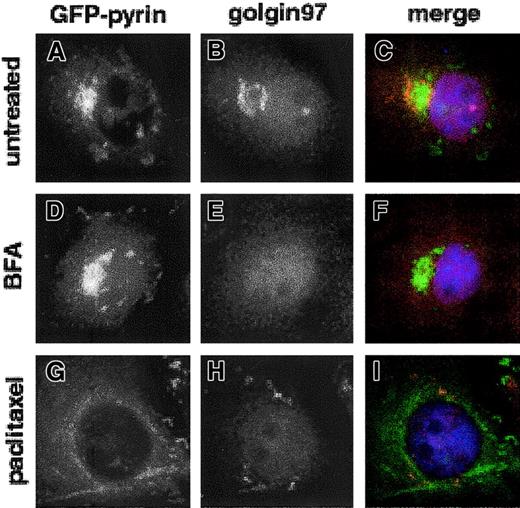
Two previous reports of the localization of GFP-pyrin in transfected cells have emphasized the aforementioned perinuclear aggregates, suggesting that pyrin may reside in the Golgi apparatus. To further investigate this possibility, we counterstained both Cos-7 and HeLa cells expressing GFP-pyrin with an antibody specific for the Golgi apparatus, anti–golgin-97.38 Two-color immunofluorescence microscopy in Cos-7 demonstrated that although some cells that expressed GFP-pyrin did contain perinuclear accumulations of tagged protein, these did not overlap the Golgi staining of anti–golgin-97 antibody, and in some cases, GFP-pyrin staining was closely juxtaposed to the Golgi in a manner suggesting that Golgi excludes pyrin (Figure3A-C). A substantial fraction of GFP-pyrin–expressing cells did not exhibit this perinuclear type of fluorescence pattern, and in no case could we identify any colocalization of GFP-pyrin with anti–golgin-97. Perinuclear staining was rarely observed in transfected HeLa cells (< 5%) and was more commonly seen in Cos-7 cells, which highly overexpress recombinant proteins.
Pyrin and anti–golgin-97 labeling do not overlap in transfected Cos-7 cells.
Cos-7 cells were transfected with pEGFP-MEFV.N plasmid and Golgi was labeled with rabbit anti–golgin-97 serum, followed by Cy3-conjugated donkey anti–rabbit IgG. Nuclei were stained with DAPI. Cells were examined by epifluorescence microscopy. (A,D,G) GFP-pyrin and (B,E,H) anti–golgin-97 staining in untreated, BFA-treated, and paclitaxel-treated cells, respectively. (C,F,I) Merged anti–golgin-97 and GFP-pyrin fluorescence images. Magnification × 1000.
Pyrin and anti–golgin-97 labeling do not overlap in transfected Cos-7 cells.
Cos-7 cells were transfected with pEGFP-MEFV.N plasmid and Golgi was labeled with rabbit anti–golgin-97 serum, followed by Cy3-conjugated donkey anti–rabbit IgG. Nuclei were stained with DAPI. Cells were examined by epifluorescence microscopy. (A,D,G) GFP-pyrin and (B,E,H) anti–golgin-97 staining in untreated, BFA-treated, and paclitaxel-treated cells, respectively. (C,F,I) Merged anti–golgin-97 and GFP-pyrin fluorescence images. Magnification × 1000.
To further examine the relationship between GFP-pyrin and the Golgi, we treated GFP-pyrin–transfected Cos-7 cells with brefeldin A, which causes dispersal of Golgi.39 This treatment did not affect the appearance of GFP-pyrin fluorescence, and cells with perinuclear aggregations of GFP-pyrin were still apparent, although Golgi staining was completely dispersed (Figure 3D-F). Incubation of transfected cells with paclitaxel and colchicine, which stabilize and disrupt cellular microtubule arrays, respectively, also alters Golgi conformation by preventing normal trafficking of membrane vesicles between the endoplasmic reticulum and the Golgi. Although paclitaxel treatment resulted in GFP-pyrin assuming a microtubular pattern (Figure 3G), anti–golgin-97 highlighted the expected dispersal of Golgi membranes to more peripheral sites on paclitaxel treatment (Figure 3H). Colchicine treatment, which depolymerized microtubules, resulted in a diffuse pattern for anti–golgin-97 but did not affect GFP-pyrin staining (not shown).
Association of mutant pyrin with microtubules
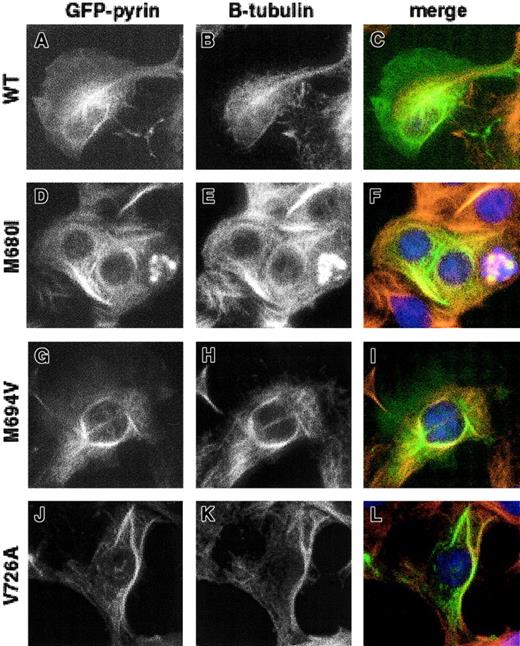
The ability of GFP-pyrin with FMF-associated mutations to bind to microtubules in paclitaxel-treated cells was also analyzed. Three of the most common FMF-associated mutations in MEFV involve missense mutations in the C-terminal B30.2 domain (M680I [2040G→C variant], M694V, and V726A). Expression of GFP-linked pyrin containing these mutations followed by paclitaxel treatment did not result in any detectable alteration of pyrin's ability to bind microtubules (Figure 4).
Effect of FMF-associated mutations on the ability of pyrin to localize with microtubules.
HeLa cells transfected with GFP-pyrin plasmid encoding 3 of the most common FMF mutations were treated with 10 μM paclitaxel before fixing. (A,D,G,J) GFP-pyrin; (B,E,H,K) staining with mouse anti–β-tubulin and Cy3-conjugated goat anti–mouse IgG; (C,F,I,L) merged images of panels A and B, D and E, G and H, and J and K, respectively. (A-C) Cells transfected with wild-type pyrin; (D-F) pyrin with M680I mutation; (G-I) pyrin with M694V mutation; (J-L) pyrin with V726A mutation. Magnification × 1000.
Effect of FMF-associated mutations on the ability of pyrin to localize with microtubules.
HeLa cells transfected with GFP-pyrin plasmid encoding 3 of the most common FMF mutations were treated with 10 μM paclitaxel before fixing. (A,D,G,J) GFP-pyrin; (B,E,H,K) staining with mouse anti–β-tubulin and Cy3-conjugated goat anti–mouse IgG; (C,F,I,L) merged images of panels A and B, D and E, G and H, and J and K, respectively. (A-C) Cells transfected with wild-type pyrin; (D-F) pyrin with M680I mutation; (G-I) pyrin with M694V mutation; (J-L) pyrin with V726A mutation. Magnification × 1000.
Pyrin binds to stablilized microtubules in vitro
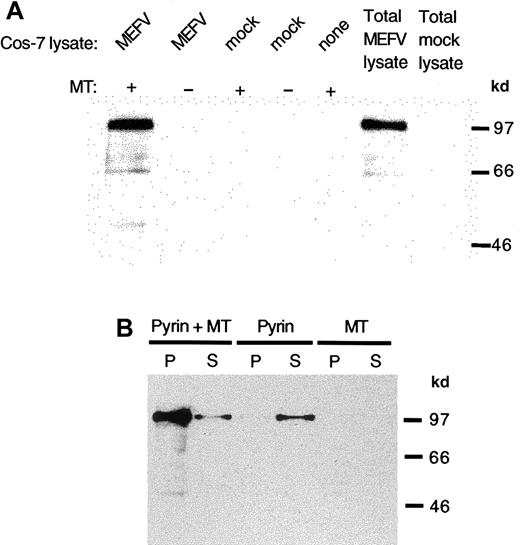
To further examine the association of pyrin with microtubules, the ability of pyrin to bind to purified, stabilized microtubules in vitro was examined. Bovine brain tubulin was polymerized in the presence of paclitaxel to form microtubules that were stable to disruption by temperature, Ca++ ions, or other depolymerizing agents. Aliquots of transfected cell lysates were mixed with the stabilized microtubules and the mixture was pelleted to sediment microtubules and any associated proteins. When pyrin-containing lysates were incubated with microtubules, and the resulting pellets analyzed by immunoblot, it was clear that pyrin could bind to microtubules in vitro, and that pyrin did not sediment to a significant degree in the absence of added microtubules (Figure 5A). The microtubule preparation itself did not contain any detectable pyrin.
Binding of pyrin to stabilized microtubules in vitro.
Lysates were prepared from MEFV cDNA or mock-transfected cells and added to bovine brain microtubules stabilized with paclitaxel. Pyrin was detected with antipyrin antibody and horseradish peroxidase–linked anti–rabbit IgG. (A) Pyrin in lysates of Cos-7 cells transfected with MEFV cDNA was sedimented in the presence of microtubules (lane 1) but not when microtubules were absent (lanes 2). Mock-transfected cell lysates did not show any pyrin sedimentation with or without microtubules (lanes 3 and 4). Neither microtubules nor mock-transfected cell lysate contain pyrin (lane 5 and 7). Lysate from cells transfected with MEFV cDNA contained pyrin (lane 6). (B) Purified recombinant pyrin cosedimented with paclitaxel-stabilized microtubules (lane 1) with some pyrin remaining in the supernatant (lane 2). In the absence of microtubules, purified pyrin did not sediment (lane 3) but remained in the supernatant (lane 4). The microtubule preparation did not contain pyrin in the pellet (lane 5) or in the supernatant (lane 6).
Binding of pyrin to stabilized microtubules in vitro.
Lysates were prepared from MEFV cDNA or mock-transfected cells and added to bovine brain microtubules stabilized with paclitaxel. Pyrin was detected with antipyrin antibody and horseradish peroxidase–linked anti–rabbit IgG. (A) Pyrin in lysates of Cos-7 cells transfected with MEFV cDNA was sedimented in the presence of microtubules (lane 1) but not when microtubules were absent (lanes 2). Mock-transfected cell lysates did not show any pyrin sedimentation with or without microtubules (lanes 3 and 4). Neither microtubules nor mock-transfected cell lysate contain pyrin (lane 5 and 7). Lysate from cells transfected with MEFV cDNA contained pyrin (lane 6). (B) Purified recombinant pyrin cosedimented with paclitaxel-stabilized microtubules (lane 1) with some pyrin remaining in the supernatant (lane 2). In the absence of microtubules, purified pyrin did not sediment (lane 3) but remained in the supernatant (lane 4). The microtubule preparation did not contain pyrin in the pellet (lane 5) or in the supernatant (lane 6).
By using purified recombinant pyrin, we examined whether the pyrin-microtubule interaction is direct or dependent on other interacting proteins. His-tagged pyrin recovered by affinity chromatography from cell lysates of transfected cells was mixed with stabilized microtubules in the absence of other cellular proteins. When the mixture was centrifuged, most of the added pyrin pelleted with microtubules (Figure 5B). When no microtubules were present, purified pyrin remained in the supernatant after centrifugation. As a further control, albumin, which does not sediment with microtubules, was used to compete with pyrin for binding to stabilized microtubules in vitro. No reduction in the level of pyrin bound to microtubules was evident in the presence of the highest amount (2 mg/mL) of albumin tested (not shown).
We also investigated whether FMF-associated mutations affect the ability of pyrin to bind microtubules. Cell lysates containing pyrin with the mutations M680I (2040G→C variant), M694V, and V726A were prepared and incubated with stabilized microtubules. Western blot analysis of the pelleted material demonstrated no difference in the ability of the mutated proteins to bind microtubules, compared to wild-type pyrin (data not shown).
Effect of wild-type and mutant pyrin on the stability of microtubules
To investigate whether pyrin has a stabilizing effect on microtubules, we tested the sensitivity of microtubules inMEFV-transfected cells to dissociation by colchicine. Cells transfected with GFP-pyrin and mutated forms of pyrin fused to GFP were treated with varying concentrations of colchicine to disrupt the microtubules. Counting of cells that both expressed GFP-pyrin and retained recognizable microtubules after treatment showed that there was no stabilizing activity of pyrin on microtubules, and that the mutated forms of pyrin did not impart any stabilizing or destabilizing qualities not found in wild-type pyrin (data not shown).
Deletion analysis of GFP-pyrin binding to microtubules in transfected cells
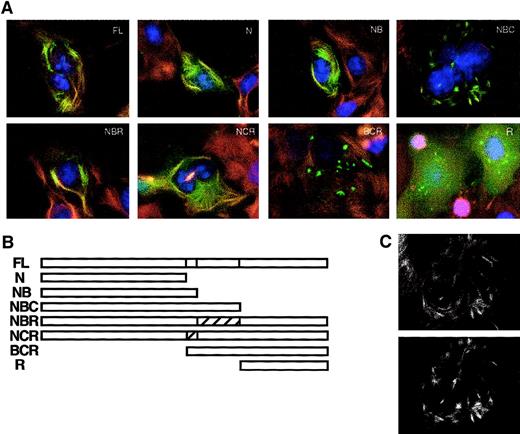
Pyrin can be divided conceptually into 4 major protein domains: the N-terminal domain (N), amino acids 1 to 374; the B-box zinc finger domain (B), amino acids 375 to 407; the coiled-coil domain (C), amino acids 408 to 576; and the B30.2 (rfp) domain (R), amino acids 577 to 781. We prepared expression constructs that encode pyrin deleted for one or more domains, with GFP fused at its N-terminus (Figure6B). Cos-7 cells transfected with these plasmids were examined by fluorescence microscopy for localization of the deleted pyrin-GFP fusions (Figure 6A). When transfected cells were treated with paclitaxel plus cold to promote formation of brushlike microtubule arrays, colocalization of N, NB, NBC, NBR, and NCR constructs with microtubules was easily detected. Conversely, the BCR and R constructs did not colocalize with microtubules. These data suggest that the region of pyrin important for microtubule association is in the N-terminal domain, comprising slightly less than half of the full-length protein. The B30.2 domain, which contains the large majority of disease-causing mutations, does not seem to be necessary or sufficient for microtubule association. This observation is consistent with the previous finding that 3 common mutations in FMF do not affect the ability of pyrin to bind to microtubules in vitro. Deletion of the B30.2 domain did result in a change in the distribution of GFP-labeled pyrin, relative to the other constructs containing the N-terminal domain (Figure 6A, NBC), but this pattern coincided with the distribution of microtubules in these cells (Figure 6C).
Analysis of the effects of pyrin domain deletions on colocalization with microtubules.
(A) Cos-7 cells were transfected with plasmids encoding GFP-pyrin deleted for one or more domains. Cells were treated with 10 μM paclitaxel before fixing. Microtubules were detected with mouse anti–β-tubulin antibody and rhodamine-labeled secondary antibody. FL indicates full-length pyrin; N, amino acids 1 to 374 of pyrin (N-terminal domain); NB, amino acids 1 to 407 of pyrin (N-terminal plus B-box zinc finger); NBC, amino acids 1 to 576 of pyrin (N-terminal, B-box zinc finger and coiled-coil); NBR, amino acids 1 to 407, 577 to 781 of pyrin (N-terminal, B-box zinc finger, RFP domain); NCR, amino acids 1 to 374, 408 to 781 of pyrin (N-terminal, coiled-coil, RFP); BCR, amino acids 374 to 781 of pyrin (B-box zinc finger, coiled-coil, RFP); R, amino acids 577 to 781 of pyrin (RFP domain). (B) Schematic of deletion/truncation series. (C) NBC construct with altered microtubule appearance shows colocalization of microtubules (upper panel) with GFP signal (lower panel). Magnification × 1000.
Analysis of the effects of pyrin domain deletions on colocalization with microtubules.
(A) Cos-7 cells were transfected with plasmids encoding GFP-pyrin deleted for one or more domains. Cells were treated with 10 μM paclitaxel before fixing. Microtubules were detected with mouse anti–β-tubulin antibody and rhodamine-labeled secondary antibody. FL indicates full-length pyrin; N, amino acids 1 to 374 of pyrin (N-terminal domain); NB, amino acids 1 to 407 of pyrin (N-terminal plus B-box zinc finger); NBC, amino acids 1 to 576 of pyrin (N-terminal, B-box zinc finger and coiled-coil); NBR, amino acids 1 to 407, 577 to 781 of pyrin (N-terminal, B-box zinc finger, RFP domain); NCR, amino acids 1 to 374, 408 to 781 of pyrin (N-terminal, coiled-coil, RFP); BCR, amino acids 374 to 781 of pyrin (B-box zinc finger, coiled-coil, RFP); R, amino acids 577 to 781 of pyrin (RFP domain). (B) Schematic of deletion/truncation series. (C) NBC construct with altered microtubule appearance shows colocalization of microtubules (upper panel) with GFP signal (lower panel). Magnification × 1000.
Colocalization of GFP-pyrin with filamentous actin in transfected cells
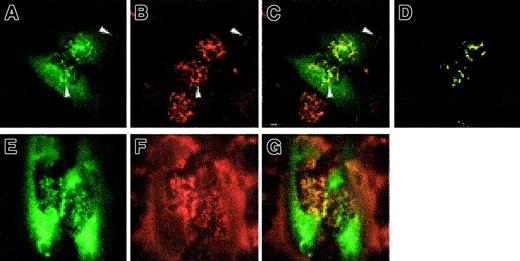
To determine whether pyrin might also colocalize with other cytoskeletal structures, we examined by confocal microscopy the localization of intracellular actin in GFP-pyrin–expressing cells by phalloidin staining of F-actin. In superficial focal planes overlying the nucleus, GFP-pyrin colocalized with the actin filaments in the lamellar ruffles on the dorsal surface of cells (Figure7A-D). Arrows in Figure 7, panels A-C, also highlight colocalization of pyrin and actin in the peripheral lamellae. No colocalization with actin stress fibers was detected, but overlap of GFP and actin signals could be detected at the lamellar edges of cells. As controls, transfected cells expressing GFP fused to Golgi or endoplasmic reticulum targeting sequences did not exhibit this pattern (not shown). Treatment of GFP-pyrin transfected cells with 1 μM cytochalasin D for 2 minutes caused partial disruption of the actin cytoskeleton. GFP-pyrin patterns closely mirrored the drug-induced actin distribution (Figure 7E,F), further supporting the observation of colocalization. In cells treated for longer periods or with higher concentrations of cytochalasin D to completely disrupt actin filaments, GFP-pyrin did not localize in a filamentous pattern (not shown). Analysis of deletion constructs (Figure 6B) did not demonstrate a clear association of actin colocalization with any specific domain of pyrin (data not shown).
Colocalization of pyrin with actin by confocal microscopy.
HeLa cells were transfected with pEGFP-MEFV.N plasmid. After fixing, cells were stained with phalloidin–Alexa Fluor 568. (A) GFP fluorescence showing staining of ruffles over the nucleus and lamellar structures (arrows). (B) Phalloidin staining of actin fibers. Shorter fibers are ruffles over the nucleus, and in lamellar structures (arrows). (C) Merged green and red channels with yellow signal at overlap. Arrows highlight overlap at nuclear and lamellar ruffles. (D) Colocalization mask computed using linear gating (see “Materials and methods”). (E-G) HeLa cells transfected with pEGFP-MEFV.N plasmid were treated with 10 μM cytochalasin D for 2 minutes, then fixed and stained with phalloidin–Alexa Fluor 568. GFP-pyrin (E) remains colocalized with the disintegrating actin scaffolding (F). Merged fluorescence images (G) demonstrate punctate colocalization overlying and surrounding the nuclear region. Magnification × 1000.
Colocalization of pyrin with actin by confocal microscopy.
HeLa cells were transfected with pEGFP-MEFV.N plasmid. After fixing, cells were stained with phalloidin–Alexa Fluor 568. (A) GFP fluorescence showing staining of ruffles over the nucleus and lamellar structures (arrows). (B) Phalloidin staining of actin fibers. Shorter fibers are ruffles over the nucleus, and in lamellar structures (arrows). (C) Merged green and red channels with yellow signal at overlap. Arrows highlight overlap at nuclear and lamellar ruffles. (D) Colocalization mask computed using linear gating (see “Materials and methods”). (E-G) HeLa cells transfected with pEGFP-MEFV.N plasmid were treated with 10 μM cytochalasin D for 2 minutes, then fixed and stained with phalloidin–Alexa Fluor 568. GFP-pyrin (E) remains colocalized with the disintegrating actin scaffolding (F). Merged fluorescence images (G) demonstrate punctate colocalization overlying and surrounding the nuclear region. Magnification × 1000.
Discussion
In this report, we present strong evidence that pyrin, the protein product of the gene for FMF (MEFV), colocalizes with both microtubules and the actin cytoskeleton, and is neither a nuclear protein nor an intrinsic Golgi protein. Fluorescence microscopy of cell lines transfected with GFP- or epitope-tagged pyrin clearly demonstrated cytoskeletal colocalization in vivo. This interaction was corroborated by experiments showing cosedimentation of paclitaxel-stabilized microtubule preparations with pyrin from cell lysates in vitro, and similar studies with purified recombinant pyrin showed that the pyrin-microtubule interaction is likely to be direct and not mediated through adaptor proteins. Finally, we found a more circumscribed pattern of colocalization of GFP-pyrin with F-actin, indicating that pyrin may be one of a group of proteins that interact with more than one cytoskeletal structure.
Based on more limited data, a number of other hypotheses regarding the likely site of pyrin action have been proposed. Prior to the positional cloning of MEFV, studies of serosal fluids from FMF patients suggested that MEFV would encode an extracellular serine protease that inhibits C5a, a powerful chemoattractant for neutrophils.40,41 To date, the gene encoding this inhibitory factor with serine protease activity has not been cloned; sequence analysis of the pyrin protein is not consistent with a serine protease enzyme. On cloning of MEFV, we and others hypothesized that the predicted protein product could be a transcriptional regulator, based on the presence of 2 overlapping nuclear-localization signals and the homology of the C-terminal half of pyrin with known transcription factors. However, it should be noted that nuclear localization signals are context dependent and that there are a number of pyrin homologs that have not been localized to the nucleus. The question of whether full-length pyrin can be found in the nucleus has now been experimentally addressed in 3 separate studies,35 36 including the analysis of perinuclear staining by confocal microscopy shown in Figure 1 of this report; in no case has full-length pyrin been found in the nucleus. Moreover, although the possibility remains that pyrin might translocate to the nucleus on cellular activation, we have not observed nuclear pyrin in transfected cell lines stimulated with 4β-phorbol 12-myristate 13-acetate or pervanadate.
Two recent reports have suggested that pyrin may be a part of the Golgi apparatus, based on the perinuclear distribution of GFP-pyrin staining in some cells35,36 and the tentative identification of an interacting protein by yeast 2-hybrid assay using the B30.2 domain of pyrin as bait.36 Neither of these studies examined colocalization of GFP-pyrin with specific Golgi markers, such as golgin-97, and neither examined the effects of pharmacologic agents that disrupt the Golgi apparatus. Although our own data indicate that pyrin does not meet these more stringent criteria, it does appear that in some cell types pyrin can aggregate in the region nearby the Golgi apparatus (Figure 3). In fact, the pyrin-interactor identified through the yeast 2-hybrid assay, a splice variant of GTC-90,42can be found in both the cytosolic and (Golgi) membrane fractions, and acts in a 13S hetero-oligomeric protein complex that stimulates in vitro Golgi transport. It is possible that pyrin associated with perinuclear cytoskeletal structures does bind to cytosolic GTC-90 in the vicinity of the Golgi apparatus, perhaps participating in the known interaction between the Golgi apparatus and the cytoskeleton in vesicle trafficking.43
Despite the fact that the colchicine responsiveness of FMF suggests a pyrin-microtubule association, this was not recognized sooner primarily for technical reasons. By fluorescence microscopy, this interaction is best visualized in transfected cells that have been treated with mild detergents to release overexpressed GFP-pyrin that obscures the cytoskeletal pattern of staining, or by stabilizing microtubules with paclitaxel. Similarly, the colocalization of pyrin with F-actin was only apparent in cells stained with phalloidin, partly due to the lack of interaction with actin stress fibers. Several other actin-associated proteins, such as Arp 2 and 3 and p21,44 that are important for proper organization of the actin cytoskeleton may, like pyrin, be found colocalized with cortical and perinuclear actin structures, and do not colocalize with actin stress fibers.
The present report therefore places FMF on a growing list of human diseases caused by altered cytoskeleton-associated proteins.33,34,45-52MEFV is highly expressed in polymorphonuclear leukocytes and activated monocytes,1,3 and microtubules have been implicated in nearly every important effector function of myelomonocytic cells, including adhesion,19 migration,53phagocytosis,54,55 and degranulation.56 57Moreover, a growing number of proteins belonging to signal-transducing pathways, including Hedgehog, Wnt, JNK, and ERK, have also been found to associate with microtubules (reviewed in reference 58). It is therefore possible that pyrin could regulate any of these processes at the level of the cytoskeleton, although signaling, adhesion, and migration would seem particularly relevant in light of the fact that colchicine is most effective early in the attacks of FMF.
Evidence that pyrin not only interacts with microtubules, but possibly also with the actin cytoskeleton, is consistent with a role for pyrin in directed migration. This process is probably achieved by capture of microtubule ends at focal adhesion points, which are also cortical actin foci, by microtubule-associated proteins that bridge the microtubule and actin cytoskeletons.59 Microtubule and F-actin growth occurs at the leading edge of the cell, coupled with retrograde flow of microtubules mediated by interaction with F-actin. The Wiskott-Aldrich syndrome protein has recently been shown to control the formation of macrophage podosomes, dynamic actin-containing adhesion structures important in migration,60 and to bind indirectly to microtubules, which may be involved in the delivery of components to the sites where podosomes form.52
The exquisite sensitivity of FMF to colchicine, and the relative ineffectiveness of a number of other anti-inflammatory agents, is consistent with the microtubule association of pyrin reported here, but does not distinguish among the possible mechanisms by which pyrin could regulate inflammation at the cytoskeleton. Early studies of the mechanism of colchicine demonstrated inhibitory effects on phagocytosis, degranulation, adhesion, and both random and directed migration (reviewed in reference 61), all of which were related to the effects of this agent on microtubule organization and dynamics. More recently, colchicine has been found to inhibit tyrosine phosphorylation in neutrophils induced by urate crystals, again in a microtubule-dependent fashion.62 Such an effect of colchicine on granulocyte signal transduction may explain the synergistic effect of colchicine and IFN-α on the induction ofMEFV expression.3 Although the accumulation of colchicine in neutrophils undoubtedly contributes to the efficacy of this agent in FMF, it is likely that additional mechanisms pertain that are dependent on the interaction of pyrin with microtubules.
Our data argue strongly that the N-terminal half of pyrin is critical for a direct association of pyrin with microtubules. This domain is relatively unique, although the first 92 amino acids encode the recently recognized “pyrin domain” (PYD) or pyrinlike motif (PLM),63-65 of unknown function. The C-terminal B30.2 domain, which harbors the FMF-associated M680I, M694V, and V726A mutations (Figure 4), is not involved in binding to microtubules, presumably subserving another as yet unknown biologic function. Disturbance of the normal localization of pyrin by colchicine prophylaxis might displace the mutation-containing domains away from their normal milieu, thus counteracting the tendency of the mutated protein to promote inflammation.
By establishing the interaction of pyrin with microtubules and its colocalization with actin filaments, the present report underscores the importance of the cytoskeleton in health and disease. Recent data showing a heightened inflammatory response in FMF carriers,66-68 and demonstrating an effect of FMF mutations on the susceptibility to other inflammatory disorders, emphasize the importance of this pathway. Nevertheless, a number of new questions regarding the biochemistry and cell biology of pyrin remain, not the least of which is the identification of additional cytoskeletal proteins that interact with pyrin. Elucidation of this inflammatory pathway will be desirable not only for its scientific importance, but also because the leukocyte cytoskeleton may provide important new targets for rational drug design in a broad range of inflammatory diseases.
The authors thank Amalia Dutra and Jeffrey Kopp for help with microscopy, Juan Bonifacino for helpful insights about the cytoskeleton, and Massimo Gadina and John J. O'Shea for useful discussions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elizabeth Mansfield, NIH-NIAMS, Bldg 10, Rm 9N210, 10 Center Dr, MSC-1820, Bethesda, MD 20892-1820; e-mail:mansfiee@arb.niams.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal