Abstract
Engagement of platelet membrane glycoprotein (GP) Ib-IX-V by von Willebrand factor triggers Ca++-dependent activation of αIIbβ3, resulting in (patho)physiological thrombus formation. It is demonstrated here that the cytoplasmic domain of GPIb-IX-V associates with cytosolic calmodulin. First, an anti-GPIbα antibody coimmunoprecipitated GPIb-IX and calmodulin from platelet lysates. Following platelet stimulation, calmodulin dissociated from GPIb-IX and, like the GPIb-IX–associated proteins 14-3-3ζ and p85, redistributed to the activated cytoskeleton. Second, a synthetic peptide based on the cytoplasmic sequence of GPIbβ, R149–L167 (single-letter amino acid codes), affinity-isolated calmodulin from platelet cytosol in the presence of Ca++ as confirmed by comigration with bovine calmodulin on sodium dodecyl sulfate–polyacrylamide gels, by sequence analysis, and by immunoreactivity with the use of an anticalmodulin antibody. The membrane-proximal GPIbβ sequence was analogous to a previously reported calmodulin-binding sequence in the leukocyte adhesion receptor, L-selectin. In addition, the cytoplasmic sequence of GPV, K529–G544, was analogous to a calmodulin-binding IQ motif within the α1c subunit of L-type Ca++ channels. Calmodulin coimmunoprecipitated with GPV from resting platelet lysates, but was dissociated in stimulated platelets. A GPV-related synthetic peptide also bound calmodulin and induced a Ca++-dependent shift on nondenaturing gels. Together, these results suggest separate regions of GPIb-IX-V can directly bind calmodulin, and this novel interaction potentially regulates aspects of GPIb-IX-V–dependent platelet activation.
Introduction
The platelet membrane glycoprotein (GP) Ib-IX-V complex binds to the adhesive ligand von Willebrand factor (vWF), an interaction that initiates platelet adhesion and aggregation at high-shear stress.1-4 Adhered platelets become activated, with elevation of cytosolic Ca++ and triggering of signaling pathways, leading to cytoskeletal rearrangements and activation of αIIbβ3 integrin (GPIIb-IIIa), which binds to fibrinogen or vWF, resulting in platelet aggregation.5-7The pathway by which ligand binding to GPIb-IX-V leads to αIIbβ3 activation and thrombus formation is poorly understood, although delineation of early signaling events mediated by GPIb-IX-V offers potential targets for therapeutic intervention.
The GPIb-IX-V complex consists of 4 membrane-spanning glycoproteins. GPIbα is disulfide-linked to GPIbβ, forming GPIb, which is noncovalently associated with GPIX in a 1:1 complex. GPIb-IX-V is composed of GPIb-IX and GPV in a 2:1 ratio.3 In extracts of platelets in the presence of Triton X-100 detergent, GPIb-IX remains a complex, while GPV is dissociated.8,9 The extracellular, N-terminal 282 residues of GPIbα contain the vWF binding site.3 10
Recent studies have identified signaling molecules associated with the cytoplasmic domain of GPIb-IX-V that appear to be important for the function of the receptor. One of these molecules is 14-3-3ζ, which directly binds to one or more sequences within the cytoplasmic domain of GPIb-IX-V.11-15 This interaction was implicated in the pathway leading from engagement of GPIb-IX-V to activation of αIIbβ3 in a model system that involved expression of both receptors in Chinese hamster ovary cells.5 Further studies have shown coassociation of the GPIb-IX–14-3-3 complex with the p85 subunit of phosphatidylinositol 3-kinase (PI 3-kinase).16The rapid translocation of these signaling proteins to the cytoskeleton following platelet activation indicated a potential role in formation of 3-position phosphoinositide-signaling molecules in this subcellular compartment.
In this study, we demonstrate that like 14-3-3ζ and p85, calmodulin is redistributed to the activated cytoskeleton following platelet stimulation. In addition, we show the novel association of calmodulin with GPIb-IX and GPV. We further demonstrate that synthetic peptides based on discrete cytoplasmic sequences of GPIbβ (R149–L167 [single-letter amino acid codes]) and GPV (K529–G544 [single-letter amino acid codes]) support calmodulin binding through conserved calmodulin-recognition motifs. The interaction of the GPIb-IX-V complex with cytosolic calmodulin may be an important component in regulating GPIb-IX-V–dependent adhesion, actin polymerization, and/or signaling.
Materials and methods
Bovine serum albumin (BSA), bovine calmodulin, prostaglandin E1 (PGE1), phenylmethylsulfonyl fluoride (PMSF), and leupeptin were purchased from Sigma (St Louis, MO).N-ethylmaleimide (NEM) was purchased from Calbiochem (San Diego, CA). Synthetic peptides based on cytoplasmic sequences of GPIbβ17 or GPV,18 in some cases containing an N-terminal cysteine to facilitate coupling, were purified by reverse-phase high-pressure liquid chromatography (HPLC) and characterized by mass spectroscopy (Chiron Mimotopes) (Clayton, Victoria, Australia). A control peptide, CLKKLIRSPSIPHQY (single-letter amino acid codes), was obtained from the same supplier.
Antibodies
Rabbit polyclonal immunoglobulin G (IgG) against the soluble extracellular portion of GPIbα (glycocalicin) was raised and purified as previously described.8 19 A rabbit polyclonal antibody against human GPV was the kind gift of Dr David Phillips (COR Therapeutics, San Francisco, CA). Rabbit polyclonal IgG against human actin-binding protein was provided by Dr Dominic Chung (University of Washington, Seattle). Rabbit polyclonal antisera against the p85 subunit of PI 3-kinase and mouse monoclonal anticalmodulin IgG were obtained from Upstate Biotechnology (Lake Placid, NY).
Preparation of washed platelets and platelet subcellular fractions
Platelets were obtained from healthy volunteers and washed by means of a previously described method.19,20 Washed platelets (5 × 108/mL) were either left unstimulated or stimulated with 1 U/mL thrombin for 5 minutes at room temperature. Following thrombin activation, platelets were lysed with 1 vol Triton X-100 lysis buffer (200 mM Tris-HCl, pH 7.4, 10% Triton X-100, 50 mM ethyleneglycotetraacetic acid [EGTA], 4 mM leupeptin, 4 mM aprotinin, 2.5 mg/mL PMSF) to 9 vol platelets and rocked at 4°C for 1 hour. Under these conditions, GPV dissociates from GPIb-IX.8,9Lysates were centrifuged at 15 400g for 10 minutes to separate the Triton X-100 soluble and insoluble (actin cytoskeletal) extracts, and the platelet subcellular fractions were isolated as previously described.19 20 The cytoskeletal pellet was solubilized by incubation with 2 × radioimmunoprecipitation-assay buffer (20 mM NaH2PO4, pH 7.0, 0.15 M NaCl, 2 mM EDTA, 2 mM PMSF, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) for 1 hour at 4°C, followed by centrifugation at 15 400g for 10 minutes.
Immunoprecipitation of platelet lysates
Immunoprecipitation from lysates with rabbit nonimmune IgG, anti-GPIbα (glycocalicin) IgG, or anti-GPV IgG was carried out as previously described.13,19 For some experiments, platelets were pretreated with a final concentration of either 3 nM PGE1 or 1 mM NEM prior to lysis and immunoprecipitation. For Western blotting, samples were electrophoresed on SDS–polyacrylamide gels under reducing conditions, electrotransferred to nitrocellulose, blocked with 5% skim milk powder in TS buffer (0.02 M Tris-HCl, 0.15 M NaCl, pH 7.4), and immunoblotted with anti-GPIbα, anti-p85, anti–actin-binding protein, or anticalmodulin antibodies, also as described elsewhere.13 19 Blots were visualized by means of the appropriate horseradish peroxidase–coupled antirabbit or antimouse second antibody (Silenus, Hawthorn, Australia), and the electrogenerated chemiluminescence (ECL) detection system (Amersham, Buckinghamshire, United Kingdom).
Preparation of peptide-affinity resins
Synthetic peptides corresponding to the GPIbβ sequence, R149–L167, the GPV sequence, K529–G544, or the control peptide CLKKLIRSPSIPHQY were coupled to BSA (0.25 mg peptide per 10 mg BSA) with m-maleimidobenzoyl-N-hydroxysuccinimide (Pierce, Rockford, IL) as previously described.13 The peptide-BSA conjugate or peptide alone was coupled to a 1:1 mixture of Affi-gel 10 and 15 (0.25 mg peptide per 5 mL resin, and 10 mg peptide-BSA per 5 mL resin, respectively) according to the manufacturer's instructions (Bio-Rad, Richmond, CA). Resins coupled to peptide and peptide-BSA were pooled and washed with TS buffer.
Peptide-affinity chromatography of platelet cytosol
Cytosol from 10 U day-old washed human platelets was prepared as previously described.8 The cytosol was dialyzed into TS buffer, CaCl2 added to give a final concentration of 1 mM, and loaded at 25 mL/h onto 10 × 1 cm GPIbβ-peptide, GPV-peptide, or control-peptide columns and washed with TS buffer containing 1 mM CaCl2. Bound protein was eluted by 10 mM EGTA in TS buffer, and fractions (2 mL) were analyzed on SDS–5%-to-20% polyacrylamide gels under reducing conditions as described elsewhere.13
Nondenaturing gel shift assay
The effect that a synthetic peptide based on the GPV sequence, K529–G544, had on the migration of bovine calmodulin was analyzed on nondenaturing gels as previously described.21-23Calmodulin (approximately 0.3 nmol) was mixed with 0 to 3.0 nmol GPV-related peptide or a control peptide (CLKKLIRSPSIPHQY) in 0.1 M Tris-HCl, pH 7.5, containing 4 M urea in the presence of either 1 mM Ca++ or 10 mM EGTA. After incubating for 30 minutes at 22°C, 0.5 vol 50% glycerol was added; samples were electrophoresed on 12.5% polyacrylamide gels containing 4 M urea at 50 mA for 3 hours; and gels were stained with Coomassie blue.
Amino acid sequence analysis
For sequence analysis, protein was dialyzed into distilled water and digested with trypsin (0.1 mg trypsin per milligram protein) overnight at 37°C. Tryptic fragments were separated by reverse-phase HPLC eluted by a linear 0% to 60% (vol/vol) acetonitrile gradient and sequenced as previously described.24
Results
Coprecipitation of calmodulin with GPIb-IX or GPV from platelet lysates
Prior to investigating whether the GPIb-IX-V complex bound calmodulin, platelet lysates were first immunoblotted with an anticalmodulin antibody. As shown in Figure1, calmodulin was present in the cytosolic fraction of both untreated and thrombin-stimulated platelets. Interestingly, activation of platelets by thrombin resulted in a redistribution of cytosolic calmodulin and markedly increased association with the actin cytoskeleton (Figure 1). For comparison, Figure 1 also shows the same samples immunoblotted with an antibody against the p85 subunit of PI 3-kinase, a protein previously demonstrated as becoming associated with the cytoskeleton following platelet activation.16 From the relative intensity of the blots and the protein loaded per lane (4.5-fold more in the nonstimulated cytosol compared with the stimulated cytoskeletal fraction), it may be estimated that up to 25% of total calmodulin could be translocated to the cytoskeleton following stimulation.
Translocation of platelet proteins to the activated cytoskeleton.
Platelets that were untreated or stimulated with α-thrombin (1 U/mL, final concentration) for 5 minutes at room temperature were lysed, and cytosolic and actin cytoskeletal (CYSK) fractions were isolated and immunoblotted with antibodies to p85, calmodulin, GPIbα, or GPV. Blots were visualized by means of a peroxidase-coupled second antibody and the ECL reagent. Results are typical of 3 separate experiments.
Translocation of platelet proteins to the activated cytoskeleton.
Platelets that were untreated or stimulated with α-thrombin (1 U/mL, final concentration) for 5 minutes at room temperature were lysed, and cytosolic and actin cytoskeletal (CYSK) fractions were isolated and immunoblotted with antibodies to p85, calmodulin, GPIbα, or GPV. Blots were visualized by means of a peroxidase-coupled second antibody and the ECL reagent. Results are typical of 3 separate experiments.
When platelets were extracted with Triton X-100, a condition in which GPV is dissociated from GPIb-IX,8,9 immunoprecipitation of GPIb-IX by an anti-GPIbα polyclonal IgG showed a specific coimmunoprecipitation of a 22-kd band as detected by Western blot analysis with an anticalmodulin antibody (Figure2A). Calmodulin was also coimmunoprecipitated by anti-GPV polyclonal IgG (Figure 2B). These experiments were carried out in the presence of EGTA, suggesting that the association of calmodulin with GPIb-IX or GPV could occur even in the absence of Ca++. Calmodulin specifically coimmunoprecipitated with GPIb-IX or GPV from the Triton X-100 soluble fraction of unstimulated platelets, but was drastically diminished in thrombin-stimulated platelets, suggesting that complexes of GPIb-IX or GPV and calmodulin were dissociated in activated platelets (Figure 2). Under these conditions, the amount of GPIb-IX or GPV in the platelet cytosol is essentially unchanged (Figure 1).16Interestingly, no association between calmodulin and either GPIb-IX or GPV was observed in the cytoskeletal fraction, suggesting that calmodulin's association with the actin cytoskeleton in activated platelets was independent of GPIb-IX or GPV (Figure 2, cf Figure 1) or that it dissociated from GPIb-IX and GPV owing to the treatment with cytoskeletal extraction buffer. Although we cannot exclude the latter possibility, both 14-3-3ζ and the p85 subunit of PI 3-kinase remain associated with GPIb-IX under these extraction conditions.16 Calmodulin was not immunoprecipitated from either fraction by nonimmune IgG (Figure 2). Together, these results are consistent with colocalization of calmodulin and GPIb-IX-V in resting platelets, rather than coprecipitation due to indirect association mediated by the cytoskeleton.
Coassociation of calmodulin and GPIb-IX-V in platelets.
Triton X-100 soluble and actin cytoskeletal (CYSK) fractions isolated from platelets either unstimulated or stimulated with thrombin for 5 minutes were immunoprecipitated (IP) by means of GPIb antibodies (A) or GPV antibodies (B) compared with nonimmune sera (NonI), and immunoblotted by means of antibodies to calmodulin. Blots were visualized by means of a peroxidase-coupled second antibody and the ECL reagent. Results are typical of 3 separate experiments.
Coassociation of calmodulin and GPIb-IX-V in platelets.
Triton X-100 soluble and actin cytoskeletal (CYSK) fractions isolated from platelets either unstimulated or stimulated with thrombin for 5 minutes were immunoprecipitated (IP) by means of GPIb antibodies (A) or GPV antibodies (B) compared with nonimmune sera (NonI), and immunoblotted by means of antibodies to calmodulin. Blots were visualized by means of a peroxidase-coupled second antibody and the ECL reagent. Results are typical of 3 separate experiments.
It has previously been shown that phosphorylation of GPIbβ at S166 enhances binding of 14-3-3ζ.13,15 Treatment of platelets with PGE1 results in protein kinase A (PKA)–dependent phosphorylation of platelet GPIbβ and enhanced 14-3-3ζ binding.15 25 We therefore assessed whether PGE1 treatment of platelets affected association of calmodulin. As shown in Figure 3A, the extent of calmodulin that coimmunoprecipitated with GPIb-IX from the Triton X-100 soluble fraction of platelet lysates was not significantly affected by PGE1 treatment, suggesting calmodulin association was not competing with 14-3-3ζ binding to GPIbβ.
Effect of PGE1 and NEM treatment on calmodulin association with GPIb-IX.
(A) Triton X-100 soluble fraction of platelets either untreated or treated with 3 nM PGE1 for 5 minutes at room temperature were immunoprecipitated by means of nonimmune sera (NonI) or GPIb antibodies and were immunoblotted by means of antibodies to calmodulin. (B) Triton X-100 soluble fractions of platelets either untreated or treated with 1 mM NEM for 5 minutes at room temperature were immunoprecipitated by means of nonimmune sera (NonI) or GPIb antibodies and were immunoblotted by means of antibodies to actin-binding protein (ABP, upper panel) or calmodulin (lower panel). Blots were visualized by means of a peroxidase-coupled second antibody and the ECL reagent. Results are representative of 3 separate experiments.
Effect of PGE1 and NEM treatment on calmodulin association with GPIb-IX.
(A) Triton X-100 soluble fraction of platelets either untreated or treated with 3 nM PGE1 for 5 minutes at room temperature were immunoprecipitated by means of nonimmune sera (NonI) or GPIb antibodies and were immunoblotted by means of antibodies to calmodulin. (B) Triton X-100 soluble fractions of platelets either untreated or treated with 1 mM NEM for 5 minutes at room temperature were immunoprecipitated by means of nonimmune sera (NonI) or GPIb antibodies and were immunoblotted by means of antibodies to actin-binding protein (ABP, upper panel) or calmodulin (lower panel). Blots were visualized by means of a peroxidase-coupled second antibody and the ECL reagent. Results are representative of 3 separate experiments.
It has also previously been shown that treatment of platelets with NEM causes dissociation of actin-binding protein from GPIbα.8 This is demonstrated by immunoblotting of anti-GPIb immunoprecipates with an anti–actin-binding protein antibody in Figure 3B. Treatment of platelets with NEM also resulted in a reduction in the level of calmodulin associated with GPIb-IX (Figure3B). The level of calmodulin associated with GPIb-IX in NEM-treated compared with untreated platelets, however, was reduced by 40% to 50% in 3 separate experiments. These data suggest that calmodulin association with the GPIb-IX complex is not dependent on coassociation of actin-binding protein but may be stabilized in part by its coassociation.
Binding of calmodulin to a synthetic peptide based on the cytoplasmic sequence of GPIbβ
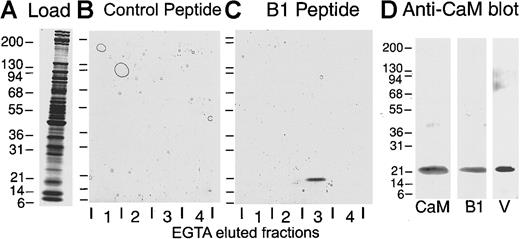
To investigate potential calmodulin binding sites within GPIb-IX-V, we observed that a sequence within the cytoplasmic tail of GPIbβ (R149–L167) was analogous to calmodulin-binding motifs found in other proteins (Figure 4). A synthetic peptide corresponding to the GPIbβ sequence, R149–L167 (B1 peptide), was coupled to agarose. Platelet cytosol (Figure5A) was loaded onto the affinity resin in the presence of 1 mM Ca++, and bound protein was eluted by 10 mM EGTA. While no detectable proteins eluted from a control peptide (CLKKLIRSPSIPHQY) column (Figure 5B), a protein eluted from the B1 peptide column was approximately 22 kd on SDS–polyacrylamide gel electrophoresis under both reducing (Figure 5C) and nonreducing (not shown) conditions. Two lines of evidence suggested that the protein eluted from the B1 column was calmodulin. First, it comigrated with bovine calmodulin on SDS–polyacrylamide gels and was immunoblotted by an anticalmodulin antibody (Figure 5D). Second, the amino acid sequence of a tryptic fragment of the purified protein revealed a sequence (R/K)XVMXNLGE that was conserved in human calmodulin (RHVMTNLGE).29
Sequences within GPIbβ compared with other calmodulin-recognition sequences.
(A) Sequence alignment of a cytoplasmic sequence of GPIbβ, R149–L167,17 with calmodulin-binding sequences in a model calmodulin-binding control peptide CBCP,23,26L-selectin,27 and m7A.28 Identical amino acids or conserved substitutions are boxed. (B) Helical wheel representation of the GPIbβ sequence (R149–L167) compared with the amphipathic α-helical peptide CBCP.
Sequences within GPIbβ compared with other calmodulin-recognition sequences.
(A) Sequence alignment of a cytoplasmic sequence of GPIbβ, R149–L167,17 with calmodulin-binding sequences in a model calmodulin-binding control peptide CBCP,23,26L-selectin,27 and m7A.28 Identical amino acids or conserved substitutions are boxed. (B) Helical wheel representation of the GPIbβ sequence (R149–L167) compared with the amphipathic α-helical peptide CBCP.
Affinity chromatography of whole-platelet lysates.
(A) SDS–5%-to-20% polyacrylamide gel of platelet lysate electrophoresed under reducing conditions and stained with Coomassie blue. (B-C) Protein from platelet lysate (A) that had bound to a control peptide column (B) or a GPIbβ (R149–L167) peptide-agarose column (B1 peptide) (C) was eluted with 10 mM EGTA in the washing buffer. Eluted 2-mL fractions1-4 were analyzed on SDS–5%-to-20% polyacrylamide gels under reducing conditions and stained with Coomassie blue. (D) Immunoblot with anticalmodulin monoclonal antibody of purified bovine calmodulin (CaM), the 22-kd protein isolated from platelet cytosol by EGTA elution from the GPIbβ peptide column (B1) or a GPV (K529–G544) peptide-agarose column (V). The proteins were electrophoresed on SDS–5%-to-20% polyacrylamide gels, electrotransferred to nitrocellulose, and visualized by means of a peroxidase-conjugated anti–mouse IgG and the ECL reagent.
Affinity chromatography of whole-platelet lysates.
(A) SDS–5%-to-20% polyacrylamide gel of platelet lysate electrophoresed under reducing conditions and stained with Coomassie blue. (B-C) Protein from platelet lysate (A) that had bound to a control peptide column (B) or a GPIbβ (R149–L167) peptide-agarose column (B1 peptide) (C) was eluted with 10 mM EGTA in the washing buffer. Eluted 2-mL fractions1-4 were analyzed on SDS–5%-to-20% polyacrylamide gels under reducing conditions and stained with Coomassie blue. (D) Immunoblot with anticalmodulin monoclonal antibody of purified bovine calmodulin (CaM), the 22-kd protein isolated from platelet cytosol by EGTA elution from the GPIbβ peptide column (B1) or a GPV (K529–G544) peptide-agarose column (V). The proteins were electrophoresed on SDS–5%-to-20% polyacrylamide gels, electrotransferred to nitrocellulose, and visualized by means of a peroxidase-conjugated anti–mouse IgG and the ECL reagent.
Interaction of calmodulin with the cytoplasmic tail of GPV
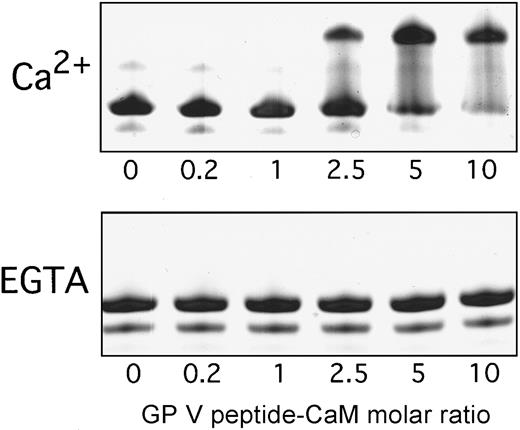
The cytoplasmic tail of GPV also contained a sequence with similarity to an IQ motif (Figure 6) previously reported to interact with calmodulin in a Ca++-dependent manner.21 As described above for the GPIbβ peptide, an affinity column of the GPV peptide coupled to agarose specifically bound a 22-kd protein from platelet cytosol in the presence of Ca++. The bound protein was eluted by EGTA, comigrated with bovine calmodulin on SDS–polyacrylamide gels, and was immunoblotted by an anticalmodulin antibody (Figure 5D). To further examine whether this sequence in GPV bound calmodulin, we also used a nondenaturing gel shift assay previously shown to specifically identify calmodulin-binding peptides.21-23 A synthetic peptide corresponding to the entire GPV cytoplasmic sequence, K529–G544, induced a concentration-dependent shift in calmodulin migration in the presence of Ca++, but not in the presence of EGTA (Figure7). In contrast to the GPV-related peptide, a positively charged control peptide, CLKKLIRSPSIPHQY, did not affect calmodulin migration in the gel shift assay when tested at concentrations up to a 10-fold molar excess over calmodulin (data not shown). Related gel shift experiments with the B1 peptide were not technically feasible owing to poor solubility of this peptide at high concentrations.
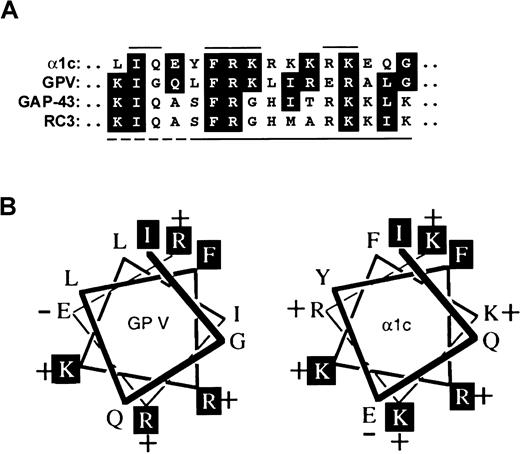
Sequence comparison of the GPV cytoplasmic tail and the IQ motif.
(A) Alignment of the cytoplasmic sequence of GPV, K529–G544,18 with the conserved IQ motif of the L-type ion channel subunit α1c,21RC3/neurogranin,30,31 and GAP-43.32,33 The solid lines above the α1c sequence indicate residues that form the IQ motif. The lower line indicates residues minimally required for calmodulin binding by RC3 (solid line), while additional upstream residues involved in the Ca++-sensitive interaction include those indicated by the dashed line.32 (B) Helical wheel representation of the GPV sequence (K529–G544) compared with the sequence of α1c.
Sequence comparison of the GPV cytoplasmic tail and the IQ motif.
(A) Alignment of the cytoplasmic sequence of GPV, K529–G544,18 with the conserved IQ motif of the L-type ion channel subunit α1c,21RC3/neurogranin,30,31 and GAP-43.32,33 The solid lines above the α1c sequence indicate residues that form the IQ motif. The lower line indicates residues minimally required for calmodulin binding by RC3 (solid line), while additional upstream residues involved in the Ca++-sensitive interaction include those indicated by the dashed line.32 (B) Helical wheel representation of the GPV sequence (K529–G544) compared with the sequence of α1c.
Interaction of the GPV cytoplasmic peptide and calmodulin.
Nondenaturing gel shift of calmodulin (0.3 nmol) in the presence of increasing concentrations of the GPV synthetic peptide, based on K529–G544. Upper and lower panels are in the presence of 1 mM Ca++ or 10 mM EGTA, respectively. Gels were stained with Coomassie blue.
Interaction of the GPV cytoplasmic peptide and calmodulin.
Nondenaturing gel shift of calmodulin (0.3 nmol) in the presence of increasing concentrations of the GPV synthetic peptide, based on K529–G544. Upper and lower panels are in the presence of 1 mM Ca++ or 10 mM EGTA, respectively. Gels were stained with Coomassie blue.
Discussion
The platelet membrane GPIb-IX-V complex binds to the adhesive ligand vWF and initiates platelet adhesion and aggregation at high-shear stress in flowing blood.1-4 Elevation of cytosolic Ca++ in activated platelets leads to actin polymerization and to cytoskeletal rearrangement and activation of αIIbβ3, resulting in platelet aggregation and contraction.5,6 Recent studies have suggested that 14-3-3ζ, which directly binds to one or more sequences within the cytoplasmic domain of GPIb-IX-V,11-13 may be involved in linking engagement of GPIb-IX-V with activation of αIIbβ3.5 The p85 subunit of PI 3-kinase has also been identified as being associated with GPIb-IX-V in platelets.16
In the current study, we have found an additional association between the cytoplasmic domain of GPIb-IX-V and the cytosolic regulatory protein calmodulin. Immunoblotting with anticalmodulin antibody showed that calmodulin was present in the soluble fraction of both resting and thrombin-activated platelets, but as with 14-3-3ζ and p85,16 there was an increased association with the cytoskeleton following activation. Calmodulin coimmunoprecipitated with GPIb-IX or GPV from the Triton X-100 soluble fraction of resting platelets, but not thrombin-stimulated platelets, suggesting the GPIb-IX-V–calmodulin complex dissociates in activated platelets.
Within the cytoplasmic domain of GPIbβ, a membrane-proximal 16-residue sequence was similar to other reported calmodulin-binding sequences (Figure 4). In Figure 4B, the GPIbβ sequence is compared with CBCP,23 a nonphysiological calmodulin-binding control peptide that is representative of a positively charged, amphipathic α-helix typical of calmodulin-recognition sites in other proteins.23,26,33,34 In addition, a sequence comprising the 16-residue cytoplasmic tail of GPV resembled the IQ motif described in the α1c subunit of the L-type Ca++ channel (Figure 6). Calmodulin is a critical Ca++ sensor for both inactivation and facilitation of L-type Ca++ channels, and mutation of I1624 within this motif dramatically affects the Ca++-dependent feedback regulation of this channel.21 Conserved motifs of the type represented in either GPIbβ (Figure 4) or GPV (Figure 6) have been reported to specifically bind calmodulin in a range of different proteins.21-23,26-34 Both motifs are net positively charged, but whether they recognize identical or overlapping sites on calmodulin has not been determined. The GPIbβ- and GPV-related peptides both affinity-isolated calmodulin from platelet cytosol in the presence of Ca++. In contrast to calmodulin's association with intact GPIb-IX or GPV in platelet extracts that contained EGTA, binding of calmodulin to synthetic peptides based on GPIbβ or GPV was observed only in the presence of Ca++. This suggests the Ca++ dependency and/or affinity of the interaction with calmodulin is different in the intact receptor. This is not inconsistent with other reports of calmodulin-binding peptides from RC3 (neurogranin). In this case, Ca++ sensitivity of interactions with calmodulin was conferred by specific residues immediately upstream of the calmodulin-binding site31(Figure 6A). The GPV peptide also induced a shift in migration of bovine calmodulin on nondenaturing gels in the presence of Ca++. Maximal shift was observed at a 10-fold molar excess of peptide to calmodulin, suggesting the interaction was of lower affinity than that reported for the α1c-related peptide, which required equimolar peptide for maximal shift (dissociation constant [KD], approximately 30 nM),21 but is of higher affinity than a peptide based on the α1a subunit of the P/Q ion channel where greater than 50-fold excess of peptide was required (KD, approximately 2 μM).22
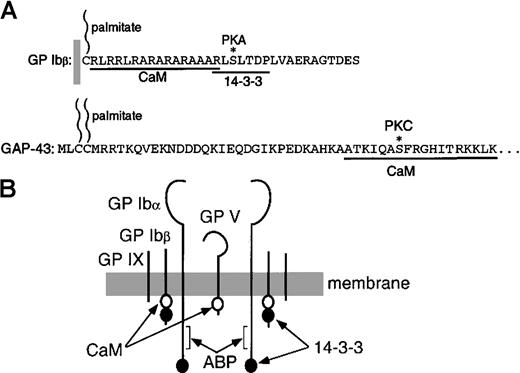
Structure-function analysis has revealed several striking similarities between platelet GPIbβ and the neural cell–specific, cytoskeletal regulatory protein GAP-43 (Figure 8A). Both GPIbβ (as part of GPIb-IX) and GAP-43 bind calmodulin independently of Ca++ (this study and Baudier et al32), are palmitylated at a site N-terminal to the calmodulin recognition sequence34,38 and are serine-phosphorylated within or proximal to the calmodulin-binding site by PKA or protein kinase C (PKC), respectively.25,35,39,40Interestingly, phosphorylation of GPIbβ by PKA inhibits the actin polymerization that normally occurs following platelet activation.25 In contrast, GAP-43 becomes concentrated at the cytoplasmic face of the membrane in growth cones during axon development and stabilizes long actin filaments when phosphorylated by PKC.40 Unphosphorylated GAP-43 reduces actin filament length, and calmodulin association potentiates this effect. It is postulated that association of calmodulin with GAP-43, myristylated alanine-rich C kinase substrates (MARCKS), and other proteins modulates their PKC-dependent phosphorylation.31,33,34,40 It has also been shown that the MARCKS-related calmodulin-binding peptide directly facilitates actin polymerization and bundle formation.41 In addition, GAP-43, MARCKS, and CAP23 have been shown to colocalize with PI(4,5)P2 and regulate actin polymerization at plasmalemmal rafts in neural cell lines.42 This process also involves colocalized PI 3-kinase,43 a signaling protein also specifically associated with GPIb-IX-V.16
Map of the calmodulin-binding subunits of the GPIb-IX-V complex.
(A) Cytoplasmic domain of GPIbβ17 showing sequences that interact with calmodulin (this study), and the RXSXTXP motif that interacts with 14-3-3ζ.13 The asterisk indicates the phosphorylation site at S166.35 The structure of GPIbβ is compared with that of GAP-43.31,32 (B) Schematic representation of GPIb-IX-V showing locations of sequences that bind calmodulin (this study), 14-3-3ζ,12-14 and actin-binding protein.36 37
Map of the calmodulin-binding subunits of the GPIb-IX-V complex.
(A) Cytoplasmic domain of GPIbβ17 showing sequences that interact with calmodulin (this study), and the RXSXTXP motif that interacts with 14-3-3ζ.13 The asterisk indicates the phosphorylation site at S166.35 The structure of GPIbβ is compared with that of GAP-43.31,32 (B) Schematic representation of GPIb-IX-V showing locations of sequences that bind calmodulin (this study), 14-3-3ζ,12-14 and actin-binding protein.36 37
In a manner similar to calmodulin's effect on PKC phosphorylation of GAP-43, calmodulin could regulate PKA access to GPIbβ and thereby influence activation-dependent actin reorganization in platelets. This possibility deserves future investigation. In this regard, the calmodulin-binding sequence of GPIbβ is immediately upstream of a conserved RXSXTXP motif that binds 14-3-3ζ and includes the PKA phosphorylation site (Figure 8A) (this study, Andrews et al,13 and Calverly et al14). Phosphorylation of this sequence inhibits actin polymerization (see above) and increases the affinity of GPIbβ for 14-3-3ζ.13,15 Our data suggest that calmodulin association with GPIb-IX is not affected by phosphorylation of GPIbβ, an event previously shown to increase 14-3-3ζ association with GPIbβ.13 15 This implies both calmodulin and 14-3-3ζ can potentially bind GPIb-IX. We also showed that NEM treatment diminished the level of GPIb-IX–associated calmodulin. This suggests not only that actin-binding protein and calmodulin can independently associate with GPIb-IX, but also that the calmodulin association with GPIb-IX may be in part stabilized by the coassociation with the GPIb-IX-V complex of actin-binding protein. Further studies are needed, however, to investigate whether NEM treatment may directly decrease the interaction of calmodulin with GPIb-IX independently of actin-binding protein association.
Like 14-3-3ζ, calmodulin binds a wide range of ligands, including calmodulin-dependent kinases I and II, and phosphatases such as calcineurin, in addition to cytoskeletal proteins.33,34GPIb-IX-V, with high copy number (greater than 10 000 per platelet) and multiple sites for calmodulin and 14-3-3ζ (Figure 8B), could therefore be involved in submembranous localization of both calmodulin- and 14-3-3ζ–associated signaling proteins. Dissociation of the Ca++-independent interactions of calmodulin and GPIb-IX-V (this study), and elevation of cytosolic Ca++ following platelet activation,1 may facilitate Ca++-dependent calmodulin activation of as-yet-unidentified signaling or cytoskeletal proteins.
In summary, this study has identified calmodulin as a GPIb-IX-V–associated protein in platelets, and its specific recognition sequences might ultimately provide novel targets for therapeutic regulation of platelet activation. Further studies are currently underway to define the precise functional consequences of the GPIb-IX-V–calmodulin interaction.
We thank Carmen Llerena, Andrea Aprico, and Catherine Upton for excellent technical assistance; Dr A. Ian Smith for sequence analysis; and Dr Walter G. Thomas, Dr Yang Shen, and Dr Elizabeth E. Gardiner for helpful discussions.
Supported in part by grants from the National Health and Medical Research Council of Australia, and the National Heart Foundation of Australia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert K. Andrews, Baker Medical Research Institute, PO Box 6492, St Kilda Rd Central, Melbourne, Victoria, Australia, 8008; e-mail: rkandrews@hotmail.com.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal