Abstract
It has been previously shown that the HIV-1 envelope glycoprotein 120 (gp120) activates cell signaling by CXCR4, independently of CD4. The present study examines the involvement of different intracellular signaling pathways and their physiopathologic consequences following the CD4-independent interaction between CXCR4 or CCR5 and gp120 in different cell types: primary T cells, CD4−/CXCR4+/CCR5+ T cells, or glioma cells. These interactions were compared with those obtained with natural ligands, stromal cell–derived factor 1 α (SDF-1α) (CXCL12) and macrophage inflammatory protein 1 β (MIP-1β) (CCL4) of their respective coreceptors. Thus, both p38 and SAPK/Jun N-terminal kinase mitogen-activated protein kinases (MAPKs) are activated on stimulation of these cells with either T- or M-tropic gp120, as well as with SDF-1α or MIP-1β. In contrast, extracellular signal-related kinase 1 and 2 MAPKs are only activated by MIP-1β but not by M-tropic gp120. Importantly, T- and M-tropic gp120 are able to induce the secretion of matrix metalloproteinase 9 (MMP-9), an extracellular metalloproteinase present in cerebrospinal fluid of patients with HIV-1 by T cells or glioma cells. Specific inhibition of MAPK p38 activation resulted in a complete abrogation of the induction of the MMP-9 pathogenic factor expression by gp120 or chemokines in both cell types. Because neurodegenerative features in acquired immune deficiency syndrome dementia may involve demyelinization by MMP-9, the specific targeting of p38 could provide a novel means to control HIV-induced cytopathogenic effects and cell homing to viral replication sites.
Introduction
AIDS results from different actions induced by HIV on its wide variety of target cells. The CD4 molecule is the major receptor of HIV-1.1 The high-affinity binding of CD4 to glycoprotein 120 (gp120)2 induces conformational changes in gp120 to allow its further binding to secondary receptors,3-5 which could result in a trimolecular complex, CD4-gp120-coreceptor.6,7 HIV coreceptors belong to the chemokine receptor family (see Baggiolini et al8for review), among which CXCR4 and CCR5 are the major attachment coreceptors for T-tropic and M-tropic isolates of HIV-1, respectively.9,10 The HIV envelope binding to target cells induces activation through receptors,11,12 eliciting functional responses that are important in AIDS pathogenesis. Although the cell activation is induced by a CD4-transconformed gp120 through the coreceptor,13-15 a CD4-independent activation occurs,16,17 particularly in T cells and cells from the central nervous system (CNS).18,19 Indeed, it has been reported that cell signaling is activated by either the X4-tropic or R5-tropic HIV-1 envelopes, leading to activation of such functional responses as proliferation, differentiation, chemotaxis,13,16 proinflammatory cytokines secretion, or apoptosis.20 21
Apoptosis or programmed cell death is one of the major physiopathologic mechanisms of AIDS. Cells continuously stimulated become sensitive to apoptosis.22 Interactions between Fas-L and CD95,23 or between tumor necrosis factor α (TNFα) and its receptor (TNFr), activation of caspases, and down-regulation of the proto-oncogene Bcl2 protein and interleukin-2 (IL2) are considered the major apoptotic events during HIV infection leading to destruction of T cells,24 macrophages, dendritic cells, and neurons.
In the CNS, another important factor contributing to the neurodegenerative process is the presence of extracellular proteases from the matrix metalloproteinase (MMP) family. MMPs have been shown to degrade constituents of the extracellular matrix such as collagens,25 favoring (1) blood-brain barrier leakage26 and (2) infiltration by activated or infected cells. Indeed, gp41, the transmembrane component of the HIV envelope, has been reported to induce MMP-2 overproduction by nerve cells, resulting in the destruction of the extracellular matrix in vivo or in vitro.27 The presence of MMP-9 was reported in cerebrospinal fluid of HIV-infected patients.26,28 In rapidly progressing simian immunodeficiency virus–infected monkeys, high expression of MMP-9 was correlated to both motor and cognitive deficits; in contrast, low progressors exhibited low levels of MMP-9, similar to uninfected control.29 In addition, in vitro studies reported evidence that HIV itself, or at least the HIV Tat or Nef proteins directly promote MMP-9 secretion.30-32
The principal aim of this study was to assess the importance of coreceptors, in some pathogenic pathways induced by their respective chemokine or gp120 ligands, without the participation of the CD4 receptor. We examined signal transduction in different cell types on cell incubation with soluble gp120s (X4- or R5-tropic HIV strains) or chemokines (stromal cell–derived factor 1 α [SDF-1α] or macrophage inflammatory protein 1 β [MIP-1β]). We also used a CD4-independent gp120 expressed at the surface of Aldrithiol-2 (AT2)-inactivated pseudotyped HIV-1 virus. More specifically, we examined the ability of soluble gp120s to activate the mitogen-activated protein kinase (MAPK) pathways (extracellular signal-related kinase 1 and 2 [ERK1/2], p38, and SAPK/Jun N-terminal kinase [JNK], with the exception of ERK1/2 activated by SDF-1α or gp120 from HIV-1 HXB2, which were already reported in our previous work16) and the secretion of MMP-9.
In this work, we show that HIV-1 gp120 activates p38, SAP/JNK MAPKs, and induces MMP-9 pathogenic factor secretion. Interestingly, gp120-mediated MMP9 production was abolished by inhibition of the p38 MAPK signaling pathway.
Materials and methods
Cell lines and plasmids
C6 rat glioma cells were obtained from the American Type Culture Collection (ATCC; Rockville, MD) and were grown in Ham F10 medium supplemented with 15% horse serum, 2.5% fetal bovine serum (FBS), and 10 mM HEPES buffer. Human leukemic T-cell line Jurkat was grown in RPMI 1640 medium supplemented with 10% FBS. The human TH1 clone CB.XI.2.12 was generated and propagated by using cloning and culture conditions as previously described33 and was grown in Yssel medium (Irvine Scientific, Santa Ana, CA) for 3 days before use in the experiment supplemented with AB+ human serum.34 Cell surface expression of CXCR4 on the latter cells was induced by the addition of 100 U/mL recombinant IL4 (rIL4) in the culture medium for at least 1 week before their use.35CEM A201 (ATCC) and Jurkat CD4−/CXCR4+, a kind gift from Dr N. Taylor (IGM, Montpellier, France), were transfected with a pEFIN3/CCR5 expression vector to obtain stable CEM CD4−/CXCR4+/CCR5+ and Jurkat CD4−/CXCR4+/CCR5+ clones. These cells were cultured in RPMI 1640 supplemented with 10% FCS and 500 μg/mL G418. Human embryonic kidney (HEK) 293T cell line was a kind gift from Dr Didier Trono (Basel, Switzerland) maintained in Iscove modified Dulbecco essential medium (IMDEM; Gibco BRL, Cergy-Pontoise, France) supplemented with 10% FCS and 1.5 mg/mL G418. Plasmid expression vector pCMVDR9 contains the majority of HIV provirus and blocked reading frames of ENV and VPU. The expression vector pHR′GFP contains the REV responsive element and green fluorescent protein gene (GFP). Both vectors were a gift from Dr Didier Trono.36The pHXB2 expression vector (National Institutes of Health [NIH] AIDS Research and Reference Reagent Program, Bethesda, MD) was mutated in our laboratory to obtain pHXB2/CD4bs−, which expresses a CD4-independent HIV envelope (manuscript in preparation).
Antibodies and reagents
Rabbit antihuman antibodies to total and to specific phosphorylated forms of p38 kinase, SAPK/JNK, and ERK/MAPK were purchased from New England Biolabs (Beverly, MA). Anti-CXCR4 12G5 monoclonal antibodies (MAbs) were purchased from BD Pharmingen (San Diego, CA). Pyridinylimidazole compound, SB 203580, a specific inhibitor of p38 kinase, and recombinant mouse and human TNF-α were purchased from Calbiochem (La Jolla, CA). Recombinant mouse and human IL1α MAbs were obtained from R & D Systems (Oxon, United Kingdom). Recombinant soluble CD4 (sCD4) was obtained from the NIH AIDS Research and Reference Reagent Program. Anisomycin, phorbol ester myristate acetate (PMA), and AT2 were purchased from Sigma-Aldrich (St Louis, MO). SDF-1α and MIP-1β were produced and kindly provided by Dr Françoise Baleux (Pasteur Institute, Paris, France). Recombinant human and mouse IL-1α and the anti-CCR5 182 MAbs were purchased from R & D Systems (Oxford, United Kingdom).
Cell stimulation and immunoblot analysis
Before stimulation, cells were cultured for 24 hours in serum-free RPMI 1640 conditioned medium. One million cells were resuspended in 100 μL serum-free RPMI 1640 medium and incubated at 37°C for 15 minutes before stimulation with either 10 μg/mL each of the gp120 (T-tropic or M-tropic), gp120-CD4 complexes (ratio 1:20), 100 nM SDF-1α, 100 nM MIP-1β, 500 ng/mL anisomycin, or 10 ng/mL PMA for different time periods. Cells were lysed in a 1% NP40 buffer. For each time point, equal amounts of protein were electrophoresed under reducing conditions and transferred electrophoretically to nitrocellulose membranes. Membranes were incubated for 30 minutes in Tris buffered saline (50 mM NaCl, 20 mM Tris HCl, pH 7.5) containing 5% bovine serum albumin (BSA) and 0.1% Tween 20 and then incubated overnight with the phosphospecific p38-kinase (T180/Y182) antibody, the phosphospecific SAPK/JNK (T183/Y185) antibody, and the anti–active MAPK that recognizes the dually phosphorylated T202/Y204 form of ERK1/2. Proteins were visualized by using the enhanced chemiluminescence system (Amersham Pharmacia Biotech, Piscataway, NJ). Blots were washed in Tris buffered saline containing 0.1% Tween 20 and incubated with horseradish peroxidase–conjugated goat antirabbit secondary antibody (Amersham Pharmacia Biotech). For reblotting with the anti-MAPK antibodies, filters were previously stripped.
HIV-1 envelopes and pseudotyped viruses
Soluble recombinant gp120/HXB2 and gp120/JRFL, respectively produced in baculovirus, as previously described37 and in Chinese hamster ovary cells (a kind gift from R. Doms, Philadelphia, PA) were used to stimulate different cell types. A pseudotyped virus carrying a CD4-independent mutated envelope was used to stimulate a human TH1 clone. A 3-plasmid expression vector was used to generate a pseudotyped HIV-1/HXB2-derived mutant (manuscript in preparation) by transient transfection HEK 293T cells. HEK 293T cells were transfected in a 15-cm diameter Petri dish with 40 μg DNA from pCMVΔR9, pHR′GFP, and pHXB2/ENV expression vectors using the CaPO4 method. Virus-containing supernatants were harvested 36 hours after transfection. Clarified virus was concentrated by centrifugation at 105 rpm for 10 minutes, using a TLA-120.2 rotor in a TL-120 centrifuge (Beckman-Coulter, Miami, FL). Viral supernatant was quantified by enzyme-linked immunosorbent assay p24 (Abbot Diagnostics), resuspended at the desired concentration, and subsequently inactivated by 1 mM AT2 treatment.38
Detection of secreted gelatinase activity
Serum-deprived cells (106) were cultured in suspension overnight with each ligand: 10 μg/mL gp120 (T-tropic or M-tropic), 100 nM chemokine (SDF-1α or MIP-1β), 0.02 μg/mL of p24 of pseudotyped HIV-1 virus, 20 ng/mL IL1α, or 20 ng/mL TNFβ. Alternatively, in some experiments cells were preincubated 45 minutes with 10 μM SB 203580, a specific inhibitor of p38 kinase, before the contact with ligands. Gelatinase secretion of cell supernatants was determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) zymography by using a gelatin substrate, as previously described.39 Briefly, equal volumes of culture supernatant (500 μL) were lyophilized, resuspended in loading buffer, and, without prior denaturation, were run on 7.5% SDS-polyacrylamide gel containing 1 mg/mL gelatin. After electrophoresis, gels were washed to remove SDS and incubated for 18 hours at 37°C in a renaturing buffer (50 mM Tris, 5 mM CaCl2, 0.02% NaN3, 1% Triton X-100). Gels were subsequently stained with Coomassie brilliant blue G-250 and destained in 30% methanol/10% acetic acid (vol/vol) to detect gelatinase secretion.
Immunofluorescence and flow cytometry analysis
Cells (200 000) were resuspended in 50 μL phosphate-buffered saline (PBS), supplemented with 3% BSA and 0.02% NaN3 in the presence of the relevant MAbs. After 1 hour of incubation with agitation at 4°C, the cells were washed twice in PBS-0.3% BSA-0.02% NaN3 and resuspended in PBS-3% BSA-0.02% NaN3in the presence of a 1/50 dilution of fluorescein isothiocyanate–conjugated goat antimouse antibody (Caltag, Burlingame, CA). After an additional hour of incubation with agitation at 4°C, cells were washed 3 times as described above, resuspended in PBS, and analyzed by single-color flow cytometry by using a FACSort (Becton Dickinson, San Jose, CA) and Lysis II software. Each datum point was represented by mean of fluorescence intensity of 10 000 gated events.
Results
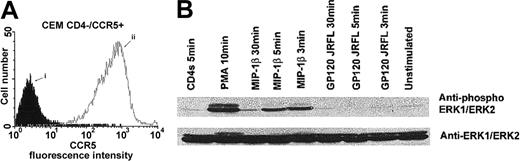
MIP-1β, but not gp120 from HIV-1 JRFL, induces phosphorylation of ERK1/2 through CCR5 in a CD4-independent manner
We have previously reported that, in CD4− T cells, the ERK1/2-MAPK pathway was activated through the CXCR4 receptor on binding by SDF-1α, but not by the T-tropic gp120 from HIV-1/HXB2.16 In this first series of experiments, we assessed the ability of MIP-1β or gp120 from the M-tropic HIV-1 strain JRFL to induce signaling through CCR5, independently of the CD4 receptor, using stable clones of CEM cells expressing high levels of CCR5 (Figure 1A). Our results showed that stimulation by PMA and MIP-1β, but not by gp120/JRFL, induced a significant and transient increase in the phosphorylation of the ERK/MAPK (Figure 1B). These results extend and converge with our previous results.16 These observations on the ERK1/2 phosphorylation could be extended to a general dissociated mechanism of ERK activation by chemokines and gp120s through their common chemokine receptor.
Binding of gp120 JRFL to CCR5 does not involve activation of the ERK1/ERK2 MAPK pathway.
Cell surface expression of CCR5 on CD4− CEM cells was analyzed by flow cytometry (A), after staining of the cells with an isotype-matched control MAb (i) or the anti-CCR5 182 MAb (ii). CD4−/CCR5+ CEM cells were preincubated in medium alone or in the presence of gp120 JRFL (10 μg/mL), MIP-1β (100 nM), recombinant soluble CD4 (10 μg/mL), or PMA (1 μM) for 1 hour on ice. Stimulations were then performed at 37°C for the indicated time periods before lysis (B). ERK1/ERK2 activation was assessed by using a polyclonal anti–active MAPK antibody. The immunoblot was then stripped and reblotted with ERK1/ERK2 antibodies detecting total level of these kinases.
Binding of gp120 JRFL to CCR5 does not involve activation of the ERK1/ERK2 MAPK pathway.
Cell surface expression of CCR5 on CD4− CEM cells was analyzed by flow cytometry (A), after staining of the cells with an isotype-matched control MAb (i) or the anti-CCR5 182 MAb (ii). CD4−/CCR5+ CEM cells were preincubated in medium alone or in the presence of gp120 JRFL (10 μg/mL), MIP-1β (100 nM), recombinant soluble CD4 (10 μg/mL), or PMA (1 μM) for 1 hour on ice. Stimulations were then performed at 37°C for the indicated time periods before lysis (B). ERK1/ERK2 activation was assessed by using a polyclonal anti–active MAPK antibody. The immunoblot was then stripped and reblotted with ERK1/ERK2 antibodies detecting total level of these kinases.
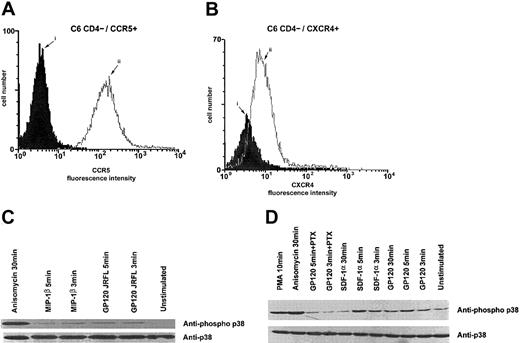
Gp120 from HIV-1 HXB2 or JRFL and the chemokines SDF-1α or MIP-1β induce activation of the p38 MAPK pathway through chemokine receptors in CD4− cells
Because the interaction between gp120 and CXCR4 has been shown to induce the phosphorylation of Pyk-2 protein tyrosine kinase,16 we next tested whether gp120 from HIV T- or M-tropic strains could induce the activation of the p38/MAPK pathway. The activation through the CCR5 receptor was tested by using stable clones of Jurkat T CD4− cells expressing CCR5 (Figure2A). We found that binding of gp120/HXB2 and gp120/JFRL to, respectively, CD4−/CXCR4+/CCR5+ Jurkat cells and CEM T cells resulted in the activation of the p38/MAPK pathway (Figure2B,D). Interestingly, preincubation of gp120 with sCD4 did not increase the ability of the gp120 to phosphorylate p38/MAPK (Figure 2B,C). Slightly different activation levels of the p38/MAPK pathway were observed on incubation with the MIP-1β and gp120/JRFL (Figure 2C,D). Similar results were obtained by stimulating CD4−/CXCR4+ Jurkat cells with gp120/HXB2 and SDF-1α, respectively (Figure 2B).
R5- or X4-tropic HIV-1 gp120 promotes p38 kinase phosphorylation in a CD4-independent manner.
Cell surface expression of CCR5 of CD4− Jurkat cells was analyzed by flow cytometry (A), after staining of the cells with an isotype-matched control MAb (i) or the anti-CCR5 182 MAb (ii). CD4−/CXCR4+ Jurkat cells (B), CD4−/CCR5+ Jurkat cells (C), or CD4−/CCR5+ CEM cells (D) were incubated in medium alone or in the presence of gp120 JRFL (10 μg/mL), MIP-1β (100 nM), recombinant soluble CD4 (10 μg/mL), anisomycin (500 ng/mL), PMA (1 μM), gp120 HXB2 (10 μg/mL), SDF-1α (100 nM), gp120-CD4s complexes (molar ratio 1:20), or recombinant soluble CD4 (10 μg/mL) for the indicated time period at 37°C. Immediately after incubation, cells were lysed; proteins were separated by SDS-PAGE and analyzed by immunoblotting with anti-p38 kinase antiserum to verify equal loading and efficiency of protein transfer.
R5- or X4-tropic HIV-1 gp120 promotes p38 kinase phosphorylation in a CD4-independent manner.
Cell surface expression of CCR5 of CD4− Jurkat cells was analyzed by flow cytometry (A), after staining of the cells with an isotype-matched control MAb (i) or the anti-CCR5 182 MAb (ii). CD4−/CXCR4+ Jurkat cells (B), CD4−/CCR5+ Jurkat cells (C), or CD4−/CCR5+ CEM cells (D) were incubated in medium alone or in the presence of gp120 JRFL (10 μg/mL), MIP-1β (100 nM), recombinant soluble CD4 (10 μg/mL), anisomycin (500 ng/mL), PMA (1 μM), gp120 HXB2 (10 μg/mL), SDF-1α (100 nM), gp120-CD4s complexes (molar ratio 1:20), or recombinant soluble CD4 (10 μg/mL) for the indicated time period at 37°C. Immediately after incubation, cells were lysed; proteins were separated by SDS-PAGE and analyzed by immunoblotting with anti-p38 kinase antiserum to verify equal loading and efficiency of protein transfer.
Taking into account the biological relevance of the contact between gp120 and nerve cells in the absence of CD4, we investigated whether the activation of the p38/MAPK pathway by gp120 through the chemokine receptors was T-cell specific. Then, we examined the ability of chemokines and gp120 to activate the C6 glioma cells. These cells do not express CD4 but constitutively express both CXCR4 and CCR5 receptors (Figure 3A,B, respectively). Indeed, similar phosphorylation levels of p38/MAPK were observed on incubation with the M- or T-tropic gp120 proteins, as well as with the chemokines, although the kinetics of the activation were slightly different in glioma cells. We also observed a slightly lower activation with M-tropic gp120 or MIP-1β (Figure 3C). Intensity and kinetic differences of p38 MAPK activation respectively induced by gp120s or chemokines could probably be due to differences in binding epitopes of each ligand.
P38 activation in C6 glioma cells by R5- or X4-tropic HIV-1 gp120.
Cell surface expression of CCR5 (A) and CXCR4 (B) on CD4−C6 glioma cells was analyzed by flow cytometry after staining of the cells with an isotype-matched control MAb (i) or the appropriate antichemokine receptor MAb (ii). CD4−/CCR5+/CXCR4+ C6 glioma cells were incubated in the presence of gp120 JRFL (10 μg/mL), MIP-1β (100 nM) (C), or gp120 HXB2 (10 μg/mL) and SDF-1α (100 nM) (D). Controls were done by using medium alone, anisomycin (500 ng/mL), or PMA (1 μM) for the indicated time period at 37°C. In some experiments, cells were pretreated for 16 hours at 37°C with pertussis toxin (100 ng/mL) before stimulation with gp120s. P38 kinase tyrosine phosphorylation was assessed as described in the legend to Figure 2.
P38 activation in C6 glioma cells by R5- or X4-tropic HIV-1 gp120.
Cell surface expression of CCR5 (A) and CXCR4 (B) on CD4−C6 glioma cells was analyzed by flow cytometry after staining of the cells with an isotype-matched control MAb (i) or the appropriate antichemokine receptor MAb (ii). CD4−/CCR5+/CXCR4+ C6 glioma cells were incubated in the presence of gp120 JRFL (10 μg/mL), MIP-1β (100 nM) (C), or gp120 HXB2 (10 μg/mL) and SDF-1α (100 nM) (D). Controls were done by using medium alone, anisomycin (500 ng/mL), or PMA (1 μM) for the indicated time period at 37°C. In some experiments, cells were pretreated for 16 hours at 37°C with pertussis toxin (100 ng/mL) before stimulation with gp120s. P38 kinase tyrosine phosphorylation was assessed as described in the legend to Figure 2.
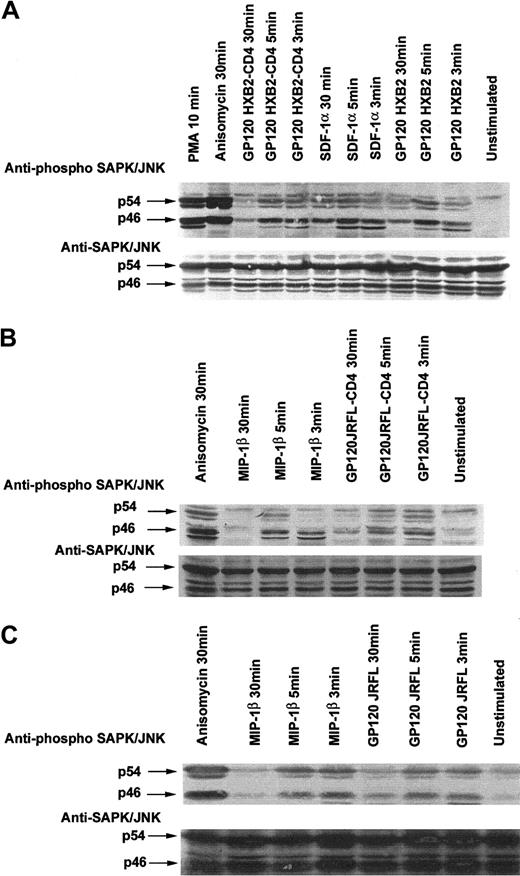
Gp120 from HIV-1 HXB2 or JRFL and SDF-1α or MIP-1β induce activation of the SAP/JNK MAPK pathway
We next tested whether the CD4−/CXCR4+ or CD4−/CCR5+ T cells on incubation with their appropriate ligands (ie, either gp120 from T- or M-tropic HIV) or chemokines could activate the SAPK/JNK pathway. Our results indeed showed that incubation of Jurkat or CEM cells with the appropriate ligands induced the tyrosine phosphorylation of SAPK/JNK in a time-dependent manner, similar to that previously observed with the p38/MAPK (Figure 4). A peak of phosphorylation was detected at approximately 3 to 5 minutes after stimulation. Interestingly, preincubation of gp120 with sCD4 did not increase the ability of the gp120 to phosphorylate SAPK/JNK (Figure4A), suggesting that the CD4-transconformation gp120/HXB2 is not needed to activate the MAPK pathway, and a lower affinity contact could be enough to transduce cell signaling.
Binding of R5- and X4-tropic HIV-1 gp120 to T-cell lines expressing the corresponding chemokine receptor stimulate phosphorylation of SAPK/JNK.
CD4−/CXCR4+ Jurkat cells (A), CD4−/CCR5+ Jurkat cells (B), or CD4−/CCR5+ CEM cells (C) were incubated with medium alone or in the presence of either 10 μg/mL gp120 (R5- or X4-tropic), MIP-1β (100 nM), SDF-1α (100 nM), anisomycin (500 ng/mL), gp120-CD4s complexes (molar ratio 1:20), or PMA (1 μM) for the indicated time period at 37°C. Cells were then lysed, proteins were separated by SDS-PAGE, and SAPK/JNK tyrosine phosphorylation was assessed. The immunoblot was stripped and reprobed with anti-JNK serum to verify equal loading and efficiency of protein transfer.
Binding of R5- and X4-tropic HIV-1 gp120 to T-cell lines expressing the corresponding chemokine receptor stimulate phosphorylation of SAPK/JNK.
CD4−/CXCR4+ Jurkat cells (A), CD4−/CCR5+ Jurkat cells (B), or CD4−/CCR5+ CEM cells (C) were incubated with medium alone or in the presence of either 10 μg/mL gp120 (R5- or X4-tropic), MIP-1β (100 nM), SDF-1α (100 nM), anisomycin (500 ng/mL), gp120-CD4s complexes (molar ratio 1:20), or PMA (1 μM) for the indicated time period at 37°C. Cells were then lysed, proteins were separated by SDS-PAGE, and SAPK/JNK tyrosine phosphorylation was assessed. The immunoblot was stripped and reprobed with anti-JNK serum to verify equal loading and efficiency of protein transfer.
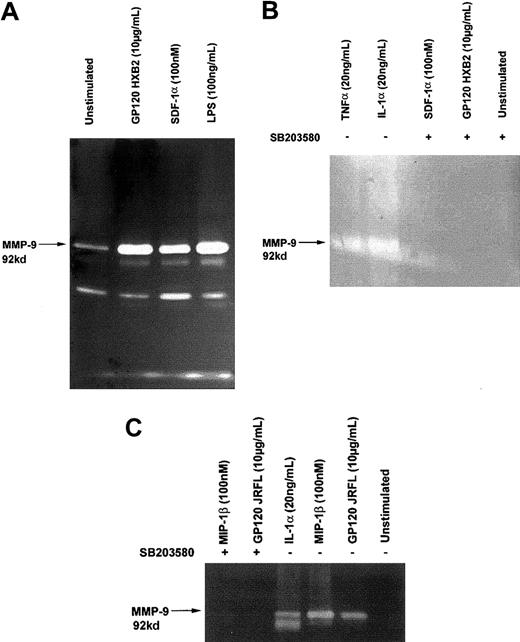
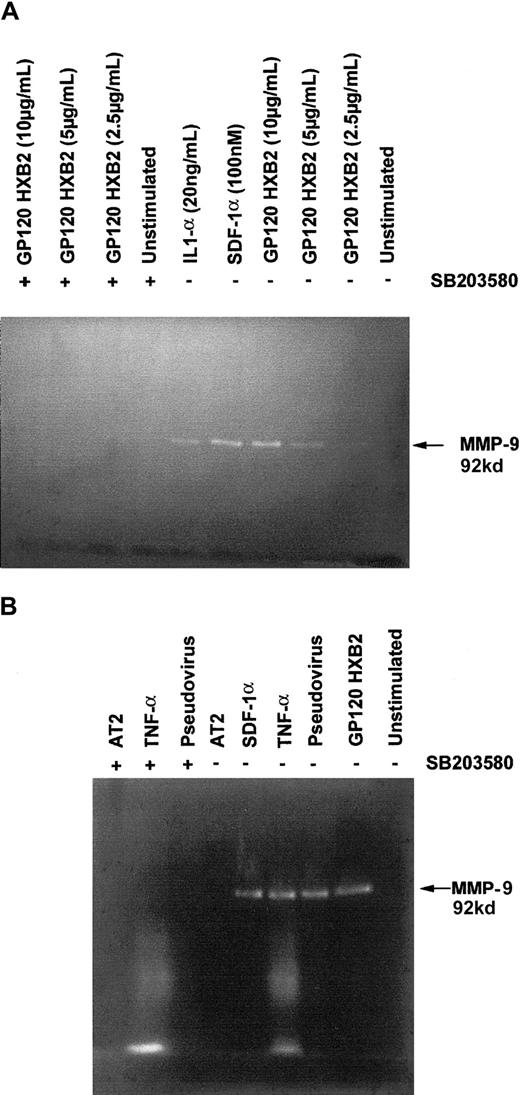
Gp120 from HIV-1 HXB2 or JRFL and SDF-1α or MIP-1β induce MMP-9 secretion
Because MMP-9 has been associated with a number of neurodegenerative processes, including demyelinization,40and has also been found in the cerebrospinal fluid of patients with AIDS,29 we investigated whether binding of gp120 could induce the production of MMP-9 by C6 glioma cells and T cells. Our results show that stimulation with either gp120 or chemokines significantly up-regulated the secretion of MMP-9 by both glioma cells (Figure 5) and T cells (Figure6), as detected by gelatin zymography. The up-regulation of MMP-9 secretion in both cell types by either gp120 or the chemokines was abolished in the presence of 10 μM SB 203580, a specific inhibitor of p38/MAPK (Figure 5B,C and Figure 6), suggesting that p38/MAPK is essential for the up-regulation of MMP-9 through the chemokine receptors, independently of their ligands. Interestingly, we also observed the same phenomenon using a pseudotyped T-tropic virus carrying “native” CD4-independent gp120 at its surface (Figure 6B). It should be noteworthy that proinflammatory cytokines (IL1α and TNFα) up-regulated the MMP-9 production. These results suggest that this situation could be found in patients with neuronal AIDS and, therefore, specific targeting of p38 could provide novel means to control HIV-induced cytopathogenic effects.
Gp120-induced MMP-9 secretion in C6 glioma cells.
C6 glioma cells were treated for 18 hours with or without (unstimulated) the indicated concentration of gp120/HXB2, gp120/JRFL, SDF-1α, MIP-1 β, IL-1 α, TNF-α, or lipopolysaccharide. Supernatants were collected, lyophilized, and assayed for their gelatinase content by zymography (A,B,C). In experiments marked with the symbol +, cells were preincubated with 10 μM SB 203580, a specific inhibitor of p38 kinase, 45 minutes at 37°C before the contact with ligands (B,C).
Gp120-induced MMP-9 secretion in C6 glioma cells.
C6 glioma cells were treated for 18 hours with or without (unstimulated) the indicated concentration of gp120/HXB2, gp120/JRFL, SDF-1α, MIP-1 β, IL-1 α, TNF-α, or lipopolysaccharide. Supernatants were collected, lyophilized, and assayed for their gelatinase content by zymography (A,B,C). In experiments marked with the symbol +, cells were preincubated with 10 μM SB 203580, a specific inhibitor of p38 kinase, 45 minutes at 37°C before the contact with ligands (B,C).
T cells produce MMP-9 on stimulation with gp120 or pseudotyped virus in a CD4-independent manner.
Analysis of MMP-9 secretion by CD4−/CXCR4+Jurkat cells (A) and human TH1 clone CB.X.212 (B) in response to stimulation by different ligands, gp120 HXB2, SDF-1α, or IL1-α at the indicated concentration or gp120 HXB2 (10 μg/mL) inactivated AT2 pseudotyped virus containing a CD4-independent mutated envelope (20 ng p24), TNF-α (20 ng/mL), SDF-1α (100 nM), or AT2. In these experiments, cells were pretreated or not with 10 μM SB 203580 (marked with the symbol +) prior to exposure with the different ligands for 16 hours at 37°C. Supernatants were collected, lyophilized, and assayed for their gelatinase content by zymography.
T cells produce MMP-9 on stimulation with gp120 or pseudotyped virus in a CD4-independent manner.
Analysis of MMP-9 secretion by CD4−/CXCR4+Jurkat cells (A) and human TH1 clone CB.X.212 (B) in response to stimulation by different ligands, gp120 HXB2, SDF-1α, or IL1-α at the indicated concentration or gp120 HXB2 (10 μg/mL) inactivated AT2 pseudotyped virus containing a CD4-independent mutated envelope (20 ng p24), TNF-α (20 ng/mL), SDF-1α (100 nM), or AT2. In these experiments, cells were pretreated or not with 10 μM SB 203580 (marked with the symbol +) prior to exposure with the different ligands for 16 hours at 37°C. Supernatants were collected, lyophilized, and assayed for their gelatinase content by zymography.
Discussion
In the present work, we show that p38, JNK/SAPK MAPKs, and the MMP-9 cytopathogenic factor were triggered on cell stimulation either by gp120s (T- or M-tropic HIV-1 strain) or by chemokines (SDF-1α or MIP-1β) through their respective chemokine receptors and independently of CD4. In CCR5+ cells, ERK1/2-MAPK was induced by the MIP-1β but not by gp120.
Cell signaling is characterized by a complex array of interconnected intracellular cascades that play a critical role in the regulation of all cellular functions, including proliferation and cell survival, and are controlled by a broad variety of extracellular stimuli. MAPKs are among the key elements that regulate this intracellular signaling network. They are highly conserved kinases that regulate cell growth, differentiation, and stress responses.41 At least 3 distinct families of MAPKs exist in mammalian cells: (1) the p42/p44 ERK MAPKs, (2) the c-Jun NH2-terminal kinases (SAPK/JNKs), and (3) the p38 MAPKs. The members of the ERK group regulate cell growth in response to growth factors and could be activated during HIV infection to enhance replication of R5, but not X4, HIV strains.42Originally described by Han et al,43 p38 is generally regarded as a stress-activated enzyme and has been shown to have downstream effects on transcription factor activation44 and actin filament rearrangement.45
During HIV infection, cell activation has been well studied on gp120-CD4 interaction.46 In a previous work,16 we have demonstrated that gp120 from HIV-1/HXB2, or SDF-1α, was able to induce cell signaling and chemotaxis through CXCR4, independently of CD4, and we have further shown that gp120 from the T-tropic HIV-1/HXB2 can induce the activation of Pyk-2. Moreover, gp120 can inhibit the Ca++ signaling induced by SDF-1α (data not shown). Pyk-2 is highly expressed in the CNS, is induced by HIV envelopes, and is involved in the regulation of MAPKs.14,47 Previous work has shown that SDF-1α and MIP-1β induce MAPKs.48 In the present work, we have extended these findings by showing induction of p38 and SAPK/JNK by the corresponding gp120 (X4- or R5-tropic) as well as the corresponding chemokine (SDF-1α or MIP-1β), whereas in CD4−/CCR5+ cells, ERK1/2 activation was induced by MIP-1β but not by gp120 JRFL. In addition, SDF-1α and gp120 induced a differential cell signaling through CXCR4 in rat cortical neurons, as reported by Lazarini et al.49 These results confirm our previous data demonstrating the ability of chemokines, but not gp120, to trigger ERK1/2. Interestingly, preincubation of gp120 with sCD4 did not increase the ability of gp120 to phosphorylate MAPKs, suggesting that the CD4-transconformation gp120/HXB2 is not needed to activate the MAPK pathway and a simple contact that does not need a high affinity could be enough to transduce cell signaling.
Apoptosis, or programmed cell death, is considered one of the major cytopathogenic processes involved in HIV infection. Interactions between Fas-L and CD95,23 TNF and TNFr50activation of caspase-3,21,51,52 and down-regulation of the proto-oncogene Bcl2 protein or IL253,54 are considered the major apoptotic pathways during HIV infection. Gp120-induced apoptosis pathways lead to destruction of T cells, dendritic cells, and cells from CNS.55 56 However, data involving gp120 on different pathways of cell activation and apoptosis are highly controversial, depending on cells, receptors involved, and experimental conditions.
More clearly, MMPs are involved in a number of physiologic and pathologic processes, including tissue remodeling, wound healing, tumor invasion, and rheumatoid arthritis. By degrading extracellular matrix proteins, such as fibronectin and collagen IV,26 MMPs contribute to demyelinization processes in the CNS, the breaking of the blood-brain barrier, and leukocyte migration through the endothelial barrier and tissues.25 These observations agree with the reported data in which MIP-1α, MIP-1β, and, to a lesser extent, RANTES promote the secretion of pro–MMP-9 by T lymphocytes.57 The presence of MMP-9 was recently reported in the cerebrospinal fluid in HIV-infected patients31 and in the rapid progressors group of simian immunodeficiency virus–infected monkeys. High expression of MMP-9 was correlated to both motor and cognitive deficits. In contrast, low progressors exhibit low levels of MMP-9, similar to uninfected controls.29 In addition, in vitro studies reported that HIV or the HIV Tat protein could promote MMP-9 secretion from monocytes,32 whereas MMP-2 secretion could be stimulated by gp41-derived peptides.27 Because chemokine receptors are expressed in normal brain and in brain from AIDS patients,58 our results raise the hypothesis that secretion of MMP-9 in the CNS could not only be induced by MIP-1α (CCL3) and MIP-1β, and, to a lesser extent, RANTES (CCL5),59 but could also be up-regulated by gp120 through the CXCR4 or CCR5 coreceptors independently of CD4.
Interestingly, we found that the activation cascade leading to MMP-9 secretion in either T cells or glial cells induced by chemokines or gp120 through coreceptor and in a CD4-independent manner was in all cases mediated by the p38/MAPK pathway. This finding is of considerable importance for the future treatment of patients with AIDS dementia, as specific p38 inhibitors could be used as potential drugs against MMP cytopathogenic effects during HIV infection. MMP-9 production was also obtained by preincubating human TH1 cells with a CD4-independent T-tropic HIV-1 pseudotyped virus, suggesting that gp120 carried at the virus surface could be able to induce MMP-9 to favor cell homing to viral replication sites.
Taken together, these results suggest that binding of chemokine receptors in vivo by either gp120 or chemokines induces activation of the p38/MAPK pathway, leading to increased secretion of the MMP-9 cytopathogenic factor by local glial cells or infiltrated T cells, thereby favoring the neurodegenerative processes observed in cases of AIDS disease.
We thank Dr Françoise Baleux from the Pasteur Institute for her kindness in providing us with chemokines used in this work. We are indebted to the AIDS Research and Reference Reagent Program of the National Institutes of Health, Dr Natalie Chazal, and Prof Didier Trono for their gift of efficient expression vectors to construct pseudotyped HIV-1/GFP viruses. We are grateful to Prof Gérard Devauchelle for his suggestions regarding this manuscript and to Mme Jean-Anne Ville for her continuous support.
Supported by the Agence Nationale de Recherche sur le SIDA, SIDACTION, the Actions de Recherche Concertées of the CommunautéFrançaise de Belgique, the Centre de Recherche Inter-universitaire en Vaccinologie, the Belgian program on Interuniversity Poles of attraction initiated by the Belgian State, Prime Minister's Office, Science Policy Programming, the BIOMED and BIOTECH programmes of the European Community (grants BIO4-CT98-0543 and BMH4-CT98-2343), and the Fonds de la Recherche Scientifique Médicale of Belgium to M.P. D.M. is a SIDACTION fellow. B.R. is an Aspirant of the Belgian Fonds National de la Recherche Scientifique.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Francisco Veas, Laboratoire d'Immunologie Rétrovirale et Moléculaire, Institut de Recherche pour le Développement, Centre National de la Recherche Scientifique IRD UR34/CNRS UMR5087, EFS, 240 Av E. Jeanbrau, 34094 Montpellier, France; e-mail: veas@mpl.ird.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal