Nonresponse to remission-induction chemotherapy, which remains a major problem in acute myeloblastic leukemia (AML), has been linked to cellular resistance to apoptosis. Because the apoptosis induced by chemotherapeutic drugs is mediated by loss of mitochondrial transmembrane potential (MTP), it was postulated that sensitivity to mitochondrial membrane depolarization might be heterogeneous in AML. Using the uncoupling agent carbonyl cyanide m-chlorophenylhydrazone (mClCCP), the mitochondrial membrane sensitivity to depolarization (mClCCP concentrations that inhibit 50% of the transmembrane potential [IC50]) in AML blasts was measured and demonstrated marked interclonal heterogeneity, with the existence of comparatively sensitive (median mClCCP IC50, 4 μM) and resistant (median mClCCP IC50, 10 μM) clones. Furthermore, the mClCCP IC50 was inversely associated with spontaneous in vitro apoptosis (P = .001). It was high in cases with mutant TP53 and correlated with the total cellular level of the multidrug resistance–associated protein (P = .019) but not of bcl-2, bax, or bcl-x. It was also found that the dithiol oxidant diamide, in contrast to the monovalent thiol oxidant diethyl maleate, increased the sensitivity of mitochondrial membranes to mClCCP. To confirm that TP53 directly affects MTP in leukemic cells and to establish the role of vicinal thiol oxidation in the TP53-dependent pathway, CEM 4G5 leukemia cells with forced, temperature-dependent expression of TP53 were studied. Monobromobimane, which inhibits mitochondrial membrane depolarization by preventing dithiol cross-linking, inhibited depolarization and apoptosis in 4G5 cells. It was concluded that in leukemia, TP53 and vicinal thiol/disulfide status are determinants of mitochondrial membrane sensitivity to depolarization, which is in turn associated with spontaneous apoptosis.
Introduction
Acute myeloblastic leukemia (AML) is a heterogeneous malignant disease in which resistance to induction chemotherapy occurs in 20% to 50% of patients.1,2Chemoresistance may be mediated by overexpression of drug-efflux molecules such as p-glycoprotein (pgp) or multidrug resistance–associated protein (MRP), as demonstrated in several clinical studies.3 Model systems have predicted that chemoresistance may also be mediated by apoptosis inhibitory pathways, such as those involving bcl-2, bax, or bcl-xL.4,5 We previously showed that a bcl-2 antisense oligonucleotide sensitized a proportion of AML blasts to the drug cytosine arabinoside.6 Others found that bcl-2 antisense oligonucleotides induced apoptosis in clinical samples that initially expressed low but not high levels of the protein.7 However, attempts to find associations between overexpression of any individual member of the bcl family proteins and clinical response in AML have been inconclusive or contradictory,8-13 reflecting the complexity of the interactions involved.
Redox mechanisms also have a role in modulating apoptosis.14 Glutathione, the predominant intracellular reducing thiol, is expressed at high levels in AML,15 and expression of the glutathione-metabolizing enzyme glutathione transferase π has been associated with poor clinical outcome.16 The role of other thiol-containing molecules in apoptosis of AML blasts has been little studied. For example, the antioxidant thioredoxin is known to affect cellular sensitivity to apoptosis in adult T-cell leukemia,17-19 but its role in AML has not been explored.
Mutations of the oncogene TP53 are associated with poor outcome in AML.20 TP53 is normally involved in the regulation of apoptosis in damaged cells. This may involve both bcl-2 family and redox pathways: up-regulation of bax by TP53 has been documented,21,22 and TP53 is known to regulate expression of several genes involved in redox mechanisms including MRP, glutathione peroxidase, and thioredoxin reductase.23-25
Mitochondrial transmembrane potential (MTP) is considered to have a key role in the control of apoptotic responses, including responses mediated by chemotherapy.26 Loss of MTP can trigger opening of the permeability transition (PT) pore.27 PT pore opening has been implicated as a critical stage in apoptosis in isolated mitochondria28 and several cellular models.29-31 PT pore opening allows release of factors that initiate the final, degradative phase of apoptosis. Although PT pore opening does not necessarily accompany membrane depolarization,32 a reduction in MTP lowers the threshold of pore transition.32,33 Both redox pathways and bcl proteins help to control MTP.32-40 Oxidants and changes in bcl proteins that decrease MTP also induce apoptosis, and conversely, antioxidants and changes in bcl proteins that prevent depolarization also inhibit apoptosis.4,14 TP53 may play a pivotal role in mitochondrial depolarization. TP53 null murine thymocytes failed to depolarize their mitochondrial membranes in response to both oxidative insult and the drug etoposide.35
Given the large number of bcl family genes and redox pathway genes that possibly modulate MTP in leukemia, it is difficult to unravel which pathways have major importance in resistance to apoptosis. To address this issue, we developed a functional assay of mitochondrial membrane sensitivity to depolarization in cases of primary AML. We then studied the effect of overexpression of bax, bcl-2, and bcl-xL on sensitivity to mitochondrial membrane depolarization in AML, along with the role of cellular redox status with particular reference to TP53. Because the data we obtained suggested roles for both TP53 and vicinal thiol oxidation in mitochondrial membrane depolarization in AML blasts, we also used a TP53 conformational mutant leukemic cell line21 to investigate the role of vicinal thiol oxidation in the depolarization and apoptosis pathways induced by TP53.
Materials and methods
Materials
The fluorescent dye 3,3′-dihexyloxacarbocyanine iodide (DiOC6) was obtained from Molecular Probes (Eugene, OR). Stock solution (1 mM) in ethanol was stored at room temperature. Working solution (400 nM) in phosphate-buffered saline (PBS) was stored at 4°C and used at a low concentration (40 nM) as recommended previously.41 Carbonyl cyanide m-chlorophenylhydrazone (mClCCP; Sigma, Poole, United Kingdom) was diluted to a concentration of 10 mM in water and stored at −20°C. Additional dilutions in PBS were made immediately before use. PSC 833 was a gift from Novartis, Basel, Switzerland. It was stored as 1-mM stock in ethanol at 4°C and diluted in medium before use. Monobromobimane (MBB; Sigma) was stored at −20°C as a 1-mM stock in ethanol. Diazene dicarboxylic acid bis N, N-dimethylamide (diamide; Sigma) was diluted to a concentration of 200 mM in water and stored at −20°C. Diethyl maleate (DEM) and t-butyl hydroperoxide (tBHP), both from Sigma, were stored undiluted. The DNA-binding vital dyes propidium iodide (PI; Sigma) and 7-amino-actinomycin D (7-AAD, Sigma) were each stored as 50-μg/mL stock at 4°C in PBS and used at a concentration of approximately 10 μg/mL.
Cells
Both leukemic cell lines and samples from patients were used in these experiments. The mutant p53 compound heterozygote CEM-C7H2, a subclone of CCRF CEM, was reconstituted with PvuI-linearized pLTRTP53V135 as described previously.21 The resultant stably transfected 4G5 subclone expresses a mutant conformation of TP53 at 37°C that adopts a wild-type conformation at 32°C. The MRP-positive HL60/ADR cell line was a gift from Dr M. Center, Kansas State University. The pgp-positive KG1a and MDR-negative U937 and HL60 myeloid leukemia cell lines were from the European Collection of Animal Cell Cultures (Salisbury, United Kingdom). All cell lines used were grown in a culture medium (CM) of RPMI 1640 with 10% fetal-calf serum (FCS) and 2 mM l-glutamine (CM). Blood or bone marrow samples were obtained with informed consent from patients presenting to Nottingham hospitals with de novo (24 cases) or relapsed (3 cases) AML. White blood cells were isolated with a standard density gradient centrifugation method using Histopaque (Sigma) and were cryopreserved in liquid nitrogen. Because several analyses were planned for each case, samples were chosen in which the original blast count was high—more than 20 × 106/mL in peripheral blood or more than 80% blasts in marrow samples. For analysis, cryopreserved samples were thawed and allowed to rest in CM enriched to 20% FCS for 90 minutes before the experimental procedures. Only samples with greater than 90% postrest viability were used.
Mitochondrial membrane depolarization with mClCCP
Several experiments to optimize assay conditions are described in the results section. For interpatient comparisons, leukemic cells were suspended at a concentration of 106/mL in fresh CM (pH 7.3-7.5). The cells were then incubated in sterile Falcon tubes for 90 minutes at 37°C in 5% carbon dioxide (CO2) with 40 nM DiOC6, with and without mitochondrial toxins. Each condition was set up in triplicate. DiOC6 fluorescence was measured by using the FL1 channel of a FACScan (Becton Dickinson, Cowley, United Kingdom). In preliminary experiments, we determined that 30 μM mClCCP induced maximum depolarization in primary leukemia samples (data not shown). To calculate the patient mClCCP concentration that inhibits 50% of the transmembrane potential (IC50), we first calculated a 50% loss of mean fluorescence intensity (MFI) by using the following formula: [(DiOC6 MFI without mClCCP − DiOC6 MFI at 30 μM mClCCP)/2] + DiOC6 MFI at 30 μM mClCCP. We then constructed a dose-response curve by using Microsoft Excel software and read the concentration of mClCCP at which a 50% loss of fluorescence occurred.
Measurement of bcl-2, bax, bcl-x, and MRP
For measurement of bcl-2, bax, and bcl-x, thawed and rested leukemic cells were fixed and permeabilized in 35% ethanol for 10 minutes, rinsed twice in PBS containing 1% bovine serum albumin and 0.1% sodium azide, and resuspended in the same buffer. Aliquots were then stained with unconjugated antibodies to bcl-x (S-18) or bax (both from Santa Cruz Biotechnology, Santa Cruz, CA) or with appropriate controls as described previously 42 by using a fluorescein isothiocyanate, conjugated (FITC)–labeled secondary antibody. A single batch of each polyclonal antibody was used, and a reproducible concentration was established as described previously.42Bcl-2 was measured by using 10 μL FITC anti–bcl-2 or control antibody (both from Dako, High Wycombe, United Kingdom). The unconjugated antibody MRPr1 (Monosan, Uden, The Netherlands) was used according to the manufacturer's instructions, with the modification that a blocking solution of 20% rabbit serum in PBS was added 30 minutes before the second-layer incubation in FITC rabbit antirat secondary antibodies.
Flow cytometry
For flow cytometric analysis, a Becton Dickinson FACScalibur was used with a logarithmic amplifier and Cellquest software. The following variables were measured. For bcl-2, bcl-x, and bax, where the test and control distributions tended to be nonoverlapping, Quantum beads were used to standardize interassay fluorescence and allow quantitation of mean bound fluorochrome molecules as described previously.42 MRP was measured by using the Kolmogoroff-Smirnoff D statistic, which we previously showed to be a sensitive measure of inhibited drug uptake in AML blasts.43
Flow cytometric measurement of apoptosis
Primary leukemia clones were incubated in 5% CO2 at a concentration of 5 × 105/mL in RPMI suspension culture with 10% FCS and 2 mM l-glutamine. 7-AAD was mixed with cultured cells to yield a final concentration of approximately 10 μg/mL. Cultures were incubated for 20 minutes in the dark at room temperature before processing for flow cytometry. 7-AAD was measured in FL3. Cells undergoing apoptotic cell death are represented by 7-AAD–high cells with low forward scatter.44 45 For PI-mediated determination of sub-G0 cells, cultured cells were first fixed in 70% ethanol at −20°C for 15 minutes. Cells were then rinsed twice in PBS, resuspended in PI at a concentration of approximately 10 μg/mL, and analyzed by flow cytometry.
TP53
Mutations of the TP53 gene in AML samples were investigated by direct sequencing of full-length TP53 complementary DNA as reported previously.46
Statistical procedures
SPSS software (SPSS, Chicago, IL) was employed. The Spearman rank correlation was used to assess the significance of associations between mClCCP IC50 and expression of cellular proteins. The Wilcoxon signed-rank test was used to investigate the significance of the effect of antioxidants on MTP.
Results
Methodology
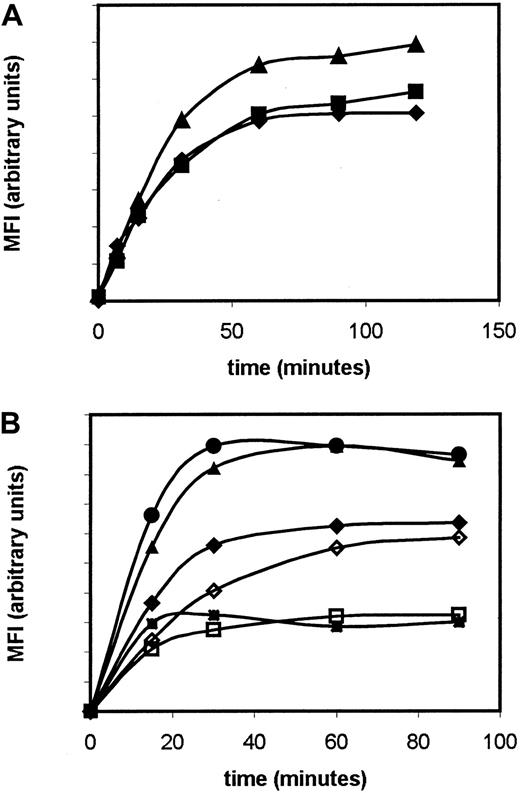
The potentiometric fluorescent dye DiOC6 can be used to measure mitochondrial membrane depolarization. We investigated use of this dye to measure mitochondrial membrane sensitivity to the protonophore mClCCP. This experimental system was previously used by others37,47 to demonstrate that monovalent thiol reagents or ectopic expression of bcl-2 can block the depolarizing effect of mClCCP. For clinical studies, we sought to develop this system into an assay that would be reproducible, sufficiently sensitive to detect differences between clones, and sensitive to mitochondrial but not plasma membrane depolarization. We first determined the saturation plateau for DiOC6 in leukemic cells after incubation at 37°C; this occurred at 60 to 120 minutes in primary AML cells and cell lines (Figure 1A). Others showed that after prolonged (> 5 hours) incubation with DiOC6, the plasma membrane as well as the mitochondrial membrane can affect DiOC6 fluorescence,48 so we performed experiments using potassium chloride to depolarize the plasma membranes of cells from patients and thus ensured that this would not be a confounding factor in our system (Figure 1B).
Saturation curves for DiOC6.
(A). Time-course saturation curves for DiOC6 (MFI, mean fluorescence intensity on an arbitrary scale) in the MDR-negative HL60 (squares), pgp-positive KG1a (diamonds), and MRP-positive HL60/ADR (triangles) cell lines. The MDR-positive cell lines were chosen to determine whether drug-efflux pumps altered the saturation plateau for DiOC6. (B) Time-course saturation curves for DiOC6 in the presence (solid symbols) and absence (open symbols) of 125 mM potassium chloride in 3 primary leukemia samples.
Saturation curves for DiOC6.
(A). Time-course saturation curves for DiOC6 (MFI, mean fluorescence intensity on an arbitrary scale) in the MDR-negative HL60 (squares), pgp-positive KG1a (diamonds), and MRP-positive HL60/ADR (triangles) cell lines. The MDR-positive cell lines were chosen to determine whether drug-efflux pumps altered the saturation plateau for DiOC6. (B) Time-course saturation curves for DiOC6 in the presence (solid symbols) and absence (open symbols) of 125 mM potassium chloride in 3 primary leukemia samples.
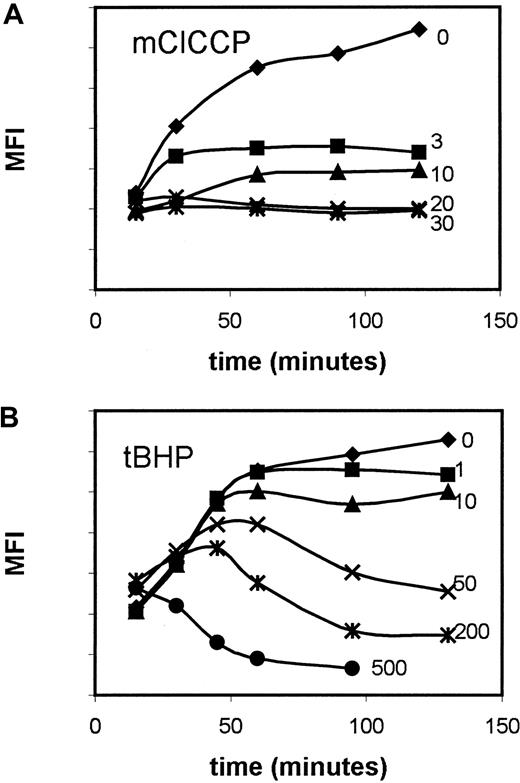
We evaluated use of the pro-oxidant tBHP and the uncoupler mClCCP as agents to depolarize the mitochondria of primary leukemia cells. The tBHP was found to hyperpolarize the mitochondrial membrane before depolarizing (Figure 2). This may be associated with continued formation of a hydrogen ion gradient despite the failure of adenosine diphosphate/adenosine triphosphate exchange as described previously.49 For practical purposes, the curve obtained with tBHP was not considered suitable for interpatient comparisons because no saturation plateau was reached, whereas a plateau was reached by using mClCCP.
Dose- and time-response curves for DiOC6modulation in primary leukemia samples.
Results with (A) mClCCP and (B) tBHP are shown; curves show a saturation plateau for the probe with the former but not the latter agent. Numbers next to the curves refer to micromolar doses of modulators.
Dose- and time-response curves for DiOC6modulation in primary leukemia samples.
Results with (A) mClCCP and (B) tBHP are shown; curves show a saturation plateau for the probe with the former but not the latter agent. Numbers next to the curves refer to micromolar doses of modulators.
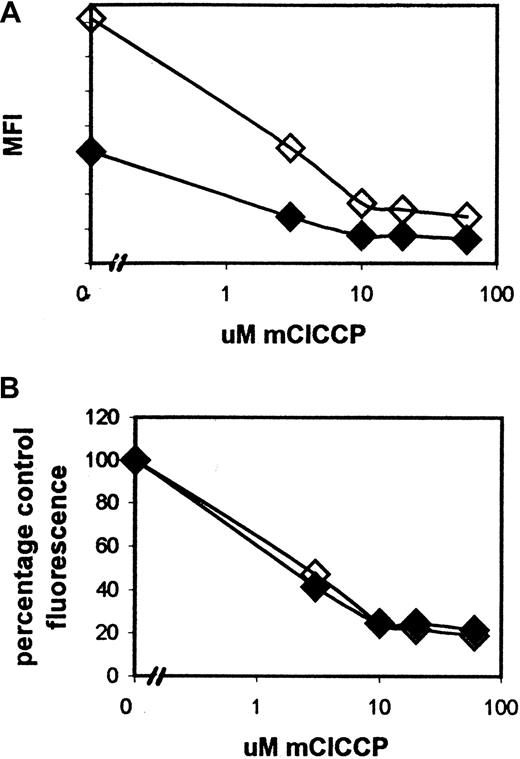
Because primary leukemia samples may be pgp positive or negative, we were concerned that pgp-mediated efflux of the cationic fluorescent dye DiOC6 would alter the intracellular availability of the dye and hence the kinetics of its binding to the mitochondrial membrane. We therefore measured DiOC6 uptake in pgp-positive primary leukemia cells with and without the pgp modulator PSC 833. As shown in Figure 3, at 90 minutes, DiOC6 fluorescence was greater in the presence of PSC833 than in its absence, a finding compatible with reduced dye efflux. However, when expressed in terms of percentage of control fluorescence, the dose of mClCCP required to induce loss of fluorescence was independent of whether PSC833 was present or absent, indicating that DiOC6 binding kinetics are unaffected when the overall intracellular dye concentration is reduced by efflux.
The effect of the pgp reversal agent PSC833 on the MFI of DiOC6 after treatment with a range of mClCCP doses.
PSC833 (2 μM) was added to cultures immediately before mClCCP. Although the total MFI of PSC-833–treated cells (open diamonds) was increased compared with that in untreated cells (solid diamonds; plot A), treated cell fluorescence as a percentage of control fluorescence was the same in treated and untreated samples (plot B). These results are representative of 3 similar experiments on AML samples selected as having high pgp expression from a bank of more than 200 assayed samples. The pgp was measured by using the MRK-16 antibody and the rhodamine modulation assay as described previously.45
The effect of the pgp reversal agent PSC833 on the MFI of DiOC6 after treatment with a range of mClCCP doses.
PSC833 (2 μM) was added to cultures immediately before mClCCP. Although the total MFI of PSC-833–treated cells (open diamonds) was increased compared with that in untreated cells (solid diamonds; plot A), treated cell fluorescence as a percentage of control fluorescence was the same in treated and untreated samples (plot B). These results are representative of 3 similar experiments on AML samples selected as having high pgp expression from a bank of more than 200 assayed samples. The pgp was measured by using the MRK-16 antibody and the rhodamine modulation assay as described previously.45
MTP sensitivity to mClCCP in primary AML blasts and cell lines
The depolarizing response to mClCCP was measured in 27 samples from patients with AML. Examples of dose-response curves are shown in Figure 4. The mClCCP dose required to reduce the fluorescence to 50% of untreated control values (the mClCCP IC50) was bimodally distributed in patient clones with modal values at 4 μM and at 10 μM (range, 1-15 μM; overall median, 6.5 μM; Figure 4). Several cell lines were also studied (Figure 4), and K562 cells were found to have the highest IC50 measured.
Sensitivity of AML blasts to mitochondrial membrane depolarization induced by mClCCP.
Each dose was assayed in triplicate. (Ai and Bi) Flow cytometric histograms for DiOC6 fluorescence at 10 μM mClCCP (unshaded histogram) and untreated control (shaded histogram). Ai illustrates a sensitive sample, and the corresponding dose-response curve is shown in Aii. Bi illustrates a resistant sample, and the corresponding dose-response curve is shown in plot Bii. The horizontal line markers on the dose-response curves indicate 50th percentile fluorescence, and the perpendicular lines indicate the mClCCP IC50. (C) Summary scatterplot showing the mClCCP IC50 for 27 samples from patients with AML and the CEM C7H2, KG1a, and K562 leukemic cell lines. The cell line data include error bars (± SEM) derived from at least 3 separate experiments.
Sensitivity of AML blasts to mitochondrial membrane depolarization induced by mClCCP.
Each dose was assayed in triplicate. (Ai and Bi) Flow cytometric histograms for DiOC6 fluorescence at 10 μM mClCCP (unshaded histogram) and untreated control (shaded histogram). Ai illustrates a sensitive sample, and the corresponding dose-response curve is shown in Aii. Bi illustrates a resistant sample, and the corresponding dose-response curve is shown in plot Bii. The horizontal line markers on the dose-response curves indicate 50th percentile fluorescence, and the perpendicular lines indicate the mClCCP IC50. (C) Summary scatterplot showing the mClCCP IC50 for 27 samples from patients with AML and the CEM C7H2, KG1a, and K562 leukemic cell lines. The cell line data include error bars (± SEM) derived from at least 3 separate experiments.
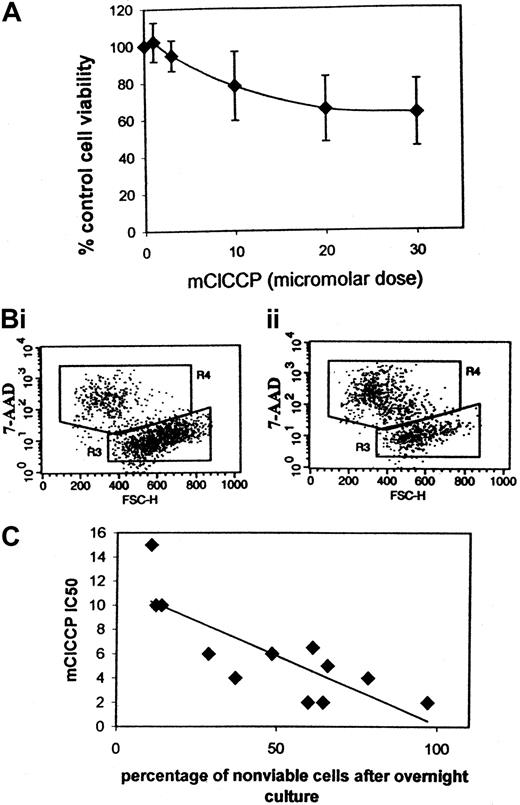
Because mitochondrial membrane depolarization does not always result in PT opening,32 we cultured AML blasts overnight with mClCCP to determine the effects of the ionophore on apoptosis. We found that doses in the same range as the IC50 values for mitochondrial membrane depolarization induced apoptosis in primary leukemia clones (Figure 5). Analysis of apoptotic cell death based on 7-AAD was previously validated against other methods by us and others.44 45
Loss of cell viability in primary AML blasts after overnight culture.
Apoptotic cell death was determined by flow cytometry using 7-AAD. (A) Dose-response curve for mClCCP. The data plotted represent the mean (SD) value from 5 different cases analyzed in separate experiments. (B) Dot plots for an individual case. Two gated regions are shown. R3 encompasses cells with high forward scatter that exclude 7-AAD (viable cells). Apoptotic cells, which take up 7-AAD and have a reduced forward scatter, indicative of shrinking, are shown in R4. (i) Control cells (21% in R4). (ii) Cells cultured overnight with 30 μM mClCCP (59% in R4). (C) The mClCCP IC50 for 12 AML cases plotted against the percentage of cells that did not survive 21 to 23 hours of suspension culture without added growth factors.
Loss of cell viability in primary AML blasts after overnight culture.
Apoptotic cell death was determined by flow cytometry using 7-AAD. (A) Dose-response curve for mClCCP. The data plotted represent the mean (SD) value from 5 different cases analyzed in separate experiments. (B) Dot plots for an individual case. Two gated regions are shown. R3 encompasses cells with high forward scatter that exclude 7-AAD (viable cells). Apoptotic cells, which take up 7-AAD and have a reduced forward scatter, indicative of shrinking, are shown in R4. (i) Control cells (21% in R4). (ii) Cells cultured overnight with 30 μM mClCCP (59% in R4). (C) The mClCCP IC50 for 12 AML cases plotted against the percentage of cells that did not survive 21 to 23 hours of suspension culture without added growth factors.
AML samples are highly heterogeneous in their need for growth factors to support both clonogenic growth50 and survival in suspension culture.51 In some cases of AML, growth factors may be needed to maintain pathways that protect the mitochondrial membrane from depolarization. We therefore hypothesized that samples that undergo the most spontaneous apoptosis in vitro have mitochondrial membranes that depolarize the most readily. We correlated spontaneous in vitro apoptosis (ie, apoptosis in suspension culture in 10% serum without added growth factors) with the mClCCP IC50 in 12 samples and established that a strong correlation existed (r = 0.8;P = .001; Figure 5C).
Associations between mClCCP IC50 and the expression of bcl-2, bax, and bcl-xL in AML blasts
Control of the MTP by transfected bcl-2, bax, or bcl-xL was previously demonstrated in model systems,36,37,39,40,52,53 but the importance of protein overexpression in controlling depolarization in primary leukemia cells has not been explored. Bcl-2, bax, and bcl-xL were measured in our patient samples. (Since we failed to find the bcl-xsisoform in a previous study, we have assumed that the isoform measured is bcl-xL.42) Expression of none of these molecules correlated with the depolarizing response to mClCCP (Table1). The bcl-x to bax ratio and the bcl-2 to bax ratio were also calculated and determined not to correlate with mClCCP sensitivity (data not shown).
Sensitivity to carbonyl cyanide m-chlorophenylhydrazone (mCICCP); bcl-2, bax, and bcl-x expression; and p53 status in 16 samples from patients with acute myeloblastic leukemia
| Patient no. . | mClCCP IC50 . | Mean bcl2 (BFM × 105) . | Mean bax (BFM × 106) . | Mean bcl-x (BFM × 105) . | p53 status . |
|---|---|---|---|---|---|
| 154R | 6 | 3.1 | 2.7 | 6.1 | wt |
| 16 | 10 | 0.54 | 1.5 | 3.9 | wt |
| 161 | 2 | 0.81 | 1.2 | 3.4 | wt |
| 145 | 10 | 2.0 | 2.3 | 5.5 | wt |
| 139 | 4 | 0.98 | 0.5 | 2.4 | wt |
| 178 | 2 | 4.6 | 7.8 | 3.5 | wt |
| 179 | 2 | 1.4 | 2.2 | 2.1 | wt |
| 71 | 4 | 2.6 | 2.9 | Failed | wt |
| 177 | 4 | 1.4 | 1.8 | 2.9 | wt |
| 71 | 10 | 0.54 | 0.57 | 1.1 | 273cgt-cat |
| 136 | 7 | 0.29 | 0.16 | 0.91 | 249agg-tgg |
| 157 | 10 | 2.3 | 1.6 | 1.9 | 273cgt-cat |
| 95 | 1 | 0.66 | 0.43 | 1.8 | wt |
| 184 | 11 | 1.26 | 2.74 | 2.2 | Not done |
| 134 | 6 | 3.1 | 2.3 | 1.7 | Not done |
| 76 | 4 | 2.7 | 0.97 | 2.66 | Not done |
| Patient no. . | mClCCP IC50 . | Mean bcl2 (BFM × 105) . | Mean bax (BFM × 106) . | Mean bcl-x (BFM × 105) . | p53 status . |
|---|---|---|---|---|---|
| 154R | 6 | 3.1 | 2.7 | 6.1 | wt |
| 16 | 10 | 0.54 | 1.5 | 3.9 | wt |
| 161 | 2 | 0.81 | 1.2 | 3.4 | wt |
| 145 | 10 | 2.0 | 2.3 | 5.5 | wt |
| 139 | 4 | 0.98 | 0.5 | 2.4 | wt |
| 178 | 2 | 4.6 | 7.8 | 3.5 | wt |
| 179 | 2 | 1.4 | 2.2 | 2.1 | wt |
| 71 | 4 | 2.6 | 2.9 | Failed | wt |
| 177 | 4 | 1.4 | 1.8 | 2.9 | wt |
| 71 | 10 | 0.54 | 0.57 | 1.1 | 273cgt-cat |
| 136 | 7 | 0.29 | 0.16 | 0.91 | 249agg-tgg |
| 157 | 10 | 2.3 | 1.6 | 1.9 | 273cgt-cat |
| 95 | 1 | 0.66 | 0.43 | 1.8 | wt |
| 184 | 11 | 1.26 | 2.74 | 2.2 | Not done |
| 134 | 6 | 3.1 | 2.3 | 1.7 | Not done |
| 76 | 4 | 2.7 | 0.97 | 2.66 | Not done |
IC50 indicates the dose of mCICCP which induces 50% loss of mitochondrial membrane potential; BFM, bound fluorochrome molecules.
Correlation coefficients (Spearman ρ for mClCCP IC50 with bcl family expression) were −0.172 for bcl2, 0.178 for bax, and −0.282 for bcl-x; none of these correlations were significant at theP < .05 level.
The role of TP53 in mitochondrial membrane depolarization
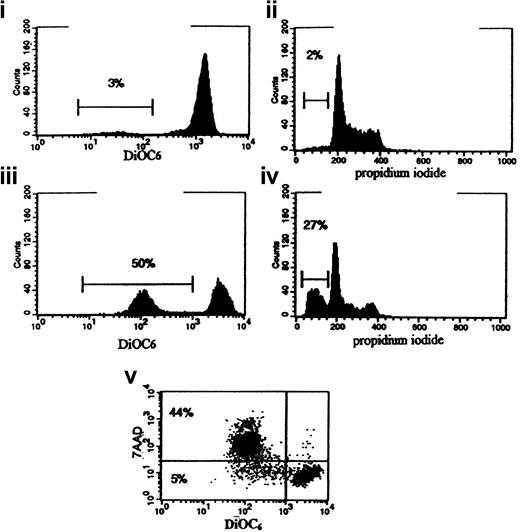
A role for TP53 in depolarizing the mitochondrial membrane has been described.35 TP53 mutation occurs in approximately 15% of AML cases.20 Cells from 3 patients with AML with a known TP53 mutation were available for inclusion in this study.46 All 3 had an mClCCP IC50 above the median level (6.5 μM) for the group as a whole (the specific values were 7, 10, and 10 μM, respectively; Table 1). This finding suggested that lack of p53 expression may be associated with resistance to mitochondrial membrane depolarization, but because of the infrequency of the TP53 mutation in patients with AML, we were unable to obtain enough clinical material for a statistical analysis of significance. Therefore, to confirm that TP53 directly affects MTP in leukemic cells, we studied CEM cells with a forced temperature-sensitive expression of wild-type TP53.21 After 18 of hours culture at the permissive temperature of 32°C, apoptosis was apparent on PI analysis of decreased DNA in 30% (SE, 4.4%) of cells from the TP53 subline 4G5, and mitochondrial membranes were depolarized at a higher percentage—47% (SE, 4%) of cells (Figure6)—thereby showing that wild-type p53 expression does induce mitochondrial membrane depolarization. Using the vital dye 7-AAD, which is taken up by apoptotic cells, we also demonstrated that mitochondrial membrane depolarization preceded loss of plasma membrane integrity.
Flow cytometric histograms for DiOC6 and apoptotic probes in the TP53 temperature-sensitive mutant 4G5 cell line cultured at the permissive temperature (32°C) for 9 and 18 hours.
At 9 hours, few DiOC6-low (depolarized, plot i) or PI-low (sub-G0, plot ii) cells were apparent. At 18 hours, DiOC6-low (depolarized cells, plot iii) were observed. PI-low sub-G0 cells were also found at this time (plot iv) but in a smaller proportion of cells than had depolarized. These results are representative of 5 similar experiments. Dual staining with DiOC6 and the vital dye 7-AAD (plot v) showed a subset of cells that had depolarized but were impermeable to 7-AAD (lower left quadrant), indicating that loss of MTP preceded loss of membrane integrity in the apoptotic process.
Flow cytometric histograms for DiOC6 and apoptotic probes in the TP53 temperature-sensitive mutant 4G5 cell line cultured at the permissive temperature (32°C) for 9 and 18 hours.
At 9 hours, few DiOC6-low (depolarized, plot i) or PI-low (sub-G0, plot ii) cells were apparent. At 18 hours, DiOC6-low (depolarized cells, plot iii) were observed. PI-low sub-G0 cells were also found at this time (plot iv) but in a smaller proportion of cells than had depolarized. These results are representative of 5 similar experiments. Dual staining with DiOC6 and the vital dye 7-AAD (plot v) showed a subset of cells that had depolarized but were impermeable to 7-AAD (lower left quadrant), indicating that loss of MTP preceded loss of membrane integrity in the apoptotic process.
The mClCCP IC50 is associated with MRP expression in primary AML blasts
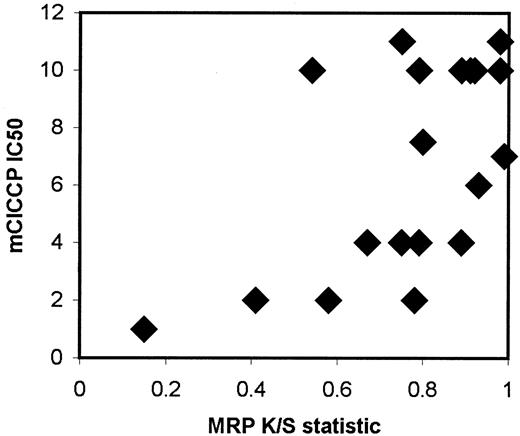
There is some evidence that TP53 controls genes involved in detoxification of cells affected by oxidative stress.23-25,54 Although most of the mammalian TP53 inducible genes have been only partly identified, control of the MRP promoter has been demonstrated.25 Moreover, we previously found that MRP overexpression is associated with the presence of TP53 mutations in AML cells.55 This led us to examine the correlation between MRP expression and mClCCP IC50 in cases of AML, and we found that there was a significant association (rs = 0.58; P = .019; Figure7).
Scatterplot for mClCCP IC50 plotted against MRP expression for cases of primary AML.
Scatterplot for mClCCP IC50 plotted against MRP expression for cases of primary AML.
Effects of antioxidant inhibitors on mitochondrial membrane depolarization
Because MRP is associated with redox status,56,57 we wondered whether other components of redox status affected the MTP in AML. Permeability transition can be induced by depletion of antioxidants within the mitochondrial space.58-60 However, because antioxidants are not evenly distributed between the mitochondrion and the cytoplasm,61 we decided not to measure total antioxidant levels in AML cells but to take the functional approach of measuring the effects of antioxidant inhibition on mitochondrial membrane depolarization. DEM is a monovalent thiol reagent that is commonly used to deplete cellular glutathione62 and can induce subsequent apoptosis,63 whereas diamide is a divalent thiol reagent that induces apoptosis through the formation of disulfide bonds in molecules with vicinal thiol groups.47 We investigated whether sensitivity to mitochondrial membrane depolarization could be augmented by antioxidant depletion through thiol or dithiol oxidation. Thus, we measured DiOC6 fluorescence in the presence of a low dose of mClCCP (3 μM) in the presence and absence of DEM or diamide. For both compounds, 100 μM doses were chosen because preliminary 7-AAD analysis of AML blasts cultured overnight with the compounds indicated that some apoptosis was induced at these doses, whereas higher doses could lead to the death and disintegration of entire clones (data not shown). Others64 65 demonstrated previously that death induced by a high dose of antioxidant inhibitors may be necrotic. Diamide, which alone induced a mean of 17.3% depolarization in the 10 samples tested, increased the depolarization induced by low-dose mClCCP from 22% to 53.6% (P = .047; Figure 8), indicating that cross-linking of thiols can sensitize AML blasts to depolarization. In contrast, the monovalent thiol reagent DEM increased the membrane potential in 6 of 8 cases and had no significant effect on depolarization in response to mClCCP (Figure 8).
Effects of diamide and DEM on depolarization.
Depolarization induced in 10 primary AML cases by 3 μM mClCCP and 100 μM diamide and in 8 cases by 3 μM mClCCP and 100 μM DEM. Cells were preincubated with diamide or DEM for 2 hours before addition of mClCCP. Results are expressed as the mean percentage of control cell depolarization on a scale calibrated by calculating that 100% depolarization is induced by 30 μM mClCCP. Error bars represent SDs. Significance levels refer to Wilcoxon signed-rank analysis of comparisons with the depolarization induced by 3 μM mClCCP alone. Two of 10 samples were not sensitized with diamide, and the range of responses was large: +5% to +43% for mClCCP, −25% to +55% for diamide alone, and −4% to +107% for the combination of the 2. DEM enhanced MTP in 6 of 8 cases (range −176% to +38%) and had no significant effect on mClCCP-induced depolarization.
Effects of diamide and DEM on depolarization.
Depolarization induced in 10 primary AML cases by 3 μM mClCCP and 100 μM diamide and in 8 cases by 3 μM mClCCP and 100 μM DEM. Cells were preincubated with diamide or DEM for 2 hours before addition of mClCCP. Results are expressed as the mean percentage of control cell depolarization on a scale calibrated by calculating that 100% depolarization is induced by 30 μM mClCCP. Error bars represent SDs. Significance levels refer to Wilcoxon signed-rank analysis of comparisons with the depolarization induced by 3 μM mClCCP alone. Two of 10 samples were not sensitized with diamide, and the range of responses was large: +5% to +43% for mClCCP, −25% to +55% for diamide alone, and −4% to +107% for the combination of the 2. DEM enhanced MTP in 6 of 8 cases (range −176% to +38%) and had no significant effect on mClCCP-induced depolarization.
To further define the role of vicinal thiol oxidation in depolarization of mitochondrial membranes and to determine whether this effect is TP53 dependent, we examined the effect of preventing dithiol oxidation on MTP and apoptosis in temperature-sensitive p53 mutant CEM cells. MBB is a monovalent thiol-reactive agent that prevents vicinal thiol cross-linking, mitochondrial membrane depolarization, and apoptosis.47 60 We found that apoptosis was inhibited in the CEM 4G5 cells incubated overnight at the TP53 permissive temperature when these cells were cultured with MBB (Figure9). In 3 similar experiments, we observed 35.7% ± 1.5% depolarized cells and 21% ± 1% sub-G0cells after overnight culture without MBB and 14.7% ± 4.0% depolarized cells and 1.7% ± 2.1% sub-G0 cells after culture with MBB (values corrected for depolarized and sub-G0 C7H2 cells). We used PI on permeabilized cells as our primary method for detecting apoptosis in this experiment. However, because this method may fail to discriminate loss of DNA content from cells dying in S or G2M phases, we also examined DiOC6 data plots for evidence of reduced forward scatter as an indicator of cell shrinkage in apoptotic cells. Using this method, we confirmed that apoptosis in 4G5 cells decreased when the cells were cultured with MBB (data not shown). The results of this experiment support a role for vicinal thiol–mediated pathways in TP53-induced apoptosis.
Inhibition of depolarization and apoptosis by MBB.
Flow cytometric histograms for DiOC6 and PI staining of the CEM C7H2 cell or its daughter cell CEM 4G5, whose mutant TP53 adopts a wild-type conformation when cultured at the permissive temperature (32°C). The cells were cultured for 18 hours with or without 4.5 μM MBB. Percentages of depolarized (DiOC6-low) cells or cells with less DNA than the G0/G1 peak (PI-low cells) are indicated on the histograms. Results are representative of 3 similar experiments.
Inhibition of depolarization and apoptosis by MBB.
Flow cytometric histograms for DiOC6 and PI staining of the CEM C7H2 cell or its daughter cell CEM 4G5, whose mutant TP53 adopts a wild-type conformation when cultured at the permissive temperature (32°C). The cells were cultured for 18 hours with or without 4.5 μM MBB. Percentages of depolarized (DiOC6-low) cells or cells with less DNA than the G0/G1 peak (PI-low cells) are indicated on the histograms. Results are representative of 3 similar experiments.
Discussion
Using a sensitive flow cytometric assay, we demonstrated differential sensitivity to mitochondrial membrane depolarization by mClCCP in AML blasts. This assay formed a basis for studying pathways contributing to depolarization resistance that could thus affect apoptosis in AML. Furthermore, we determined that depolarization can be enhanced by diamide, an agent that cross-links vicinal thiols. Surprisingly, sensitivity to mitochondrial membrane depolarization was not significantly associated with total protein levels of bcl-2, bax, or bcl-x but was associated with mutant TP53 and with high expression of MRP. We also determined that sensitivity to mitochondrial membrane depolarization correlates with spontaneous apoptosis in vitro. A high mClCCP IC50 may thus be indicative of autocrine growth factor–mediated—or of growth factor–independent—rescue from apoptosis in AML, which may be clinically relevant in light of the previously established association between autonomous growth of blast cells and reduced patient survival.50
An obligate role for mitochondrial membrane depolarization in TP53-mediated apoptosis has been shown in thymocyte and DLD-1 (colorectal cancer) models.23,35 Using the CEM C7H2 subclone 4G5 with temperature-sensitive TP53 expression, we showed that wild-type TP53 directly induces mitochondrial membrane depolarization in leukemic cells. The regulation of bcl family proteins, particularly bax, by TP53 is well documented,21,22,66,67 but it was also found that TP53 may also control apoptosis through activation of redox pathway genes.22-24,54,68 Up-regulation of bax, which is strongly associated with TP53 expression,21,22may not be sufficient to induce apoptosis, as was clearly shown in a temperature-sensitive mutant TP53 erythroleukemia model in which rescue from apoptosis could be achieved by using the receptor tyrosine kinase KIT, although TP53-dependent bax production was high in both rescued and unrescued cells.22 Antioxidants have been shown to inhibit TP53-induced membrane depolarization68 and to suppress apoptosis.68,69 The antiapoptotic effect of the thiol cross-linking inhibitor MBB on TP53-specific pathways was not directly demonstrated previously, although it is entirely consistent with the antiapoptotic effect of MBB on drug-treated thymocytes.47 The critical molecular targets of the dithiol cross-linking compound diamide are unclear, but the adenine nucleotide translocator27,70,71 is a strong contender. Thioredoxin is also known to be oxidized by diamide.17Diamide overcomes thioredoxin-mediated drug resistance in adult T-cell leukemia cells,18 but its importance in AML is not known and merits exploration.
The central role for mitochondrial depolarization in triggering apoptosis was established in studies showing that apoptosis was abolished when depolarization was blocked.72 Although mitochondrial membrane depolarization is not part of all apoptotic pathways, it is known to be part of the death pathway of cells exposed to chemotherapeutic drugs such as those commonly used to treat leukemia.47,52 Several proteins at the inner mitochondrial membrane or contact sites between inner and outer membranes can affect depolarization,4 and an aim of this study was to work toward identifying the crucial molecules in AML. The relative contributions of most individual molecules to therapy resistance in clinical samples cannot be assessed easily because there are so many contending molecules (at least 16 bcl proteins alone), and their differential deployment is likely to depend on complex activation and inhibitory pathways.73 Indeed, in simplified models, factors that affect the sensitivity of the mitochondrial pore were shown to interact: bcl-2 inhibited oxidative stress–induced apoptosis,74 and conversely, cellular thiol depletion reversed bcl-2–induced resistance to oxidative stress.63 75 A quantitative functional assay of sensitivity to a depolarization agonist is therefore useful because it enabled us to measure resistance modulation in primary tumor cells and to determine associations with levels of candidate modulatory molecules.
We found that all 3 cases of AML with mutant TP53 were resistant to mClCCP. Moreover, using the temperature-sensitive CEM sublines, we demonstrated that depolarization is at least partly under TP53 control. Specific genomic targets of TP53 have not been fully characterized.23 However, one known target is MRP, which is involved in cellular detoxification25 and is elevated in patients with AML who have TP53 mutations.55 We do not know why MRP expression was high in cases with a high mitochondrial mClCCP IC50. It may be that MRP is one of several detoxification-related molecules under common regulatory control and should thus be viewed simply as a marker of mitochondrial membrane sensitivity to depolarization. MRP, which associates with glutathione to function as an efflux pump, is coexpressed with γ-glutamylcysteine synthetase, a rate-limiting enzyme in the formation of glutathione,76 77 so it is plausible that MRP expression reflects the intracellular concentration of glutathione.
Expression of redox-related and bcl family proteins is highly heterogeneous in AML.13,15,42,78,79 In several cellular models, bcl proteins transfected into hematopoietic cells affected sensitivity to mitochondrial depolarization, but our data do not support the idea sometimes extrapolated from these studies that variations in total expression of bcl-2, bcl-x, or bax are responsible for clinical variability in chemoresistance. In our experiments, absolute levels of bcl-2, bax, or bcl-x did not appear to be important. However, posttranslational differences between bcl proteins that affected the ability of these proteins to associate and move between intracellular compartments cannot be ruled out as factors contributing to differential sensitivity to depolarization and apoptosis.5 Furthermore, our data should not be interpreted as negating the importance of this protein family and should be evaluated along with our previous finding that bcl-2 oligonucleotides can increase in vitro chemosensitivity in some primary AML blasts.6 Furthermore, bcl-2 is known to have a fundamental role in maintaining MTP in the presence of depolarizing stimuli.39
The model that appears most consistent with our patient data is that described by Costantini and colleagues,71 who demonstrated that oxidation of the adenine nucleotide translocator by thiol cross-linking agents can induce permeability transition by a mechanism independent of bcl-2. By moving away from a model system and establishing a functional assay for primary leukemia clones, we have shown that the vicinal thiol/disulfide rheostat is a variable but significant determinant of MTP in AML. This has important implications in the search for treatments that aim to suppress apoptosis-resistance pathways.
Supported by a grant from the Austrian Science Fund (SFB-F002) to R.K.; M.P. and N.R. are supported by the Leukaemia Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Monica Pallis, Academic Haematology, Clinical Sciences Building, Nottingham City Hospital, Nottingham NG5 1PB, United Kingdom.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal