This study demonstrates that in vivo exposure to cigarette smoke (CS) and in vitro treatment of long-term bone marrow cultures (LTBMCs) with nicotine, a major constituent of CS, result in inhibition of hematopoiesis. Nicotine treatment significantly delayed the onset of hematopoietic foci and reduced their size. Furthermore, the number of long-term culture-initiating cells (LTC-ICs) within an adherent layer of LTBMCs was significantly reduced in cultures treated with nicotine. Although the production of nonadherent mature cells and their progenitors in nicotine-treated LTBMCs was inhibited, this treatment failed to influence the proliferation of committed hematopoietic progenitors when added into methylcellulose cultures. Bone marrow stromal cells are an integral component of the hematopoietic microenvironment and play a critical role in the regulation of hematopoietic stem cell proliferation and self-renewal. Exposure to nicotine decreased CD44 surface expression on primary bone marrow–derived fibroblastlike stromal cells and MS-5 stromal cell line, but not on hematopoietic cells. In addition, mainstream CS altered the trafficking of hematopoietic stem/progenitor cells (HSPC) in vivo. Exposure of mice to CS resulted in the inhibition of HSPC homing into bone marrow. Nicotine and cotinine treatment resulted in reduction of CD44 surface expression on lung microvascular endothelial cell line (LEISVO) and bone marrow–derived (STR-12) endothelial cell line. Nicotine treatment increased E-selectin expression on LEISVO cells, but not on STR-12 cells. These findings demonstrate that nicotine can modulate hematopoiesis by affecting the functions of the hematopoiesis-supportive stromal microenvironment, resulting in the inhibition of bone marrow seeding by LTC-ICs and interfering with stem cell homing by targeting microvascular endothelial cells.
Introduction
Proliferation, differentiation, and self-renewal of hematopoietic bone marrow cells are strictly regulated and complex processes. They are controlled by a number of soluble factors, including cytokines, interleukins, and chemokines, as well as by extracellular matrix (ECM) and adhesion molecules. The cellular compartment of the bone marrow is represented by a heterogeneous population of mature cells, including hematopoietic progenitor cells at different stages of differentiation and stromal cells. Stromal cells are an integral part of the regulatory network within the bone marrow microenvironment and play a critical role in hematopoiesis. Stromal cells elaborate various positive and negative regulators as well as ECM and adhesion molecules that contribute to the formation of “stem cell niches.” These stem cell niches regulate proliferation, differentiation, and self-renewal of hematopoietic stem cells.1-4 Stromal cells are also a major component of the adherent layer of long-term bone marrow cultures (LTBMCs). Several bone marrow–derived stromal cell lines substituting primary stromal cells in in vitro cultures have been described.5-7 These cell lines, as well as primary stromal cells, give rise to stem cell regulatory signals via production of cytokines, ECM, and expression of adhesion molecules.
Stromal cells are sensitive to extrinsic regulatory factors. For example, steroid hormones and cytokines8-11 can alter the production of ECM and cytokines by stromal cells. The expression pattern of adhesion molecules and their ligand-binding activity can be influenced by exposure to different chemicals and cytokines. In addition, platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and macrophage colony-stimulating factor (M-CSF) regulate stromal cell function by an autocrine mechanism.12-14 The function of stromal cells is also dramatically altered in a number of hematologic disorders.15-19 These observations imply that the hematopoietic microenvironment, as a target for extrinsic factors, may have a significant effect on hematopoiesis during both normal and pathologic conditions.
Exposure to certain pathophysiologic factors, including cigarette smoke (CS) and tobacco smoke (TS) and their byproducts, could lead to an imbalance in hematopoietic homeostasis.20,21 Exposure to TS results in the reduction of functionally active immunocompetent cells.22 Recent studies have revealed that the hematopoietic system is one of the targets of TS. A number of constituents of TS that are distributed throughout the bloodstream directly affect target cells. For example, bone marrow–derived macrophages incubated with the gas phase of CS demonstrated a dose-dependent decrease of tissue-type (thrombin-independent) transglutaminase activity, although viability or adherence of these cells was not affected.23 Moreover, exposure to components of CS such as acrolein and acetaldehyde inhibits proliferation and migration of fibroblasts,24,25 a part of the bone marrow stromal microenvironment. Furthermore, CS inhibits the production of fibronectin and collagen by fibroblasts.25-27 These ECM molecules play an important role in hematopoietic stem/progenitor cells (HSPC) behavior.28 These studies suggest that bone marrow cells are a target for components of TS, including nicotine.
In the present study, the effects of CS, nicotine, and its metabolite cotinine on LTBMCs and their influence on HSPC homing were determined. Our findings suggest that in addition to other deleterious effects of CS and nicotine on the immune and vascular systems, the development of hematopoietic tissue in individuals exposed to TS could also be affected through altered function of the hematopoietic microenvironment.
Materials and methods
Endothelial and stromal cell lines
Human umbilical vein endothelial cells (HUVECs) were obtained from Clonetics (Walkersville, MD) and cultured according to the manufacturer's instructions. STR-12, a murine bone marrow endothelial cell line, and LEISVO, a murine lung endothelial cell line (kindly provided by Dr Masanobu Kobayashi, Hokkaido School of Medicine, Sapporo, Japan), were cultured in RPMI supplemented with 10% fetal calf serum (FCS).29 The MS-5 murine stromal cell line was cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FCS.7
Flow cytometry
The cell-surface expression of human CD44 (monoclonal antibody [mAb] 25-32), α4 (mAb P4C2), β2 (mAb 60.3), and β7 (mAb FIB 504) adhesion molecules on HUVECs was determined by flow cytometry. Similarly, the surface expression of murine CD44 (mAb IM7; American Type Culture Collection TIB235), vascular cell adhesion molecule (VCAM; MK 2.7), α4 (mAb PS/2), β2 (2E6), and β7 (mAb FIB504), as well as E-selectin (mAb 9A9) and P-selectin (mAb 5H1) (obtained from Dr Eugene Butcher, Stanford University, Stanford, CA, and Dr Barry Wolitzky, Hoffmann-La Roche, Nutley, NJ), on STR-12, LEISVO, MS-5 cells, and primary bone marrow–derived cells was determined by fluorescence-activated cell sorter (FACS) analysis. Briefly, 5 × 105 cells were incubated with phycoerythrin-labeled or fluorescein isothiocyanate–labeled antibody at a concentration of 10 μg/mL for 30 minutes at 4°C and washed twice with FACS buffer (phosphate-buffered saline [PBS], 2% FCS, 0.1% bovine serum albumin [BSA], 0.01% NaN3). Fluorescence was analyzed on a FACScan (Becton Dickinson, San Jose, CA) according to standard procedures.
HSPC purification
BALB/c mice 4 to 8 weeks old were used for all experiments. The contents of femurs and tibias were flushed out from the bone with PBS supplemented with 5% FCS with a needle (21G) attached to a 1-mL syringe. Cells were kept on ice until use.30 HSPC were isolated according to the manufacturer's instructions (StemCell Technology, Vancouver, BC, Canada). Briefly, bone marrow cells were incubated with a cocktail containing lineage-specific antibodies for 15 minutes at 4°C. After washing, bone marrow cells were incubated with antibiotin tetrameric antibody complexes for 15 minutes, followed by incubation with magnetic colloid for 15 minutes. Thereafter, cells were applied on a magnetic column. Recovered cells were collected, washed, and assayed.
Methylcellulose assay
Bone marrow cells (2 × 104/mL of plating mixture) were mixed gently with semisolid methylcellulose medium supplemented with 10% FCS, 1% BSA, L-glutamine, and 2-mercaptoethanol (StemCell Technology). Corresponding lineage-specific factors, including interleukin (IL)-7 (1 ng/mL), IL-5 (10 ng/mL), granulocyte macrophage colony-stimulating factor (GM-CSF; 10 ng/mL), or M-CSF, were added to induce growth of B-lymphoid (CFU-B), eosinophil (CFU-eos), granulocyte-macrophage, and macrophage colonies, respectively. To induce the growth of early erythroid cell progenitors, IL-3 and erythropoietin (1 U/mL) were added. The conditioned medium from cell line WEHI-3B was used as a source of IL-3 (15% vol/vol). The conditioned medium from cell line L929 was used as a source of M-CSF (15% vol/vol).30-32 The cultures were incubated in a humidified incubator with 5% CO2 at 37°C for 7 to 14 days. Colonies (a group of more than 50 cells) were scored under the microscope.
Long-term bone marrow cultures
Bone marrow cells (1 × 106 cells/mL) were cultured in 6-well or 24-well plates in DMEM supplemented with 20% horse serum (StemCell Technology) and hydrocortisone (10−6M; Sigma) at 33°C in a humidified atmosphere at 5% CO2. Cultures were fed weekly by replacing half of the culture medium. Nonadherent cells were collected, counted, and used for the CFU assay.30
Long-term culture-initiating cell assay
The assay was performed according to the manufacturer's instructions (StemCell Technology). Briefly, cell populations to be examined were plated in DMEM supplemented with 20% horse serum and hydrocortisone (10−6 M) in limiting dilution into 96-well plates containing the stromal cell line S17. Cultures were fed weekly, and the numbers of wells containing colonies were evaluated after 14 days of culture.33
Bone marrow–derived macrophage cultures
Bone marrow–derived macrophages were cultured according to a protocol described previously.34 Bone marrow cells at a concentration of 1 × 106 cells/mL were cultivated in DMEM containing 2 mM glutamine, 0.37% (wt/vol) NaHCO3, 10% (vol/vol) heat-inactivated FCS, and 10% (vol/vol) L929 cell-conditioned medium as a source of M-CSF. Cultures were maintained at 37°C in a 5% CO2 atmosphere for 8 days. Ninety-nine percent of the cells then on the culture dish were phagocytic for latex particles.
Primary bone marrow–derived fibroblastlike stromal cell cultures
Bone marrow cells (106/mL) were incubated in DMEM supplemented with 20% horse serum (StemCell Technology) and hydrocortisone (10−6 M; Sigma) at 33°C in a humidified atmosphere at 5% CO2 for 24 hours. Thereafter, nonadherent cells were washed out and the remaining cells were further cultured under the same conditions. Each week, cultures were passaged into the new culture flasks with fresh media. After 28 days, monolayers containing stromal cells were used for experiments.
Nicotine and cotinine treatment
Nicotine and cotinine (both from Sigma, St Louis, MO; dissolved in ethanol, 10−1 M stock) were diluted in PBS, sterile filtered, and used immediately in a light-protected cell culture hood. A total of 75% to 90% confluent cells were treated with varying concentrations of nicotine or cotinine (10−4 M to 10−8 M) and incubated at 37°C for 5 minutes, 30 minutes, 1 hour, 2 hours, and 4 hours. Thereafter, the cells were detached using Enzyme Free Cell Dissociation Solution (Speciality Media, Phillipsburg, NJ) and assayed for cell-surface molecule expression by flow cytometry. For LTBMC and CFU assays, nicotine or cotinine was diluted in cell culture medium and sterile filtered before use.
CS exposure, repopulation assay, and spleen CFU assay
To determine the effect of CS on hematopoiesis, we placed BALB/c mice (n = 10) in a hermetically closed chamber filled with CS (n = 10 cigarettes). Animals (n = 10) were killed after 21 days of intermittent exposure to CS, and bone marrow cells were harvested, calculated, and assayed for hematopoietic progenitors in methylcellulose cultures. For the assay of marrow-repopulating ability, mice were lethally irradiated with 8 Gy, and after 24 hours reconstituted with 1 × 106 bone marrow cells injected intravenously.30 Animals were killed after 14 days, and bone marrow cells were collected from a femur of each mouse, calculated, and transplanted into lethally irradiated recipients (4 × 104/mouse) to determine the number of CFU in spleens after 12 days (CFUs-12).35 Spleens were fixed in Tellesnicky's solution, and colonies were counted after several hours of fixation.
Results
CS decreases the number of committed progenitors in the bone marrow
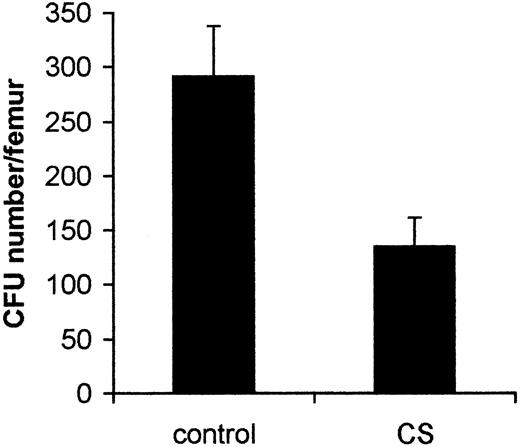
To determine the effect of CS on hematopoiesis, we exposed BALB/c mice (n = 10) to CS (n = 10 cigarettes) in a hermetically closed chamber. Compared with control animals, exposure to CS had no impact on the total numbers of bone marrow cells (25.8 ± 3.1 × 106/femur in control mice and 22.5 ± 4.7 × 106/femur in CS-exposed mice). However, in mice exposed to CS, the total number of myeloid progenitors in the femur was decreased by 2.1-fold (from 291.5 ± 46.4 in control mice to 135.0 ± 27 in CS-exposed mice; P < .01) (Figure1).
CS affects the number of myeloid progenitors in bone marrow.
Experimental and control groups of mice were kept in the hermetically closed chamber with or without CS (one cigarette per mouse), respectively, for 5 hours daily for 2 weeks. Bone marrow cells from control mice (n = 10 mice) and those exposed to CS (n = 10 mice) were plated into methylcellulose cultures in the presence of IL-3. Colonies were counted after 7 days of incubation. Data presented are from one representative experiment (mean ± SD).
CS affects the number of myeloid progenitors in bone marrow.
Experimental and control groups of mice were kept in the hermetically closed chamber with or without CS (one cigarette per mouse), respectively, for 5 hours daily for 2 weeks. Bone marrow cells from control mice (n = 10 mice) and those exposed to CS (n = 10 mice) were plated into methylcellulose cultures in the presence of IL-3. Colonies were counted after 7 days of incubation. Data presented are from one representative experiment (mean ± SD).
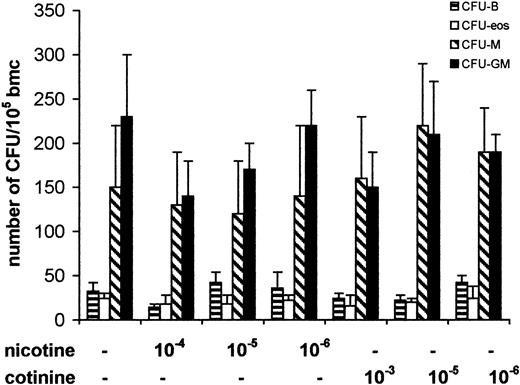
Next, to determine the direct effect of nicotine on colony-forming ability of committed hematopoietic progenitors, we cultured freshly isolated bone marrow cells in methylcellulose semisolid cultures supplemented with lineage-specific factors with varying concentrations of nicotine and its metabolite cotinine. Recent studies have demonstrated the nonspecific and high levels of intracellular uptake of nicotine by fibroblasts.36 Because the intracellular uptake of nicotine is likely to vary depending upon the extent of exposure,36 we tested a wide range of concentrations of nicotine and cotinine on colony-forming ability of hematopoietic progenitor cells. Treatment of cultures with nicotine and cotinine at concentrations of 10−4 to 10−6 M failed to induce a significant change in the number of colonies. These results suggest that treatment of freshly isolated intact bone marrow cells with nicotine or cotinine does not affect the ability of committed progenitors to proliferate and form colonies (Figure2).
Influence of nicotine and cotinine on colony formation of myeloid and lymphoid progenitors.
Freshly isolated bone marrow cells (bmc) were cultivated in semisolid methylcellulose cultures with lineage-specific cytokines. Bone marrow cells were cultured with GM-CSF or M-CSF to assay for granulocyte-macrophage or macrophage progenitors (CFU-GM, CFU-M), with IL-7 to assay for B-cell progenitors (B-cell colony-forming unit, CFU-B), and with IL-5 to assay monopotent progenitors for eosinophils (CFU-eos). Nicotine and cotinine were diluted in PBS, sterile filtered, and added into cultures at final concentrations indicated on each graph. Data shown are means ± SD of the colony numbers from 4 different wells, representing 1 of 3 independent experiments.
Influence of nicotine and cotinine on colony formation of myeloid and lymphoid progenitors.
Freshly isolated bone marrow cells (bmc) were cultivated in semisolid methylcellulose cultures with lineage-specific cytokines. Bone marrow cells were cultured with GM-CSF or M-CSF to assay for granulocyte-macrophage or macrophage progenitors (CFU-GM, CFU-M), with IL-7 to assay for B-cell progenitors (B-cell colony-forming unit, CFU-B), and with IL-5 to assay monopotent progenitors for eosinophils (CFU-eos). Nicotine and cotinine were diluted in PBS, sterile filtered, and added into cultures at final concentrations indicated on each graph. Data shown are means ± SD of the colony numbers from 4 different wells, representing 1 of 3 independent experiments.
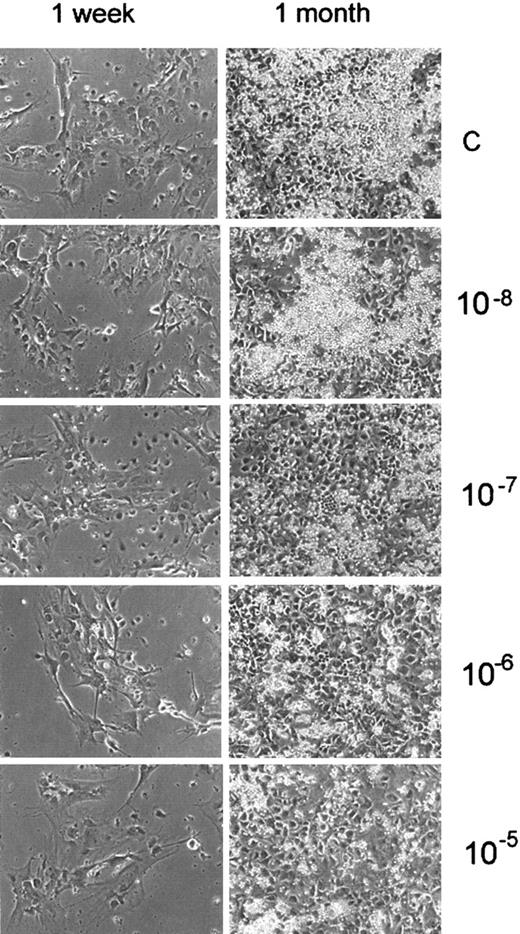
Nicotine inhibits the formation of an adherent layer in LTBMCs
We next determined whether nicotine or cotinine might inhibit hematopoiesis by affecting the hematopoietic microenvironment. To examine this, we added physiologic concentrations of nicotine or cotinine (10−5 M to 10−8 M) to LTBMCs from the first day of culture. Treatment of the cultures with nicotine resulted in inhibition of adherent-layer formation in a dose-dependent manner (Figure 3). Although the formation of a confluent adherent layer was not significantly affected in LTBMCs treated with nicotine or cotinine, the formation of loci of active hematopoiesis in LTBMCs, “cobblestone areas,” in cultures treated with nicotine (10−5 to 10−7 M) was inhibited (Figure 3). These areas were found to be smaller in cultures treated with nicotine than in control cultures or those treated with cotinine (data not shown). In support of this finding, we observed that neither nicotine nor cotinine affected the adhesion of fibroblast precursors (CFU-F) to culture dishes (data not shown).
Nicotine inhibits the formation of “cobblestone areas” in LTBMCs.
LTBMCs were treated with different concentrations of nicotine (10−5 to 10−8 M). Nicotine was added to the culture medium weekly during feedings, starting from day 0. Formation of the adherent layer was monitored under an inverted microscope. Photographs were taken after 1 week and 1 month of culture.
Nicotine inhibits the formation of “cobblestone areas” in LTBMCs.
LTBMCs were treated with different concentrations of nicotine (10−5 to 10−8 M). Nicotine was added to the culture medium weekly during feedings, starting from day 0. Formation of the adherent layer was monitored under an inverted microscope. Photographs were taken after 1 week and 1 month of culture.
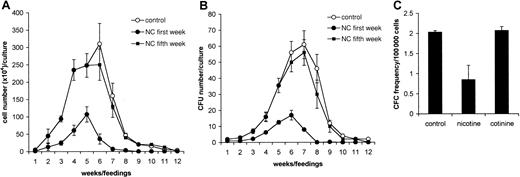
Nicotine inhibits nonadherent cell production in LTBMCs
Next, the influence of nicotine and cotinine on hematopoiesis in LTBMCs was examined. Nonadherent cells from LTBMCs were harvested weekly during feedings and counted. No differences in the nonadherent cell numbers were observed in cultures treated with 10−4to 10−6 M nicotine compared with controls after 1 week in culture (P > .1). At weeks 2 and 3, the numbers of nonadherent cells in short-term cultures treated with varying concentrations of nicotine were significantly lower than in controls (P < .01). The inhibition of nonadherent cell production was dose dependent (data not shown). Long-term administration of 10−5 M nicotine resulted in significant inhibition of nonadherent cell production during the entire period of culture (Figure4A). Interestingly, addition of cotinine at concentrations from 10−5 to 10−8 M did not affect hematopoiesis in LTBMCs (data not shown). Because the population of nonadherent cells in LTBMCs is heterogeneous and contains hematopoietic progenitors and mature cells, it is conceivable that the decreased number of total cells in LTBMCs could be due to the low number of progenitor cells generated in LTBMCs. Therefore, cells harvested during feedings were assayed for a number of myeloid progenitors. Although cotinine failed to affect the production of myeloid progenitors in LTBMCs (data not shown), treatment with nicotine resulted in a significant reduction in the numbers of myeloid progenitors (Figure 4B). This observation could be explained by low numbers of long-term culture-initiating cells (LTC-ICs) in nicotine-treated cultures. To examine this, we harvested adherent layers of LTBMCs treated with nicotine or cotinine and assayed them for LTC-ICs. We found that the numbers of LTC-ICs in nicotine-treated cultures were 2.5-fold lower than in control cultures or cultures treated with cotinine (Figure 4C).
Nicotine inhibits hematopoiesis in LTBMCs.
LTBMCs were treated weekly without (○) or with 10−5 M nicotine added from the first week of culture (●) and the fifth week of culture (▪). (A) Nonadherent cells were harvested weekly during feedings of the cultures (n = 4) and calculated, and numbers were expressed as mean ± SD. (B) Nonadherent cells harvested from LTBMCs treated with nicotine were plated at a concentration of 104 cells/mL in methylcellulose cultures (n = 4) supplemented with GM-CSF (10 ng/mL). After 7 days in culture, the number of colonies was counted, recalculated for a total number of cells for each culture, and expressed as mean ± SD. (C) LTBMCs were cultured with nicotine or cotinine for 3 weeks. Thereafter, the adherent layer of LTBMCs was harvested, calculated, and tested for LTC-IC content in a 14-day limiting-dilution assay on the S17 cell line. Data shown are means ± SD of the LTC-IC content of 2 experiments.
Nicotine inhibits hematopoiesis in LTBMCs.
LTBMCs were treated weekly without (○) or with 10−5 M nicotine added from the first week of culture (●) and the fifth week of culture (▪). (A) Nonadherent cells were harvested weekly during feedings of the cultures (n = 4) and calculated, and numbers were expressed as mean ± SD. (B) Nonadherent cells harvested from LTBMCs treated with nicotine were plated at a concentration of 104 cells/mL in methylcellulose cultures (n = 4) supplemented with GM-CSF (10 ng/mL). After 7 days in culture, the number of colonies was counted, recalculated for a total number of cells for each culture, and expressed as mean ± SD. (C) LTBMCs were cultured with nicotine or cotinine for 3 weeks. Thereafter, the adherent layer of LTBMCs was harvested, calculated, and tested for LTC-IC content in a 14-day limiting-dilution assay on the S17 cell line. Data shown are means ± SD of the LTC-IC content of 2 experiments.
We further investigated whether nicotine affects LTBMCs in steady state. Cultures were initially set up in the absence of nicotine for 3 weeks. After 5 weeks, we observed a well-formed adherent layer with cobblestone areas. Before the addition of nicotine (10−5M), cells from all cultures were harvested and enumerated weekly (Figure 4A). The number of nonadherent cells from LTBMCs did not differ between groups before nicotine treatment. The addition of nicotine at each subsequent feeding (ie, after the initial 5 weeks) failed to alter the production of nonadherent cells, as demonstrated by cell numbers harvested during the following period of culture (P > .1). Harvested cells were evaluated for the number of hematopoietic progenitors derived from cultures treated with and without nicotine (Figure 4B). We observed that the numbers of committed progenitors in LTBMCs were not significantly altered after treatment with nicotine.
These studies suggest that nicotine may affect the formation of hematopoietic “niches” within the adherent layer of LTBMCs, and this in turn leads to a reduced number of HSPC seeded.
Effect of nicotine on adhesion molecule expression by stromal cells
We next examined whether nicotine affects the expression of adhesion molecules by stromal cells because several of these cell-surface receptors, including CD44, α4, β2, β7, and VCAM, may participate in the formation of hematopoietic niches.3,4We incubated freshly isolated bone marrow cells with varying concentrations of nicotine and cotinine and then evaluated cell-surface expression of CD44, α4, β2, β7, and VCAM by flow cytometry. We observed that within 4 gated populations of bone marrow cells, only a small population (18%) of large and granulated cells (population number 3) demonstrated a decrease in the intensity of CD44 expression after treatment with nicotine, but not cotinine. Interestingly, the expression levels of α4, β2, β7, and VCAM were not affected by either nicotine or cotinine (Table 1). Because these cells might constitute the bone marrow hematopoietic microenvironment, we treated steady-state LTBMCs with nicotine or cotinine, harvested cells of the adherent layer, and examined adhesion molecule expression by FACS. Our findings consistently demonstrated a significant reduction in the cell-surface expression of CD44 on a population of large and granulated cells treated with nicotine, but not cotinine. These cells could be either bone marrow macrophages or bone marrow fibroblastlike stromal cells, a major cell population forming the bone marrow microenvironment. Therefore, we further developed primary cultures of bone marrow–derived macrophages and treated these cells with varying concentrations of nicotine and cotinine. This treatment did not affect the expression patterns of any of the adhesion molecules we examined. We next established primary cultures of bone marrow–derived fibroblastlike stromal cells. These cells demonstrated a significant reduction (2.3-fold) in the intensity of CD44 cell-surface expression after treatment with nicotine, but not cotinine. However, expression of other adhesion molecules remained unaffected. Finally, the MS-5 stromal cell line was used to study the effects of nicotine because it supports the growth of hematopoietic progenitors in vitro.7 Cell-surface expression levels of CD44, α4, β2, β7, and VCAM on harvested stromal cell line MS-5 after treatment with nicotine and cotinine were examined by FACS analysis. Exposure to nicotine resulted in a 3.5-fold decrease in the intensity of CD44 expression and a 3.5-fold increase in β7-integrin expression. In contrast, the expression of other adhesion molecules remained unaffected by this treatment.
Effects of nicotine and cotinine on expression of adhesion molecules in bone marrow–derived cells
| Cell population . | FSC/SSC . | Treatment . | Control . | CD44 . | VCAM . | α4 . | β2 . | β7 . |
|---|---|---|---|---|---|---|---|---|
| BMCs | 323 ± 53/185 ± 2 | NT | 4 ± 2.6/6.8 ± 1.8 | 73 ± 11/46 ± 6.5 | 8.5 ± 6.8/7.4 ± 0.3 | 38 ± 0.5/16 ± 0.7 | 5.7 ± 5.3/6.6 ± 1 | 8.4 ± 4.6/7 ± 1.4 |
| NC | 4.5 ± 2.8/6.9 ± 1.8 | 75 ± 14/52 ± 1.4 | 14.6 ± 4/9.2 ± 1.7 | 48 ± 5/23 ± 2.6 | 13 ± 6/9 ± 1.8 | 11 ± 9/7.7 ± 0.5 | ||
| CN | 5 ± 1.3/6.7 ± 1.6 | 74 ± 2/49 ± 20 | 13 ± 4.5/8 ± 1 | 39 ± 12/15 ± 5 | 6.5 ± 1.8/6.5 ± 1 | 10 ± 2.4/5 ± 3 | ||
| 528 ± 76/204 ± 13 | NT | 7.3 ± 2/59 ± 8 | 97.7 ± 2.8/480 ± 83 | 18.3 ± 5/82 ± 24 | 94.8 ± 2.4/330 ± 16 | 17 ± 9/86 ± 9 | 9.4 ± 3.6/56 ± 15 | |
| NC | 2 ± 1.4/49 ± 10.5 | 99 ± 0.06/448 ± 29 | 16.3 ± 1.4/72 ± 1.3 | 96 ± 2/298 ± 17 | 16.6 ± 5.8/67 ± 18 | 8.7 ± 1.6/45 ± 10 | ||
| CN | 3.5 ± 2/47.6 ± 3.4 | 98 ± 1.6/470 ± 44 | 20 ± 2.5/78 ± 3.3 | 94.7 ± 2/308 ± 26 | 15 ± 2.3/83 ± 2 | 7.2 ± 5/45 ± 16 | ||
| 836 ± 69/624 ± 4 | NT | 4.2 ± 1/82 ± 13 | 99 ± 1.2/2534 ± 116 | 22 ± 8.4/119 ± 45 | 41 ± 8.6/232 ± 10 | 57.5 ± 15/263 ± 35 | 6 ± 2/49 ± 9 | |
| NC | 7.7 ± 0.05/78 ± 11 | 99 ± 0.3/2148 ± 75 | 39 ± 13/109 ± 33 | 68 ± 11/250 ± 2.5 | 85 ± 2/293 ± 40 | 8.7 ± 1/38.2 ± 5.7 | ||
| CN | 6.3 ± 0.6/78 ± 6 | 99 ± 0.04/2677 ± 96 | 34 ± 9/110 ± 20 | 57.3 ± 1/240 ± 18 | 81 ± 7/260 ± 28 | 5.9 ± 3/42 ± 4.7 | ||
| 328 ± 23/572 ± 60 | NT | 5 ± 4/5.6 ± 1.5 | 78.3 ± 13/122 ± 1.2 | 9.5 ± 9/6.2 ± 0.6 | 27.9 ± 14/10 ± 0.6 | 6.2 ± 7/5.4 ± 1 | 7.5 ± 5.6/6 ± 1.6 | |
| NC | 5.3 ± 4.8/5.3 ± 1.5 | 77.4 ± 11/120 ± 8 | 9.9 ± 6.9/5.7 ± 0.7 | 27.5 ± 12/9.6 ± 0.4 | 10 ± 7/6 ± 1 | 8.6 ± 5.5/5.6 ± 1.2 | ||
| CN | 5.5 ± 0.7/5.2 ± 1.3 | 79 ± 2.8/117 ± 16 | 10 ± 6.8/5.8 ± 1 | 28 ± 8/10 ± 0.4 | 6 ± 3/5.7 ± 1 | 8 ± 2/6 ± 0.7 | ||
| LTBMCs | 625 ± 110/368 ± 150 | NT | 3 ± 1.2/4 ± 0.1 | 99 ± 0.9/203 ± 10 | 20.4 ± 3/7.2 ± 0.5 | 82 ± 15/26 ± 8 | 90.2 ± 5/31.5 ± 7.6 | 10 ± 2.8/5 ± 0.4 |
| NC | 3 ± 2/3.7 ± 0.4 | 97 ± 3.2/159 ± 8 | 19.3 ± 1.2/6.7 ± 0.5 | 84.5 ± 14/26 ± 7 | 90.5 ± 6/30.5 ± 5.29 | 9 ± 0.1/4.8 ± 0.04 | ||
| CN | 2.6 ± 2.2/3.9 ± 0.2 | 99 ± 0.6/189 ± 12 | 20.3 ± 1.5/7 ± 0.5 | 81 ± 14.8/24.6 ± 6 | 92.6 ± 0.7/32 ± 0.3 | 7.6 ± 1.5/4.7 ± 0.8 | ||
| 92 ± 10/124 ± 53 | NT | 2.3 ± 1.5/8.3 ± 2.6 | 24.4 ± 6.8/24 ± 6 | 27 ± 2.8/23 ± 6.3 | 17 ± 3.5/15 ± 3.7 | 18 ± 3/16 ± 4.4 | 7.7 ± 4/11 ± 2.3 | |
| NC | 1.6 ± 0.4/8.7 ± 3.8 | 24 ± 3.8/23 ± 6.7 | 23 ± 0.4/20 ± 7.2 | 16.8 ± 0.4/16 ± 5.4 | 17.6 ± 0.5/16.5 ± 6 | 7 ± 2.7/12 ± 3.8 | ||
| CN | 3 ± 1.6/9.5 ± 4 | 23.3 ± 4/22 ± 6.9 | 26.4 ± 6.3/21 ± 5.6 | 14.5 ± 0.4/14 ± 5.7 | 19 ± 4.7/17 ± 2.9 | 7.3 ± 3/11 ± 3.8 | ||
| PBMDMs | 495 ± 67/561 ± 26 | NT | 3.2 ± 0.04/7 ± 1.4 | 83 ± 2/27.9 ± 5.6 | 8 ± 1.2/9.4 ± 1.2 | 30.4 ± 5.2/14 ± 1.4 | 82 ± 3.6/25 ± 2.6 | 3.6 ± 0.4/7.6 ± 1.7 |
| NC | 2 ± 0.4/5.8 ± 2 | 83.7 ± 18/24 ± 8 | 7.7 ± 3.3/7.5 ± 2 | 27.6 ± 9/11 ± 2.5 | 71 ± 14/19 ± 6 | 3.2 ± 0.6/6.3 ± 1.6 | ||
| CN | 3.3 ± 0.3/6 ± 1.2 | 84.5 ± 0.9/27 ± 2.5 | 8.3 ± 0.1/9 ± 1.2 | 23 ± 12/12 ± 6 | 83 ± 10/26 ± 11 | 7 ± 5/8.2 ± 2.8 | ||
| PBMDFs | 188 ± 59/480 ± 120 | NT | 2.5 ± 0.7/2.6 ± 7.3 | 66 ± 4/355 ± 58 | 16 ± 0.6/53 ± 13 | 5 ± 1.3/32 ± 10 | 6.5 ± 1.4/30 ± 13 | 7 ± 2.4/36 ± 9 |
| NC | 2.7 ± 1.7/21 ± 7 | 48 ± 32.4/156 ± 22 | 12 ± 0.2/44 ± 9 | 6 ± 2/27 ± 13 | 5 ± 0.2/25 ± 11 | 4.3 ± 0.3/33 ± 2.8 | ||
| CN | 2.6 ± 0.8/24 ± 6.4 | 59 ± 5.4/296 ± 13 | 13 ± 2/52 ± 0.1 | 6.8 ± 0.05/30 ± 7 | 6 ± 0.3/28 ± 0.6 | 6 ± 0.9/33 ± 5 | ||
| MS-5 | 460 ± 61/358 ± 44 | NT | 3 ± 1.8/4 ± 0.5 | 81 ± 10/76.9 ± 28 | 3.5 ± 1.2/3.9 ± 0.4 | 5 ± 3.4/4 ± 0.6 | 1.4 ± 1.4/3.5 ± 0.3 | 7 ± 4.8/4 ± 0.7 |
| NC | 2.7 ± 1.4/4 ± 0.2 | 59.6 ± 3/22 ± 12 | 7 ± 5.4/4.7 ± 0.9 | 10 ± 11/5 ± 1.4 | 2 ± 1.3/4 ± 0.4 | 17.4 ± 5.4/14 ± 0.5 | ||
| CN | 3.2 ± 1.2/3.8 ± 0.6 | 71 ± 17/77 ± 9 | 7.5 ± 4.8/4.6 ± 0.5 | 3.4 ± 2.5/4.2 ± 0.9 | 2.5 ± 0.8/3.6 ± 0.6 | 19 ± 4.2/10 ± 7 |
| Cell population . | FSC/SSC . | Treatment . | Control . | CD44 . | VCAM . | α4 . | β2 . | β7 . |
|---|---|---|---|---|---|---|---|---|
| BMCs | 323 ± 53/185 ± 2 | NT | 4 ± 2.6/6.8 ± 1.8 | 73 ± 11/46 ± 6.5 | 8.5 ± 6.8/7.4 ± 0.3 | 38 ± 0.5/16 ± 0.7 | 5.7 ± 5.3/6.6 ± 1 | 8.4 ± 4.6/7 ± 1.4 |
| NC | 4.5 ± 2.8/6.9 ± 1.8 | 75 ± 14/52 ± 1.4 | 14.6 ± 4/9.2 ± 1.7 | 48 ± 5/23 ± 2.6 | 13 ± 6/9 ± 1.8 | 11 ± 9/7.7 ± 0.5 | ||
| CN | 5 ± 1.3/6.7 ± 1.6 | 74 ± 2/49 ± 20 | 13 ± 4.5/8 ± 1 | 39 ± 12/15 ± 5 | 6.5 ± 1.8/6.5 ± 1 | 10 ± 2.4/5 ± 3 | ||
| 528 ± 76/204 ± 13 | NT | 7.3 ± 2/59 ± 8 | 97.7 ± 2.8/480 ± 83 | 18.3 ± 5/82 ± 24 | 94.8 ± 2.4/330 ± 16 | 17 ± 9/86 ± 9 | 9.4 ± 3.6/56 ± 15 | |
| NC | 2 ± 1.4/49 ± 10.5 | 99 ± 0.06/448 ± 29 | 16.3 ± 1.4/72 ± 1.3 | 96 ± 2/298 ± 17 | 16.6 ± 5.8/67 ± 18 | 8.7 ± 1.6/45 ± 10 | ||
| CN | 3.5 ± 2/47.6 ± 3.4 | 98 ± 1.6/470 ± 44 | 20 ± 2.5/78 ± 3.3 | 94.7 ± 2/308 ± 26 | 15 ± 2.3/83 ± 2 | 7.2 ± 5/45 ± 16 | ||
| 836 ± 69/624 ± 4 | NT | 4.2 ± 1/82 ± 13 | 99 ± 1.2/2534 ± 116 | 22 ± 8.4/119 ± 45 | 41 ± 8.6/232 ± 10 | 57.5 ± 15/263 ± 35 | 6 ± 2/49 ± 9 | |
| NC | 7.7 ± 0.05/78 ± 11 | 99 ± 0.3/2148 ± 75 | 39 ± 13/109 ± 33 | 68 ± 11/250 ± 2.5 | 85 ± 2/293 ± 40 | 8.7 ± 1/38.2 ± 5.7 | ||
| CN | 6.3 ± 0.6/78 ± 6 | 99 ± 0.04/2677 ± 96 | 34 ± 9/110 ± 20 | 57.3 ± 1/240 ± 18 | 81 ± 7/260 ± 28 | 5.9 ± 3/42 ± 4.7 | ||
| 328 ± 23/572 ± 60 | NT | 5 ± 4/5.6 ± 1.5 | 78.3 ± 13/122 ± 1.2 | 9.5 ± 9/6.2 ± 0.6 | 27.9 ± 14/10 ± 0.6 | 6.2 ± 7/5.4 ± 1 | 7.5 ± 5.6/6 ± 1.6 | |
| NC | 5.3 ± 4.8/5.3 ± 1.5 | 77.4 ± 11/120 ± 8 | 9.9 ± 6.9/5.7 ± 0.7 | 27.5 ± 12/9.6 ± 0.4 | 10 ± 7/6 ± 1 | 8.6 ± 5.5/5.6 ± 1.2 | ||
| CN | 5.5 ± 0.7/5.2 ± 1.3 | 79 ± 2.8/117 ± 16 | 10 ± 6.8/5.8 ± 1 | 28 ± 8/10 ± 0.4 | 6 ± 3/5.7 ± 1 | 8 ± 2/6 ± 0.7 | ||
| LTBMCs | 625 ± 110/368 ± 150 | NT | 3 ± 1.2/4 ± 0.1 | 99 ± 0.9/203 ± 10 | 20.4 ± 3/7.2 ± 0.5 | 82 ± 15/26 ± 8 | 90.2 ± 5/31.5 ± 7.6 | 10 ± 2.8/5 ± 0.4 |
| NC | 3 ± 2/3.7 ± 0.4 | 97 ± 3.2/159 ± 8 | 19.3 ± 1.2/6.7 ± 0.5 | 84.5 ± 14/26 ± 7 | 90.5 ± 6/30.5 ± 5.29 | 9 ± 0.1/4.8 ± 0.04 | ||
| CN | 2.6 ± 2.2/3.9 ± 0.2 | 99 ± 0.6/189 ± 12 | 20.3 ± 1.5/7 ± 0.5 | 81 ± 14.8/24.6 ± 6 | 92.6 ± 0.7/32 ± 0.3 | 7.6 ± 1.5/4.7 ± 0.8 | ||
| 92 ± 10/124 ± 53 | NT | 2.3 ± 1.5/8.3 ± 2.6 | 24.4 ± 6.8/24 ± 6 | 27 ± 2.8/23 ± 6.3 | 17 ± 3.5/15 ± 3.7 | 18 ± 3/16 ± 4.4 | 7.7 ± 4/11 ± 2.3 | |
| NC | 1.6 ± 0.4/8.7 ± 3.8 | 24 ± 3.8/23 ± 6.7 | 23 ± 0.4/20 ± 7.2 | 16.8 ± 0.4/16 ± 5.4 | 17.6 ± 0.5/16.5 ± 6 | 7 ± 2.7/12 ± 3.8 | ||
| CN | 3 ± 1.6/9.5 ± 4 | 23.3 ± 4/22 ± 6.9 | 26.4 ± 6.3/21 ± 5.6 | 14.5 ± 0.4/14 ± 5.7 | 19 ± 4.7/17 ± 2.9 | 7.3 ± 3/11 ± 3.8 | ||
| PBMDMs | 495 ± 67/561 ± 26 | NT | 3.2 ± 0.04/7 ± 1.4 | 83 ± 2/27.9 ± 5.6 | 8 ± 1.2/9.4 ± 1.2 | 30.4 ± 5.2/14 ± 1.4 | 82 ± 3.6/25 ± 2.6 | 3.6 ± 0.4/7.6 ± 1.7 |
| NC | 2 ± 0.4/5.8 ± 2 | 83.7 ± 18/24 ± 8 | 7.7 ± 3.3/7.5 ± 2 | 27.6 ± 9/11 ± 2.5 | 71 ± 14/19 ± 6 | 3.2 ± 0.6/6.3 ± 1.6 | ||
| CN | 3.3 ± 0.3/6 ± 1.2 | 84.5 ± 0.9/27 ± 2.5 | 8.3 ± 0.1/9 ± 1.2 | 23 ± 12/12 ± 6 | 83 ± 10/26 ± 11 | 7 ± 5/8.2 ± 2.8 | ||
| PBMDFs | 188 ± 59/480 ± 120 | NT | 2.5 ± 0.7/2.6 ± 7.3 | 66 ± 4/355 ± 58 | 16 ± 0.6/53 ± 13 | 5 ± 1.3/32 ± 10 | 6.5 ± 1.4/30 ± 13 | 7 ± 2.4/36 ± 9 |
| NC | 2.7 ± 1.7/21 ± 7 | 48 ± 32.4/156 ± 22 | 12 ± 0.2/44 ± 9 | 6 ± 2/27 ± 13 | 5 ± 0.2/25 ± 11 | 4.3 ± 0.3/33 ± 2.8 | ||
| CN | 2.6 ± 0.8/24 ± 6.4 | 59 ± 5.4/296 ± 13 | 13 ± 2/52 ± 0.1 | 6.8 ± 0.05/30 ± 7 | 6 ± 0.3/28 ± 0.6 | 6 ± 0.9/33 ± 5 | ||
| MS-5 | 460 ± 61/358 ± 44 | NT | 3 ± 1.8/4 ± 0.5 | 81 ± 10/76.9 ± 28 | 3.5 ± 1.2/3.9 ± 0.4 | 5 ± 3.4/4 ± 0.6 | 1.4 ± 1.4/3.5 ± 0.3 | 7 ± 4.8/4 ± 0.7 |
| NC | 2.7 ± 1.4/4 ± 0.2 | 59.6 ± 3/22 ± 12 | 7 ± 5.4/4.7 ± 0.9 | 10 ± 11/5 ± 1.4 | 2 ± 1.3/4 ± 0.4 | 17.4 ± 5.4/14 ± 0.5 | ||
| CN | 3.2 ± 1.2/3.8 ± 0.6 | 71 ± 17/77 ± 9 | 7.5 ± 4.8/4.6 ± 0.5 | 3.4 ± 2.5/4.2 ± 0.9 | 2.5 ± 0.8/3.6 ± 0.6 | 19 ± 4.2/10 ± 7 |
Cells were not treated or were treated with 10−4 M (for 2 hours) nicotine or cotinine. Expression of adhesion molecules was evaluated by FACS and expressed as means ± SD of the percentage of the gated cell population/mean fluorescence intensity. Data from 2 to 5 independent experiments are presented.
FSC indicates forward light scatter; SSC, side light scatter; VCAM, vascular cell adhesion molecule; BMCs, bone marrow cells; NT, no treatment; NC, nicotine; CN, cotinine; LTBMCs, long-term bone marrow cultures; PBMDMs, primary bone marrow–derived macrophages; PBMDFs, primary bone marrow–derived fibroblasts.
Nicotine inhibits HSPC homing and platelet recovery
To investigate the effect of nicotine on HSPC homing, we reconstituted lethally irradiated mice with bone marrow cells and exposed them to CS for 5 hours daily for 14 days. The numbers of leukocytes and erythrocytes in peripheral blood of mice exposed to CS were not significantly different from those in the control group. However, the reconstitution of platelets was significantly delayed. One week after transplantation, the number of platelets in the control group was 576 ± 82/mL of blood, whereas in the CS group, it reached only 363 ± 65/mL of blood. By week 2, the number of platelets in the CS group (356 ± 30/mL of blood) was still lower than in the control group (639 ± 82/mL of blood).
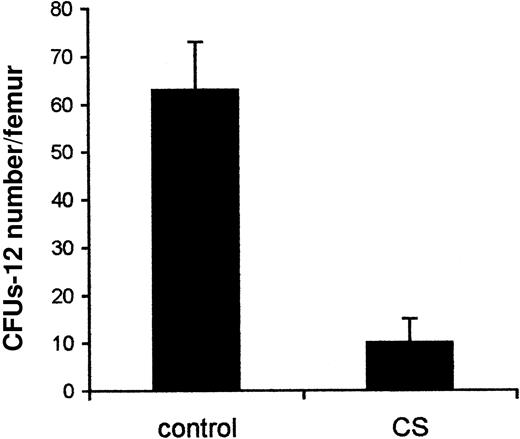
We next examined whether CS affects the trafficking of HSPC into the bone marrow. We especially assessed whether hematopoietic precursors from mice exposed to CS would reconstitute bone marrow with efficiency comparable to that of hematopoietic precursors from untreated mice. The number of CFUs-12 in the bone marrow of mice exposed to CS was 6-fold lower than in the control animals (P < .01) (Figure5). These findings suggest that CS significantly affects HSPC homing.
CS inhibits HSPC homing into the bone marrow.
Lethally irradiated mice were reconstituted with bone marrow cells and exposed to CS in a hermetically closed chamber (5 hours daily for 14 days). The control group was kept in a similar chamber without CS. Thereafter, the mice were killed and numbers of CFUs-12 in the bone marrow were evaluated. Data presented are from one representative experiment and are expressed as the number of CFU per 4 × 105 injected cells (mean ± SD).
CS inhibits HSPC homing into the bone marrow.
Lethally irradiated mice were reconstituted with bone marrow cells and exposed to CS in a hermetically closed chamber (5 hours daily for 14 days). The control group was kept in a similar chamber without CS. Thereafter, the mice were killed and numbers of CFUs-12 in the bone marrow were evaluated. Data presented are from one representative experiment and are expressed as the number of CFU per 4 × 105 injected cells (mean ± SD).
Nicotine decreases CD44 expression on endothelial cells
Extravasation of HSPC is a complex process involving a number of adhesion molecules. CD44 adhesion molecule is expressed by both endothelial cells and HSPC and has been shown to play a critical role in HSPC homing.37 38 We therefore examined the effect of nicotine or cotinine treatment on CD44 expression on both endothelial and progenitor cells by flow cytometry. CD44 expression on enriched populations of progenitor cells was not altered by nicotine or cotinine treatment (data not shown). Similarly, murine or human endothelial cells were treated with nicotine and harvested, and the cell-surface expression levels of CD44 along with VCAM, β7, α4, and P- and L-selectins were determined. Treatment of HUVECs and the murine bone marrow–derived endothelial cell line STR-12 with nicotine or cotinine resulted in a significant reduction of CD44 expression (Table2). Treatment of HUVECs with nicotine and cotinine resulted in 2-fold and 1.8-fold decreases in CD44 expression, respectively. We also observed a decrease (1.5-fold) in the intensity of CD44 expression on STR-12 endothelial cells after exposure to nicotine and cotinine. In contrast, both nicotine and cotinine failed to alter the surface expression of VCAM, β7, α4, and P- and L-selectins on HUVECs and STR-12. Interestingly, a comparison of E-selectin expression on lung endothelial cell line (LEISVO) and STR-12 cells revealed that a 4-hour exposure to nicotine induced E-selectin expression on LEISVO, but not on STR-12 (Figure6). However, no significant change in cell-surface VCAM expression on LEISVO and STR-12 was observed after exposure to nicotine for varying intervals.
Effects of nicotine and cotinine on adhesion molecule expression in endothelial cells
| Cell population . | FSC/SSC . | Treatment . | Control . | CD44 . | VCAM . | α4 . | β2 . | β7 . | P-selectin . | L-selectin . |
|---|---|---|---|---|---|---|---|---|---|---|
| HUVECs | 369 ± 23/414 ± 12 | NT | 5.7 ± 2/5.3 ± 2 | 95 ± 4/167 ± 63 | 8 ± 3/6 ± 2 | 12 ± 3.4/7 ± 2.3 | 5.7 ± 5.3/7 ± 1 | 8.4 ± 5/7 ± 1.4 | 5.7 ± 1/7 ± 0.3 | 7 ± 4/7 ± 0.7 |
| NC | 4 ± 2/6.7 ± 2.6 | 82 ± 17/79 ± 47 | 7 ± 3/7.5 ± 2.5 | 9.5 ± 2/8.5 ± 3 | 7 ± 4/7 ± 2.6 | 8.6 ± 7/7.8 ± 3 | 5.6 ± 3/9 ± 1.7 | 6 ± 3.5/9 ± 1 | ||
| CN | 4.8 ± 1/6 ± 2 | 88 ± 4/90 ± 48 | 9 ± 7/7 ± 2.8 | 12 ± 5.5/8 ± 2.7 | 7 ± 5/6.6 ± 3 | 10 ± 7/7.3 ± 3 | 6.4 ± 5/8.3 ± 1 | 6 ± 1.8/7 ± 0.2 | ||
| STR-12 | 575 ± 27/385 ± 16 | NT | 2 ± 0.8/4 ± 1 | 96 ± 4/63 ± 4 | 97 ± 1/56 ± 19 | 7 ± 6/4.7 ± 1 | 5 ± 3/4 ± 0.5 | 3.7 ± 2/4 ± 0.5 | 4 ± 2.5/4.3 ± 1 | 2.7 ± 1.4/4 ± 1 |
| NC | 2 ± 0.1/4 ± 0.6 | 95 ± 6/49 ± 3 | 96 ± 1/57 ± 6 | 4 ± 3/4 ± 0.4 | 4 ± 2.6/4 ± 0.1 | 3 ± 1.4/4 ± 0.4 | 3 ± 1/4 ± 0.3 | 3 ± 0.4/3 ± 0.5 | ||
| CN | 3 ± 0.8/3 ± 0.3 | 98 ± 2/50 ± 5 | 97 ± 1/48 ± 14 | 12 ± 9/4.4 ± 0.7 | 7 ± 3.5/4 ± 0.4 | 4 ± 0.8/4 ± 0.4 | 4.5 ± 1/4 ± 0.5 | 4 ± 2/4 ± 0.6 |
| Cell population . | FSC/SSC . | Treatment . | Control . | CD44 . | VCAM . | α4 . | β2 . | β7 . | P-selectin . | L-selectin . |
|---|---|---|---|---|---|---|---|---|---|---|
| HUVECs | 369 ± 23/414 ± 12 | NT | 5.7 ± 2/5.3 ± 2 | 95 ± 4/167 ± 63 | 8 ± 3/6 ± 2 | 12 ± 3.4/7 ± 2.3 | 5.7 ± 5.3/7 ± 1 | 8.4 ± 5/7 ± 1.4 | 5.7 ± 1/7 ± 0.3 | 7 ± 4/7 ± 0.7 |
| NC | 4 ± 2/6.7 ± 2.6 | 82 ± 17/79 ± 47 | 7 ± 3/7.5 ± 2.5 | 9.5 ± 2/8.5 ± 3 | 7 ± 4/7 ± 2.6 | 8.6 ± 7/7.8 ± 3 | 5.6 ± 3/9 ± 1.7 | 6 ± 3.5/9 ± 1 | ||
| CN | 4.8 ± 1/6 ± 2 | 88 ± 4/90 ± 48 | 9 ± 7/7 ± 2.8 | 12 ± 5.5/8 ± 2.7 | 7 ± 5/6.6 ± 3 | 10 ± 7/7.3 ± 3 | 6.4 ± 5/8.3 ± 1 | 6 ± 1.8/7 ± 0.2 | ||
| STR-12 | 575 ± 27/385 ± 16 | NT | 2 ± 0.8/4 ± 1 | 96 ± 4/63 ± 4 | 97 ± 1/56 ± 19 | 7 ± 6/4.7 ± 1 | 5 ± 3/4 ± 0.5 | 3.7 ± 2/4 ± 0.5 | 4 ± 2.5/4.3 ± 1 | 2.7 ± 1.4/4 ± 1 |
| NC | 2 ± 0.1/4 ± 0.6 | 95 ± 6/49 ± 3 | 96 ± 1/57 ± 6 | 4 ± 3/4 ± 0.4 | 4 ± 2.6/4 ± 0.1 | 3 ± 1.4/4 ± 0.4 | 3 ± 1/4 ± 0.3 | 3 ± 0.4/3 ± 0.5 | ||
| CN | 3 ± 0.8/3 ± 0.3 | 98 ± 2/50 ± 5 | 97 ± 1/48 ± 14 | 12 ± 9/4.4 ± 0.7 | 7 ± 3.5/4 ± 0.4 | 4 ± 0.8/4 ± 0.4 | 4.5 ± 1/4 ± 0.5 | 4 ± 2/4 ± 0.6 |
Cells were not treated or were treated with 10−4 M (for 2 hours) nicotine or cotinine. Expression of adhesion molecules was evaluated by FACS and expressed as means ± SD of the percentage of the gated cell population/mean fluorescence intensity. Data from 2 to 5 independent experiments are presented.
FSC indicates forward light scatter; SSC, side light scatter; VCAM, vascular cell adhesion molecule; HUVECs, human umbilical vein endothelial cells; NT, no treatment; NC, nicotine; CN, cotinine; STR-12, murine bone marrow–derived endothelial cell line.
Induction of E-selectin expression on lung microvascular endothelial cells.
Lung microvascular endothelial cells (LEISVO) and bone marrow–derived endothelial cells (STR-12) were exposed to nicotine for 4 hours. Expression of CD44, VCAM, and E-selectin was evaluated by FACS analysis. Adhesion molecule expression on untreated cells (dark field) was compared with that on nicotine-treated cells (transparent field).
Induction of E-selectin expression on lung microvascular endothelial cells.
Lung microvascular endothelial cells (LEISVO) and bone marrow–derived endothelial cells (STR-12) were exposed to nicotine for 4 hours. Expression of CD44, VCAM, and E-selectin was evaluated by FACS analysis. Adhesion molecule expression on untreated cells (dark field) was compared with that on nicotine-treated cells (transparent field).
Discussion
Tobacco use, especially smoking, has been associated with an increased risk of cancer and pulmonary and cardiovascular diseases. Exposure to TS is associated with altered immune responses, including lymphocyte proliferation and neutrophil and macrophage functions, leading to tobacco-related immunodeficiency. Moreover, TS may result in imbalance of the hematopoietic system, such as changes in the erythrocyte-leukocyte ratio and the composition of mature leukocytes in the peripheral blood.39 Most of the undesirable effects of TS have been associated with nicotine. For example, exposure of various types of cells to nicotine results in a decrease in DNA synthesis, inhibition of cell proliferation, alteration of cytokine and ECM molecule production, and changes in adhesion molecule expression.40-47 Although these studies indicate the negative effects of nicotine on various tissues, the potential deleterious effects of TS or nicotine on bone marrow hematopoiesis and HSPC trafficking have not been investigated. We demonstrate for the first time that exposure to nicotine or CS results in inhibition of hematopoiesis in LTBMCs as well as homing of HSPC into the bone marrow.
The results of our study indicate that the numbers of hematopoietic progenitors in the bone marrow of mice exposed to CS are significantly lower than those observed in control mice. To examine the cellular mechanisms involved in this phenomenon, we treated LTBMCs with varying concentrations of nicotine and cotinine. The concentration of nicotine used in the present study is comparable to the physiologic concentration of nicotine observed in the serum of smokers.48 Nicotine either specifically binds to cell-surface receptors49,50 or undergoes nonspecific uptake, leading to high levels of intracellular buildup.36Therefore, the concentration of nicotine required to functionally modulate cellular responses is likely to vary depending on the cell type, cell-surface or intracellular binding, tissue of origin, and duration of exposure. We have therefore examined the effect of nicotine exposure on hematopoiesis in LTBMCs at physiologic concentrations varying from 10−5 to 10−8 M. We observed that treatment with nicotine, but not its metabolite cotinine (10−5 to 10−6 M), resulted in a significant decrease in nonadherent cell and progenitor cell production in LTBMCs. Interestingly, nicotine treatment of LTBMCs in steady state affected neither progenitor cell nor mature cell production. A possible cause for this effect could be that nicotine does not inhibit proliferation of the bone marrow hematopoietic progenitors, but instead alters the supportive functions of bone marrow stroma. In support of this hypothesis, we have demonstrated that the addition of nicotine into the methylcellulose assay at a concentration of 10−4 to 10−6 M does not affect colony formation by bone marrow hematopoietic progenitors. Furthermore, nicotine or cotinine does not interfere with adhesion and proliferation of CFU-F, progenitor cells for bone marrow stroma. As a part of the bone marrow regulatory network, stromal cells participate in hematopoietic niche formation by expressing a variety of adhesion molecules. Alteration of this could lead to an inhibition of initial adhesion of hematopoietic stem cells to the stroma and establishment of cobblestone areas, which is essential for HSPC self-renewal. Lack of stromal cell–stem cell interactions may result in initiation of stem cell differentiation and a significant reduction in early stem cell numbers. Indeed, treatment of LTBMCs with nicotine, but not cotinine, significantly decreased the number of LTC-ICs within
the adherent layer and affected the formation of cobblestone areas. As a consequence of this, we observed decreased numbers of hematopoietic progenitors and mature cells in nicotine-treated cultures. Interestingly, nicotine treatment of LTBMCs in steady state did not affect cobblestone areas. These findings support the idea that nicotine affects initial stromal cell–stem cell interactions and HSPC seeding of the stromal layer. This results in a decreased number of stem cells that could colonize the adherent layer and produce progeny. In turn, this process could lead to secondary immunosuppression in smokers.
We anticipated that stromal cells are the targets for intracellular uptake of nicotine. The interaction between HSPC and stromal cells is dependent upon the engagement of several cell-surface adhesion molecules.51-56 Therefore, we further postulated that stromal cells treated with nicotine may fail to support HSPC by changing the profile of adhesion molecules expressed by stromal cells. CD44 is one of the adhesion molecules that plays a critical role in normal hematopoiesis, and anti-CD44 mAbs significantly affect both the formation of cobblestone areas within the adherent layer of LTBMCs and the production of mature cells.37,57 Furthermore, we have previously demonstrated that CD44 contributes to the adhesion of myeloid progenitor cells to the bone marrow stromal cell line MS-5.58 Here we show that the expression level of CD44 adhesion molecule was 3.5-fold down-regulated on bone marrow–derived stromal cell line MS-5 after exposure to nicotine, but not cotinine, which could reflect events taking place in the primary stroma. Indeed, exposure of primary bone marrow–derived fibroblastlike stromal cells, but not bone marrow macrophages, to nicotine resulted in a 2.3-fold reduction of cell-surface CD44. Because CD44 is required for interactions between stromal cells and HSPC, the nicotine-induced decrease of CD44 expression could lead to a decreased number of HSPC seeded on the stroma, and consequently, reduced hematopoietic activity. In addition to the effects on CD44 expression, treatment of MS-5 cells with nicotine results in a 3.5-fold increase in β7-integrin, but not VCAM, α4-, or β2-integrin expression. Although β7 integrin is expressed on hematopoietic cells,59-62 the expression of β7 integrin on bone marrow stromal cells has not been reported previously. Based on gene-targeting experiments, it was concluded that β7 integrin is not involved in the regulation of hematopoiesis.63 In line with this study, we did not observe an increase in β7-integrin expression on primary stromal cells or on the adherent layer of LTBMCs after exposure to nicotine.
Homing of HSPC into the bone marrow is a complex process mediated by various adhesion molecules expressed on HSPC and on bone marrow endothelial cells. Because nicotine inhibits the repopulation of irradiated bone marrow by intravenously injected HSPC, we speculated that nicotine may target bone marrow endothelial cells. This, in turn, may result in alteration of HSPC extravasation and homing. We therefore examined the effects of nicotine and its major metabolite cotinine on bone marrow endothelial cells (STR-12) in comparison with lung microvascular endothelial cells (LEISVO). Exposure of STR-12 and LEISVO to nicotine resulted in a decrease of cell-surface CD44 expression. Interestingly, in sharp contrast to its lack of any effect on hematopoiesis and expression of adhesion molecules by bone marrow stromal cells, cotinine was observed to inhibit CD44 expression by HUVECs as well as bone marrow endothelial cells. Previous studies including ours37,38 have demonstrated that blockade of cell-surface CD44 expression on HSPC with anti-CD44 mAb results in the inhibition of HSPC homing into the bone marrow. The molecular basis of this phenomenon is still not well understood. Furthermore, the endothelial cell ligand for CD44 expressed on HSPC is not known. Because CD44–CD44 homotypic interactions could be involved in cell–cell adhesion,64 it is conceivable that CD44 expressed on the endothelium may serve as a ligand for CD44 expressed on HSPC. Therefore, reduction of CD44 expression on endothelial cells would lead to a decreased adhesion of HSPC to the endothelium and, as a consequence, inhibition of HSPC homing. In contrast to the reduced CD44 expression, treatment with nicotine for 4 hours resulted in up-regulation of E-selectin on LEISVO, but not on STR-12 cells, suggesting an organ-specific targeting effect of nicotine. Although previous studies have demonstrated that treatment of HUVECs with nicotine containing CS condensate resulted in increased expression of E-selectin, which is associated with increased monocyte adhesion in vitro,45 the effect of nicotine-induced E-selectin expression on HSPC homing is not known. Our observations suggest that E-selectin induction is restricted to lung endothelium and not bone marrow–derived endothelial cells, and, as such, may not influence HSPC homing to the bone marrow.
Up-regulation of E-selectin on endothelial cells and β7 integrin on MS-5 suggests that nicotine targets signal-transduction pathways leading to the activation of E-selectin and β7-integrin genes. In support of this observation, an inverse correlation between CD44 and E-selectin as well as CD44 and β7 integrin has been reported previously.65-67 This finding indicates cross-linking of signal-transduction pathways, leading to the activation or inhibition of the transcription factors regulating gene expression. It is conceivable that the reduction of CD44 on the cell surface is due to shedding or receptor internalization. The molecular basis for nicotine's action on bone marrow cells remains largely unknown. Although nicotinic acetylcholine receptor (nAChR) knockout mice do not demonstrate any hematologic abnormalities68-70 and the expression of nAChR on stromal cells has not yet been reported, we speculate that nicotine undergoes nonspecific uptake by bone marrow fibroblastlike stromal cells,36 binds intracellular targets, and may interfere with the interplay of positively and negatively acting transactivating factors regulating gene expression. Consistent with our observations, recent studies have revealed that cotinine does not interfere with carrier-mediated nicotine transport within cells.71 Therefore, it is conceivable that nicotine and cotinine differ in their cellular uptake and result in differential effects on changes of cell phenotype and behavior.
Overall, these studies identify for the first time the differential cellular effects of nicotine, cotinine, and CS exposure in vivo and in vitro and demonstrate that long-term exposure to nicotine affects both hematopoiesis and HSPC homing, whereas cotinine is likely to inhibit only cell trafficking.
Supported by National Institutes of Health grant AI35796 and Tobacco Research Disease Related Program (TRDRP) grant 7RT-0197 to P.S.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
P. Sriramarao, Division of Vascular Biology, La Jolla Institute for Molecular Medicine, 4570 Executive Dr, San Diego, CA 92121; e-mail: rao@ljimm.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal