Abstract
Dendritic cells (DCs) are responsible for the initiation of immune responses. Two distinct subsets of blood DCs have been characterized thus far. Myeloid DCs (MDCs) and plasmacytoid monocytes (PDCs) were shown to be able to promote polarization of naive T cells. This study shows a dramatic quantitative imbalance in both circulating blood DC subsets in 37 patients with acute myeloid leukemias. Eleven patients (30%) displayed a normal quantitative profile (MDC mean, 0.37% ± 0.21%; range, 0.01% to 0.78%; PDC mean, 0.21% ± 0.24%; range, 0.04% to 0.62%), whereas 22 (59%) showed a tremendous expansion of MDCs (9 patients: mean, 16.76% ± 14.03%; range, 1.36% to 41%), PDCs (4 patients: mean, 7.28% ± 6.84%; range, 1% to 14%), or both subsets (9 patients: MDC mean, 10.86% ± 12.36%; range, 1.02% to 37.1%; PDC mean, 4.25% ± 3.78%; range, 1.14% to 13.04%). Finally, in 4 patients (11%), no DC subsets were detectable. Both MDC and PDC subsets exhibited the original leukemic chromosomal abnormality. Ex vivo, leukemic PDCs, but not leukemic MDCs, had impaired capacity for maturation and decreased allostimulatory activity. Also, leukemic PDCs were altered in their ability to secrete interferon-α. These data provide evidence that DC subsets in vivo may be affected by leukemogenesis and may contribute to leukemia escape from immune control.
Introduction
Dendritic cells (DCs) are bone marrow–derived leukocytes that are responsible for the initiation of immune responses and exert a sentinel-like function.1 DCs are phenotypically and functionally heterogeneous in vivo. In humans, 2 distinct subsets of blood DCs have been characterized based on the differential expression of CD11c.2 Myeloid DCs (MDCs) and plasmacytoid monocytes (PDCs) were shown to be able to promote polarization of naive T cells into Th1 or Th2.3-6 Th1 and Th2 cells are cross-regulatory in vitro. The balance of these cells in vivo determines the character of cell-mediated immune and inflammatory responses.7 Furthermore, PDCs are crucial effectors in antiviral innate immunity. They can also induce Th1 development through secretion of type 1 interferon (IFN).4 5 Thus, blood circulating DCs may play a key role in promoting antitumor immunity.
Tumor-associated DCs have been shown to have a low allostimulatory capacity, particularly if isolated from progressing metastatic lesions, as in malignant melanoma.8 DC numbers were shown to be increased in certain hematologic diseases, such as Hodgkin disease,9 or decreased in others, such as myelomonocytic leukemia,10 but the significance of this is unknown. We and others reported previously that myeloid leukemic cells were able to differentiate into mature DCs in vitro.11-16Because the relation between the various DC differentiation pathways and the hematopoietic progenitors remains unclear, the development of blood circulating DCs in vivo might be affected by leukemic cell proliferation. In this study, we investigated whether circulating DCs could be detected in the blood of patients with acute myeloid leukemia (AML). We show here a dramatic quantitative imbalance in blood DC subsets among the majority of 37 patients with myeloid leukemias. Both MDC and PDC subsets were found to exhibit the original leukemic chromosomal abnormality. Leukemic PDCs have impaired capacity for maturation, decreased allostimulatory activity, and alteration in their ability to secrete IFN-α. Thus, these data provide evidence that DC subsets in vivo may be affected by leukemogenesis and may contribute to the escape of leukemia from immune control.
Patients, materials, and methods
Patient samples
AML cell samples were obtained after informed consent from 37 patients at diagnosis and before any chemotherapy. AML peripheral blood mononuclear cells (PBMCs) analyzed in this study were derived from patients with ages ranging from 15 to 86 years treated at the Institut Paoli-Calmettes (Marseille, France) between 1993 and 2000. AMLs were subtyped according to the FAB classification. PBMCs from patients and healthy controls (Regional Transfusion Center, Marseille, France), to which the patients' samples have been compared, were isolated by standard density gradient centrifugation with Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) before cryopreservation. At all times, leukemic samples and healthy controls were handled similarly to prevent functional differences that would result from differences in cryopreservation and cell separation.
Cell lines
Murine L cells transfected with human CD40L were kindly provided by Schering-Plough (Laboratory for Immunological Research, Dardilly, France)2 and used after 75 Gy irradiation.
Blood DC detection and sorting
Blood DCs were identified by 3-color staining performed on PBMCs using the following monoclonal antibodies: ZM3.8-PC5 (mAb against ILT3, an immunoglobulinlike transcript recently used to isolate DCs from the blood, mouse IgG1),17 BU15-phycoerythrin (PE) (mAb against CD11c), and fluorescein isothiocyanate (FITC)–labeled mAbs against lineage markers CD14, CD16, CD19, and CD56 (Beckman-Coulter, Marseille, France). Cells that did not label with these lineage markers were designated as lin−. The purity of each DC subpopulation after cell sorting was always greater than 98% and was assessed by fluorescence-activated cell sorting (FACS) before subsequent fluorescence in situ hybridization (FISH) and functional experiments. The following mAbs were used for flow cytometry studies: HLA-DR, CD13, CD33, CD45RA, CD45RO, CD4, CD5, CD116, CD1a, and CD83 (Beckman-Coulter); and CD80, CD86, CD123, and CD40 (Pharmingen, San Diego, CA). Stained cells were analyzed on a FACSCalibur cytometer using Cellquest software (Becton Dickinson, San Jose, CA). DCs were sorted on a FACSVantage cytometer (Becton Dickinson).
FISH analysis
Interphase FISH was performed as described previously on cytospin preparations with sorted MDCs and PDCs from 4 patients selected for detection of their cytogenetic abnormality.18For detection of trisomy 8 (unique patient number 156 [UPN156] and UPN79) and monosomy 7 (UPN223), metaphases were analyzed with a specific probe for chromosome 8 (D8Z2 probe) or chromosome 7 (D7Z1 probe), both purchased from Oncor (Gaithersburg, MD). For the detection of translocations associated with the q23 region of chromosome 11 (UPN109), LSI MLL dual-color DNA probe (Vysis, Downers Grove, IL) was used. At least 200 nuclei were examined under fluorescence microscopy by 2 independent observers.
Confocal microscopy
Cells were adhered to polylysine-coated glass slides for 30 minutes at room temperature, fixed in 4% paraformaldehyde, and permeabilized in 0.1% Triton in phosphate-buffered saline. Cells were then labeled with different primary mAbs and revealed by species-specific Alexa 488, tetramethylrhodamine isothiocyanate (TRITC; Molecular Probes, Eugene, OR), or cyanin 5–labeled secondary antibodies (Jackson Immunoresearch, West Baltimore Pike, PA). DC-LAMP mAb was kindly provided by Serge Lebecque (Schering-Plough, Laboratory for Immunological Research).19 Slides were then mounted using fluorescent mounting medium (Dako, Trappes, France). Confocal analysis was performed with a TCS NT microscope equipped with argon and krypton ion lasers and a X100 1.3NA PL fluotar objective (Leica Microsystem, Heidelberg, Germany).
Reverse transcriptase–polymerase chain reaction analysis
Expression of pre-Tα transcript on freshly sorted MDCs and PDCs was analyzed as described previously.20 Briefly, total cellular RNA was extracted from the FACS-sorted cells using TRIzol reagent (Gibco BRL, Cergy-Pontoise, France). First-strand cDNAs were prepared using oligo(dT) primers and murine Moloney leukemia virus reverse transcriptase (RT; Gibco BRL). Polymerase chain reaction (PCR) amplification was performed for 35 cycles (1 minute at 94°C, 1 minute at 55°C, and 2 minutes at 72°C) with Taq DNA polymerase (Gibco BRL). Oligonucleotide primers for pre-Ta were 5′-GGCACACCCTTTCCTTCTCTG-3′ and 5′-GCAGGTCCTGGCTGTAGAAGC-3′ for sense and antisense primers, respectively.
Functional analysis
To evaluate T-cell proliferation capacity, we sorted ILT3+CD11c− (PDCs) and ILT3+CD11c+ (MDCs) from peripheral blood from healthy volunteers or leukemic patients and cultured them in 48-well culture plates in RPMI 1640 medium containing 10% fetal calf serum (BioWhittaker, Verviers, Belgium) in the presence of 100 ng/mL granulocyte macrophage colony-stimulating factor (GM-CSF; kindly provided by Novartis, Berne, Switzerland) and 20 ng/mL interleukin-4 (IL-4) (kindly provided by Schering-Plough Research Institute, Kenilworth, NJ) for MDCs or 10 ng/mL IL-3 (Genzyme, Cergy St Christophe, France) for PDCs. Cells were stimulated by adding 75 Gy–irradiated CD40L-transfected cells (1 × 104/well). Freshly isolated and 3-day–cultured DCs were cocultured with 1 × 105 allogeneic naive CD4+ T cells in 96-well flat-bottom plates for 6 days. Proliferation of T cells was monitored by measuring methyl-[3H]thymidine (Amersham, Little Chalfont, United Kingdom) incorporation during the last 12 hours of culture on a gas-phase β counter (Matrix 9600; Packard, Downers Grove, IL). Naive CD4+ T cells were prepared from adult donor PBMCs negatively depleted of CD8, CD14, CD19, CD56, and CD45RO+ cells using goat anti–mouse Ig-coated magnetic beads (Beckman-Coulter). Fully 98% of the resulting cells were CD4+CD45RA+ as controlled by FACS analysis. To determine cytokine production, we cultured freshly sorted DCs in 96-well flat-bottom plates at a concentration of 2 × 104 cells/well and stimulated them with either CD40L-transfected cells (2000 cells/well) or 106 PFU/mL herpes simplex virus 1 (HSV). Supernatants were collected after 24, 48, and 72 hours and tested for their cytokine contents. IFN-α levels were measured by enzyme-linked immunosorbent assay (Beckman-Coulter).
Results
Imbalance of blood DC subsets in myeloid leukemia patients
Circulating blood DC subsets from 37 leukemic patients were analyzed at diagnosis and before treatment by flow cytometry after staining with lineage markers (CD14, CD16, CD19, and CD56), CD11c, and ILT3, an immunoglobulinlike transcript recently used to isolate DCs from the blood.6 In healthy individuals, 2 distinct populations of lin−/ILT3+ cells were observed with respect to the expression of CD11c, with the phenotypes of lin−/CD11c−/ILT3+ (PDCs) and lin−/CD11c+/ILT3+ (MDCs) (Figure1). The MDC and PDC subsets represented, respectively, 0.26% ± 0.23% (range, 0.01% to 0.8%) and 0.24% ± 0.18% (range, 0.01% to 0.7%) of PBMCs in the different healthy volunteers (HVs) that we analyzed (n = 15) and are in accordance with those already published21 (Table1). Leukemic samples representing the different subgroups of the FAB classification (3 M1, 2 M2, 3 M3, 8 M4, 18 M5, 1 M6, and 2 M7) were analyzed and revealed an important quantitative imbalance in the proportions of circulating MDC and PDC subsets, as exemplified by the 2 samples shown for patients UPN109 and UPN223 (Figure 1). Eleven patients (30%, group I) displayed a normal quantitative profile (MDC mean, 0.37% ± 0.21%; range, 0.01% to 0.78%; PDC mean, 0.21% ± 0.24%; range, 0.04% to 0.62%), whereas 22 (59%, group II) showed an expansion of MDCs (9 patients: mean, 16.76% ± 14.03%; range, 1.36% to 41%), PDCs (4 patients: mean, 7.28% ± 6.84%; range, 1% to 14%), or both subsets (9 patients: MDC mean, 10.86% ± 12.36%; range, 1.02% to 37.1%; PDC mean, 4.25% ± 3.78%; range, 1.14% to 13.04%). Finally, in 4 patients (11%, group III), no DC subsets were detectable (Table 1). Hereafter, all immunophenotypic and functional assays were performed in different patients taken in both groups I and II.
Detection of circulating DC subsets in the peripheral blood.
PBMCs isolated from healthy volunteers or AML patients were analyzed by flow cytometry after 3-color staining with a combination of FITC-labeled mAbs against lineage markers (CD14, CD16, CD19, and CD56), PE-labeled anti-CD11c, and PC5-labeled ILT3. Two distinct populations of lin−/ILT3+ cells were observed with respect to the expression of CD11c, with the phenotypes of lin−/CD11c+/ILT3+ (MDCs) and lin−/CD11c−/ILT3+ (PDCs). Examples of expansion of the MDC subset for patient UPN109 or the PDC subset for patient UPN223 are shown.
Detection of circulating DC subsets in the peripheral blood.
PBMCs isolated from healthy volunteers or AML patients were analyzed by flow cytometry after 3-color staining with a combination of FITC-labeled mAbs against lineage markers (CD14, CD16, CD19, and CD56), PE-labeled anti-CD11c, and PC5-labeled ILT3. Two distinct populations of lin−/ILT3+ cells were observed with respect to the expression of CD11c, with the phenotypes of lin−/CD11c+/ILT3+ (MDCs) and lin−/CD11c−/ILT3+ (PDCs). Examples of expansion of the MDC subset for patient UPN109 or the PDC subset for patient UPN223 are shown.
Quantitative imbalance in the proportions of circulating MDC and PDC subsets in patients with acute myeloid leukemia
| Group . | n . | % MDCs . | % PDCs . |
|---|---|---|---|
| Healthy donors | 15 | 0.26 ± 0.23 | 0.24 ± 0.18 |
| (0.01-0.8) | (0.01-0.7) | ||
| Group I | 11 | 0.37 ± 0.21 | 0.21 ± 0.24 |
| (1 M2, 2 M4, 7 M5, 1 M7) | (0.01-0.78) | (0.04-0.62) | |
| Group II | |||
| MDC expansion | 9 | 16.76 ± 14.03 | 0.39 ± 0.23 |
| (1 M2, 1 M4, 6 M5, 1 M6) | (1.36-41) | (0.22-0.82) | |
| PDC expansion | 4 | 0.37 ± 0.39 | 7.28 ± 6.84 |
| (1 M1, 1 M3, 2 M5) | (0.04-0.78) | (1-14) | |
| MDC and PDC expansion | 9 | 10.86 ± 12.36 | 4.25 ± 3.78 |
| (2 M3, 3 M4, 3 M5, 1 M7) | (1.02-37.1) | (1.14-13.04) | |
| Group III | 4 | 0 | 0 |
| (2 M1, 2 M4) |
| Group . | n . | % MDCs . | % PDCs . |
|---|---|---|---|
| Healthy donors | 15 | 0.26 ± 0.23 | 0.24 ± 0.18 |
| (0.01-0.8) | (0.01-0.7) | ||
| Group I | 11 | 0.37 ± 0.21 | 0.21 ± 0.24 |
| (1 M2, 2 M4, 7 M5, 1 M7) | (0.01-0.78) | (0.04-0.62) | |
| Group II | |||
| MDC expansion | 9 | 16.76 ± 14.03 | 0.39 ± 0.23 |
| (1 M2, 1 M4, 6 M5, 1 M6) | (1.36-41) | (0.22-0.82) | |
| PDC expansion | 4 | 0.37 ± 0.39 | 7.28 ± 6.84 |
| (1 M1, 1 M3, 2 M5) | (0.04-0.78) | (1-14) | |
| MDC and PDC expansion | 9 | 10.86 ± 12.36 | 4.25 ± 3.78 |
| (2 M3, 3 M4, 3 M5, 1 M7) | (1.02-37.1) | (1.14-13.04) | |
| Group III | 4 | 0 | 0 |
| (2 M1, 2 M4) |
Thirty-seven leukemic samples were analyzed for distribution of circulating MDC and PDC subsets. Eleven patients (group I) displayed a quantitative profile similar to that of healthy donors; 22 patients (group II) showed an expansion of MDCs, PDCs, or both subsets; 4 patients (group III) had no detectable DC subsets. Data are presented as mean ± SD (range).
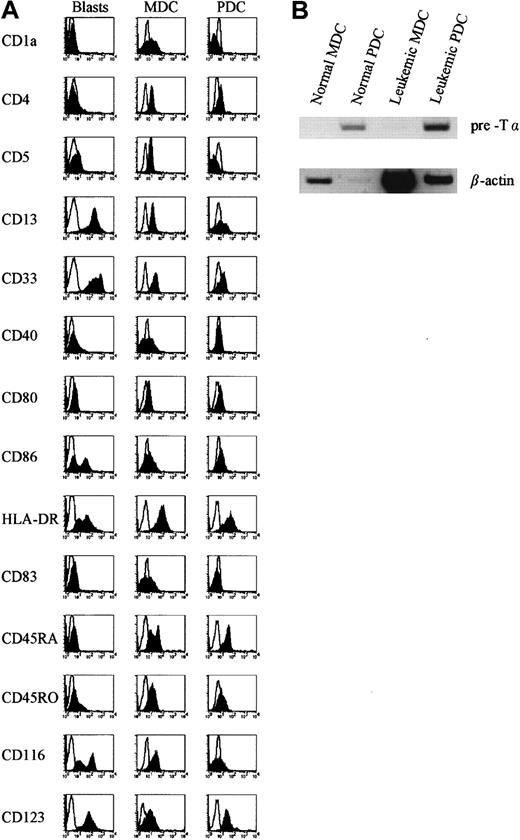
Phenotypic comparisons of freshly isolated MDCs and PDCs from HVs and from patients were performed, indicating their similarity to the 2 previously described subsets of precursor DCs, one lacking and one expressing CD11c.6,22 MDC populations isolated from patients expressed the myeloid markers CD11c, CD13, and CD33, as well as CD5, CD45RO, and the GM-CSFRα (CD116), and thus display a similar phenotype to MDCs isolated from HVs. PDC populations expressed little CD13 and CD33, were positive for CD45RA, and expressed IL-3Rα (CD123) but little or no CD116, confirming the identity of this subset as the PDC precursors.2 In addition, they were moderately positive for HLA-DR, as were their normal counterparts (Figure2A). Furthermore, an extensive phenotypic analysis was performed to compare leukemic blasts, MDCs, and PDCs from the same patient. This phenotypic analysis demonstrated that MDCs and PDCs were phenotypically different from the parental clone (Figure 2A). In addition, as previously described for normal PDCs,20 23-25 RT-PCR analysis revealed that PDCs isolated from leukemic patients expressed the lymphoid-associated transcript pre-Tα (Figure 2B).
Immunophenotype of freshly isolated MDCs, PDCs, and leukemic blasts and expression of pre-Tα by leukemic PDCs.
(A) Leukemic MDCs and PDCs were sorted as lin−/CD11c+/ILT3+ (MDCs) and lin−/CD11c−/ILT3+ (PDCs) and compared with blasts obtained from the same patient for the expression of a number of lymphoid, myeloid, costimulatory, and cytokine receptor markers. Open histograms represent cells stained with isotype-matched control mAbs. Data shown were obtained from patient UPN243 and are representative of more than 5 patients with or without DC expansion. (B) Freshly isolated normal and leukemic MDCs and PDCs were analyzed for expression of mRNA for pre-Tα. Data shown are representative of 3 patients.
Immunophenotype of freshly isolated MDCs, PDCs, and leukemic blasts and expression of pre-Tα by leukemic PDCs.
(A) Leukemic MDCs and PDCs were sorted as lin−/CD11c+/ILT3+ (MDCs) and lin−/CD11c−/ILT3+ (PDCs) and compared with blasts obtained from the same patient for the expression of a number of lymphoid, myeloid, costimulatory, and cytokine receptor markers. Open histograms represent cells stained with isotype-matched control mAbs. Data shown were obtained from patient UPN243 and are representative of more than 5 patients with or without DC expansion. (B) Freshly isolated normal and leukemic MDCs and PDCs were analyzed for expression of mRNA for pre-Tα. Data shown are representative of 3 patients.
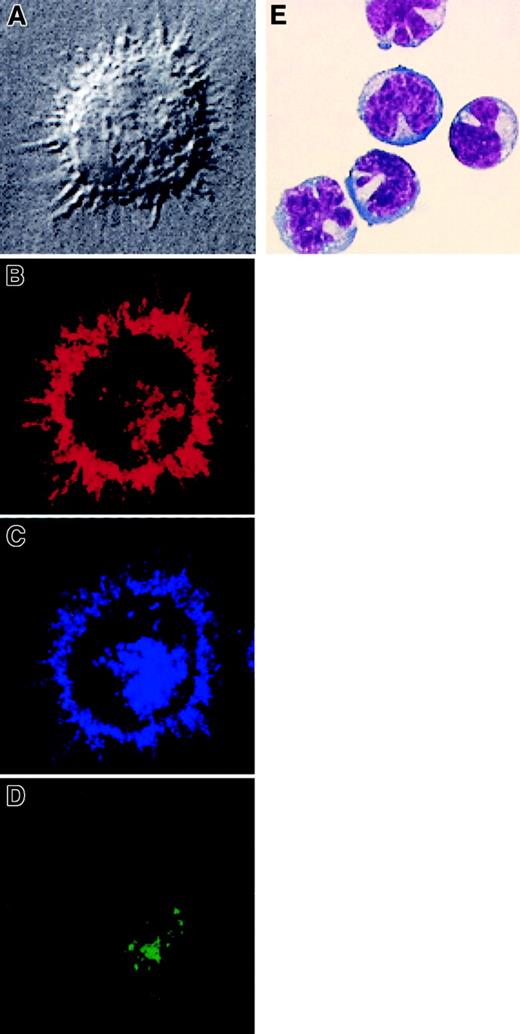
Morphology of the 2 blood DC subsets was assessed after culture in the presence of GM-CSF, IL-4, and CD40L for MDCs and IL-3 and CD40L for PDCs. MDCs from both HVs and patients showed large clusters with many long and fine dendrites (Figure 3 and data not shown), whereas freshly isolated PDCs displayed a characteristic lymphoid or plasma cell–like shape with no or fewer polarized dendrites, resembling the previously described plasmacytoid T cells (Figure 3E).2 Confocal microscopy of cultured MDCs isolated from blood of patients revealed their typical DC morphology (Figure 3).
Morphologic and confocal microscopy analysis of MDCs and PDCs isolated from AML patients.
CD11c+ MDCs were isolated from patient UPN71 and cultured in vitro for 3 days with GM-CSF, IL-4, and CD40L. (A) They display dendritic morphology as shown by interferential contrast transmission microscopy (×100). Three-color immunofluorescence staining was performed. (B) HLA-DR expression is shown in red, (C) CD83 in blue, and (D) DC-LAMP in green. (E) Giemsa staining of freshly isolated PDCs from patient UPN109. Original magnification × 1000.
Morphologic and confocal microscopy analysis of MDCs and PDCs isolated from AML patients.
CD11c+ MDCs were isolated from patient UPN71 and cultured in vitro for 3 days with GM-CSF, IL-4, and CD40L. (A) They display dendritic morphology as shown by interferential contrast transmission microscopy (×100). Three-color immunofluorescence staining was performed. (B) HLA-DR expression is shown in red, (C) CD83 in blue, and (D) DC-LAMP in green. (E) Giemsa staining of freshly isolated PDCs from patient UPN109. Original magnification × 1000.
Leukemic origin of circulating blood DC subsets
The leukemic origin of freshly isolated MDC and PDC populations from patients with AML was investigated by examining the presence of cytogenetic abnormalities by FISH experiments. MDCs and PDCs from 4 patients taken in both groups I and II (UPN79, UPN109, UPN156, and UPN223) displaying cytogenetic abnormalities on their leukemic blasts at diagnosis were analyzed. All freshly sorted MDCs and PDCs from these patients, with or without quantitative expansion of DC subsets, exhibited the initial cytogenetic abnormalities. Examples of MDCs from one AML patient with trisomy 8 at diagnosis (UPN156) are depicted in Figure 4. A red hybridization signal on all of the analyzed nuclei showed the presence of trisomy 8 on sorted MDCs. These results demonstrated the leukemic status of blood circulating DC subsets in patients with myeloid leukemia.
Leukemic status of MDCs and PDCs isolated from the blood of AML patients.
MDCs from patient UPN156 were sorted and analyzed by FISH for the presence of trisomy 8 using a specific probe for chromosome 8. This result is representative of 4 experiments performed with MDCs and PDCs isolated from 4 different patients.
Leukemic status of MDCs and PDCs isolated from the blood of AML patients.
MDCs from patient UPN156 were sorted and analyzed by FISH for the presence of trisomy 8 using a specific probe for chromosome 8. This result is representative of 4 experiments performed with MDCs and PDCs isolated from 4 different patients.
Impaired in vitro maturation of PDCs isolated from patients
Freshly sorted MDCs and PDCs isolated either from HVs or from leukemic patients were cultured for 3 days in the presence of GM-CSF and IL-4 for MDCs and IL-3 for PDCs. We examined their capacity to undergo maturation after either simultaneous in vitro culture with appropriate cytokines and CD40L for 3 days or 3 days with cytokines followed by 2 days of stimulation by CD40L. Phenotypic analysis indicated that both subsets isolated from HVs acquired the expression of CD83 and expressed the costimulatory molecules CD80 and CD86. The expression of HLA-DR was up-regulated, especially on the MDC subset (Figure 5A,C). In leukemic patients, the MDC subset could acquire CD83 and the costimulatory molecules CD80 and CD86 (Figure 5B). Acquisition of CD83 and distribution of HLA-DR molecules on leukemic MDCs were further confirmed by confocal microscopy staining (Figure 3B,C). Furthermore, confocal staining of MDCs indicated that they expressed DC-LAMP after maturation in culture (Figure 3D), which is a newly described DC-specific lysosomal marker specifically induced in maturing DCs.19 In contrast, PDCs from leukemic patients never acquired CD83 or costimulatory molecules, even after prolonged culture or with the addition of tumor necrosis factor-α (TNF-α; Figure 5D and data not shown). These results strikingly indicate that PDCs isolated from leukemic patients display altered capacities for maturation as compared with their healthy counterparts.
Maturation capacities of MDCs and PDCs isolated from patients after in vitro culture.
(A,C) The 2 DC subsets CD11c+ILT3+ (MDCs) and CD11c−ILT3+ (PDCs) were sorted from the blood of healthy individuals or (B,D) from leukemic patients and analyzed by flow cytometry after 72 hours of culture with either GM-CSF, IL-4, and CD40L (MDCs) or IL-3 and CD40L (PDCs) for their expression of DC marker CD83 and costimulatory molecules. (A) MDCs and (C) PDCs isolated from healthy volunteers acquired the expression of CD83 and expressed the costimulatory molecules (CD80 and CD86). (B) In leukemic patients, the MDC subset could acquire CD83 and the costimulatory molecules CD80 and CD86. (D) PDCs from leukemic patients never acquired CD83 or costimulatory molecules. Open histograms represent cells stained with isotype-matched control mAbs. Results indicated are representative of those obtained from 4 healthy donors and 5 patients.
Maturation capacities of MDCs and PDCs isolated from patients after in vitro culture.
(A,C) The 2 DC subsets CD11c+ILT3+ (MDCs) and CD11c−ILT3+ (PDCs) were sorted from the blood of healthy individuals or (B,D) from leukemic patients and analyzed by flow cytometry after 72 hours of culture with either GM-CSF, IL-4, and CD40L (MDCs) or IL-3 and CD40L (PDCs) for their expression of DC marker CD83 and costimulatory molecules. (A) MDCs and (C) PDCs isolated from healthy volunteers acquired the expression of CD83 and expressed the costimulatory molecules (CD80 and CD86). (B) In leukemic patients, the MDC subset could acquire CD83 and the costimulatory molecules CD80 and CD86. (D) PDCs from leukemic patients never acquired CD83 or costimulatory molecules. Open histograms represent cells stained with isotype-matched control mAbs. Results indicated are representative of those obtained from 4 healthy donors and 5 patients.
Functional properties of MDCs and PDCs from leukemic patients
MDCs and PDCs were assessed for their ability to stimulate naive CD4+ T cells in an allogeneic mixed lymphocyte reaction. Freshly isolated and 3-day–cultured MDCs obtained either from HVs or leukemic patients could efficiently stimulate the proliferation of naive CD4+ T cells (Figure6A and data not shown). As described previously,2 26 freshly isolated PDCs from HVs induced weak, if any, proliferation of naive allogeneic T cells (Figure 6B). After culture in the presence of IL-3 and CD40L, there was an increase in the allostimulatory activity of PDCs from HVs (Figure 6B). In marked contrast, even after culture, leukemic PDCs were inefficient to induce proliferative responses by naive T cells (Figure 6A,B).
T-cell stimulatory capacity of MDCs and PDCs isolated from AML patients.
MDCs and PDCs were sorted from the blood of healthy individuals or leukemic patients and cultured for 3 days in the presence of GM-CSF, IL-4, and CD40L (MDCs) or IL-3 and CD40L (PDCs). (A) Allostimulatory activity of 3-day–cultured MDCs and PDCs was monitored by their ability to induce the proliferation of CD4+ naive T cells. The ratio between the proliferative response measured by [3H]thymidine incorporation of 105 T cells induced by 3 × 103 leukemic MDCs and PDCs and normal MDCs and PDCs is represented. Results are represented as the mean of the ratio obtained from 4 experiments performed with DCs isolated from patients UPN109, UPN156, UPN223, and UPN90. (B) Allostimulatory activity of freshly isolated and 3-day–cultured PDCs. Graded numbers of freshly isolated PDCs from healthy donor (▵) or patient UPN109 (▴), or 3-day–cultured PDCs from healthy donor (○) or patient UPN109 (●), were cocultured with 105 CD4+naive T cells for 6 days. CD14+ monocytes (purified by magnetic separation using CD14 microbeads; Myltenyi Biotec GmbH, Bergisch Gladbach, Germany) from healthy donor (▪) were prepared and used as control stimulators. Representative data of 3 experiments are shown.
T-cell stimulatory capacity of MDCs and PDCs isolated from AML patients.
MDCs and PDCs were sorted from the blood of healthy individuals or leukemic patients and cultured for 3 days in the presence of GM-CSF, IL-4, and CD40L (MDCs) or IL-3 and CD40L (PDCs). (A) Allostimulatory activity of 3-day–cultured MDCs and PDCs was monitored by their ability to induce the proliferation of CD4+ naive T cells. The ratio between the proliferative response measured by [3H]thymidine incorporation of 105 T cells induced by 3 × 103 leukemic MDCs and PDCs and normal MDCs and PDCs is represented. Results are represented as the mean of the ratio obtained from 4 experiments performed with DCs isolated from patients UPN109, UPN156, UPN223, and UPN90. (B) Allostimulatory activity of freshly isolated and 3-day–cultured PDCs. Graded numbers of freshly isolated PDCs from healthy donor (▵) or patient UPN109 (▴), or 3-day–cultured PDCs from healthy donor (○) or patient UPN109 (●), were cocultured with 105 CD4+naive T cells for 6 days. CD14+ monocytes (purified by magnetic separation using CD14 microbeads; Myltenyi Biotec GmbH, Bergisch Gladbach, Germany) from healthy donor (▪) were prepared and used as control stimulators. Representative data of 3 experiments are shown.
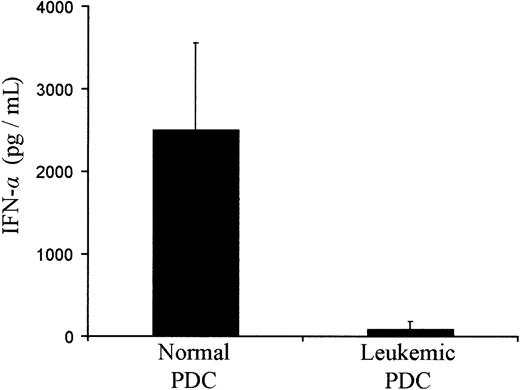
PDCs proved recently to be the main producers of type I IFN-α, an important cytokine in immune responses, after microbial challenge.4-6 27 To investigate the functional properties of PDCs from leukemic patients, we tested their capacity to produce IFN-α when stimulated with HSV. PDCs from HVs could produce large amounts of IFN-α. In contrast, PDCs from leukemic patients produced small amounts of IFN-α (25-fold less) compared with that produced by PDCs from HVs (Figure 7). Thus, decreased production of IFN-α in response to viral stimuli appears to be another prominent feature of PDCs from leukemic patients.
IFN-α production by PDCs isolated from healthy donors and AML patients after in vitro stimulation with HSV.
PDCs were sorted by FACS and stimulated with HSV without additional cytokine. Results represent the mean of cytokine concentrations contained in the culture supernatant obtained from 2 different donors and patients UPN90, UPN109, UPN156, UPN223, and UPN243.
IFN-α production by PDCs isolated from healthy donors and AML patients after in vitro stimulation with HSV.
PDCs were sorted by FACS and stimulated with HSV without additional cytokine. Results represent the mean of cytokine concentrations contained in the culture supernatant obtained from 2 different donors and patients UPN90, UPN109, UPN156, UPN223, and UPN243.
Discussion
Our results show that the 2 circulating DC subsets can be identified in the blood of patients with AML. No specific marker is currently available for positive identification of circulating DCs. A method based on a lin−/HLA-DR+ DC phenotype has been used to isolate circulating blood DCs.28,29 This approach is not applicable in patients with AML because leukemic cells can express HLA-DR. Furthermore, expression of HLA-DR on activated cells during illness may also complicate the issue, creating a potential for error when expression of HLA-DR is used to monitor blood DCs.21 In this study, ILT3 was the reference antibody because it has already been proved to be able to identify the 2 circulating DC subsets in fresh blood without any previous culture technique.6,17 However, our definition of leukemic MDCs and PDCs was not exclusively based on the expression of the ILT3 antigen. Leukemic MDCs and PDCs presented many specific features that could clearly distinguish them from leukemic blasts. Leukemic MDCs exhibited after culture a potent allostimulatory activity toward naive T cells. This allostimulatory activity of leukemic MDCs could also be demonstrated when we used freshly isolated normal and leukemic MDCs without any further differentiation or activation, in comparison with monocytes and leukemic blasts from the same patient. The ability to stimulate naive T cells is not a common feature of freshly isolated leukemic blasts. Exhaustive phenotypic analysis of the sorted DC subpopulations was performed using a large panel of cell-surface markers expressed by human DCs.30 These markers could distinguish MDCs and PDCs as populations distinct from the leukemic blasts. In addition, leukemic MDCs expressed DC-LAMP, which is a DC-specific lysosomal marker specifically induced in maturing DCs.19 This marker is not currently known to be expressed by fresh leukemic blasts. Although leukemic cells can express some markers of unknown significance, the high number of leukemic samples we studied (37 different AMLs) argues against an aberrant expression of the ILT3 antigen, which is another specific marker of antigen-presenting cells.6,17,31 On the morphologic level, leukemic MDCs and PDCs had a distinctive morphology. Although this feature is insufficient to define a DC, DCs were named initially because of their distinctive morphology, and leukemic MDCs in our study exhibited in vitro a typical dendritic morphology as soon as 24 hours after culture. This was not the case for leukemic blasts. On the other hand, freshly isolated leukemic PDCs exhibited a lymphoid or plasma cell–like shape, which could clearly distinguish them among blasts with common myeloid morphology. Moreover, these leukemic PDCs were shown to express lymphoid-associated pre-Tα transcript. At present, little is known about the origin of PDCs, although some evidence suggests their lymphoid origin. Recently, it has been shown that PDCs in the thymus20 and human committed DC precursors that are present in adult peripheral blood and thymus23,25 share with T-cell precursors the expression of pre-Tα mRNA.24We extended these observations by showing that the PDC subset we isolated from leukemic patients expressed pre-Tα transcript. The finding of a transcript associated with the T-cell differentiation pathway adds support to the putative lymphoid origin of the leukemic PDC subset we have analyzed. The latter finding is a major feature that can distinguish the PDC subset from leukemic blasts that are of myeloid origin in patients with AML. Taken together, the aforementioned features of leukemic MDCs and PDCs can rigorously help to define these cells toward the DC lineage.
Our results among 37 AML patients show an important quantitative imbalance for MDCs and PDCs. The clinical relevance of increased blood DC numbers is not yet clear. Recently, it has been proposed that PDCs might be responsible for maintaining peripheral T-cell tolerance to self-antigens.3 The balance of these cells in vivo influences the character of cell-mediated immune and inflammatory responses and may interfere in the mechanisms regulating antitumor immunity versus tolerance. FISH analysis confirmed that MDCs and PDCs had the same cytogenetic abnormalities expressed by freshly isolated leukemic cells. Therefore, DCs in leukemic patients may be part of the malignant clone, or “dendritopoiesis” is affected by the leukemic process. The latter does not exclude that normal MDC and PDC subsets without cytogenetic abnormalities might circulate in the blood of these leukemic patients. Because of the quantitative predominance of leukemic MDC and PDC subsets, such normal subsets could not be detected. Furthermore, at present, the different differentiation pathways of DCs are not clearly established. At this stage, though MDC and PDC development appears to be affected by the leukemic process, we cannot confirm whether circulating DCs in leukemic patients originate exclusively from the leukemic clone or from another stem cell with expression of leukemic cytogenetic markers.
The recognized characteristics of DCs include the ability to present antigens and to stimulate specific T-cell responses. Leukemic MDCs and PDCs might express at least some leukemia-related proteins associated with the cytogenetic abnormality, but this does not necessarily mean that they will be efficiently presented to T cells. The crucial role of costimulatory molecules in the generation of an antileukemic response has been shown in murine leukemia models.32,33 In the current study, we have demonstrated that PDCs from leukemic patients could not acquire CD80 and CD86. The same held true for HLA-DR, which was weakly expressed on leukemic PDCs after culture. On the other hand, MDCs could achieve increased expression of the costimulatory molecules, which correlated with their capacity to induce naive CD4+T-cell proliferation. This maturation process was associated with the expression of CD1a and CD83. These properties and markers helped to define the maturation pattern of these cells toward the DC lineage.30
Our results established a clear difference in the function of leukemic circulating DCs compared with their normal counterparts. Stimulation through the CD40 pathway failed to enhance the allostimulatory activity of PDCs from leukemic patients, which is compatible with the absence or low expression of HLA-DR and costimulatory molecules.
It has been shown recently that PDCs produce large amounts of type I IFN after microbial challenge.4-6,27 The in vivo effects of type I IFN are associated with promoting an antiviral state, including a broad spectrum of cellular targets.34 Type I IFN plays an essential role in antiviral immunity and is widely used to treat viral hepatitis and various types of malignancies, especially chronic myeloid leukemia.35 These effects appear to be due to direct inhibition of viral replication in infected cells and to the pleiotropic immunomodulating activity of type I IFN.35Type I IFN may act by enhancing the cytotoxic activity of natural killer cells and macrophages,35 by inducing T-cell activation,36 or by maintaining the survival of activated T cells.37 It has also been shown that IFN-α can induce Th1 development in human CD4+ T cells,38,39and HSV infection results in the development of IFN-γ–secreting CD4+ T cells with cytolytic activity.40Furthermore, type I IFN can induce the expression of TNF-related apoptosis-inducing ligand on T cells and thereby enhance T-cell cytotoxicity.41 Taken together, all of these findings illustrate the critical role of PDCs for linking innate and adaptive immune responses against tumors. The dramatically decreased secretion of IFN-α by leukemic PDCs would have serious consequences on the induction of antileukemic immune responses.
In summary, in AML patients, both circulating DC subsets exhibit quantitative abnormalities and exhibit leukemic cytogenetic features. The leukemic process, through interference with the DC system, appears to contribute to creating a state of profound immune suppression in AML patients. Although leukemic, MDCs retain the ability to differentiate, whereas PDCs show altered immunogenicity. The latter suggests that PDCs may play a key role in AML and other cancers where the immune system appears to be suppressed or otherwise “tolerized” to the tumors. Further studies are warranted to investigate the clinical relevance of altered circulating blood DC numbers in leukemic patients and the potential role of PDCs in the induction of tolerance to leukemic cells.
We thank N. Bendriss-Vermare (Schering-Plough, Laboratory for Immunological Research, Dardilly) for helpful discussions and L. Leserman (Centre d'Immunologie de Marseille Luminy), D. Blaise, D. Maraninchi (Institut Paoli-Calmettes), and C. Mawas (INSERM, Marseille) for their critical reading of the manuscript. We also thank R. Galindeau for assistance in cell sorting and S. Just-Landi and N. Baratier for excellent technical assistance.
Supported by grant “Mitjavile” from the Academie Nationale de Medecine, Paris, France (to M.M.).
D.J. has declared a financial interest in Beckman-Coulter, whose product was studied in the present work. D.J. was employed by Beckman-Coulter (Marseille) at the time of this study.
D.O. and B.G. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Béatrice Gaugler, Immunologie des Tumeurs, Institut Paoli-Calmettes, 232 Bd Ste Marguerite, 13273 Marseille Cedex 09, France; e-mail: gauglerb@marseille.fnclcc.fr.




![Fig. 6. T-cell stimulatory capacity of MDCs and PDCs isolated from AML patients. / MDCs and PDCs were sorted from the blood of healthy individuals or leukemic patients and cultured for 3 days in the presence of GM-CSF, IL-4, and CD40L (MDCs) or IL-3 and CD40L (PDCs). (A) Allostimulatory activity of 3-day–cultured MDCs and PDCs was monitored by their ability to induce the proliferation of CD4+ naive T cells. The ratio between the proliferative response measured by [3H]thymidine incorporation of 105 T cells induced by 3 × 103 leukemic MDCs and PDCs and normal MDCs and PDCs is represented. Results are represented as the mean of the ratio obtained from 4 experiments performed with DCs isolated from patients UPN109, UPN156, UPN223, and UPN90. (B) Allostimulatory activity of freshly isolated and 3-day–cultured PDCs. Graded numbers of freshly isolated PDCs from healthy donor (▵) or patient UPN109 (▴), or 3-day–cultured PDCs from healthy donor (○) or patient UPN109 (●), were cocultured with 105 CD4+naive T cells for 6 days. CD14+ monocytes (purified by magnetic separation using CD14 microbeads; Myltenyi Biotec GmbH, Bergisch Gladbach, Germany) from healthy donor (▪) were prepared and used as control stimulators. Representative data of 3 experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/13/10.1182_blood.v98.13.3750/6/m_h82411869006.jpeg?Expires=1765896190&Signature=xfcFjs49TNuxIC2lV4Y-pYXtYh3vwY3EzfHrMNsEa7Y7Y2HuJUw8aJv4-Uf-bfI2dVIuD5bXFwHPQXxIKMcPoDM9SmnlRJOxvoaCXHb~9MH--XLkA~gdi6Vry62lL8hJl3K8-YNCLL5LWORF2cwgqMs9jy2WJLaFjZe01pFzz0lv2SK~gLXf31IS-GQdIKJLSBo-v8RJJoBKdGSHhYS6qilFEZmuTjPeXDwpMJAIZcmc5pl8Xt5GlYDVqiSCZN7kHMnSPOgx~xxKvqTquCV46Bz9F51cIJ69niWsHsPj66lb9n6NA8aMwnbJtyJU6mMtii21Ln-7XD-lMz10gckYRg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal