Abstract
Primary infection with the human herpesvirus, Epstein-Barr virus (EBV), may result in subclinical seroconversion or may appear as infectious mononucleosis (IM), a lymphoproliferative disease of variable severity. Why primary infection manifests differently between patients is unknown, and, given the difficulties in identifying donors undergoing silent seroconversion, little information has been reported. However, a longstanding assumption has been held that IM represents an exaggerated form of the virologic and immunologic events of asymptomatic infection. T-cell receptor (TCR) repertoires of a unique cohort of subclinically infected patients undergoing silent infection were studied, and the results highlight a fundamental difference between the 2 forms of infection. In contrast to the massive T-cell expansions mobilized during the acute symptomatic phase of IM, asymptomatic donors largely maintain homeostatic T-cell control and peripheral blood repertoire diversity. This disparity cannot simply be linked to severity or spread of the infection because high levels of EBV DNA were found in the blood from both types of acute infection. The results suggest that large expansions of T cells within the blood during IM may not always be associated with the control of primary EBV infection and that they may represent an overreaction that exacerbates disease.
Introduction
Epstein-Barr virus (EBV) is a γ herpesvirus that persists for life in humans as a latent infection of B cells under the immunosurveillance of CD8+ αβ cytotoxic T lymphocytes (CTLs) (reviewed in Khanna and Burrows1). An interesting enigma in EBV biology is the variable nature of primary infection. At one extreme, seroconversion can occur asymptomatically, whereas at the other extreme, mild to severe clinical manifestations of the lymphoproliferative disease infectious mononucleosis (IM) develop. Little is known about the immunologic and virologic parameters of silent infection except that it occurs most commonly in early childhood2,3 without significant blood lymphocytosis and with reduced and delayed titers of viral antibodies.2 In contrast, the overt clinical signs of IM (fever, lymphadenopathy, tonsillitis, and splenomegaly) have provided an experimental window for studying this form of primary infection. This self-limiting disease is typified by an increased systemic viral load4-6 and, in common with many other acute viral infections (eg, human immunodeficiency virus [HIV], lymphocytic choriomeningitis virus [LCMV], simian immunodeficiency virus),7-10 by a vigorous immune response of proliferating CD8+αβ-expressing T cells.11 Tracking studies using EBV-specific major histocompatibility complex class 1 tetramers have confirmed that most of these T-cell expansions are virus specific.12 It is unclear, however, whether these T cells play a pathologic or a protective role in IM. Resolution of the disease and transition to the long-term state of virus carrier are accompanied by a decrease in the numbers of virus-specific cytotoxic T lymphocytes (CTLs).
Precisely what factors (eg, preimmune precursor CTLs, burst size, affinity maturation, viral load) govern the extent of CTL expansion development, not only in IM but in other acute viral infections, remains largely unresolved. Infectious dose and degree of viral spread (systemic vs localized infection) appear to be important. Size or clonal burst of the antigen-specific CD8+ T-cell expansions in acute LCMV infection are determined by antigen load alone,13 whereas productive influenza A infection, which is largely restricted to the lung, elicits lower numbers of CTLs.14 In EBV, viral load may be important—high levels of virus are characteristic of IM, but levels are substantially lower in the healthy virus carrier state,6 and viral load is a prognosticator of lymphoproliferative disease progression in patients who have undergone transplantation.15 In addition, strong antibody responses to viral replication antigens are seen in acute IM, consistent with increased levels of virus replication in the oropharynx, but they are only rarely found among healthy EBV-seropositive donors (reviewed in Rickinson and Kieff16). Indeed, it has previously been hypothesized that IM donors receive high doses of EBV at the time of virus exposure and that they mobilize greater T-cell responses to control infection.16
The variable clinical manifestations of acute EBV infection (asymptomatic vs mild to severe IM) offer an ideal opportunity to assess various virologic and immunologic parameters and their relation to the course of infection. Such comparative studies have been virtually unachievable, however, given the difficulty in finding donors without clinical symptoms at the time of EBV seroconversion. In the current study, a panel of patients undergoing silent seroconversion was serendipitously identified during screening for a phase 1 EBV vaccine trial. Using this unique cohort, we show that in contrast to the massive T-cell expansions elicited during the disease stage of acute IM, asymptomatic donors showed little evidence of blood repertoire perturbations. Furthermore, viral load studies revealed that this occurred despite high levels of circulating EBV at the time of seroconversion.
Materials and methods
Donors
Peripheral blood samples were taken from 3 different donor groups: healthy EBV seropositive control donors (designated HC), IM donors (designated IM), and asymptomatic donors undergoing primary EBV infection (designated AS). A panel of healthy donors was obtained by randomly selecting 6 healthy, HLA-unmatched subjects with lymphocyte counts, after Ficoll-Hypaque (Pharmacia Biotechnology, Melbourne, Australia) separation (see below), ranging from 1 to 2 × 106 cells/mL whole blood. From one of the HC donors, 2 consecutive samples were taken several months apart to test for T-cell repertoire stability. IM donors were initially bled (day 0) during the first 3 to 7 days of illness and were classified on clinical symptoms and serologic profiles consistent with early acute infection (immunoglobulin M+ [IgM+] viral capsid antigen [VCA] and IgG− Epstein-Barr nuclear antigen [EBNA]). AS donors were serendipitously identified and exhibited serologic profiles consistent with early primary EBV infection (IgM+ VCA and IgG− EBNA) in the absence of clinical symptoms. AS donors were also prospectively surveyed to confirm that IM symptoms did not subsequently develop. In IM and AS donors, the transition from primary to persistent infection was indicated by serology (IgM− VCA; IgG+EBNA, IgG+VCA, or both). Note that in some patients with past EBV infection, EBNA IgG antibodies do not develop. Specific descriptions of the IM and AS donors are outlined in Table1. Peripheral blood mononuclear cells (PBMCs) were isolated from sodium-heparinized blood by centrifugation over Ficoll-Hypaque (Pharmacia Biotechnology). CD4+ and CD8+ lymphocyte levels within PBMCs were determined by FACS analysis using anti–human CD4 monoclonal antibody (mAb) conjugated with fluorescein isothiocyanate (clone SK4; Becton Dickinson, Sydney, Australia) and anti–human CD8 mAb conjugated with phycoerythrin (clone SK1; Becton Dickinson).
IM and AS donor descriptions
| Donor . | Day(s) after diagnosis . | PBMC count† . | CD8 (%)‡ . | CD4 (%)1-153 . | Anti-VCA IgM . | Anti-VCA IgG . | Anti-EBNA IgG . | Health status . | Infection stage1-155 . |
|---|---|---|---|---|---|---|---|---|---|
| IM1 | 0 | 6 × 106/mL | 77 | 8 | + | − | − | IM symptoms | Primary |
| 132 | 1 × 106/mL | ND | ND | − | + | + | Recovery | Persistent | |
| IM2 | 0 | 2.3 × 106/mL | 70 | 24 | + | + | − | IM symptoms | Primary |
| 115 | 1 × 106/mL | ND | ND | − | − | + | Recovery | Persistent | |
| IM3* | 0 | 10 × 106/mL | 67 | 24 | + | − | − | IM symptoms | Primary |
| IM4* | 0 | 6 × 106/mL | 53 | 7 | + | + | − | IM symptoms | Primary |
| AS1 | 0 | 1 × 106/mL | ND | ND | + | + | − | Asymptomatic | Primary |
| 300 | 1 × 106/mL | ND | ND | − | + | − | Asymptomatic | Persistent | |
| AS2 | 0 | 0.8 × 106/mL | 32 | 14 | + | + | − | Asymptomatic | Primary |
| 73 | 1.6 × 106/mL | 48 | 24 | − | + | − | Asymptomatic | Persistent | |
| AS3 | 0 | 1.5 × 106/mL | 27 | 53 | + | − | − | Asymptomatic | Primary |
| 17 | 1.2 × 106/mL | 28 | 45 | + | − | − | Asymptomatic | Primary | |
| AS4* | 0 | 2 × 106/mL | ND | ND | + | − | − | Asymptomatic | Primary |
| Donor . | Day(s) after diagnosis . | PBMC count† . | CD8 (%)‡ . | CD4 (%)1-153 . | Anti-VCA IgM . | Anti-VCA IgG . | Anti-EBNA IgG . | Health status . | Infection stage1-155 . |
|---|---|---|---|---|---|---|---|---|---|
| IM1 | 0 | 6 × 106/mL | 77 | 8 | + | − | − | IM symptoms | Primary |
| 132 | 1 × 106/mL | ND | ND | − | + | + | Recovery | Persistent | |
| IM2 | 0 | 2.3 × 106/mL | 70 | 24 | + | + | − | IM symptoms | Primary |
| 115 | 1 × 106/mL | ND | ND | − | − | + | Recovery | Persistent | |
| IM3* | 0 | 10 × 106/mL | 67 | 24 | + | − | − | IM symptoms | Primary |
| IM4* | 0 | 6 × 106/mL | 53 | 7 | + | + | − | IM symptoms | Primary |
| AS1 | 0 | 1 × 106/mL | ND | ND | + | + | − | Asymptomatic | Primary |
| 300 | 1 × 106/mL | ND | ND | − | + | − | Asymptomatic | Persistent | |
| AS2 | 0 | 0.8 × 106/mL | 32 | 14 | + | + | − | Asymptomatic | Primary |
| 73 | 1.6 × 106/mL | 48 | 24 | − | + | − | Asymptomatic | Persistent | |
| AS3 | 0 | 1.5 × 106/mL | 27 | 53 | + | − | − | Asymptomatic | Primary |
| 17 | 1.2 × 106/mL | 28 | 45 | + | − | − | Asymptomatic | Primary | |
| AS4* | 0 | 2 × 106/mL | ND | ND | + | − | − | Asymptomatic | Primary |
ND indicates not done.
No follow-up bleed was available.
Normal range for healthy control donors, 1-2 × 106 cells/mL blood.
Normal range for healthy control donors, 19% to 48% PBMCs.
Normal range for healthy control donors, 28% to 58% PBMCs.
Based on serologic profile (see “Materials and methods”).
T-cell receptor Vβ amplification and repertoire diversity analysis
PBMCs (1-2 × 106) were used for RNA extraction (Total RNA Isolation Reagent; Advanced Biotechnologies, London, United Kingdom). First-strand cDNA was synthesized using an antisense TCR Cβ primer (Cb1), as described previously.17 TCR Vβ-rearranged sequences were amplified with each of 26 different 5′ Vβ-specific primers (Vβ1-5.1, Vβ5.2-25) and a 3′ TCR Cβ constant primer.18 19 Amplifications were performed in 25-μL reactions using 0.5 μL cDNA, 5 pmol each primer, 200 μM dNTP, 1.5 mM MgCl2, 1.25 U Taq polymerase (Ampli-Taq Gold), and a GeneAmp PCR 9600 system (Perkin-Elmer Cetus, Norfolk, CT). Polymerase chain reaction (PCR) conditions consisted of an initial denaturation at 95°C for 9 minutes followed by 35 cycles of denaturation at 95°C for 15 seconds, annealing at 55°C for 40 seconds, extension at 72°C for 40 seconds, and a final extension at 72°C for 5 minutes.
The technique of CDR3 length determination and distribution to analyze TCR repertoire diversity is based on the methodology described previously.20 TCR Vβ PCR products were labeled with a nested 3′-FAM fluorophore-labeled primer specific for the TCR Cβ gene (CβP*: 5′-FAM-TTCTGATGGCTCAAACAC-3′; Research Genetics, Huntsville, AL) in a PCR run-off reaction. PCR conditions were identical to those described above except that 8% of TCR Vβ product was used as a template for 7 cycles of elongation (run-off) and a 5-minute final extension at 72°C. Fluorescent PCR run-off products were heat-denatured at 95°C for 5 minutes and were separated on a 6% acrylamide gel together with size standards (Genescan-1000 Rox; Applied Biosystems, Brisbane, Australia) on an Applied Biosystems 373A DNA sequencer. Data were processed using the Genescan Analysis 2.1 Software (Applied Biosystems), which records the fluorescence intensity in each peak. CDR3 length, defined by Chothia et al,21 was deduced from the fragment size.
Statistical analysis
CDR3 expansion was only considered significant if it satisfied the following criteria—at least a 2-fold magnitude change from the acute to the persistent infection phase and probability indicating statistical significance (P < .05) against a control background. The control background was calculated from data generated from the HC donors.
T-cell receptor V-D-J junctional region sequencing
Qiaex-purified TCR Vβ PCR products were ligated into the pGEM-T Vector System (Promega, Madison, WI) and were used to transform Epicurian Coli Sure Competent Cells (Stratagene, La Jolla, CA) according to the respective manufacturers' instructions. Plasmid inserts were amplified by PCR and were sequenced in both directions using the respective Vβ primers and Cβ with a Prism Ready Reaction Dyedeoxy Terminator Cycle Sequencing Kit and an ABI377 DNA sequencer (Applied Biosystems). Monoclonal expansions were also identified by direct sequencing of TCR Vβ PCR products, which showed that only a single nucleotide sequence was amplified.
Semiquantitative determination of EBV DNA load by polymerase chain reaction–enzyme-linked immunosorbent assay
EBV DNA load was determined by amplification of a 304-bp segment of the BMLF1 region of the EBV genome using 5′ and 3′ primers, GTCAACCAACAAGGACACAT and CACCACCTTGTTTTGACGGG, respectively.22 The oligonucleotide probe, CCGCGGGAGCTAGGGGCAGG, specific for an internal region of the 304-bp product,22 was biotinylated at the 5′ end during synthesis. Amplification of the BMLF1 region was carried out in a 25-μL reaction consisting of Ampgold buffer (Perkin-Elmer Cetus), 2 mM MgCl2, 0.5 μM each primer, and 200 μM dATP, dCTP, dGTP, 5.7 μM dUTP, 0.3 μM digoxigenin-dUTP (Boehringer-Mannheim, Castle Hill, Australia), 2.5 U Amplitaq Gold (Perkin-Elmer Cetus), and 100 ng genomic DNA as template. Genomic DNA was extracted (Qiagen Blood Kit; Qiagen, Clifton Hill, Australia) from donor PBMCs and from the EBV-positive cell line, Raji, which harbors 50 copies of EBV per cell. Raji DNA was added to 100 ng EBV seronegative PBMC DNA such that EBV copy numbers ranged from 1000 copies to 7.8 copies in doubling dilutions. These standards were used in the same PCR and acted as reference standards to determine EBV DNA load, which then was quantitatively assessed by using a PCR–enzyme-linked immunosorbent assay kit (DIG-detection; Boehringer-Mannheim) according to the manufacturer's instructions. All samples were made in a masked fashion, and in duplicate and negative controls they included water and PBMCs from an EBV-seronegative donor (EBV− PBMC) and from the EBV− B-cell line BJAB−.
Results
Presence of blood lymphocytosis in infectious mononucleosis but not AS donors
Primary infection with EBV can occur either asymptomatically, when only serologic evidence confirms infection, or symptomatically, when serology and clinical symptoms consistent with IM confirm diagnosis. The current study compares several features of the asymptomatic and symptomatic states. Four IM and 4 AS donors were included in the analysis. Peripheral blood samples from patients with IM were taken within 3 to 7 days of the onset of clinical symptoms (day 0). Consistent with early acute infection, a serologic pattern of primary infection (IgM+ VCA and IgG− EBNA) was detectable in IM and AS donors at the time of initial sampling (day 0) (Table 1). For IM donors, the transition from primary to persistent infection was accompanied by resolution of disease, and for IM and AS donors it was accompanied by the disappearance of anti-VCA IgM antibodies and the emergence of either an EBNA-specific or a VCA-specific IgG response (Table 1). Total blood lymphocyte counts differed significantly between IM and AS donors during the seroconversion period. IM donors displayed striking lymphocytosis and elevated levels of CD8+ lymphocytes at day 0 that were observed to resolve to normal total lymphocyte levels (≤2 × 106/mL) on disease recovery (Table 1). In contrast, primary infection in the 4 AS seroconvertants occurred in the absence of significant lymphocytosis, with PBMC levels and CD8+ lymphocyte counts falling within the normal range (Table 1). Donor AS2 did show unusually low levels of circulating CD4+ lymphocytes (Table 1), but this was not associated with EBV seroconversion because the decrease was consistently observed on serial measurements taken before day 0 sampling, when the donor was EBV seronegative (data not shown).
Major T-cell receptor expansions are common in infectious mononucleosis
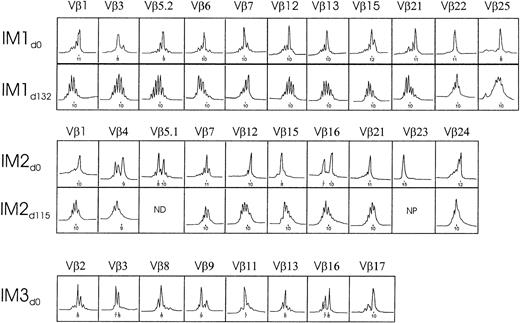
To determine the global impact of EBV infection on PBMC repertoire diversity, PBMCs from 3 donors undergoing acute symptomatic infection were subjected to TCR analysis by immunoscope. This is a PCR-based technique that determines the distribution of CDR3 lengths within a given TCR Vβ family. Gaussian-shaped profiles represent a diverse array of clonotypes of varying CDR3 lengths, whereas oligoclonal peak profiles have been shown to represent clonally expanded T cells (reviewed in Pannetier et al23). Because the uniquely rearranged CDR3 region directly contacts the peptide antigen,24,25 this technique provides a sensitive indicator of antigen-specific clonal expansion. CDR3 profiles were compared between primary infection and after disease recovery to confirm whether they represented EBV-induced changes or formed part of the normal TCR repertoire for the patient. Because the Vβ repertoire of CD8+ cells26 and CD8+CD45RO+ memory cells27 in healthy adults is often skewed, presumably as a result of previous antigen challenge (eg, after EBV infection),28 repertoire perturbations were monitored at the PBMC level, which rarely showed any deviation from a polyclonal, Gaussian-shaped profile. As shown in Figure1, dramatic distortions in repertoire distribution were evident for at least 50% of the TCR Vβ families for each of the IM donors. These perturbations represented at least a 2-fold magnitude change and were statistically significant (P < .05) against a normal control background. For donor IM3, in whom a follow-up bleed was unavailable, expansions were confirmed based on statistical significance to normal control background levels. Overall, as expected for an HLA-unmatched cohort of IM donors, each donor exhibited a largely unique pattern of Vβ expansions of a particular CDR3 length that were found to resolve to normal Gaussian-like TCR profiles after disease recovery.
TCR Vβ repertoire analysis of PBMCs from IM donors during acute infection and recovery.
Depicted are the Vβ values of the known 1 to 25 families with significant expansions. Significance was based on at least a 2-fold magnitude change and P < .05 against a control background (see “Materials and methods”). Profiles are displayed of fluorescence intensity (x-axis, arbitrary units) as a function of CDR3 size (y-axis, amino acids). CDR3 peaks are spaced 3 nucleotides apart. ND indicates not done; NP, not present.
TCR Vβ repertoire analysis of PBMCs from IM donors during acute infection and recovery.
Depicted are the Vβ values of the known 1 to 25 families with significant expansions. Significance was based on at least a 2-fold magnitude change and P < .05 against a control background (see “Materials and methods”). Profiles are displayed of fluorescence intensity (x-axis, arbitrary units) as a function of CDR3 size (y-axis, amino acids). CDR3 peaks are spaced 3 nucleotides apart. ND indicates not done; NP, not present.
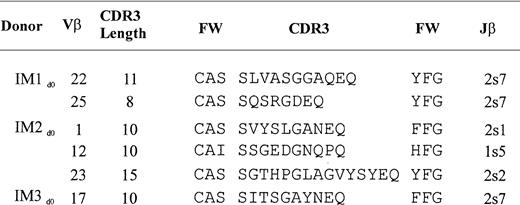
A single expanded peak in any given Vβ profile may represent either a monoclonal expansion of a single TCR clonotype or an oligoclonal expansion of different TCR clonotypes. To define the clonal nature of the observed perturbations, the TCR V-D-J junctional region sequences of recombinant clones that contained select TCR Vβ inserts from the 3 IM donors were examined (Figure 2). Expansions of Vβ 22 and 25 from IM1, Vβ 1, 12, and 23 from IM2, and Vβ 17 from IM3 were found to be clonal, indicating that the expansions resulted from antigen-driven T-cell selection.
TCR clonotype sequences from acute IM donors.
TCR CDR3 region sequences of selected Vβ expansions that were found by direct sequencing and recombinant cloning to be monoclonal (see “Materials and methods”). TCR Vβ and Jβ gene elements were assigned according to Arden et al52 and Toyonaga et al,53 respectively. FW signifies framework branches that putatively support the CDR3 region. Nucleotide sequences of each these clones are available from EMBL/GenBank/DDBJ under accession numbersAJ308532 to AJ308537.
TCR clonotype sequences from acute IM donors.
TCR CDR3 region sequences of selected Vβ expansions that were found by direct sequencing and recombinant cloning to be monoclonal (see “Materials and methods”). TCR Vβ and Jβ gene elements were assigned according to Arden et al52 and Toyonaga et al,53 respectively. FW signifies framework branches that putatively support the CDR3 region. Nucleotide sequences of each these clones are available from EMBL/GenBank/DDBJ under accession numbersAJ308532 to AJ308537.
Major T-cell receptor expansions are uncommon in asymptomatic infection
To investigate whether TCR repertoire perturbations eventuate in the absence of a detectable lymphocytosis, the PBMC repertoires of the 4 donors undergoing asymptomatic primary infection were assessed by immunoscope. In stark contrast to the dramatic and selective expansions observed in IM, most asymptomatic donors showed Gaussian-like profiles that remained unperturbed from the primary to the persistent stage of infection (Figure 3). Occasionally, minor repertoire perturbations were observed, but, despite lowering the cut-off criteria from a 2-fold to a 1.75-fold change in magnitude, these were not found to be statistically significant. The exception was donor AS4, who exhibited 6 Vβ families that proved to be significantly skewed (P < .05). The TCR V-D-J junctional sequencing showed that several of these represented clonal expansions (Figure 4).
TCR Vβ repertoire analysis of PBMCs from asymptomatic donors during acute and persistent infection.
Depicted are those Vβ values of the 1 to 25 known families who demonstrated a 2-fold magnitude change (see “Materials and methods”). Expansions that were statistically significant (P < .05) are indicated by an asterisk. Profiles are displayed of fluorescence intensity (x-axis, arbitrary units) as a function of CDR3 size (y-axis, amino acids). All CDR3 peaks are spaced 3 nucleotides apart.
TCR Vβ repertoire analysis of PBMCs from asymptomatic donors during acute and persistent infection.
Depicted are those Vβ values of the 1 to 25 known families who demonstrated a 2-fold magnitude change (see “Materials and methods”). Expansions that were statistically significant (P < .05) are indicated by an asterisk. Profiles are displayed of fluorescence intensity (x-axis, arbitrary units) as a function of CDR3 size (y-axis, amino acids). All CDR3 peaks are spaced 3 nucleotides apart.
TCR clonotype sequences from acute asymptomatic donor AS4.
TCR CDR3 region sequences of selected Vβ expansions that were found by direct sequencing and recombinant cloning to be monoclonal (see “Materials and methods”). TCR Vβ and Jβ gene elements were assigned according to Arden et al52 and Toyonaga et al,53 respectively. FW signifies framework branches that putatively support the CDR3 region. Nucleotide sequences of each of these clones are available from EMBL/GenBank/DDBJ under accession numbers AJ308538 to AJ308539.
TCR clonotype sequences from acute asymptomatic donor AS4.
TCR CDR3 region sequences of selected Vβ expansions that were found by direct sequencing and recombinant cloning to be monoclonal (see “Materials and methods”). TCR Vβ and Jβ gene elements were assigned according to Arden et al52 and Toyonaga et al,53 respectively. FW signifies framework branches that putatively support the CDR3 region. Nucleotide sequences of each of these clones are available from EMBL/GenBank/DDBJ under accession numbers AJ308538 to AJ308539.
High levels of EBV genomes circulate in asymptomatic primary infection
An important virologic parameter that may regulate the clinical outcome and the expansion or mobilization of T cells during primary EBV infection is viral load. To investigate whether the absence of major T-cell expansions during subclinical primary infection was associated with a low-level underlying viral infection, EBV genome copy number per microgram total cellular DNA was determined in PBMCs by semiquantitative PCR-ELISA. Three asymptomatic seroconvertants (AS1, AS3, AS4), 3 IM donors (IM1, IM3, IM4), and 3 seropositive healthy control donors (HC1, HC2, HC3) were assessed (Figure5). Consistent with previous reports, viral genomes were low or undetectable in healthy control donors, reflective of low-level latent infection,6 29 whereas the level of circulating EBV DNA was 500- to 1000-fold increased in those with IM. Surprisingly, very high viral loads were also detected in 2 (AS1, AS4) donors undergoing asymptomatic primary EBV infection, and the third (AS3), though not as intense, showed levels well above (greater than 70-fold) those of background healthy controls. These unexpectedly high levels of EBV DNA fell to undetectable levels on establishment of persistent latent infection. Overall, the data provide compelling evidence of high systemic EBV DNA levels in silent primary infection despite the lack of detectable peripheral T-cell expansions.
EBV load.
In peripheral blood of IM patients (IM), asymptomatic seroconvertants (AS), healthy control donors (HC), and negative controls (water, EBV− PBMC, BJAB−). EBV genome copies were estimated by semiquantitative BMLF1 PCR-ELISA on PBMCs (see “Materials and methods”). UN indicates undetectable (less than 0). Specific descriptions of the IM and AS donors are outlined in Table 1.
EBV load.
In peripheral blood of IM patients (IM), asymptomatic seroconvertants (AS), healthy control donors (HC), and negative controls (water, EBV− PBMC, BJAB−). EBV genome copies were estimated by semiquantitative BMLF1 PCR-ELISA on PBMCs (see “Materials and methods”). UN indicates undetectable (less than 0). Specific descriptions of the IM and AS donors are outlined in Table 1.
Discussion
This report highlights new findings regarding the biology of primary EBV infection. First, in contrast to acute IM, subclinical infection does not evoke a massive peripheral T-cell response, as evidenced by the lack of significant blood lymphocytosis and selective expansions of Vβ-expressing T cells. Second, high levels of systemic viral infection are common in both forms of primary infection despite the stark disparity in peripheral blood repertoire profiles.
The impressive mobilization of activated CD8+ T cells in response to acute EBV infection is well documented in IM. Consistent with the results of this study, selective expansions of dominant Vβ-expressing T cells are a feature of the acute immune response and do not persist after recovery from the disease.30 As much as 50% of the Vβ blood repertoire is distorted during the symptomatic phase, which supports the known multispecificity of CTL epitope reactivities in acute IM.18,31-33 Furthermore, many of these expansions are clonal in composition, a strong indicator of antigen-driven amplification rather than the result of bystander or superantigen-induced T-cell activation. Indeed, findings from major histocompatibility complex class I tetramer studies have confirmed that most of these expansions are reactive to viral antigens12and that their decline in numbers is paralleled by a drop in EBV genome load within the blood during disease recovery.34 What is unclear is whether the magnitude of lymphocytosis and virus-specific CTL response is directly linked to viremia level during the primary infection period. Our unexpected observation that donors undergoing subclinical primary EBV infection were heavily infected with the virus yet usually showed no evidence of major expansion of virus-specific T cells when compared with IM argued against this simple linear relationship. Moreover, the notion that massive CTL expansions are linked with widespread (not localized) viral infection is unlikely, at least in the subclinical EBV setting, in view of the abundance of virus within the circulation. Interestingly, in acute HIV infection, which also shows a mononucleosislike syndrome of variable severity, the extent of peripheral blood T-cell perturbations is also independent of initial viral load levels.35-37 However, the relation between these parameters changes once persistent infection is established, with HIV-specific CTL frequencies and viral load becoming inversely correlated38 and increased plasma viremia becoming a prognosticator of faster disease progression.39 40
An important correlate with disease development in IM appears to be the development of a significant blood CD8+ T-cell lymphocytosis. Indeed, patients who undergo silent seroconversion have normal levels of blood mononuclear cells, whereas elevated levels are characteristic of symptomatic seroconversion. A similar observation is reported in children who asymptomatically contract EBV for the first time; transient lymphocytosis is noted in only a small number, and most demonstrate no hematologic disturbances.2 Although more quantitative studies are needed that measure the degree and duration of lymphocytosis with the severity of clinical symptoms, it appears that the severity of immunopathology in IM is modulated by participating T-cell numbers. In support, the disease course of IM closely parallels the lymphoproliferative phase in which activated T cells appear in the blood.41 Furthermore, our recent discovery that EBV-specific T cells cross-react with self-antigens strengthens the possibility that such cross-reactions in abundance could lead to lysis of uninfected host cells, thereby contributing to disease exacerbation.19 Also of likely importance are the as yet unidentified homeostatic regulatory mechanisms that bring the T-cell response under control. Interestingly, the presence of T-cell perturbations is not necessarily linked to increased numbers of T cells within the circulation. For example, dominant TCR expansion profiles are documented in PBMCs of HIV-infected patients without elevated levels of CD8+ cells.37 In the current study, major T-cell expansions were evident despite the absence of blood lymphocytosis in one of the asymptomatic donors studied (donor AS4; Figure 3). Therefore, homeostatic T-cell control may be maintained in some instances during active stimulation of an antiviral T-cell response. Clearly, our studies suggest that the magnitude of the CD8+ response to EBV is likely to be considerably lower in the asymptomatic form of primary EBV infection. Yet these donors responded to high levels of systemic virus infection without having clinical symptoms. Strong EBV-specific memory CTL responses to commonly targeted epitopes within several viral latent gene products have been detected in infants who previously had asymptomatic EBV infection.42 It remains to be seen whether the primary asymptomatic T-cell response is qualitatively different (eg, in breadth or specificity of CTL reactivity) than that in IM.
What then triggers the development of T-cell lymphocytosis and disease during primary EBV infection? It is highly likely that interdependent host and virologic factors are involved in the regulation of disease development. Polymorphisms of the interleukin-1 (IL-1) gene complex43 and of the IL-10 gene44have been linked to susceptibility to EBV infection and, in the case of IL-10, to lymphoproliferative disease severity. Similarly, disease severity after respiratory syncytial virus infection appears to be associated with the IL-8 gene region,45 and elevated serum levels of Fas ligand are associated with the asymptomatic stage of HIV infection.46 An important virologic parameter controlling the level of T-cell expansion development may be the type of life cycle adopted by the virus in vivo. EBV can establish latent or lytic infection, and the virus has evolved several gene regulatory mechanisms (eg, transcriptional, posttranscriptional, and posttranslational) for controlling its own gene expression (reviewed in Kieff47). Therefore, the tantalizing possibility exists that EBV establishes a different form of infection or pattern of gene expression in IM than it does during asymptomatic seroconversion. For instance, the high viral load observed in silent primary infection might result from an increase in the number of latently infected B cells in the blood, whereas in IM there may be more virus replication. Significantly, notable features of IM are the presence of lytically infected B cells within the blood,48 high titers of antibodies against lytic cycle proteins,16 and high amounts of free virus DNA in the serum.49,50 Furthermore, studies monitoring CTL responses during acute IM have established that a sizable proportion of the total CD8+ response is directed toward lytic rather than latent antigen recognition.12,32 Additional support for lytic infection, possibly contributing to disease etiology in IM, is the isolation of an EBV strain with an increased propensity for lytic rather than latent B cell infection from a patient with severe chronic active infection.51
In conclusion, we have shown that the expansion of T cells in response to acute EBV infection differs depending on whether the infection progresses without clinical symptoms or develops into IM. Furthermore, the development of large peripheral T-cell expansions during IM—though largely virus specific, as shown by previous studies—are not necessary for controlling the high load of virus-infected cells in the blood during the acute phase. In this context, T cells may become immunopathogenic when expanded beyond homeostasis in IM. The mobilization of large expansions of virus-specific CTLs that contribute to pathologic conditions during infection appear counterproductive, but possibly they arise as a result of host genetic and virologic life cycle differences that effect the activation and expansion of antigen-specific T cells.
We thank Andrew Rosenstengel and Stephanie Pye for their valuable technical assistance with the EBV phase 1 vaccine trial.
Supported by grants from the National Health and Medical Research Council of Australia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sharon L. Silins, Queensland Institute of Medical Research, PO Box Royal Brisbane Hospital, Australia 4029; e-mail:sharons@qimr.edu.au.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal